Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
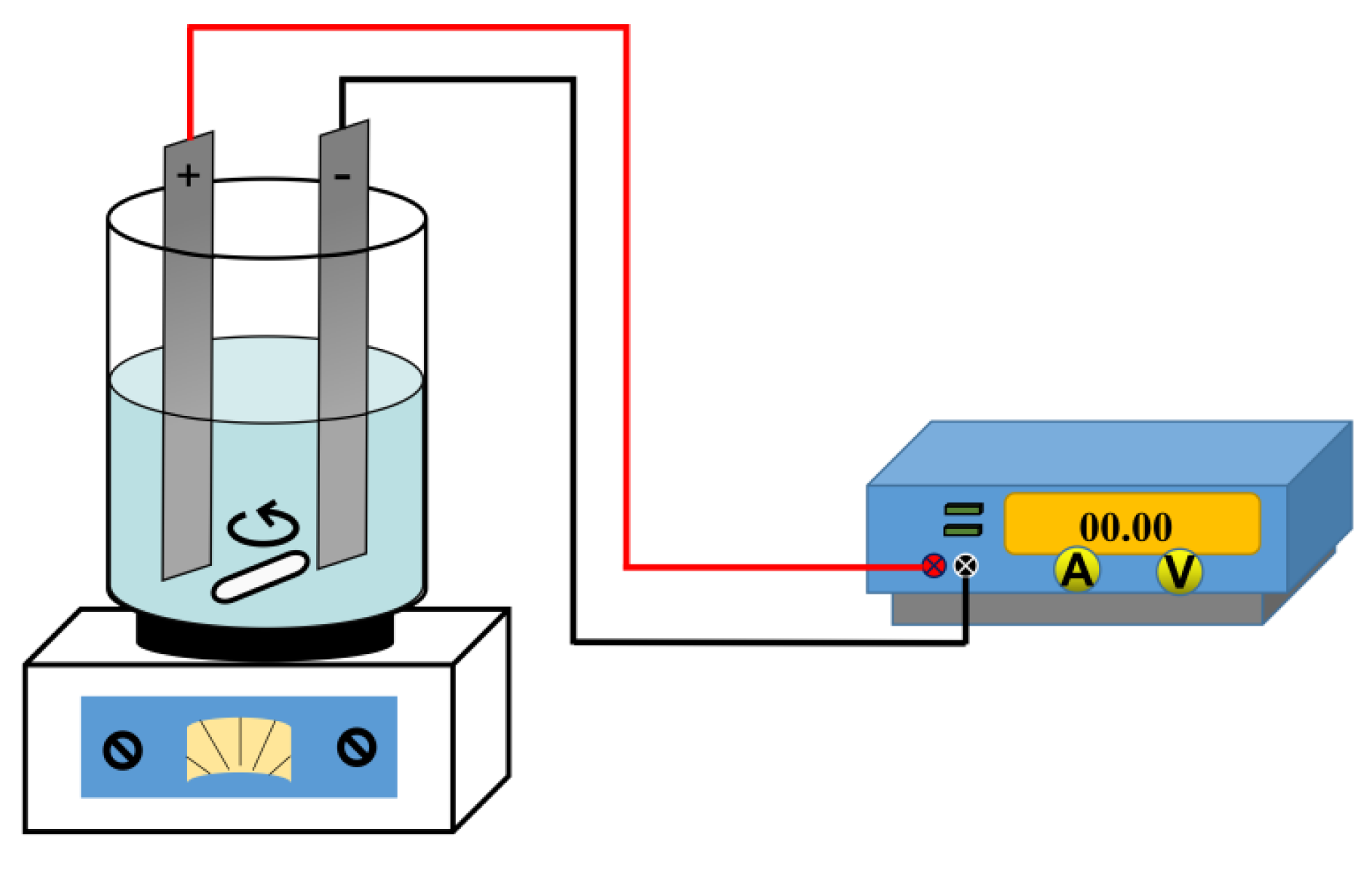
2.2. Electric Enhanced Washing
2.3. Total Heavy Metal Content
2.4. Toxicity Characteristic Leaching Procedure
2.5. Sequential Extraction Procedure
2.6. Characterization
3. Results and Discussion
3.1. Characteristics of MSWI Fly Ash
3.2. Characteristics of Heavy Metals in MSWI Fly Ash
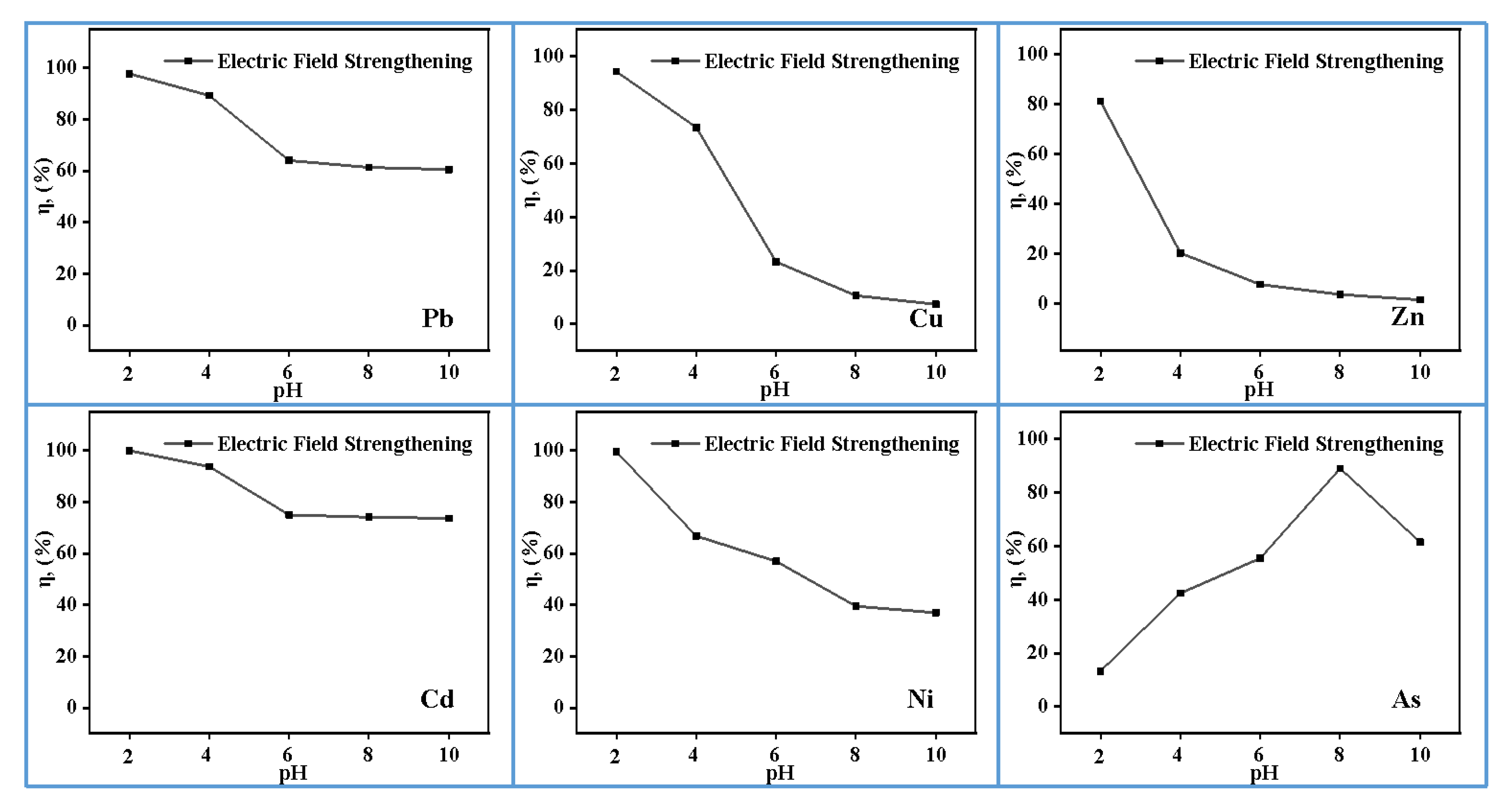
3.3. Effect of pH
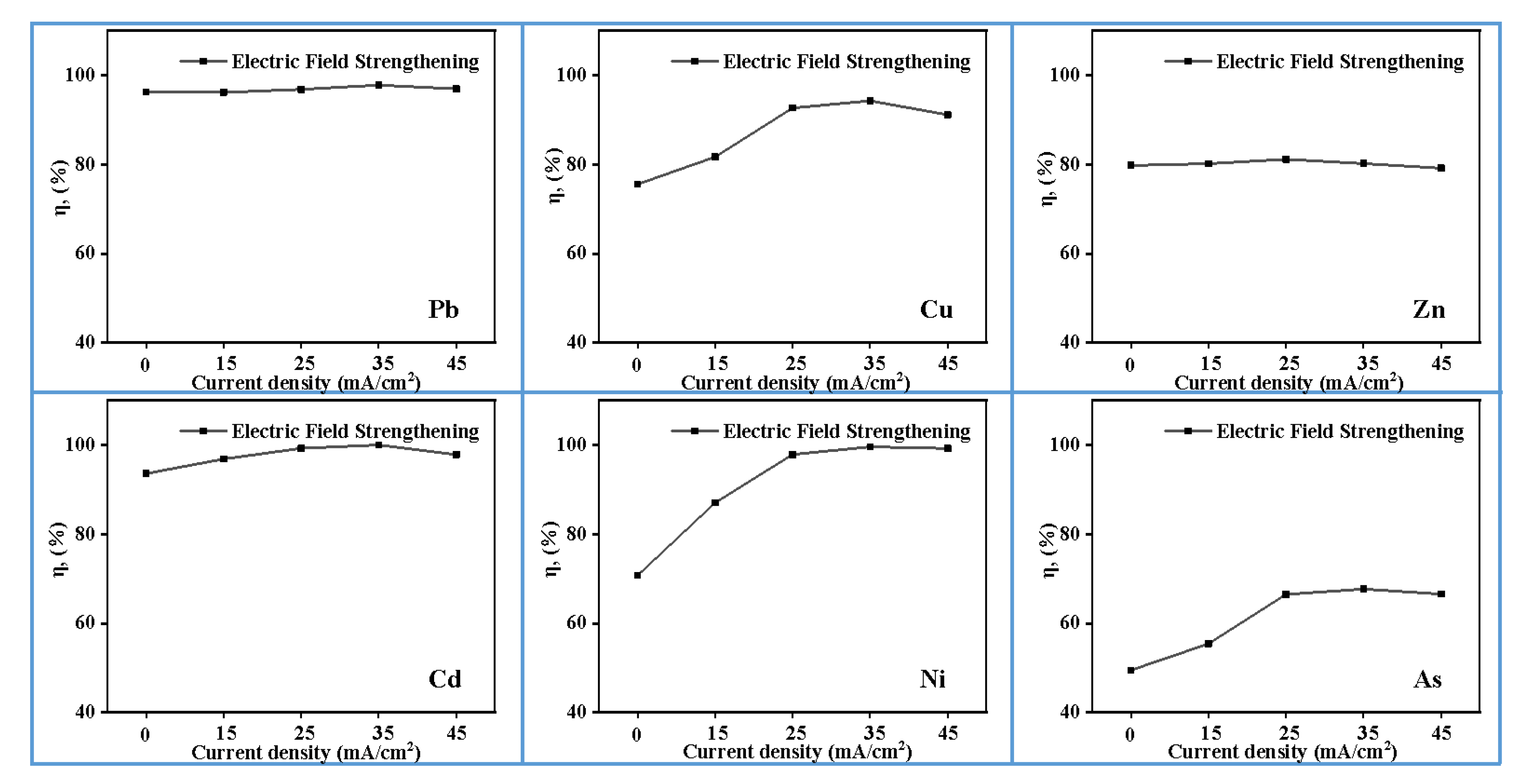
3.4. Effect of Current Density
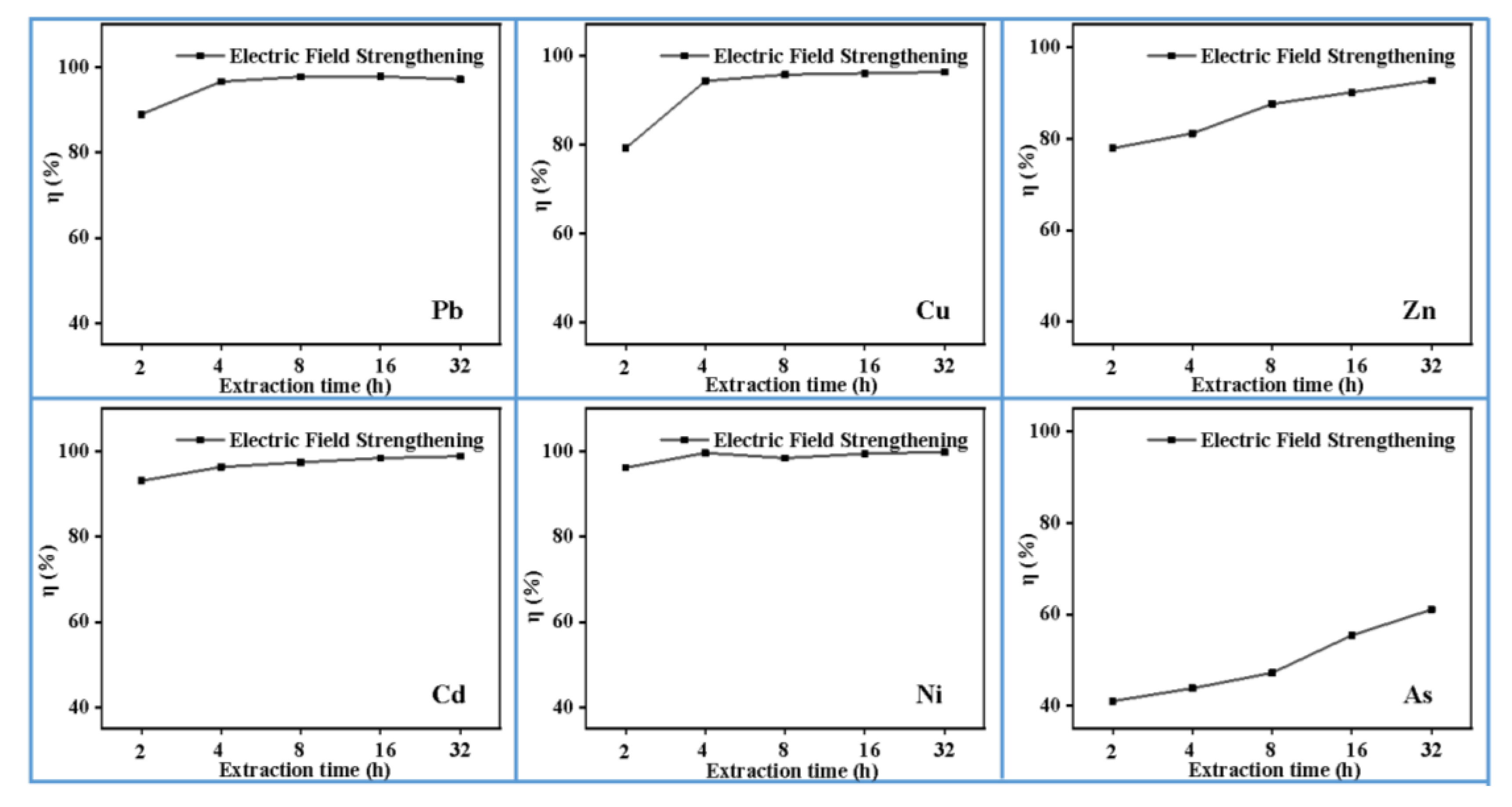
3.5. Effect of Extraction Time
3.6. Effect of Polarity Switching Frequency
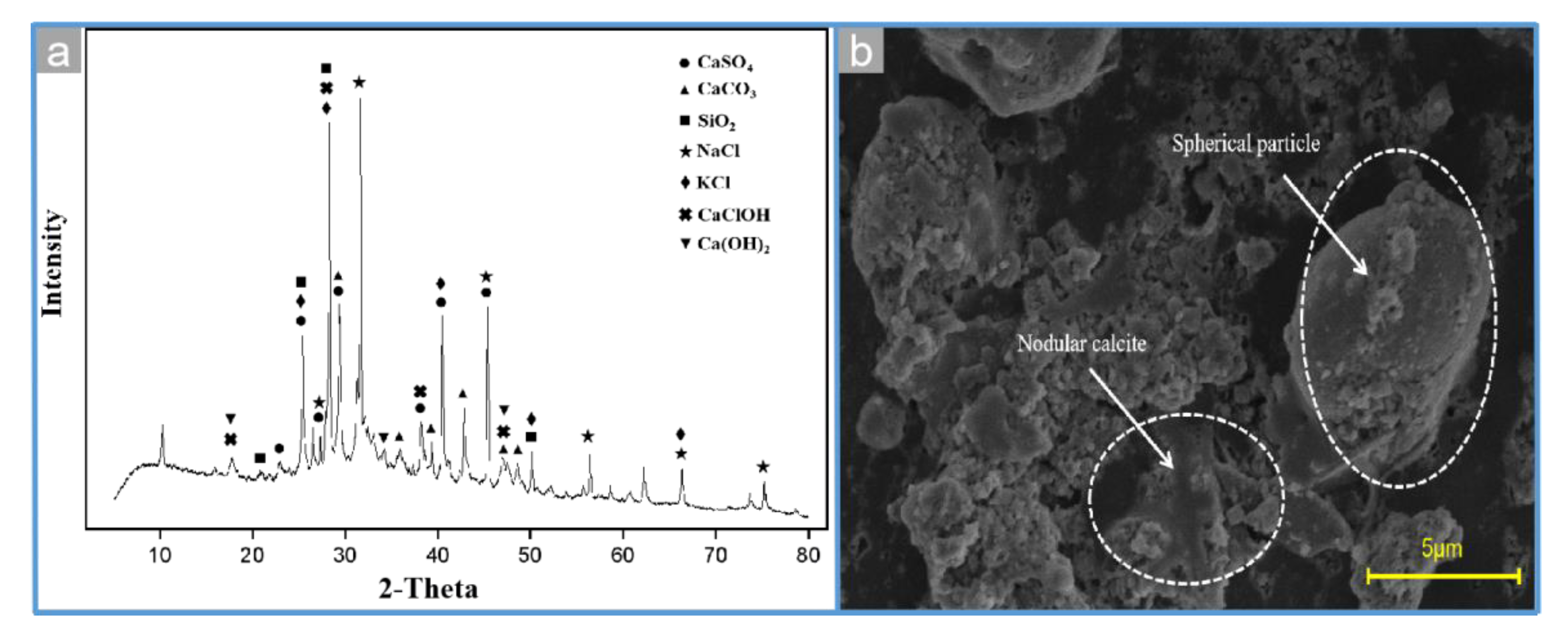
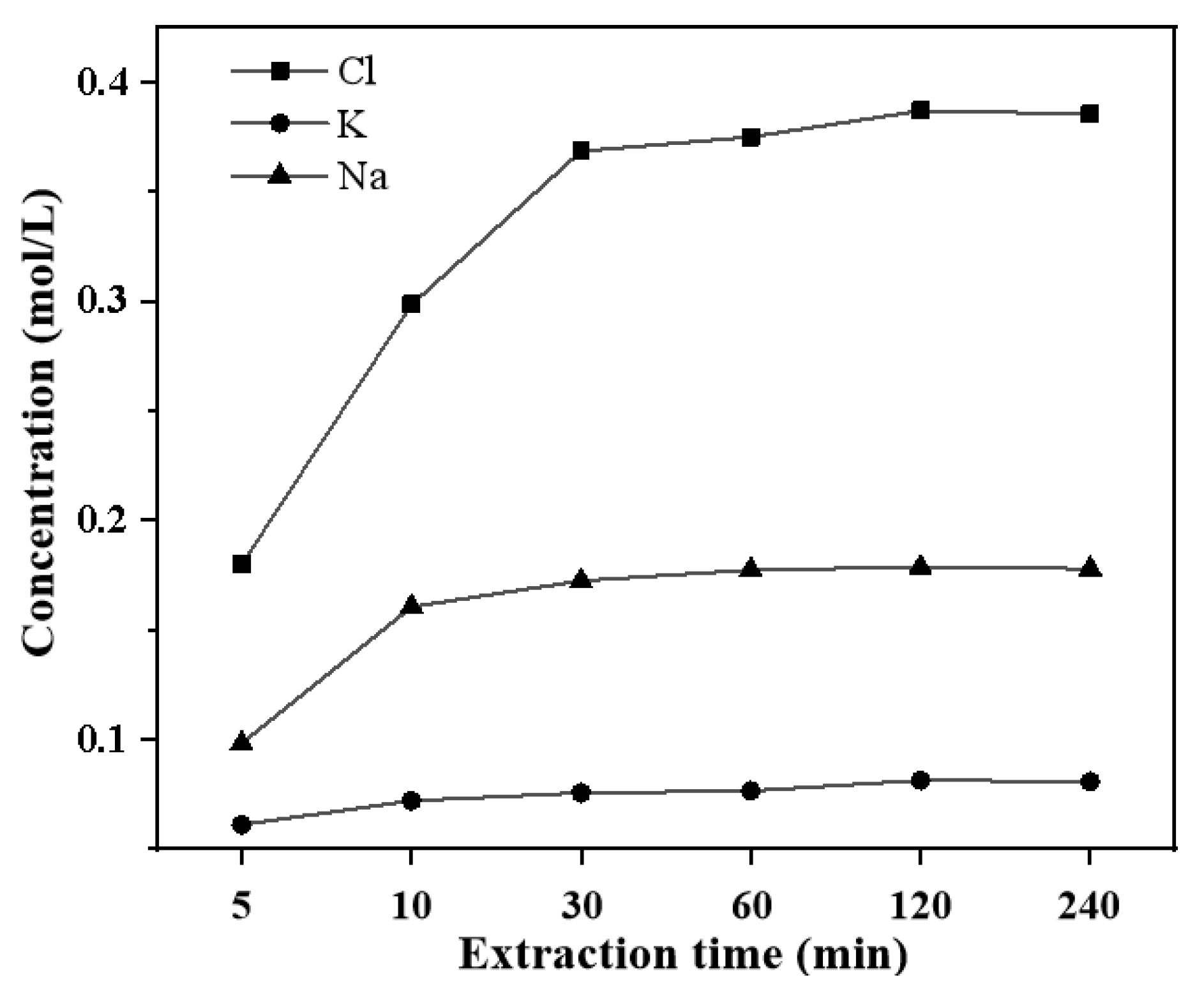
3.7. Characterization of Soluble Salts in MSWI Fly Ash During Extraction Process
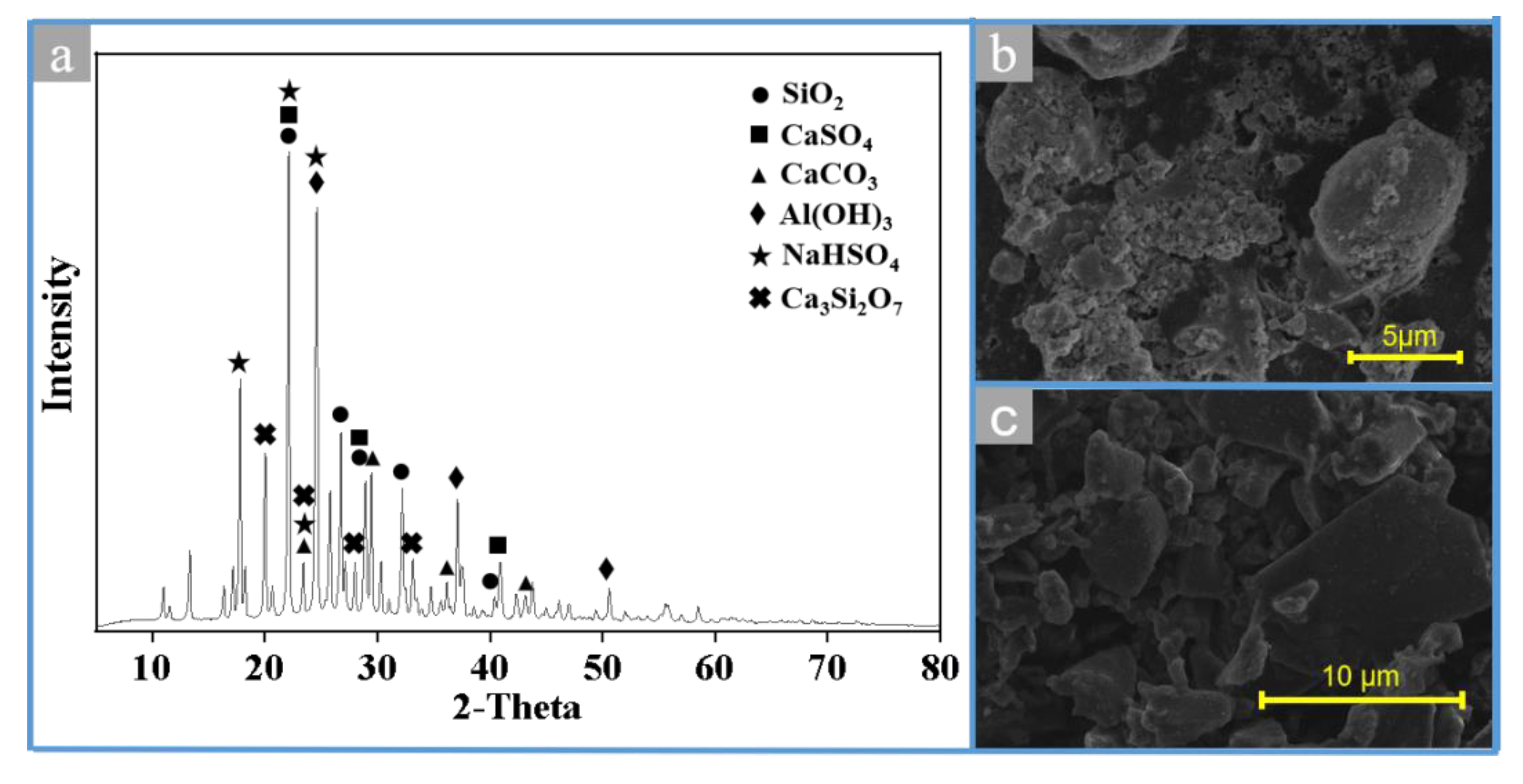
3.8. Characterization of the MSWI Fly Ash under Different Conditions
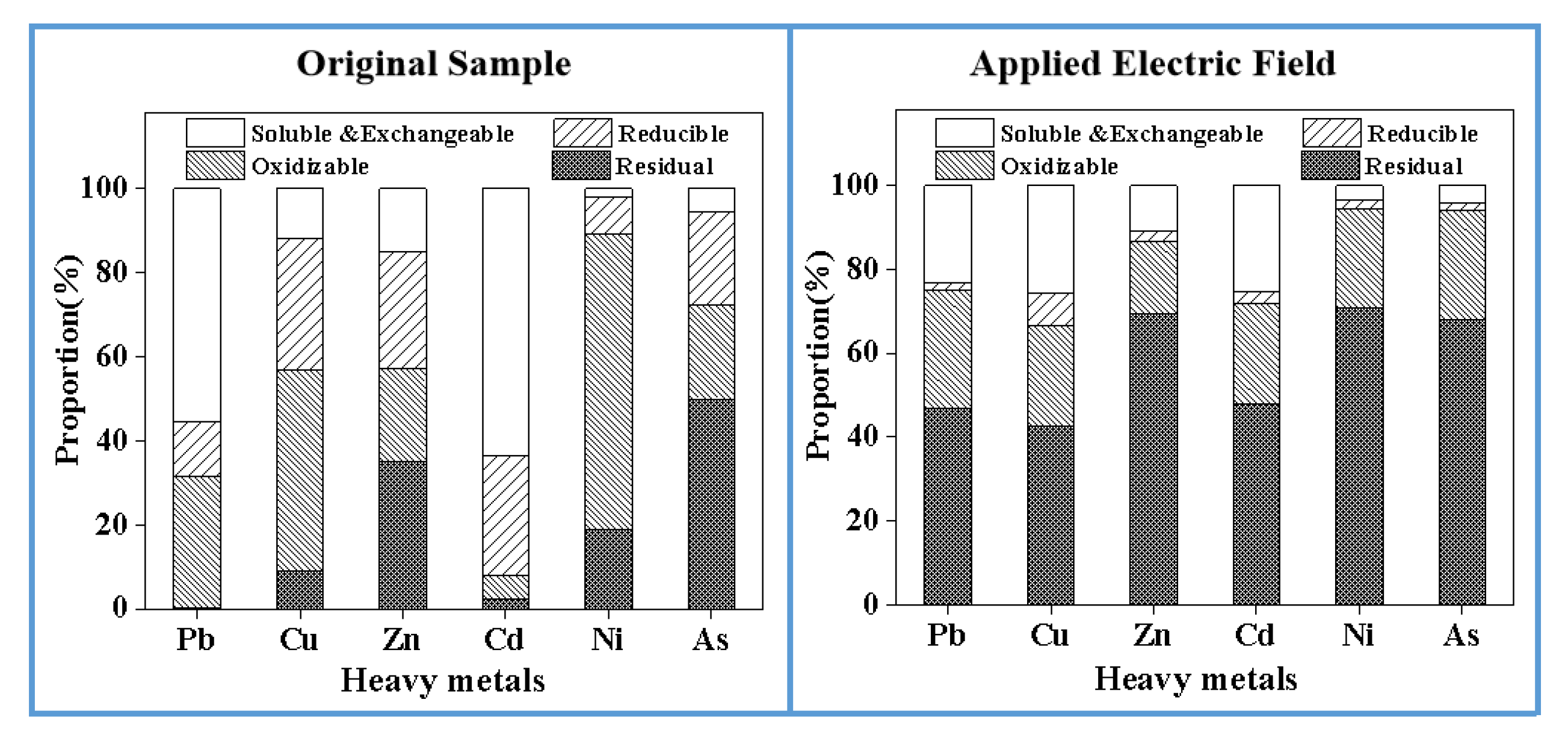
3.9. The Main Mechanism of Pollutant Migration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Zhang, C.; Li, Y.; Zhi, Q. The Status of Municipal Solid Waste Incineration (MSWI) in China and its Clean Development. Energy Procedia 2016, 104, 498–503. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, W.; Zhao, H.; Tian, Z.; Li, F.; Wu, Y. Stabilization/solidification of lead in MSWI fly ash with mercapto functionalized dendrimer Chelator. Waste Manag. 