3.1. Processing Effects on the Phase Composition
The selected processing route for the preparation of titania-based composites included the powder pressing as one of the steps, and further sintering of the ceramic samples. From the first look, this approach does not appear to be optimal for processing materials suitable as photocatalysts, where nanostructuring and a large available surface area are usually required [
27]. However, the selected method allowed to provide better contact between the rutile and SiC particles during the pre-reduction reaction and to minimize surface oxidation of silicon carbide without reaction with TiO
2. To avoid ambiguities arising from intermediate ceramics grinding, both steps of processing in quasi-inert Ar atmosphere and post-oxidation were done for ceramic samples. Preliminary tests showed that the selected range of processing conditions does not result in excessive densification (the ceramic samples were porous enough to soak water). Thus, sufficient open porosity of the pre-reduced samples was provided.
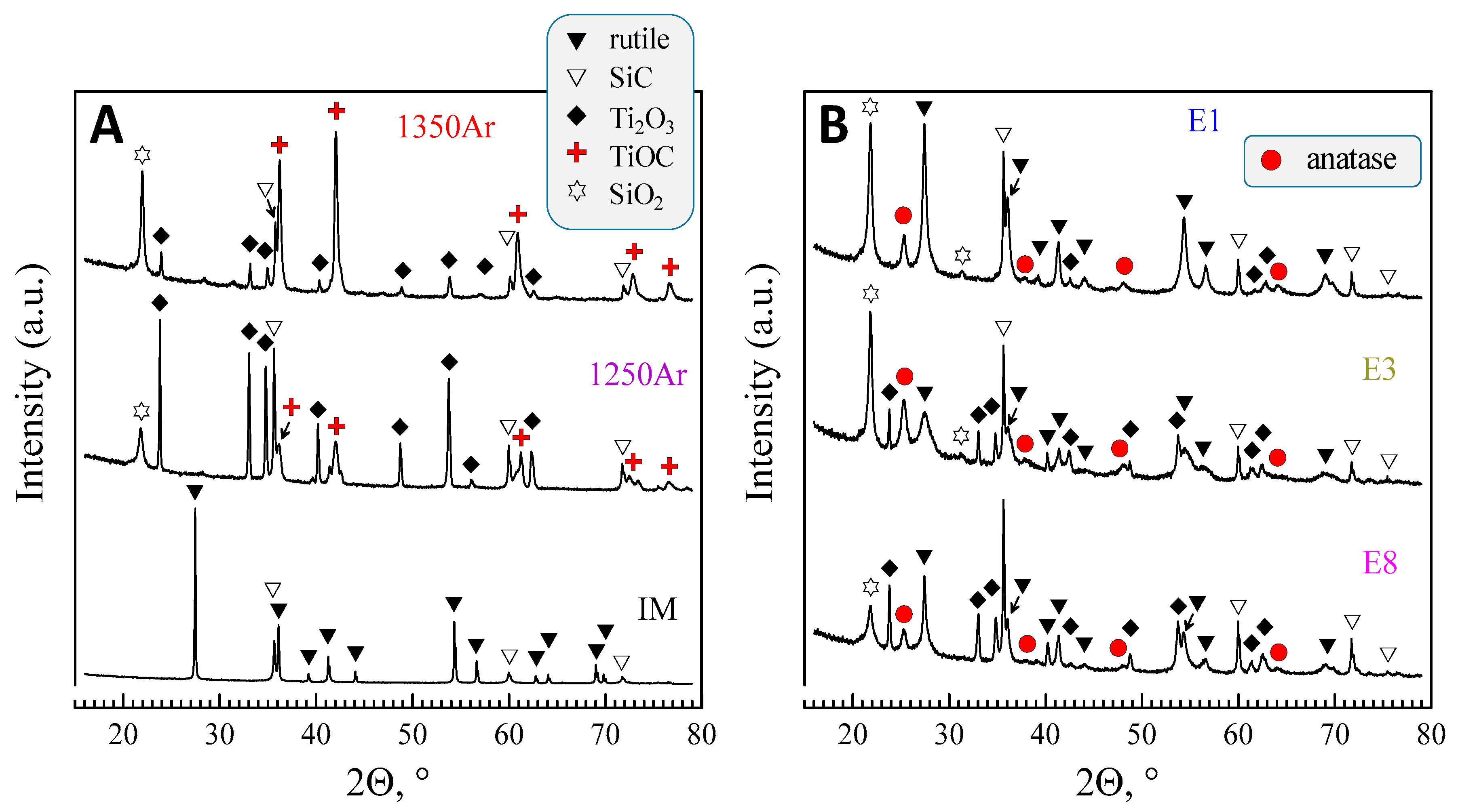
The XRD results obtained for the samples processed in Ar atmosphere are shown in
Figure 2. The results depict significant changes in the phase composition as compared to the initial mixture. The formation of at least three new phases, including reduced titanium oxide (Ti
2O
3, ICDD PDF #04-005-8760), titanium oxycarbide (TiOC, ICDD PDF #04-002-5443), and silicon oxide (SiO
2, ICDD PDF #04-008-7640) was observed.
The results of the XRD quantification are given in
Table 4.
In this table, the quantitative results in wt%. obtained by the Rietveld refinement of the XRD data were converted to mol% in order to better illustrate the reaction stoichiometry. It should be noticed that the nominal molar Ti/Si ratio of the initial mixture (1:1) is kept at an acceptable level in the results of the XRD quantification. As an example, this ratio corresponds to 0.97, 1.09, and 0.98 in the case of the samples 1250Ar, 1300Ar, and 1350Ar, accordingly. The latter is a good confirmation of the quality of the XRD quantification and its relevance for the analysis of phase transformations observed in the present work.
