First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. DF Voltammetric Analysis
2.4. DF Chromatographic Analysis
2.5. Real Sample Application
3. Results and Discussion
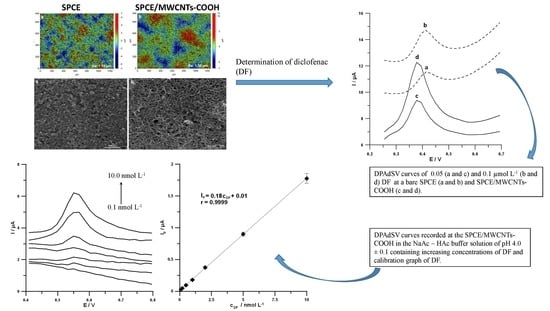
3.1. Characteristics of SPCE/MWCNTs-COOH Sensors
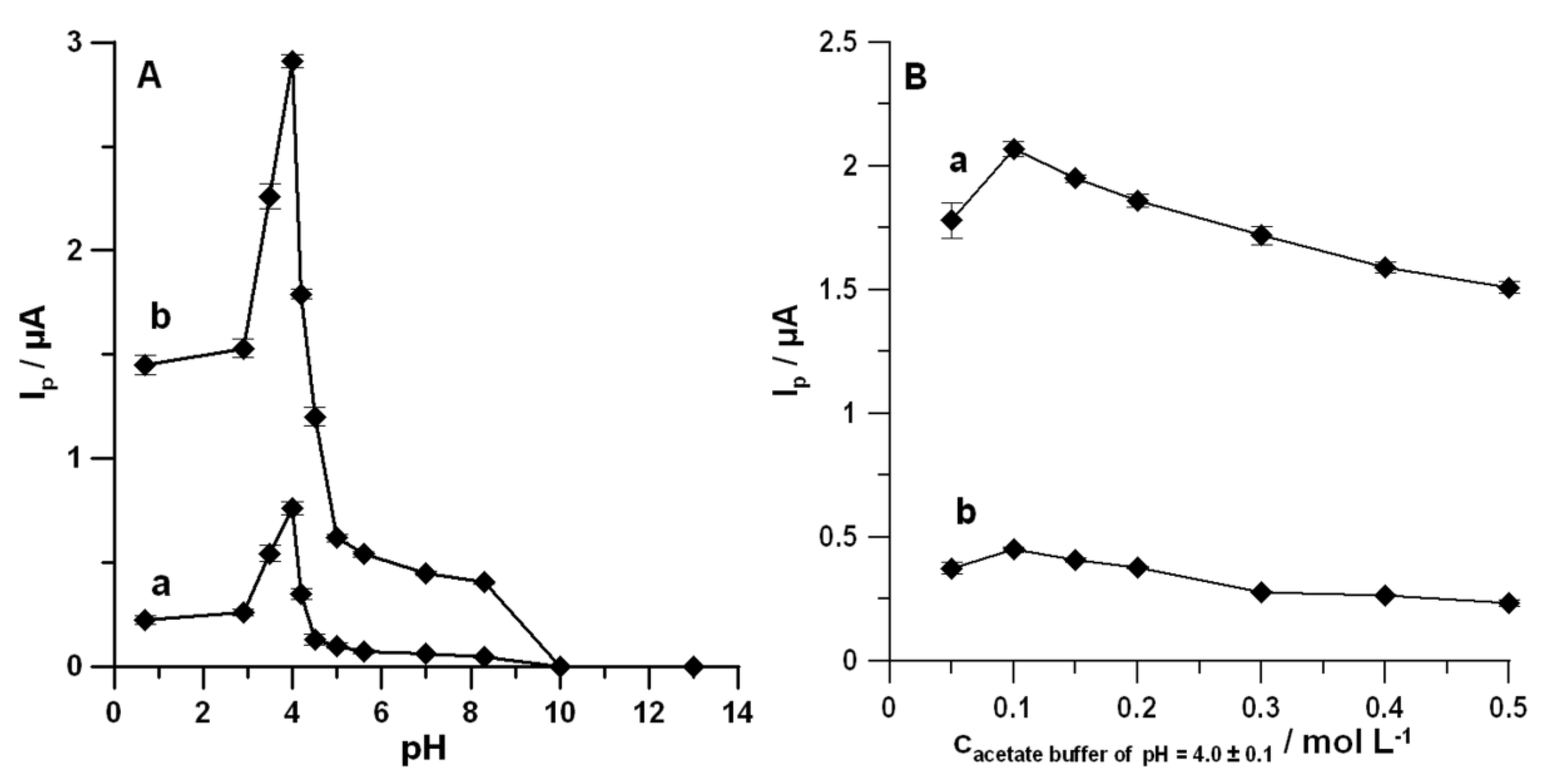
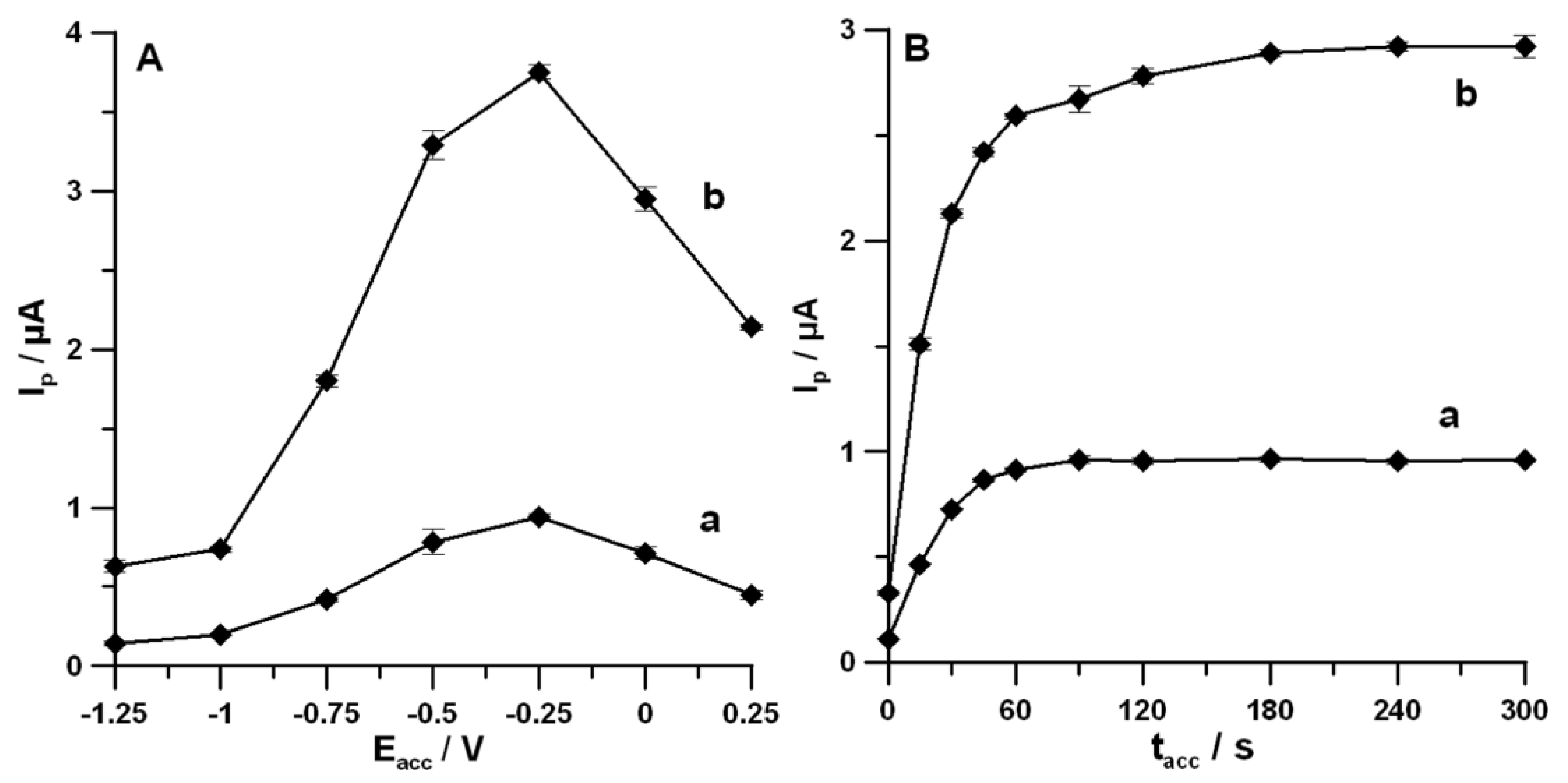
3.2. Optimization of Measurements Solution Composition
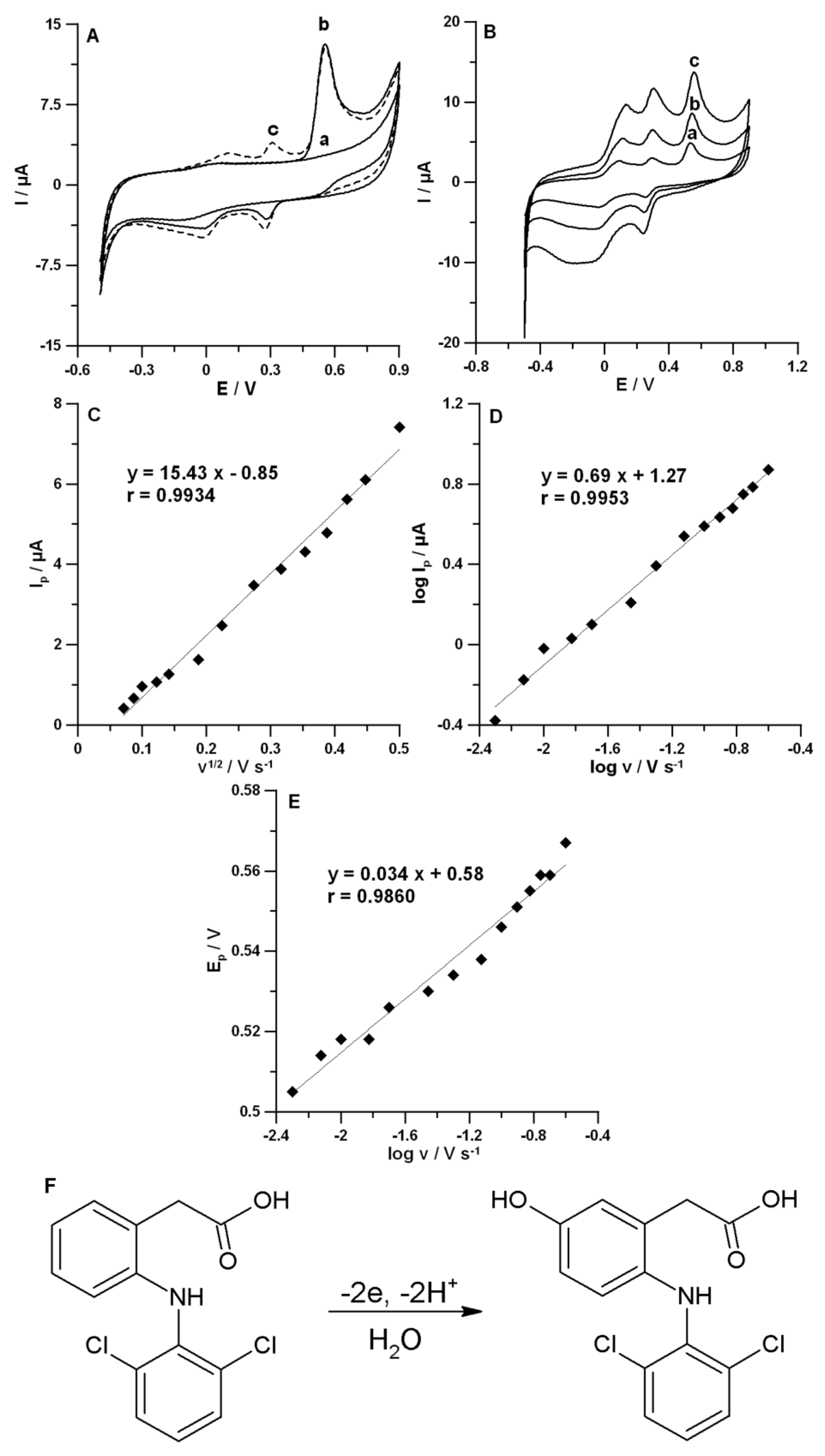
3.3. CV Behaviors of DF with the SPCE/MWCNTs-COOH
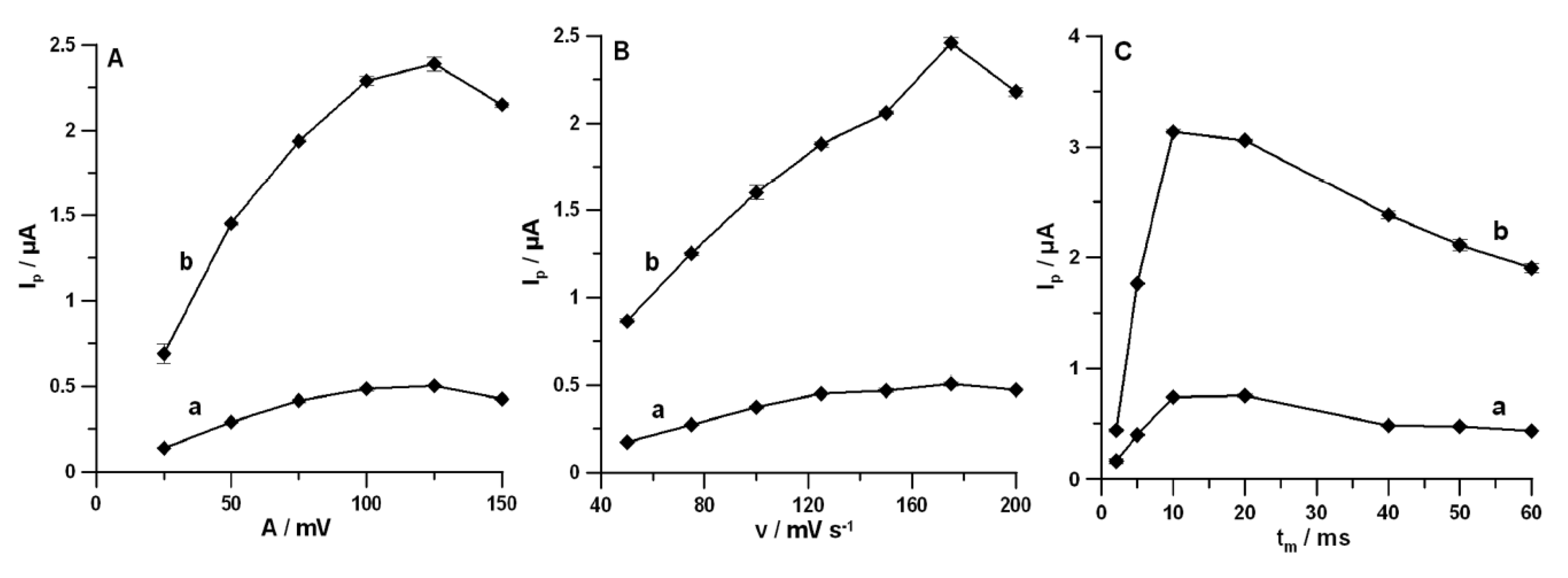
3.4. Optimization of DPAdSV Parameters
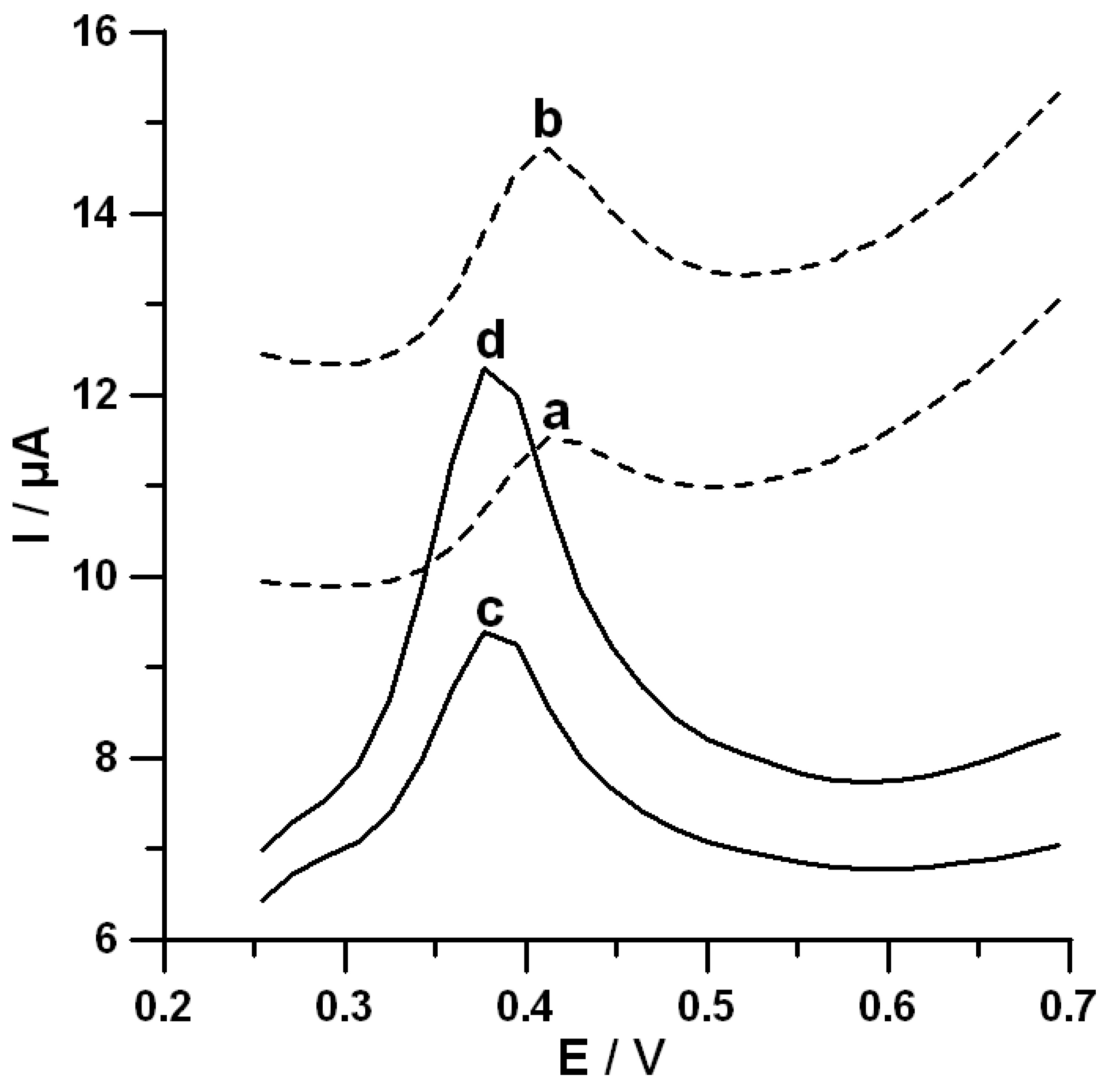
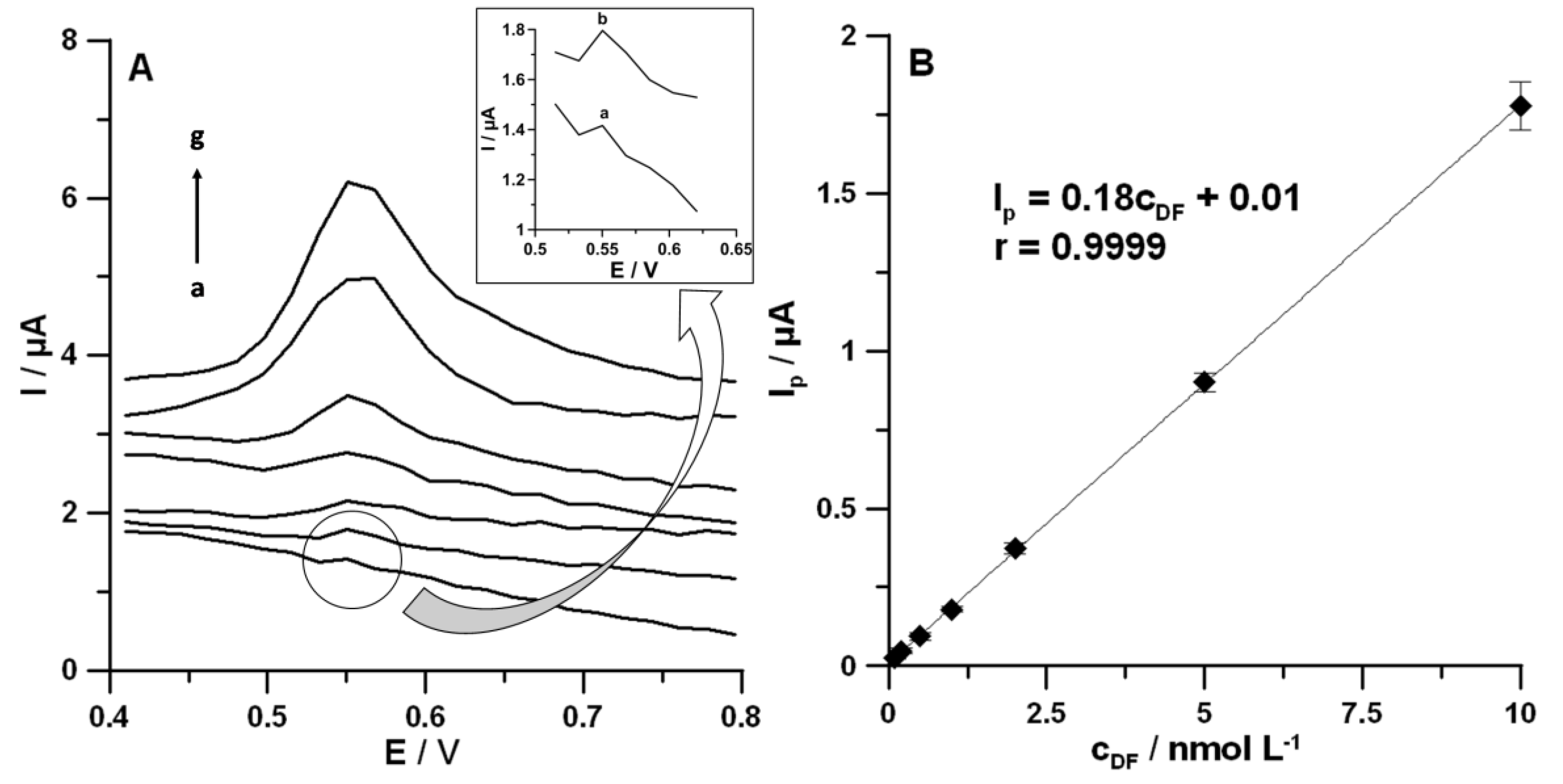
3.5. Analytical Characteristics
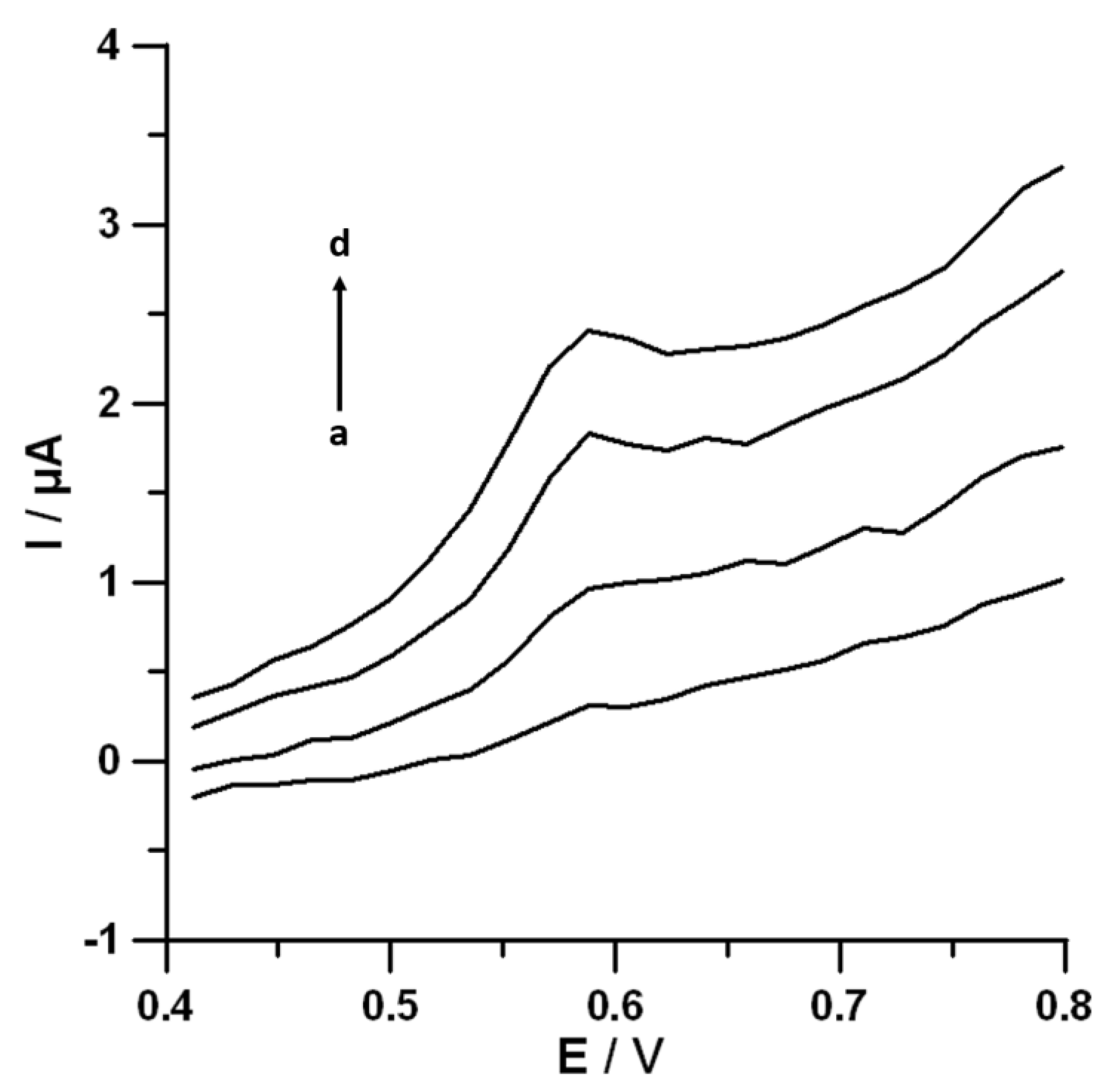
3.6. Selectivity of the SPCE/MWCNTs-COOH
3.7. Application in Environmental Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mekassa, B.; Baker, P.G.L.; Chandravanshi, B.S.; Tessema, M. Synthesis, characterization, and preparation of nickel nanoparticles decorated electrochemically reduced graphene oxide modified electrode for electrochemical sensing of diclofenac. J. Solid State Electrochem. 2018, 22, 3607–3619. [Google Scholar] [CrossRef]
- Valcarcel, Y.; Gonzales Alonso, S.; Rodriguez-Gil, J.L.; Romo Maroto, R.; Gil, A.; Catala, M. Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceutical in rivier- and drinking- water of the Madrid Region in Spain. Chemosphere 2011, 82, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E.; Krbavcic, A. Determination of non-steroidal anti-inflammatory drug (NSAIDs) residues in water samples. Environ. Int. 2005, 31, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Taggart, M.A.; Senacha, K.R.; Green, R.E.; Jhala, Y.V.; Raghavan, B.; Rahmani, A.R.; Cuthbert, R.; Pain, D.J.; Meharg, A.E. Diclofenac residues in carcasses of domestic ungulates available to vultures in India. Environ. Int. 2007, 33, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.; Wilson, C.; Brain, R.; Solomon, K. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Pandey, G. Spectrophotometric, chromatographic and spectrofluorometric methods for the determination of diclofenac: A review. Pharm. Lett. 2011, 3, 257–265. [Google Scholar]
- Gouda, A.A.; Kotb El-Sayed, M.I.; Amin, A.S.; Sheikh, R.E.L. Spectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: A review. Arab. J. Chem. 2013, 6, 145–163. [Google Scholar] [CrossRef]
- Heli, H.; Jabbari, A.; Majdi, S.; Mahjoub, M.; Moosavi-Movahedi, A.A.; Sheibani, S.H. Electrooxidation and determination of some non-steroidal anti-inflammatory drugs on nanoparticles of Ni–curcumin-complex-modified electrode. J. Solid State Electrochem. 2009, 13, 1951–1958. [Google Scholar] [CrossRef]
- Afkhami, A.; Bahiraei, A.; Madrakian, T. Gold nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium. Mater. Sci. Eng. C 2016, 59, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non-steroidal anti-inflammatory drug diclofenac. Mater. Sci. Eng. C 2012, 32, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Sarhang-Zadeh, K.; Mohammad-Rezaei, R. Electrochemical behavior and voltammetric determination of diclofenac at a multi-walled carbon nanotube-ionic liquid composite modified carbon Cceramic electrode. Anal. Lett. 2013, 46, 1885–1896. [Google Scholar] [CrossRef]
- Karuppiah, C.; Cheemalapati, S.; Chen, S.M.; Palanisamy, S. Carboxyl-functionalized graphene oxide-modified electrode for the electrochemical determination of nonsteroidal anti-inflammatory drug diclofenac. Ionics 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Jiokenga, S.L.Z.; Tonlea, I.K.; Walcariusb, A. Amino-attapulgite/mesoporous silica composite films generated by electroassisted self-assembly for the voltammetric determination of diclofenac. Sens. Actuators B Chem. 2019, 287, 296–305. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Liu, L.; Zhang, J. Simultaneous electrochemical determination of paracetamol and diclofenac based on poly(diallyldimethylammonium chloride) functionalized graphene. Electroanalysis 2016, 28, 76–82. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Hu, S. Enhanced oxidation of diclofenac sodium at a nano-structured electrochemical sensing film constructed by multi-wall carbon nanotubes–surfactant composite. Mater. Sci. Eng. C 2008, 28, 188–194. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Design and construction of a label free aptasensor for electrochemical detection of sodium diclofenac. Biosens. Bioelectron. 2012, 33, 184–189. [Google Scholar] [CrossRef]
- Kamenická, B.; Bartášková, A.; Švancara, I.; Weidlich, T. Applicability of voltammetric determination of diclofenac at carbon paste electrodes to the analysis of aqueous solutions purified by adsorption and/or ionic liquid based ion exchange. Mon. Chem. 2019, 150, 429–437. [Google Scholar] [CrossRef]
- Damiri, S.; Oskoei, Y.M.; Fouladgar, M. Highly sensitive voltammetric and impedimetric sensor based on an ionic liquid/cobalt hexacyanoferrate nanoparticle modified multiwalled carbon nanotubes electrode for diclofenac analysis. J. Exp. Nanosci. 2016, 11, 1384–1401. [Google Scholar] [CrossRef]
- Arvand, M.; Hassannezhad, M. Square wave voltammetric determination of uric acid and diclofenac on multi-walled carbon nanotubes decorated with magnetic core-shell Fe3O4@SiO2 nanoparticles as an enhanced sensing interface. Ionics 2015, 21, 3245–3256. [Google Scholar] [CrossRef]