2016, 50, 105–112. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, S.; Liu, L.; Wang, X.; Zhang, Z. Application of washed MSWI fly ash in cement composites: Long-term environmental impacts. Environ. Sci. Pollut. Res. 2018, 25, 12127–12138. [Google Scholar] [CrossRef]
- Pesonen, J.; Yliniemi, J.; Illikainen, M.; Kuokkanen, T.; Lassi, U. Stabilization/solidification of fly ash from fluidized bed combustion of recovered fuel and biofuel using alkali activation and cement addition. J. Environ. Chem. Eng. 2016, 4, 1759–1768. [Google Scholar] [CrossRef]
- Aubert, J.E.; Husson, B.; Sarramone, N. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement Part 1: Processing and characterization of MSWI fly ash. J. Hazard. Mater. 2006, 136, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hou, H.; Zhu, S.; Zha, J. Utilization of municipal solid waste incineration ash in stone mastic asphalt mixture: Pavement performance and environmental impact. Constr. Build. Mater. 2009, 23, 989–996. [Google Scholar] [CrossRef]
- Chen, C.G.; Sun, C.J.; Gau, S.H.; Wu, C.W.; Chen, Y.L. The effects of the mechanical–chemical stabilization process for municipal solid waste incinerator fly ash on the chemical reactions in cement paste. Waste Manag. 2013, 33, 858–865. [Google Scholar] [CrossRef]