In general, assuming the observed phase composition, the reaction between titania and silicon carbide can be represented as follows:
However, this only describes the partial conversion of silicon carbide, and should yield about 40 mol% of residual SiC, 20 mol% Ti
2O
3, 20 mol% TiCO, and 20 mol% SiO
2; this is only close to the results obtained at 1250 °C. Thus, one should consider the uptake of residual oxygen from the nominally inert Ar atmosphere (about 10 ppm), or due to imperfect sealing, possibly combined with slight carbon excess (or Si:C < 1), as often found in β-SiC, and also suggested by mol% SiO
2 < mol% TiOC. Thus, one may assume a generic reaction:
Equation (2) may describe departure from Equation (1), when y ≈ 1/3, and convergence to:
mainly at the highest T
Ar temperature, as indicated by the prevailing formation of TiOC and drastic decrease of the fraction of Ti
2O
3 in the sequence TiCO > SiO
2 >> Ti
2O
3.
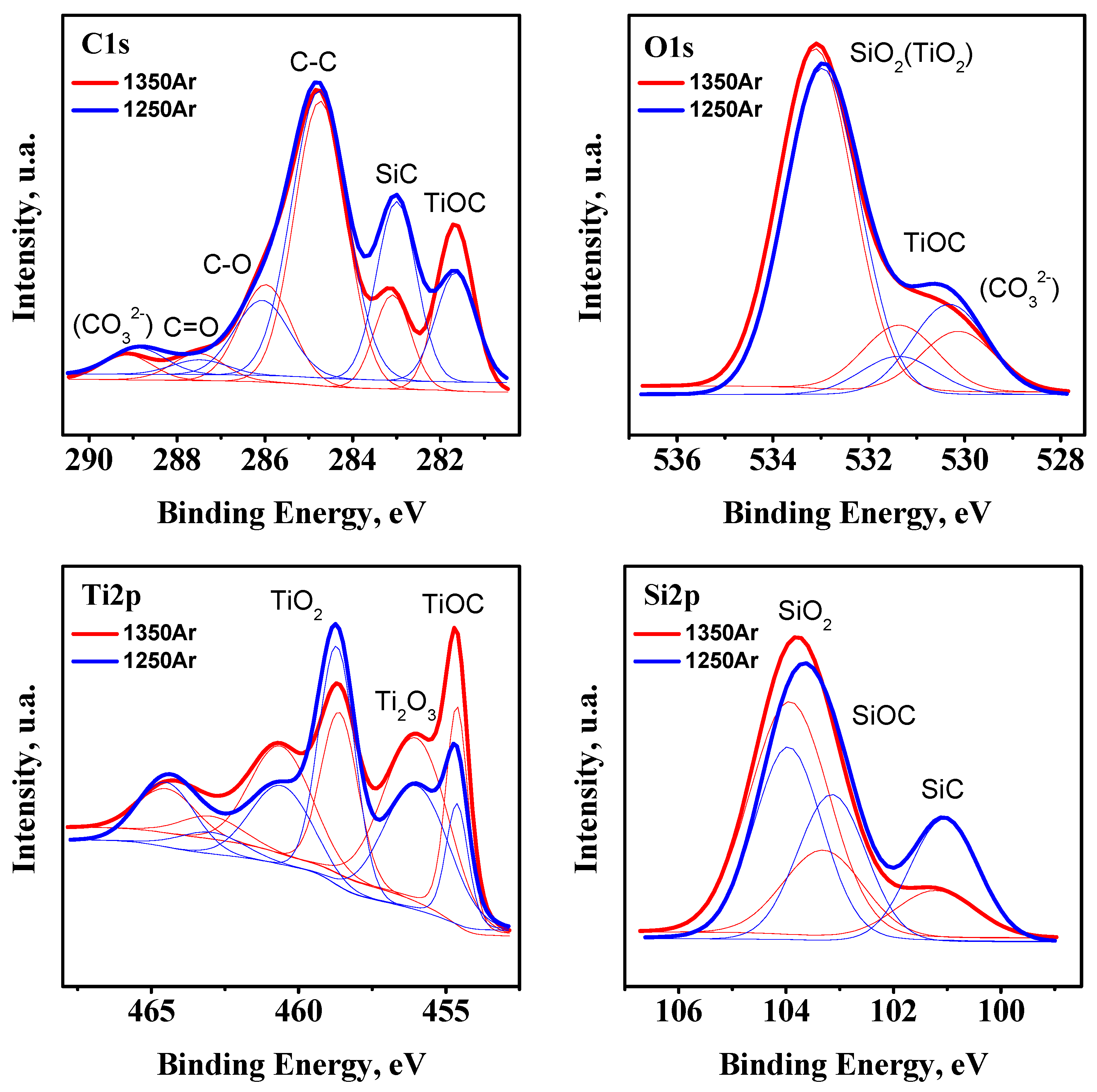
Additional XPS studies of the pre-reduced samples were performed in order to confirm the XRD observations (
Figure 3).
Since the XPS studies were performed for powdered samples produced under ambient air conditions, the C 1
s core level spectrum shows the presence of adventitious carbon species, which correspond to C-C, C-O, and C=O bonds, with binding energies (BE) ~284.7, ~286.0, and 287.5 eV, and carbonates (CO
32−) (BE ~289.0 eV) and with the corresponding O 1
s contribution at ~530.2 eV due to carbonates. The shape of the Ti 2
p core level spectrum suggests the presence of a significant fraction of TiO
2, along with the expected Ti
2O
3 (BE for Ti 2
p3/2 ~458.6 and 456.0 eV, respectively) [
28]. In addition to titanium oxide species, the results confirm the presence of TiOC, with a BE of ~454.7 eV for Ti 2
p3/2 and a contribution at ~281.7 eV in the C 1
s core level spectrum [
29]. Since XPS is a surface characterisation technique, the presence of a significant fraction of TiO
2 may arise from the partial surface oxidation of the powders before analysis. However, even more considerable uncertainties are expected from strong chemical inhomogeneity of the pre-reduced samples, including the presence of core-shell structures discussed below, with the surface shell composed mostly of silica. Further SiO
2 and SiC species are also ascribed to BE for contributions in the Si 2
p spectrum at ~103.9 and ~101.2 eV, respectively. As an example, an attempt to quantify the SiO
2/SiC molar ratio from the XPS data gives the overestimated values of 3.7 and 9.1 for 1250Ar and 1350Ar samples, respectively, as compared to the data in
Table 4 based on the results of the bulk XRD analysis. Still, in the latter case this ratio might be, in turn, partially underestimated due to possible amorphous structures of the silica shells. However, the results of XRD analysis appear to show a rather relevant picture of the phase composition, confirmed by the Ti/Si ratio close to the nominal 1:1 (
Table 4). The slight asymmetry of the main Si 2
p peak suggests the presence of the SiOC phase (BE of ~103.2 eV for Si 2
p), not detected by the XRD, but a likely amorphous or intermediate product SiC
xO
y between initial SiC and SiO
2 phases [
30], similarly to the case of TiOC.