- Mokhtaria, A.; Karimi-Malehb, H.; Ensafic, A.A.; Beitollahi, H. Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sens. Actuators B Chem. 2012, 169, 96–105. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Izadi, M.; Karimi-Maleh, H. Sensitive voltammetric determination of diclofenac using room-temperature ionic liquid-modified carbon nanotubes paste electrode. Ionics 2013, 19, 137–144. [Google Scholar] [CrossRef]
- Goodarzian, M.; Khalilzade, M.A.; Karimi, F.; Gupta, V.K.; Keyvanfard, M.; Bagheri, H.; Fouladgar, M. Square wave voltammetric determination of diclofenac in liquid phase using a novel ionic liquid multiwall carbon nanotubes paste electrode. J. Mol. Liq. 2014, 197, 114–119. [Google Scholar] [CrossRef]
- Pourghobadi, R.; Baezzat, M.R. Silica nanoparticles modified carbon paste electrode as a voltammetric sensor for determination of diclofenac. Anal. Bioanal. Chem. Res. 2017, 4, 261–268. [Google Scholar]
- Chethana, B.K.; Basavanna, S.; Naik, Y.A. Voltammetric determination of diclofenac sodium using tyrosine-modified carbon paste electrode. Ind. Eng. Chem. Res. 2012, 51, 10287–10295. [Google Scholar] [CrossRef]
- Blanco-Lopez, M.C.; Fernandez-Llano, L.; Lobo-Castanon, M.J.; Miranda-Ordieres, A.J.; Tunon-Blanco, P. Voltammetry of diclofenac at graphite, carbon Composites, and molecularly imprinted polymer-composite electrodes. Anal. Lett. 2004, 37, 915–927. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sens. Actuators B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Manea, F.; Ihos, M.; Remes, A.; Burtica, G.; Schoonmanc, J. Electrochemical determination of diclofenac sodium in aqueous solution on Cu-doped zeolite-expanded graphite-epoxy electrode. Electroanalysis 2010, 22, 2058–2063. [Google Scholar] [CrossRef]
