Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O
Abstract
1. Introduction
2. Materials and Methods
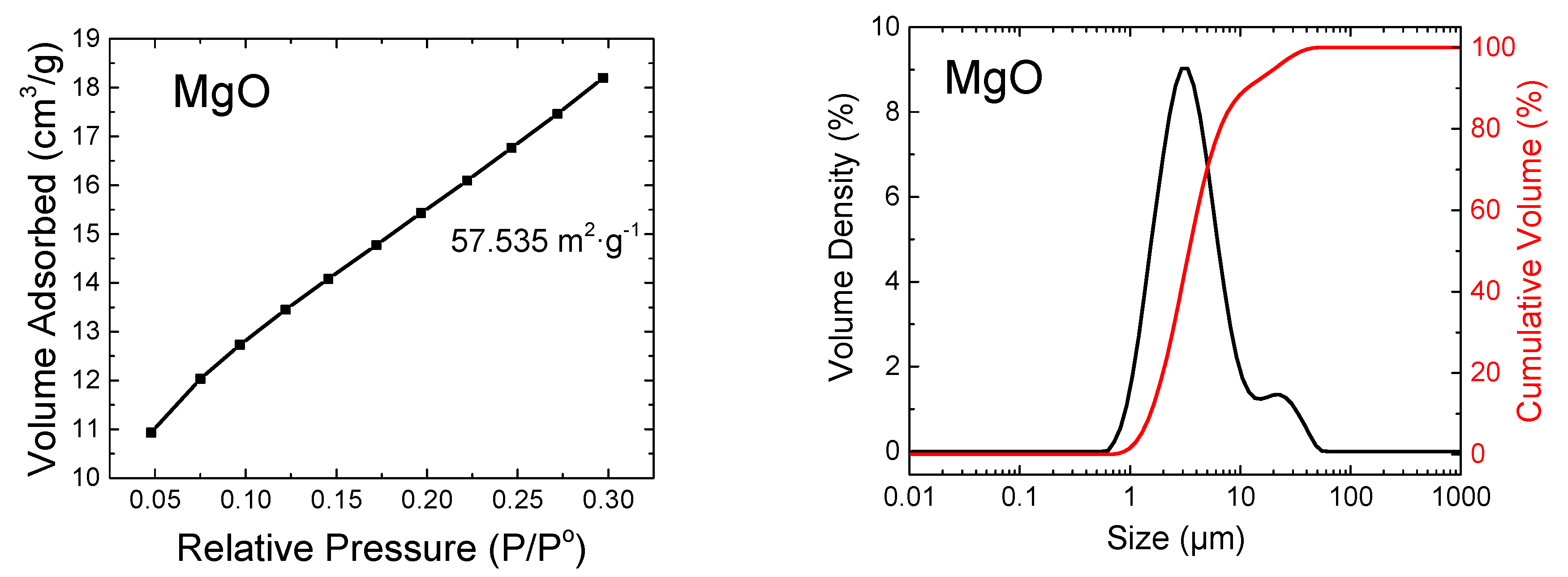
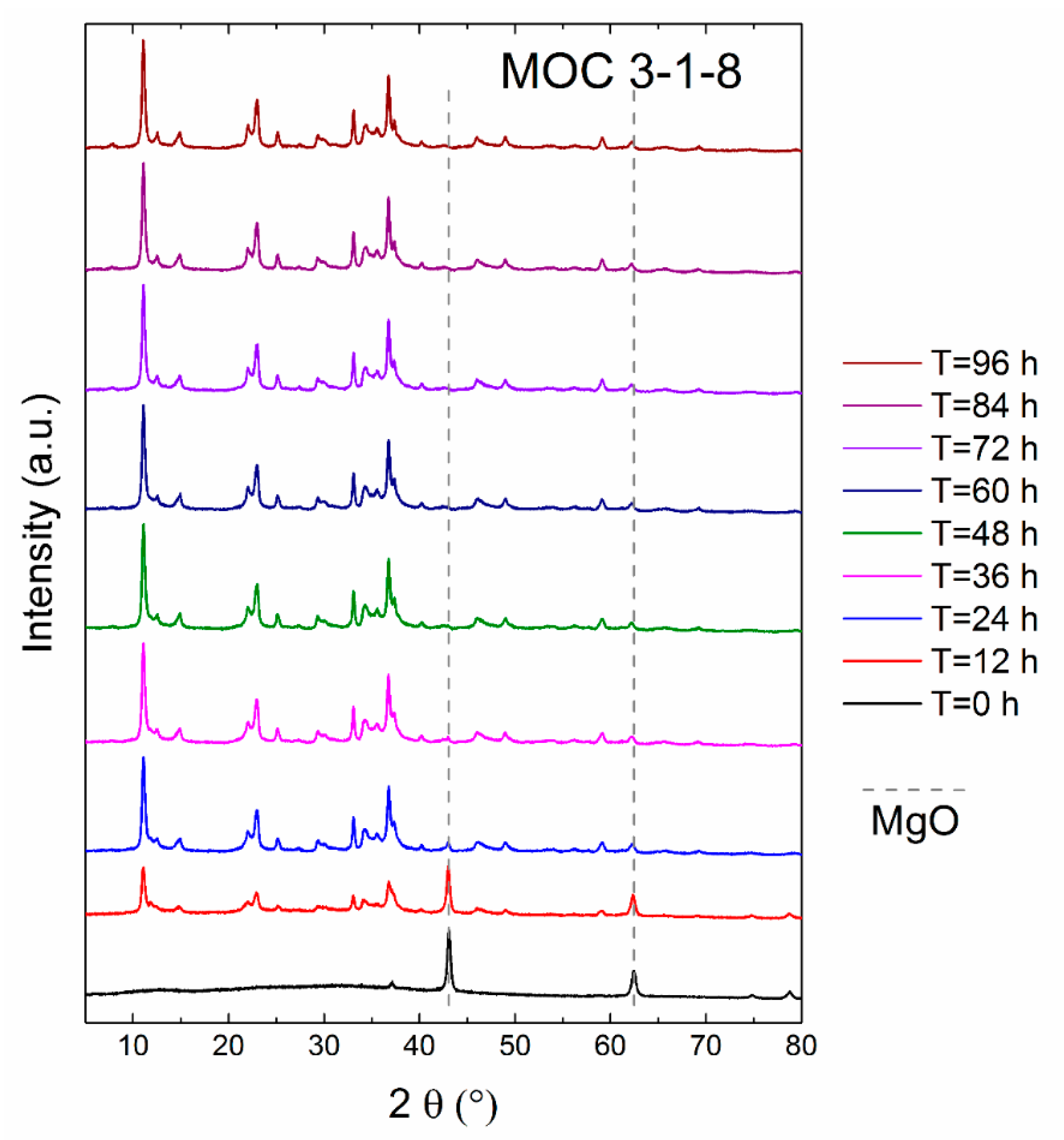
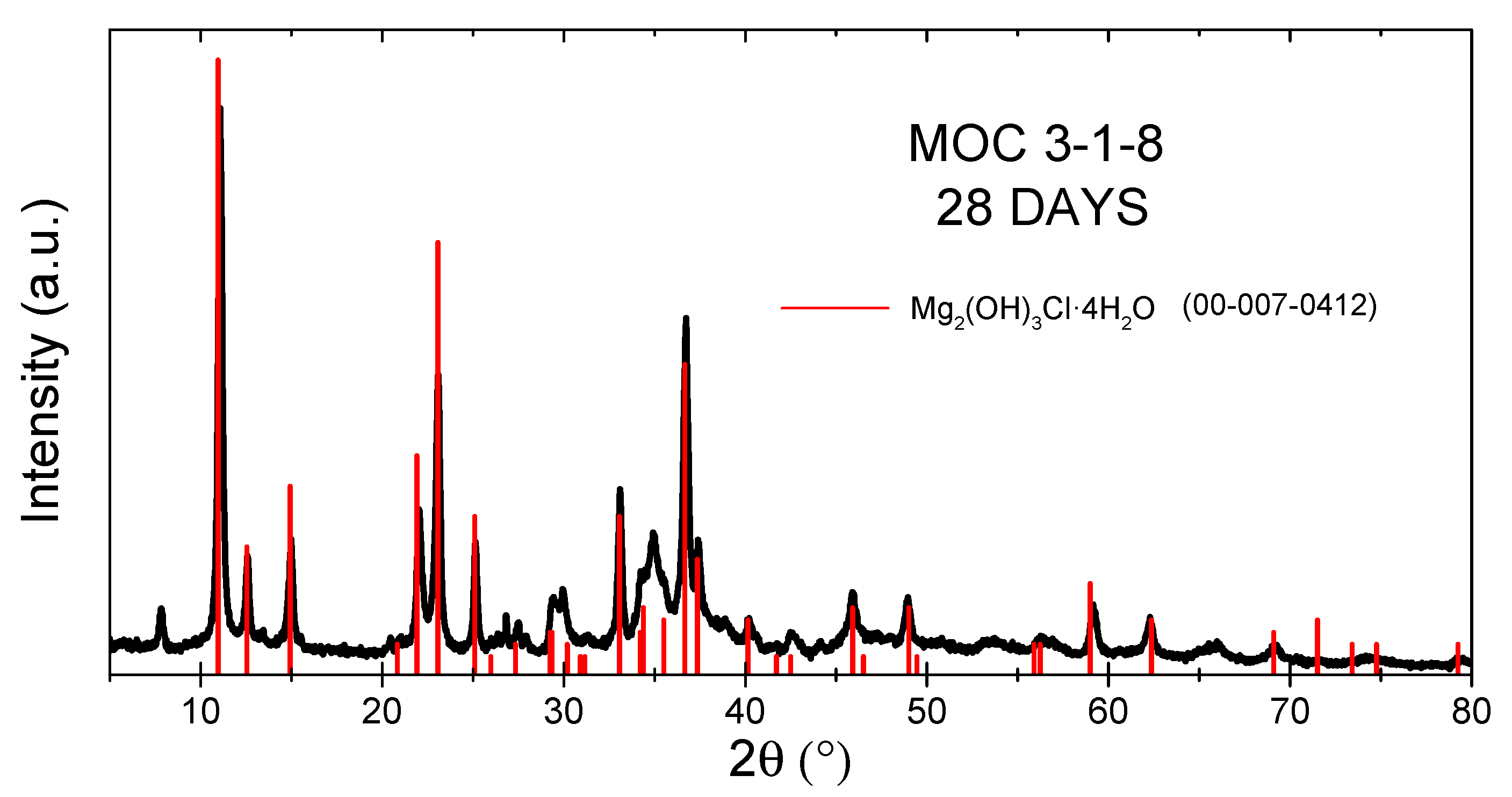
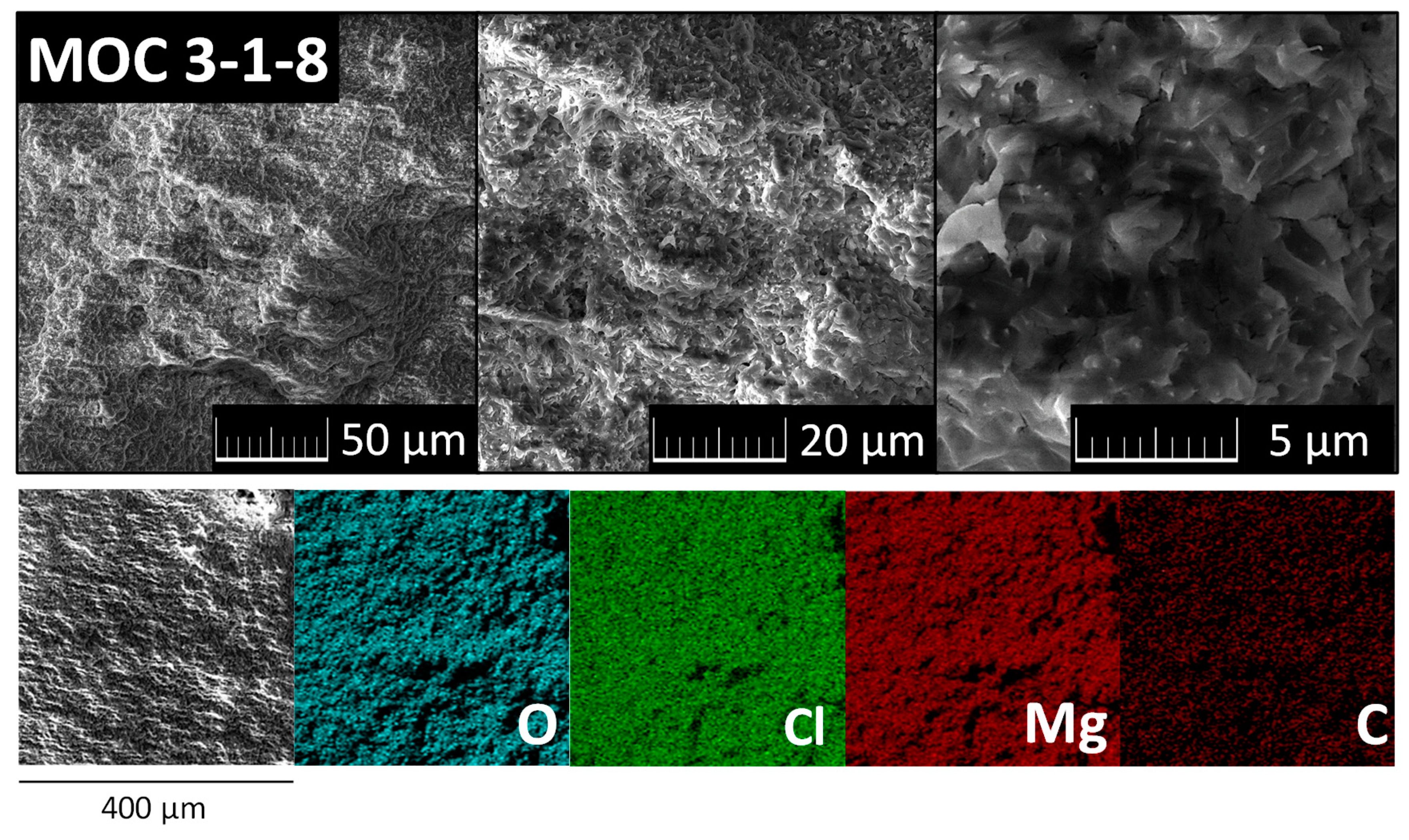
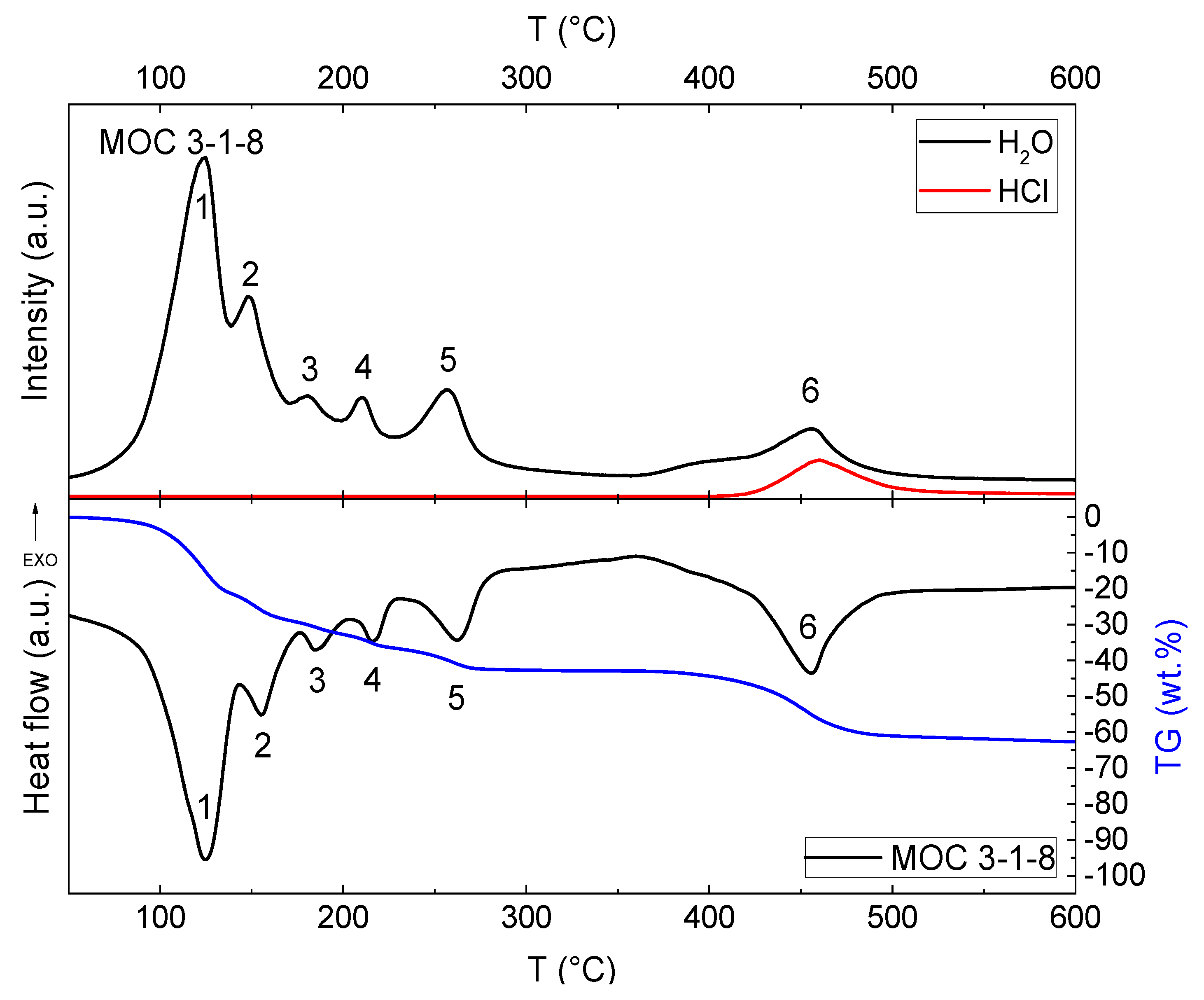
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Durability of new blended cements additioned with recycled biomass bottom ash from electric power plants. Constr. Build. Mater. 2019, 225, 429–440. [Google Scholar] [CrossRef]
- Bhagatg Singh, G.V.P.; Subramaniam, K.V.L. Production and characterization of low-energy Portland composite cement from post-industrial waste. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Souto-Martinez, A.; Arehart, J.H.; Srubar, W.V. Cradle-to-gate CO2 emissions vs. in situ CO2 sequestration of structural concrete elements. Energy Build. 2018, 167, 301–311. [Google Scholar] [CrossRef]
- Boesch, M.E.; Koehler, A.; Hellweg, S. Model for cradle-to-gate life cycle assessment of clinker production. Environ. Sci. Technol. 2009, 43, 7578–7583. [Google Scholar] [CrossRef] [PubMed]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Pivák, A.; Pavlík, Z. Experimental Analysis of MOC Composite with a Waste-Expanded Polypropylene-Based Aggregate. Materials 2018, 11, 931. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, M.; Zemanová, L.; Pokorný, J.; Záleská, M.; Jankovský, O.; Lojka, M.; Sedmidubský, D.; Pavlík, Z. Valorization of wood chips ash as an eco-friendly mineral admixture in mortar mix design. Waste Manag. 2018, 80, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Záleská, M.; Pavlík, Z.; Pavlíková, M.; Scheinherrová, L.; Pokorný, J.; Trník, A.; Svora, P.; Fořt, J.; Jankovský, O.; Suchorab, Z.; et al. Biomass ash-based mineral admixture prepared from municipal sewage sludge and its application in cement composites. Clean Technol. Environ. Policy 2018, 20, 159–171. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pavlík, Z.; Jankovský, O.; Pokorný, J.; Tydlitát, V.; Svora, P.; Černý, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Dehua, D.; Chuanmei, Z. The formation mechanism of the hydrate phases in magnesium oxychloride cement. Cem. Concr. Res. 1999, 29, 1365–1371. [Google Scholar] [CrossRef]
- Sorel, S. On a New Magnesium Cement. Comptes Rendus Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Demediuk, T.; Cole, W.F.; Hueber, H.V. Studies on magnesium and calcium oxychlorides. Aust. J. Chem. 1955, 8, 215–233. [Google Scholar] [CrossRef]