The formation of titanium oxycarbide by carbothermal reduction of titania in inert or CO-containing atmospheres at the temperatures close to those employed in this work was previously observed in many studies (for example, [
31,
32]). In agreement with the cited papers, higher temperatures of the treatments in Ar increase the fraction of TiOC formed, the corresponding fraction of SiC decreases on the temperature increase. The deviations of TiOC to SiO
2 molar ratio from 1:1, predicted in accordance with Equation (1), can be attributed to the partial oxidation of silicon carbide by oxygen residues presented in Ar and the possible presence of the amorphous phase. In general, the results suggest that the extent of the reaction between rutile and silicon carbide can be well controlled by sintering conditions. Although the time of Ar treatments was not varied in this work, kinetic control of this reaction is expected to allow flexible tuning of the phase composition and residual amount of SiC phase.
The pre-reduction step in Ar atmosphere allowed one to attain two prerequisites for further anatase formation and stabilization, aiming at tunable photocatalyst compositions. Firstly, both Ti-containing phases formed at pre-reduction (TiCO and Ti
2O
3) are capable of forming TiO
2 on oxidation, either in the form of rutile or anatase, or as a co-existence of these polymorphs by post-oxidation at intermediate temperatures. Secondly, the presence of silica is expected to stabilize the fraction of anatase, in accordance with the results [
22,
23]. Thus, relatively mild post-oxidation conditions were selected, including the temperatures in the range 400–500 °C and different times. The selected XRD patterns of the oxidised samples are shown in
Figure 2B.
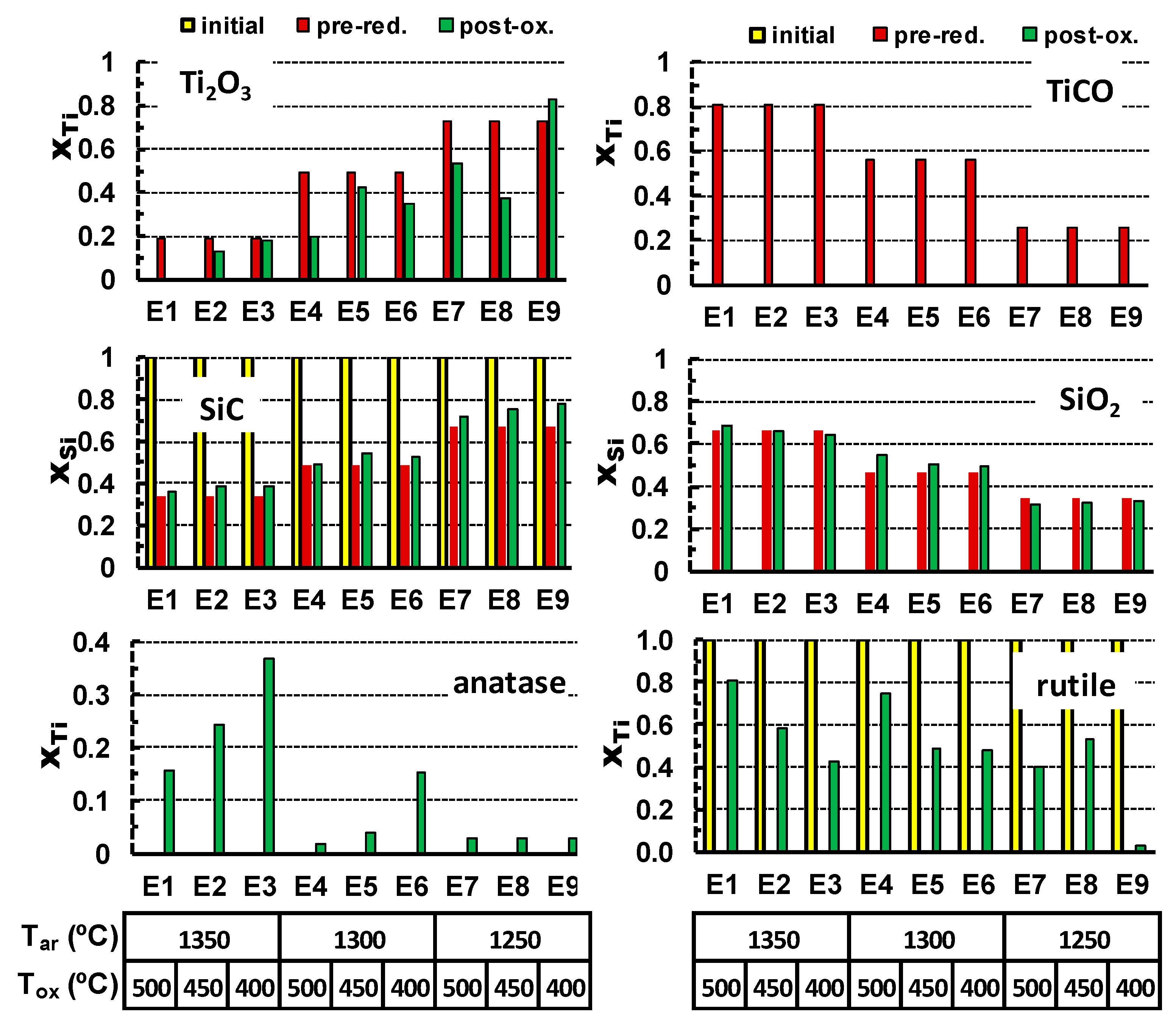
The oxidation results in the vanishing of titanium oxycarbide phase and formation of both anatase and rutile polymorphs of TiO
2. At the same time, Ti
2O
3 is still present in the samples after oxidation and disappears only in extreme oxidising conditions as 500 °C for 25 h. Corresponding results of the XRD quantification are also shown in
Table 4, and guidelines for relevant reactions are shown in
Figure 4. In this case, molar quantities of anatase, rutile (Y-axis), and other phases are converted to corresponding fractions of Ti or Si, to follow gradual changes from the initial precursors, through pre-reduction and the after post-oxidation. These results show the impact of TiCO on final yield of anatase after post-oxidation, combined with the opposite effect of the increase in oxidation temperature, which favours conversion to rutile. Note also that the residual content of SiC after the pre-reduction stage remains nearly unchanged upon oxidation at temperatures in the range 400–500 °C. The contents of SiO
2 also remain nearly unchanged during the oxidation stage.