- Sarhang-Zadeh, K.; Khatami, A.A.; Jabbari, M.; Bahari, S. Simultaneous determination of diclofenac and indomethacin using a sensitive electrochemical sensor based on multiwalled carbon nanotube and ionic liquid nanocomposite. J. Appl. Electrochem. 2013, 43, 1217–1224. [Google Scholar] [CrossRef]
- Ihosa, M.; Remesb, A.; Maneab, F. Electrochemical determination of diclofenac using boron-doped diamond Electrode. J. Environ. Prot. Ecol. 2012, 13, 2096–2103. [Google Scholar]
- Yilmaz, B.; Kaban, S.; Akcay, B.K.; Ciltas, U. Differential pulse voltammetric determination of diclofenac in pharmaceutical preparations and human serum. Braz. J. Pharm. Sci. 2015, 51, 285–294. [Google Scholar] [CrossRef]
- Ciltas, U.; Yilmaz, B.; Kaban, S.; Akcay, B.K.; Nazik, G. Square wave voltammetric determination of diclofenac in pharmaceutical preparations and human serum. Iran. J. Pharm. Res. 2015, 14, 715–722. [Google Scholar] [PubMed]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Arcos Martinez, M.J. Recent development in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Golzari Aqda, T.; Behkami, S.; Bagheri, H. Porous eco–friendly fibers for on–line micro solid–phase extraction of nonsteroidal anti–inflammatory drugs from urine and plasma samples. J. Chromatogr. A 2018, 1574, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sipa, K.; Brycht, M.; Leniart, A.; Nosal-Wiercińska, A.; Skrzypek, S. Improved electroanalytical characteristics for the determination of pesticide metobromuron in the presence of nanomaterials. Anal. Chim. Acta 2018, 1030, 61–69. [Google Scholar] [CrossRef]
- Cid-Cerón, M.M.; Guzmán-Hernández, D.S.; Ramírez-Silva, M.T.; Galano, A.; Romero-Romo, M.; Palomar-Pardavé, M. New insights on the kinetics and mechanism of the electrochemical oxidation of diclofenac in neutral aqueous medium. Electrochim. Acta 2016, 199, 92–98. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Pietrzak, K.; Sasal, A. Adsorptive stripping voltammetric method for the determination of caffeine at integrated three-electrode screen-printed sensor with carbon/carbon nanofibers working electrode. Adsorption 2019, 25, 913–921. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Medsen, K.G.; Skonberg, C.; Jurva, U.; Cornett, C.; Hansen, S.H.; Johansen, T.N.; Olsen, J. Bioactivation of diclofenac in vitro and In Vivo: Correlation to electrochemical studies. Chem. Res. Toxicol. 2008, 21, 1107–1119. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques. Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]

| Calculated parameter | SPCE | SPCE/MWCNTs-COOH |
|---|---|---|
| ΔE for v of 175 mV s−1 | 189.0 ± 1.9 mV (n = 3) | 149.0 ± 1.5 mV (n = 3) |
| χ0 for v of 175 mV s−1 | 3.26 ± 0.031 (n = 3) | 2.57 ± 0.025 (n = 3) |
| As for v of 5–500 mV s−1 | 0.061 ± 0.00058 cm2 (n = 3) | 0.10 ± 0.00097 cm2 (n = 3) |
| Parameter | DPAdSV |
|---|---|
| Linear range (nmol L−1) | 0.1–10.0 |
| Accumulation time (s) | 60 |
| Slope (b) ± SDb (n = 3) (µA/nmol L−1) | 0.18 ± 0.0070 |
| Intercept (a) ± SDa (n = 3) (µA) | 0.010 ± 0.0017 |
| Correlation coefficient (r) | 0.9999 |
| Limit of detection (LOD; nmol L−1) | 0.028 |
| Limit of quantification (LOQ; nmol L−1) | 0.094 |
| Intra-day precision (RSD, n = 10) (%) | 0.7 |
| Inter-day precision (RSD, n = 15) (%) | 2.1 |
| Reproducibility (RSD, n = 9) (%) | 2.9 |
| Electrode | Method | Linear Range (mol L−1) | Detection Limit (mol L−1) | Application | Ref. |
|---|---|---|---|---|---|
| n-GCE | CV | 2.0 × 10−4–1.5 × 10−3 | 2.8 × 10−5 | pharmaceutical formulations | [10] |
| NiNPs/ERGO/GCE | SWV | 2.5 × 10−7–1.3 × 10−4 | 9.0 × 10−8 | pharmaceutical formulations, urine samples | [1] |
| AuNP/MWCNT/GCE | SWV | 3.0 × 10−8–2.0 × 10−4 | 2.0 × 10−8 | pharmaceutical formulations, urine samples | [11] |