- Chau, C.K.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Dai, W.; Gong, C.; Lu, L.; Cheng, X. Effect of MgO on calcination and properties of belite-barium calcium sulphoaluminate cement clinker with Na2O and K2O. Ceram. Silikáty 2018, 62, 121–130. [Google Scholar] [CrossRef]
- Stephan, D.; Dikoundou, S.N.; Raudaschl-Sieber, G. Hydration characteristics and hydration products of tricalcium silicate doped with a combination of MgO, Al2O3 and Fe2O3. Thermochim. Acta 2008, 472, 64–73. [Google Scholar] [CrossRef]
- De la Torre, A.G.; De Vera, R.N.; Cuberos, A.J.M.; Aranda, M.A. Crystal structure of low magnesium-content alite: Application to Rietveld quantitative phase analysis. Cem. Concr. Res. 2008, 38, 1261–1269. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Freyer, D.; Bette, S.; Oestreich, M. 9Mg(OH)2·MgCl2·4H2O, a High Temperature Phase of the Magnesia Binder System. Inorg. Chem. 2010, 49, 9770–9779. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Oestreich, M.; Bette, S.; Freyer, D. 2Mg(OH)2·MgCl2·2H2O and 2Mg(OH)2·MgCl2·4H2O, Two High Temperature Phases of the Magnesia Cement System. Z. Anorg. Allg. Chem. 2012, 638, 628–633. [Google Scholar] [CrossRef]
- Tooper, B.; Cartz, L. Structure and Formation of Magnesium Oxychloride Sorel Cements. Nature 1966, 211, 64–66. [Google Scholar] [CrossRef]
- Matkovic, B.; Young, J.F. Microstructure of Magnesium Oxychloride Cements. Nature Phys. Sci. 1973, 246, 79–80. [Google Scholar] [CrossRef]