- Jin, M.; Zheng, Z.; Sun, Y.; Chen, L.; Jin, Z. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non-Cryst. Solids 2016, 450, 116–122. [Google Scholar] [CrossRef]
- Wei, X.; Guo, S.; Wu, B.; Li, F.; Li, G. Effects of reducing agent and approaching anodes on chromium removal in electrokinetic soil remediation. Front. Environ. Sci. Eng. 2016, 10, 253–261. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yang, G. Electric fields within clay materials: How to affect the adsorption of metal ions. J. Colloid Interface Sci. 2017, 501, 54–59. [Google Scholar] [CrossRef]
- Rojo, A.; Hansen, H.K.; Monárdez, O.; Jorquera, C.; Santis, P.; Inostroza, P. Electrical Behavior of Copper Mine Tailings during EKR with Modified Electric Fields. Bull. Environ. Contam. Toxicol. 2017, 98, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shu, J.; Chen, M.; Wang, J.; Wang, Y.; Luo, Z.; Wang, R.; Yang, F.; Xiu, F.; Sun, Z. Manganese and ammonia nitrogen recovery from electrolytic manganese residue by electric field enhanced leaching. J. Clean. Prod. 2019, 236, 117708. [Google Scholar]
- Liu, Z.; Nueraihemaiti, A.; Chen, M.; Du, J.; Fan, X.; Tao, C. Hydrometallurgical leaching process intensified by an electric field for converter vanadium slag. Hydrometallurgy 2015, 155, 56–60. [Google Scholar] [CrossRef]