Other useful guidelines for the effects of processing conditions on the phase composition of the oxidised samples can be obtained from assessing the results of Taguchi experimental planning (
Table 5).
The impact of each parameter was analysed using the linear regression model:
The correlation matrix and the values of regression coefficients obtained for the post-oxidised samples suggest the following trends. The processing conditions in Ar, which result in pre-reduction of titania, are of special importance for the anatase formation. Namely, a higher pre-reduction temperature enhances the yield of TiCO, which facilitates the formation of anatase by subsequent oxidation at much lower temperatures. Again, this correlates well with the trends in the formation of titanium oxycarbide, which, for the selected post-oxidising conditions, appears to be the main precursor for the anatase formation (
Table 4 and
Figure 4). The negative value of the correlation factor between the Ar-processing temperature and contents of Ti
2O
3 seems counterintuitive. One expects rather an increase in Ti
2O
3 concentration on increasing this temperature. However, this can be explained by the fact that the higher Ar processing temperature rather facilitates the formation of TiOC as compared to Ti
2O
3 (
Table 4).
Upon oxidation, TiOC is fully converted to anatase/rutile even at the lowest oxidation temperature/time, while the Ti2O3 residuals still remain and thus suggest this type of apparent correlation. A higher oxidising temperature (Tox) appears favourable for the rutile formation as compared to anatase. The main parameter affecting the amount of SiO2 in the post-oxidized samples is the Ar-processing temperature, as indicated by the corresponding correlation factor above 0.98, while the factors for Tox and tox are close to zero, indicating a very weak correlation with these parameters. The oxidation kinetics may be illustrated by the tox parameter, showing a moderate relevance of the oxidation time for the formation of rutile and, probably, for vanishing Ti2O3 oxide.
3.2. Microstructural Evolution
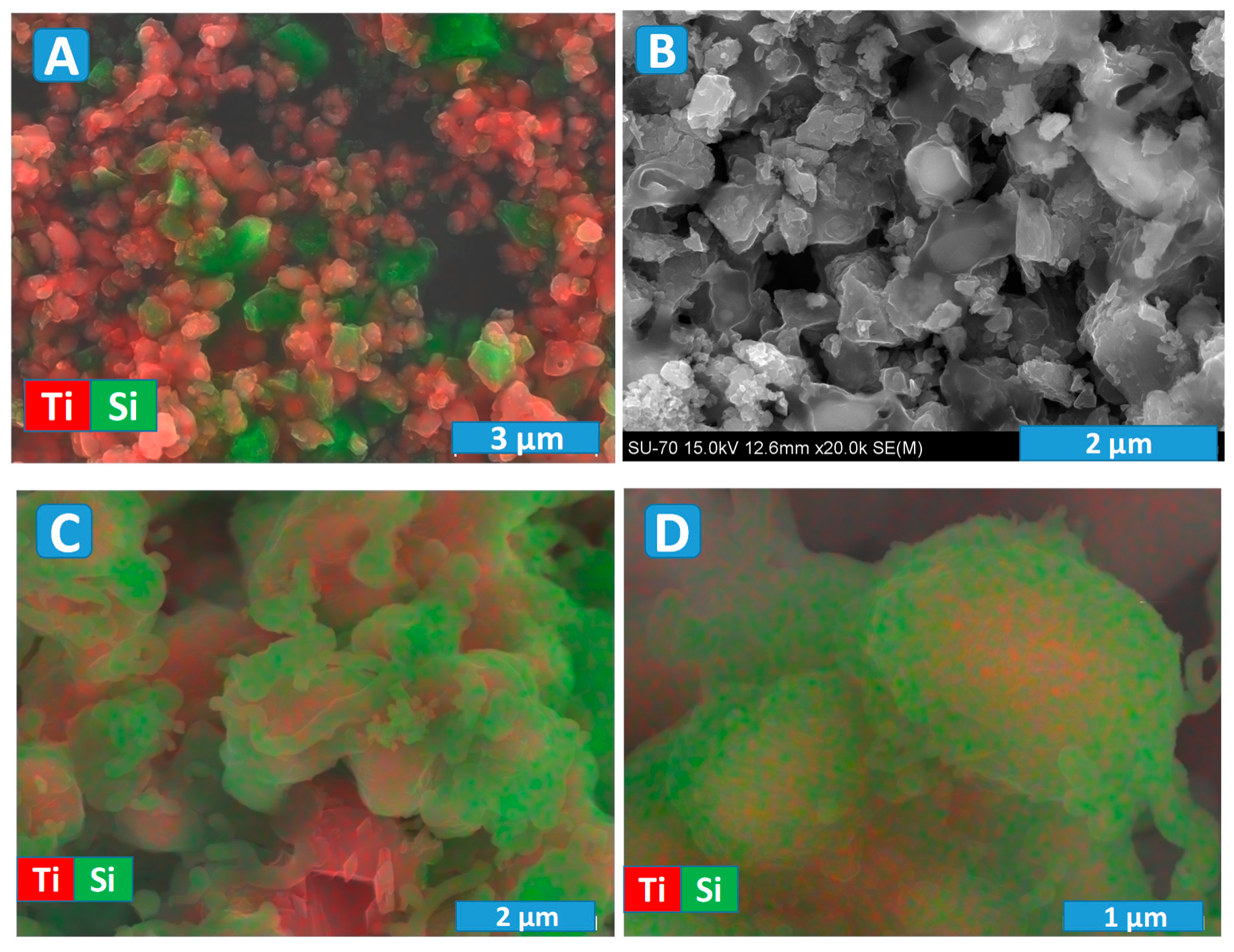
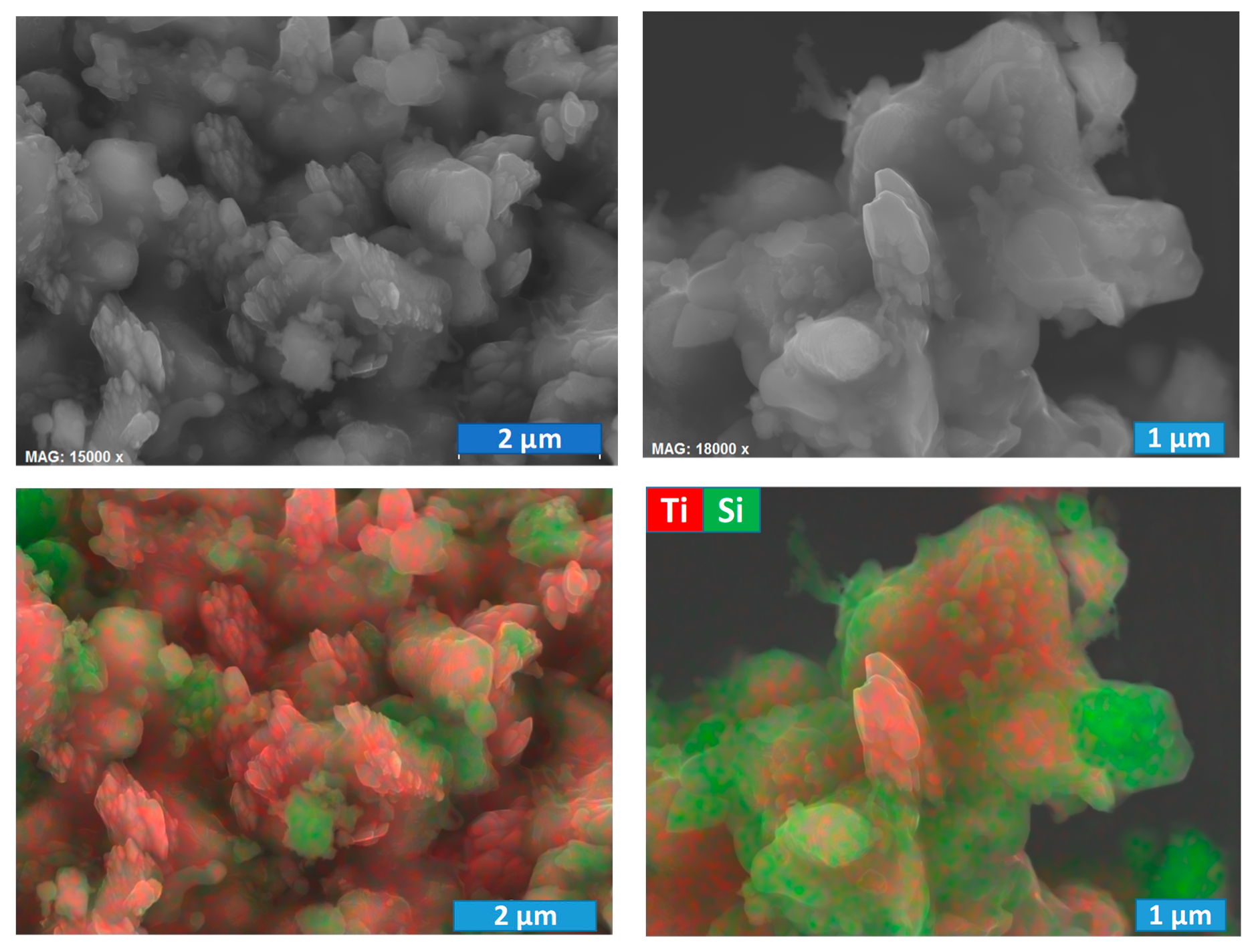
A typical microstructure of the IM powder and corresponding distribution of the titania and silicon carbide particles are presented in
Figure 5A. The particles size is similar to that for initial precursor powders, where SiC is mostly of micrometric size, while TiO
2 rutile particles are of submicrometric or even nanometric (agglomerated) size. Indeed, the silicon carbide is well-known as a very hard material, and it was impossible to reduce its particle size by the milling procedure employed in the present work. Still, the used milling allows sufficient homogenisation, as it follows from the chemical analysis map (
Figure 5A).