- Thompson, H.C. Fireproof Product Using Magnesium Oxychloride Cement. U.S. Patent No. 3,963,849, 15 June 1976. [Google Scholar]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2016, 49, 1319–1326. [Google Scholar] [CrossRef]
- Misra, A.K.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mat. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef]
- Biel, T.D.; Lee, H. Magnesium Oxychloride Cement Concrete with Recycled Tire Rubber. Transp. Res. Rec. 1996, 1561, 6–12. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Wang, L.; Wang, J. Effect of Magnesium oxychloride Cement on Stabilization/Solidification of Sewage Sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar]
- Li, G.; Yu, Y.; Li, J.; Wang, Y.; Liu, H. Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cem. Concr. Res. 2003, 33, 1663–1668. [Google Scholar] [CrossRef]
- Karimi, Y.; Monshi, A. Effect of magnesium chloride concentrations on the properties of magnesium oxychloride cement for nano SiC composite purposes. Ceram. Int. 2011, 37, 2405–2410. [Google Scholar] [CrossRef]
- Montle, J.F.; Mayhan, K.G. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C.K. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood–magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef]
- Weng, R.; Liu, N.; Liu, X.Y.; Yang, X.L. Study of water resistance of magnesium oxychloride cement reinforced by glass fiber. J. Korean Ceram. Soc. 1988, 15, 30–34. [Google Scholar]
- Xia, S.; Xing, P.; Gao, S. Studies on the basic compounds of magnesia cement: The thermal behaviour of magnesium oxychlorides. Thermochim. Acta 1991, 183, 349–363. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Hu, C.L.; Yang, S.Q.; Li, Z.J. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Sglavo, V.M.; De Genua, F.; Conci, A.; Ceccato, R.; Cavallini, R. Influence of curing temperature on the evolution of magnesium oxychloride cement. J. Mater. Sci. 2011, 46, 6726–6733. [Google Scholar] [CrossRef]
- Liu, Z.; Balonis, M.; Huang, J.; Sha, A.; Sant, G. The influence of composition and temperature on hydrated phase assemblages in magnesium oxychloride cements. J. Amer. Ceram. Soc. 2017, 100, 3246–3261. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Beaudoin, J.J.; Ramachandran, V.S. Strength development in magnesium oxychloride and other cements. Cem. Concr. Res. 1975, 5, 617–630. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Li, Z.; Li, H. Simulation of the properties of MgO-MgfCl2-H2O system by thermodynamic method. Cem. Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- Shi, E.; Ling, Z.; Wang, A. MIR, NIR and Raman Spectra of magnesium chlorides with six hydration degrees–implication for Mars and Europe. J. Raman Spectrosc. 2019, 1–14. [Google Scholar] [CrossRef]