| MWCNTs/ Cu(OH)2/IL/GCE | DPV | 1.8 × 10−7–1.2 × 10−4 | 4.0 × 10−8 | pharmaceutical formulations | [12] |
| MWCNT-IL/CCE | DPV | 5.0 × 10−8–2.0 × 10−5 | 2.7 × 10−8 | blood plasma samples | [13] |
| GO-COOH/GCE | LSV | 1.2 × 10−6–4.0 × 10−4 | 9.0 × 10−8 | urine samples, blood serum samples | [14] |
| GCE/Amino-AT | SWV | 3.0 × 10−7–2.0 × 10−5 | 2.0 × 10−7 | pharmaceutical formulations, spiked water samples | [15] |
| GCE/APTES-Amino-AT-Silica | 5.3 × 10−8 | ||||
| PDDA-GR/GCE | DPV | 1.0 × 10−5–1.0 × 10−4 | 6.1 × 10−7 | pharmaceutical formulations, spiked lake water samples | [16] |
| MWNTs–DHP/GCE | CV | 1.7 × 10−7–2.5 × 10−6 2.5 × 10−6–7.5 × 10−5 | 8.0 × 10−8 | pharmaceutical formulations | [17] |
| DBA/GCE | CV | 1.0 × 10−5–1.0 × 10−3 | 2.7 × 10−7 | blood serum samples | [18] |
| CPE | SWV | 1.0 × 10−6–1.0 × 10−5 | 2.0 × 10−7 | spiked model water samples | [19] |
| MWCNTs/CoHCF/IL/PE | DPV | 1.0 × 10−3–1.0 × 10−1 | 3.0 × 10−4 | pharmaceutical formulations, urine samples | [20] |
| Fe3O4@SiO2/MWCNTs-CPE | SWV | 5.0 × 10−7–1.0 × 10−4 | 4.0 × 10−8 | pharmaceutical formulations, blood serum samples | [21] |
| VFMCNTPE | SWV | 2.5 × 10−6–6.0 × 10−4 | 2.0 × 10−6 | pharmaceutical formulations, urine samples | [22] |
| IL/CNTPE | DPV | 5.0 × 10−7–3.0 × 10−4 | 2.0 × 10−7 | pharmaceutical formulations, urine samples | [23] |
| IL/CNTPE | SWV | 3.0 × 10−7–7.5 × 10−4 | 9.0 × 10−8 | pharmaceutical formulations, urine samples | [24] |
| Silica NPs-CPE | DPV | 1.0 × 10−7–5.0 × 10−4 | 4.6 × 10−8 | pharmaceutical formulations | [25] |
| TCPE | DPV | 1.0 × 10−5–1.4 × 10−4 | 3.3 × 10−6 | pharmaceutical formulations, urine samples | [26] |
| PTFE-G; EG; E-CB | DPV | 6.0 × 10−8–1.0 × 10−6 | 5.0 × 10−8 | pharmaceutical formulations | [27] |
| EPPG | SWV | 1.0 × 10−8–1.0 × 10−6 | 6.2 × 10−9 | pharmaceutical formulations, urine samples | [28] |