- Kim, R.; Ghahreman, A. The effect of ore mineralogy on the electrochemical gold dissolution behavior in various cyanide and oxygen concentrations; Effect of sulfidic ores containing heavy metals. Hydrometallurgy 2019, 184, 75–87. [Google Scholar] [CrossRef]
- Bas, A.D.; Safizadeh, F.; Ghali, E.; Choi, Y. Leaching and electrochemical dissolution of gold in the presence of iron oxide minerals associated with roasted gold ore. Hydrometallurgy 2016, 166, 143–153. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Ferreira, C.; Jensen, P.; Ottosen, L.; Ribeiro, A. Removal of selected heavy metals from MSW fly ash by the electrodialytic process. Eng. Geol. 2005, 77, 339–347. [Google Scholar] [CrossRef]
- Lu, C.-C.; Hsu, M.H.; Lin, Y.-P. Evaluation of heavy metal leachability of incinerating recycled aggregate and solidification/stabilization products for construction reuse using TCLP, multi-final pH and EDTA-mediated TCLP leaching tests. J. Hazard. Mater. 2019, 368, 336–344. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Huang, A. EDTA-Functionalized Covalent Organic Framework for the Removal of Heavy-Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 32186–32191. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Dong, Z.; Namioka, T.; Yamada, N.; Ninomiya, Y. Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Process. Technol. 2016, 152, 108–115. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminum additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, S.; Pan, Y.; Zhang, L.; Cao, Z.; Zhang, X.; Yonemochi, S.; Hosono, S.; Wang, Y.; Oh, K.; et al. Enrichment of heavy metals in fine particles of municipal solid waste incinerator (MSWI) fly ash and associated health risk. Waste Manag. 2015, 43, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Alphen, C.V. Automated mineralogical analysis of coal and ash products—Challenges and requirements. Miner. Eng. 2007, 20, 496–505. [Google Scholar] [CrossRef]
- Gong, B.; Deng, Y.; Yang, Y.; Tan, S.N.; Liu, Q.; Yang, W. Solidification and Biotoxicity Assessment of Thermally Treated Municipal Solid Waste Incineration (MSWI) Fly Ash. Int. J. Environ. Res. Publ. Health 2017, 14, 626. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, G.; Wang, J.; Wu, Y. Bioavailability and mobility of heavy metals in soil in vicinity of a coal mine from Huaibei, China. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1164–1177. [Google Scholar] [CrossRef]
- Chen, P.; Sun, X.; Gao, M.; Ma, J.; Guo, Q. Transformation and migration of cadmium during chemical-looping combustion/gasification of municipal solid waste. Chem. Eng. J. 2019, 365, 389–399. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Wang, Y.N.; Li, W.; Sun, Y.; Zhan, M.; Wu, G. Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J. Environ. Manag. 2017, 208, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Cetin, B.; Likos, W.J.; Edil, T.B. Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 2016, 184, 815–825. [Google Scholar] [CrossRef]
- Chen, W.; Kirkelund, G.M.; Jensen, P.E.; Ottosen, L.M. Electrodialytic extraction of Cr from water-washed MSWI fly ash by changing pH and redox conditions. Waste Manag. 2018, 71, 215. [Google Scholar] [CrossRef]
- Ke, Y.; Li, P.; Chan, W.P.; Dou, X.; Wang, J.Y. Characteristics of heavy metals leaching from MSWI fly ashes in sequential scrubbing processes. J. Mater. Cycles Waste Manag. 2018, 20, 604–613. [Google Scholar]
- Shu, J.; Liu, R.; Liu, Z.; Chen, H.; Tao, C. Enhanced extraction of manganese from electrolytic manganese residue by electrochemical. J. Electroanal. Chem. 2016, 780, 32–37. [Google Scholar] [CrossRef]
- Ng, Y.S.; Gupta, B.S.; Hashim, M.A. Remediation of Pb/Cr co-contaminated soil using electrokinetic process and approaching electrode technique. Environ. Sci. Pollut. Res. Int. 2016, 23, 546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.E.; Zhang, Q.R.; Zhang, W.R.; Jiang, K.Q.; Hou, H.B.; Li, L.; Li, H. Historical venation of chromium-contaminated soil remediation in the past 15 years based on the bibliometric analysis (2001–2015). J. Agro-Environ. Sci. 2017, 36, 409–419. [Google Scholar]