- Sugimoto, K.; Dinnebiera, R.E.; Hansonb, J.C. Structures of three dehydration products of bischofite from in situ synchrotron powder diffraction data (MgCl2nH2O; n = 1, 2, 4). Acta Cryst. 2007, B63, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Halasz, I.; Freyer, D. The crystal structures of two anhydrous magnesium hydroxychloride phases from in situ synchrotron powder diffraction data. Z. Anorg. Allg. Chem. 2011, 637, 1458–1462. [Google Scholar] [CrossRef]
- Cole, W.; Demediuk, T. X-ray, thermal, and dehydration studies on magnesium oxychlorides. Aust. J. Chem. 1955, 8, 234–251. [Google Scholar] [CrossRef]
- Lojka, M.; Jiříčková, A.; Lauermannová, A.-M.; Pavlíková, M.; Pavlík, M.; Jankovský, O. Kinetics of formation and thermal stability of Mg2(OH)3Cl·4H2O. AIP Conf. Proc. 2019, 2170. [Google Scholar] [CrossRef]


| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3434 | stretching modes (ν) of H–O–H in H2O |
| 3654, 3638 | stretching modes (ν) of O–H in Mg(OH)2 |
| 3319 | stretching modes (ν) of H–O–H in H2O |
| 2053 | bending (δ) and rocking (ρ) vibrations of H–O–H in H2O |
| 1608 | bending (δ) vibration of H–O–H in MgCl2.8H2O |