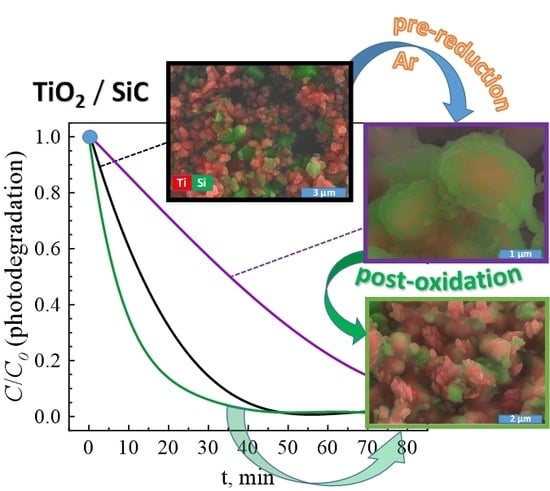
An example of the microstructure of the ceramic samples processed under Ar atmosphere is shown in
Figure 5B. These results again evidence a significant porosity, which is expected to minimise oxygen diffusion limitations during following the post-oxidation step. The observed microstructures suggest the presence of a noticeable interaction between initial composite components, in agreement with the XRD results (
Figure 2) discussed above. In particular, one can observe the formation of core-shell-like particles with a lighter core surrounded with a darker shell (
Figure 5B). Thus, the prospective photocatalytic properties might be affected by microstructural features of the prepared materials, in addition to the phase composition.
More details regarding the composition of those core-shell particles can be revealed using EDS analysis, combined with SEM (
Figure 5C,D). The images illustrate quite typical microstructural features in the sample, processed in Ar at an intermediate temperature of 1300 °C. The shell is enriched in silicon, and the corresponding morphology is very similar to a glassy/amorphous state. Since the silicon carbide particles have initially an absolutely different shape (
Figure 5A), the shell is likely composed of SiO
2, in agreement with the XRD results. Unfortunately, the used equipment and procedure do not allow tracking of the carbon contrast in the samples, since they required preliminary carbon deposition before the SEM/EDS characterisation. Thus, the core is composed of Ti
2O
3 and TiOC; the latter is rather likely formed closer to the surface in the space confined by the contact between initial TiO
2 and SiC particles. Some Ti-rich particles have the surface clear from SiO
2 (
Figure 5C), while other particles look completely blocked (
Figure 5C,D). Such blocking may impede oxidation, which is necessary for the formation of anatase-containing samples from Ar-processed materials. On the other hand, it could facilitate very delicate, kinetically controlled oxidation, which might be rather suitable for anatase formation and nanostructuring.
The microstructural studies performed for post-oxidised samples show the formation of more open structures (
Figure 6), while part of the Ti-rich particles still has a SiO
2 surface blocking layer.
Oxidation and the corresponding volume increase seem at least partially to destroy the SiO
2 shell, leading to the formation of nanostructured titania polymorphs. The latter is especially well illustrated by the results obtained for the E4 oxidised sample, where columnar submicro- and nanosized titania-based structures appear to grow through the silica shell (
Figure 6). Secondly, the observed microstructures of the oxidised samples look quite similar and do not allow revealing any tendencies with temperature/oxidation time based only on the microstructural studies. Thus, these results basically highlight that possible promoting effects on the photocatalytic performance, imposed by the formation of anatase and rutile under oxidising conditions, may be at least partially hindered by the surface blocking layers composed of SiO
2. On the other hand, silica is known to have a promoting effect on the photocatalytic performance of titania, which involves the formation of local Ti-O-Si bonds [
33]. In particular, a common strategy to benefit from this synergy includes the preparation of SiO
2@TiO
2 core-shell nanoparticles with enhanced activity [
34]. Although the core-shell structures produced in the present work have, to a certain extent, an inversed architecture comprised of a titanium-rich core and silica shell, one still expects some synergistic effects towards photocatalysis based on TiO
2-SiO
2 interaction and formation of local Ti-O-Si linkages, invisible for the XRD technique. The corresponding photocatalytic tests were performed and described in the next section.
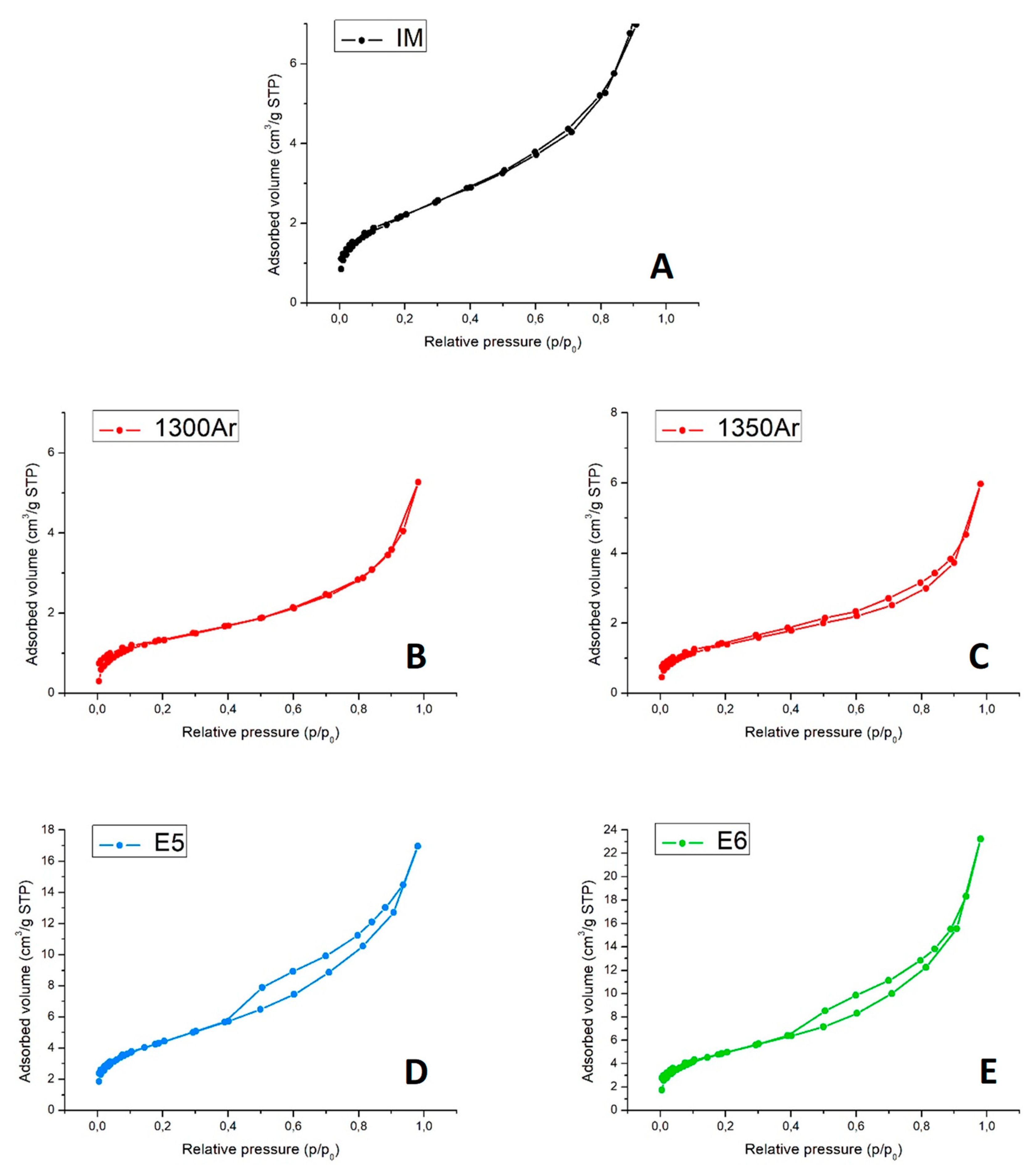
Figure 7 presents the N
2 adsorption–desorption isotherms at −196 °C.