| CuZEGE | CV, DPV | 2.0 × 10−5–3.0 × 10−7 | 5.0 × 10−8 | - | [29] |
| MWCNT-IL/CCE | DPV | 5.0 × 10−8–5.0 × 10−5 | 1.8 × 10−8 | pharmaceutical formulations, blood plasma samples | [30] |
| BDDE | DPV | 3.1 × 10−7–3.1 × 10−5 | 3.0 × 10−8 | spiked tap water samples | [31] |
| PtDE | DPV | 5.0 × 10−6–5.9 × 10−5 | 1.0 × 10−6 | pharmaceutical formulations, blood serum samples | [32] |
| PtDE | SWV | 5.1 × 10−6–5.9 × 10−5 | 1.7 × 10−6 | pharmaceutical preparations, blood serum samples | [33] |
| SPCE/MWCNTs-COOH | DPAdSV | 1.0 × 10−10–1.0 × 10−8 | 2.8 × 10−11 | river water samples | This work |
| Sample | DF concentration ± SD (nmol L–1) (n = 3) | Recovery (%) | texp | ||
|---|---|---|---|---|---|
| Added | Found with the DPAdSV procedure | Found with the HPLC/PAD method | DPAdSV | ||
| #1 | 0 | 0.42 ± 0.08 | - | - | - |
| #1 | 5.0 | 5.40 ± 0.20 | - | 99.6 | - |
| #1 | 50.0 | 50.80 ± 1.40 | 52.30 ± 4.08 | 100.5 | 0.60 |
| #2 | 0 | - | - | - | - |
| #2 | 0.4 | 0.40 ± 0.01 | - | 100.0 | - |
| #2 | 5.0 | 5.38 ± 0.33 | - | 99.6 | - |
| #2 | 50.0 | 51.0 ± 0.90 | 49.80 ± 4.25 | 100.9 | 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials 2020, 13, 781. https://doi.org/10.3390/ma13030781
Sasal A, Tyszczuk-Rotko K, Wójciak M, Sowa I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials. 2020; 13(3):781. https://doi.org/10.3390/ma13030781
Chicago/Turabian StyleSasal, Agnieszka, Katarzyna Tyszczuk-Rotko, Magdalena Wójciak, and Ireneusz Sowa. 2020. "First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac" Materials 13, no. 3: 781. https://doi.org/10.3390/ma13030781
APA StyleSasal, A., Tyszczuk-Rotko, K., Wójciak, M., & Sowa, I. (2020). First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials, 13(3), 781. https://doi.org/10.3390/ma13030781