- Tao, Z.; Zou, H.; Ji, M.; Li, X.; Li, L.; Tang, T. Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ. Sci. Pollut. Res. 2014, 21, 3126–3133. [Google Scholar]



| Factors | Levels |
|---|---|
| pH | 2, 4, 6, 8, 10 |
| Current density (mA/cm2) | 0, 15, 25, 35, 45 |
| Duration (h) | 2, 4, 8, 16, 32 |
| Switching frequency (Hz) | 0, 20, 40, 60, 80 |
| Compound | CaO | Cl | SiO2 | SO3 | Na2O | K2O | Al2O3 | MgO | Fe2O3 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Original Sample | 39.18 | 18.38 | 10.02 | 8.97 | 5.99 | 4.47 | 3.81 | 3.75 | 2.11 | 3.32 |
| Heavy Metals | Pb | Cu | Zn | Cd | Cr | Ni | As | Ba | |
|---|---|---|---|---|---|---|---|---|---|
| Original sample | Content (mg/kg) | 1143.64 | 546.12 | 3610.76 | 132.48 | 176.65 | 19.35 | 123.12 | 1047.00 |
| Extraction toxicity (mg/L) | 12.82 | 1.86 | 14.12 | 1.21 | 0.28 | 0.06 | ND | 2.77 | |
| GB 5085.3-2007 Threshold | 5 | 100 | 100 | 1 | 15 | 5 | 5 | 100 | |
| Compound | CaO | Cl | SiO2 | SO3 | Na2O | K2O | Al2O3 | MgO | Fe2O3 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Original samples | 39.18 | 18.38 | 10.02 | 8.97 | 5.99 | 4.47 | 3.81 | 3.75 | 2.11 | 3.32 |