The N
2 adsorption–desorption isotherms of the initial mixture and reduced samples (
Figure 7A–C) are of type II, which is characteristic of nonporous materials [
35]. This is somewhat expected, taking into account the nature of the composite precursors’ powders with a (sub) micrometric particle size. Moreover, the reaction between titania and silicon carbide at elevated temperatures and formation of the core-shell structures are not likely to result in the appearance of the micro- and mesoporosity. On the contrary, the isotherms of oxidized samples (
Figure 7D,E) present a hysteresis loop and are of type IV, which is characteristic of mesoporous sorbents. The results suggest that oxidising conditions induce the formation of some mesoporosity (pore width in the range 2–50 nm), very likely being promoted by the growth of nanostructured anatase.
Finally,
Table 6 presents the values of the specific surface area and total pore volume.
Overall, the materials present a low total porosity. The results are in excellent agreement with the SEM/EDS studies, discussed above. The formation of core-shell structures results even in a decrease of the specific surface area. At the same time, the nanostructuring promoted by the delicate oxidation of the samples significantly enlarges the available surface, with the expected impact on the photocatalytic activity.
3.3. Photocatalytic Activity
For the sake of comparison, the photocatalytic studies were performed for the initial IM powder, standard P25 TiO
2-based photocatalyst as a reference and the samples converted to powder after processing under Ar and air atmospheres. During the photocatalytic process, the photo-generated electrons in the materials under study are transferred to the nearby methylene blue molecules and participate in the redox reactions resulting in the decomposition of methylene blue into H
2O and CO
2 [
36,
37].
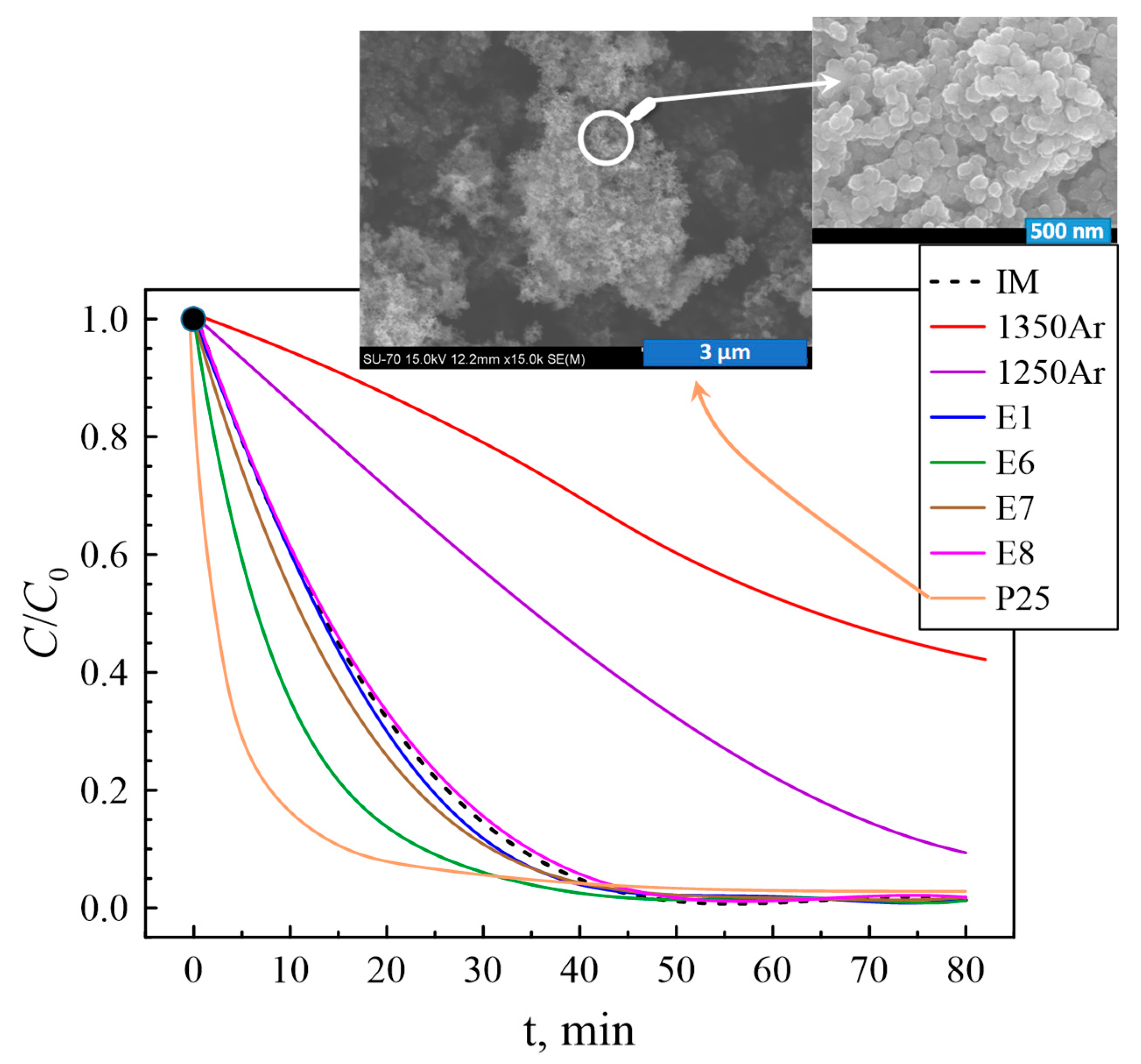
Figure 8 presents a C/C
0 plot against irradiation time for several selected compositions, where
C0 is the initial concentration after dark conditions stage and
C is the concentration of methylene blue at different test times.