| 1623, 1558, 1520, 1476, 1440 | stretching modes (ν) of Mg–O in MgCl2.8H2O |
| 845 | stretching vibration (ν) of the Mg–O cubic structure |
| 634 | stretching (ν) vibrations of Mg–O |
| 581 | deformation (δ) and stretching (ν) vibrations of Mg–Cl |
| 489 | translation vibrations of Mg/Mg–O, Mg–OH |
| 417 | vibrational modes of the lattice showing the Mg–O/Mg2+, O/O–Mg–O/O–Mg2+–O bonds |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z.; Pavlíková, M. Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O. Materials 2020, 13, 767. https://doi.org/10.3390/ma13030767
Lojka M, Jankovský O, Jiříčková A, Lauermannová A-M, Antončík F, Sedmidubský D, Pavlík Z, Pavlíková M. Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O. Materials. 2020; 13(3):767. https://doi.org/10.3390/ma13030767
Chicago/Turabian StyleLojka, Michal, Ondřej Jankovský, Adéla Jiříčková, Anna-Marie Lauermannová, Filip Antončík, David Sedmidubský, Zbyšek Pavlík, and Milena Pavlíková. 2020. "Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O" Materials 13, no. 3: 767. https://doi.org/10.3390/ma13030767
APA StyleLojka, M., Jankovský, O., Jiříčková, A., Lauermannová, A.-M., Antončík, F., Sedmidubský, D., Pavlík, Z., & Pavlíková, M. (2020). Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O. Materials, 13(3), 767. https://doi.org/10.3390/ma13030767