| Electric field strengthening | 20.35 | 1.65 | 32.71 | 1.04 | 3.11 | 3.71 | 20.52 | 0.83 | 6.61 | 9.47 |
| Heavy Metals | Pb | Zn | Cu | Cd | Cr | Ni | As | Ba |
|---|---|---|---|---|---|---|---|---|
| Original sample | 12.82 | 1.86 | 14.12 | 1.21 | 0.28 | 0.06 | ND | 2.77 |
| Electric field strengthening | 1.78 | ND | 1.12 | ND | ND | ND | ND | 0.49 |
| GB 5085.3-2007 | 5 | 100 | 100 | 1 | 15 | 5 | 5 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, R.; Luo, Z.; Wang, R.; Yang, F.; Wang, Z.; Shu, J.; Chen, M. Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing. Materials 2020, 13, 793. https://doi.org/10.3390/ma13030793
Tian Y, Wang R, Luo Z, Wang R, Yang F, Wang Z, Shu J, Chen M. Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing. Materials. 2020; 13(3):793. https://doi.org/10.3390/ma13030793
Chicago/Turabian StyleTian, Yang, Rong Wang, Zhenggang Luo, Rui Wang, Feihua Yang, Zhaojia Wang, Jiancheng Shu, and Mengjun Chen. 2020. "Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing" Materials 13, no. 3: 793. https://doi.org/10.3390/ma13030793
APA StyleTian, Y., Wang, R., Luo, Z., Wang, R., Yang, F., Wang, Z., Shu, J., & Chen, M. (2020). Heavy Metals Removing from Municipal Solid Waste Incineration Fly Ashes by Electric Field-Enhanced Washing. Materials, 13(3), 793. https://doi.org/10.3390/ma13030793