The initial IM powder shows a noticeable photocatalytic activity provided by the presence of rutile. As expected, the fastest photodegradation of the methylene blue was expectedly observed in the case of the standard P25 TiO
2 powder, containing anatase and rutile phases in a ratio of about 4:1. As an example, the insets in
Figure 8 show the typical microstructure of P25 particles, with a high degree of nanostructuring and obviously higher surface area as compared to the Ar-processed and post-oxidised samples produced in this work (
Figure 5 and
Figure 6). The Ar-processed nonoxidised samples, containing Ti
2O
3 and TiOC, demonstrate the lowest rate of photocatalytic degradation. Based on the results on specific surface areas calculated from the BET equation (
Table 6), this behaviour can be attributed to the blocking of the active area, as also revealed by combined SEM/EDS studies (
Figure 5C,D). It should be noticed that, although the presence of significant amount of Ti
3+ species may facilitate the recombination of the charge carriers and, thus, effectively suppress the photodegradation, the photoactivation process for TiOC and Ti
2O
3 phases may be actually shifted to the visible light [
28,
38,
39], while the studies in the present work were performed using an ultraviolet lamp. This means that Ar-processed samples may still possess some photocatalytic activity under visible light, although microstructural constraints imposed by the presence of silica shells are likely to counterbalance this improvement.
On the contrary, the major part of the post-oxidised samples promotes faster photodegradation of the methylene blue as compared to the initial IM mixture, in agreement with the tendencies observed for the phase composition and specific surface area. The best performance was observed for the E6 sample, prepared by processing in Ar at an intermediate temperature (1300 °C) and post-oxidised at the lowest temperature (400 °C) for the longest oxidation time of 25 h. The latter may indicate that the range of processing conditions under oxidising atmosphere should be further optimised taking into account possible kinetic limitations if aiming at further improvement of the photocatalytic properties.
Finally, the impacts of the processing conditions on photocatalytic activity of the post-oxidised samples were analysed based on the values of the apparent rate constant of the first-order kinetics (
Kap) and the half-life time of photocatalytic degradation (
t1/2), expressed by Equations (5) and (6), respectively:
The calculated values of the rate constant and half-life time are given in
Table 7, as well as the linear regression correlation coefficient (R
2).
The highest Kap value of 0.0893 min−1 and lowest t1/2 of 7.8 min were observed for the E6 sample. On the other hand, in the same testing conditions, the performance of industrial P25 photocatalyst is roughly ~1.6 better than that for the E6 sample. It should be noticed that the actual fraction of the photocatalytically-active species is significantly higher in P25, due to the presence of silica-based species in the post-oxidised samples. However, a rather important issue is that the surface area of the best-performing E6 sample is ~2.7 times lower than for P25. Thus, in terms of the area-specific photocatalytic activity, several post-oxidized materials show comparable values or even outperform the P25 photocatalyst. These results show an obvious potential for designing the performing titania-based photocatalysts using the proposed approach.
Table 8 presents the values of correlation factors for the effects of processing conditions on photocatalytic activity of the post-oxidised samples.
Surprisingly, but these results predict the major impact of the oxidation time on
Kap and
t1/2 values, while a similar analysis for the phase composition (
Table 5) suggests that the amounts of Ti
2O
3, anatase, rutile, and SiO
2 phases are determined by the Ar-processing and post-oxidation temperatures rather than by
tox. It should be noticed that the maximum difference in the measured photocatalytic activities between E1, E2, E3, E7, E8, and IM samples corresponds to ~30%. Such a moderate variable variation may increase the errors associated with the Taguchi plan analysis, while the relevance of the
tox may be simply conditioned by the fact that the highest photocatalytic performance was observed for the sample oxidized for 25 h. In addition to the phase composition, the photocatalytic activity is strongly influenced by the microstructural features and availability of the surface for the reaction, which, in turn, can be affected by the formation of core-shell particles. As an example, while in several cases the formation of anatase/rutile mixture by post-oxidation is expected to be advantageous for the photodegradation reaction rate as compared to the initial mixture, partial blocking of the active surface by silica may impair this positive effect. Additional studies are required to confirm the relevant mechanisms behind the observed effects of the processing conditions on the phase composition and photocatalytic performance. Still, in terms of the degradation efficiency compared to irradiation time, the best photocatalytic activity observed for the E6 redox-tuned sample is comparable or even exceeds the corresponding performance for several nanostructured photocatalysts found in the literature data (
Table 9).
Although some differences may originate from the variation in testing methodology, the results clearly demonstrate the potential of the proposed concept for designing titania-based multifunctional photocatalysts by controlled redox reactions. However, further tests involving different dyes/contaminants, behaviour under visible light irradiation and microwave heating, and reusability assessment are essential on the way towards practical applications.