Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PPY/rGO Hybrid Coatings by CV Mode
2.3. Synthesis of PPy/rGO Hybrid Coating by Galvanostatic Mode
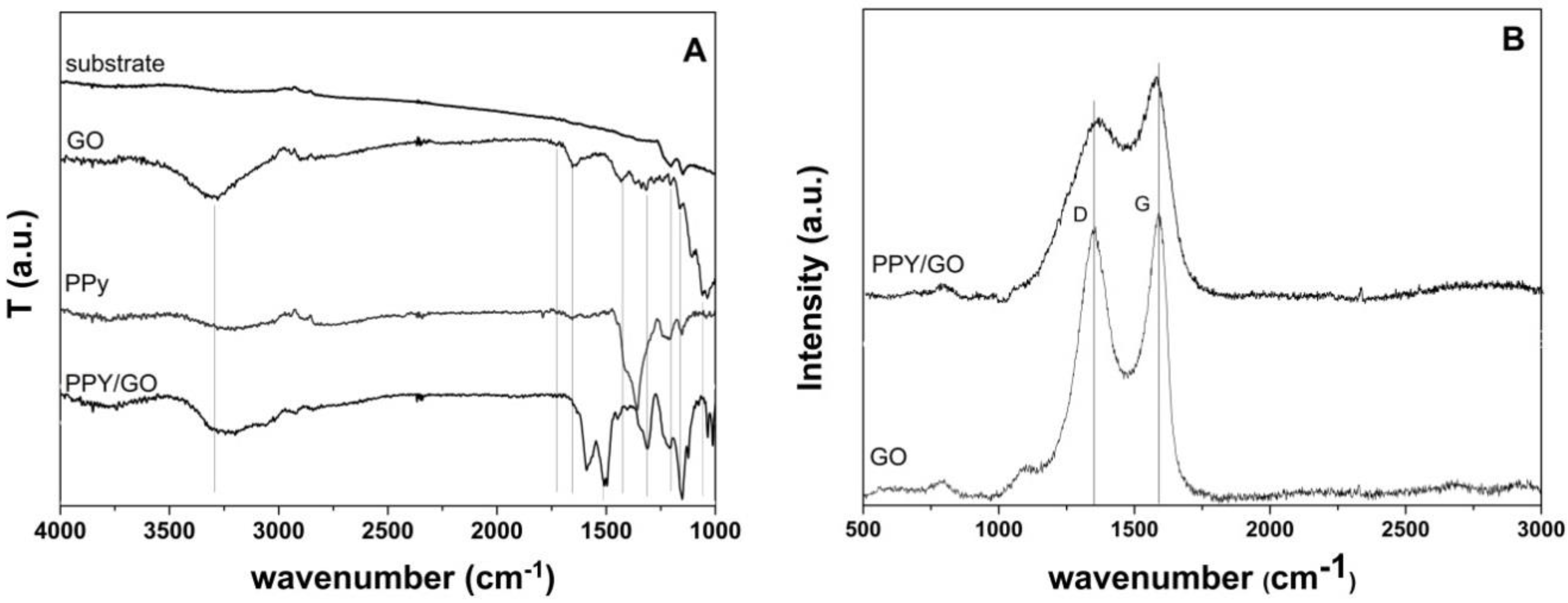
2.4. Characterization
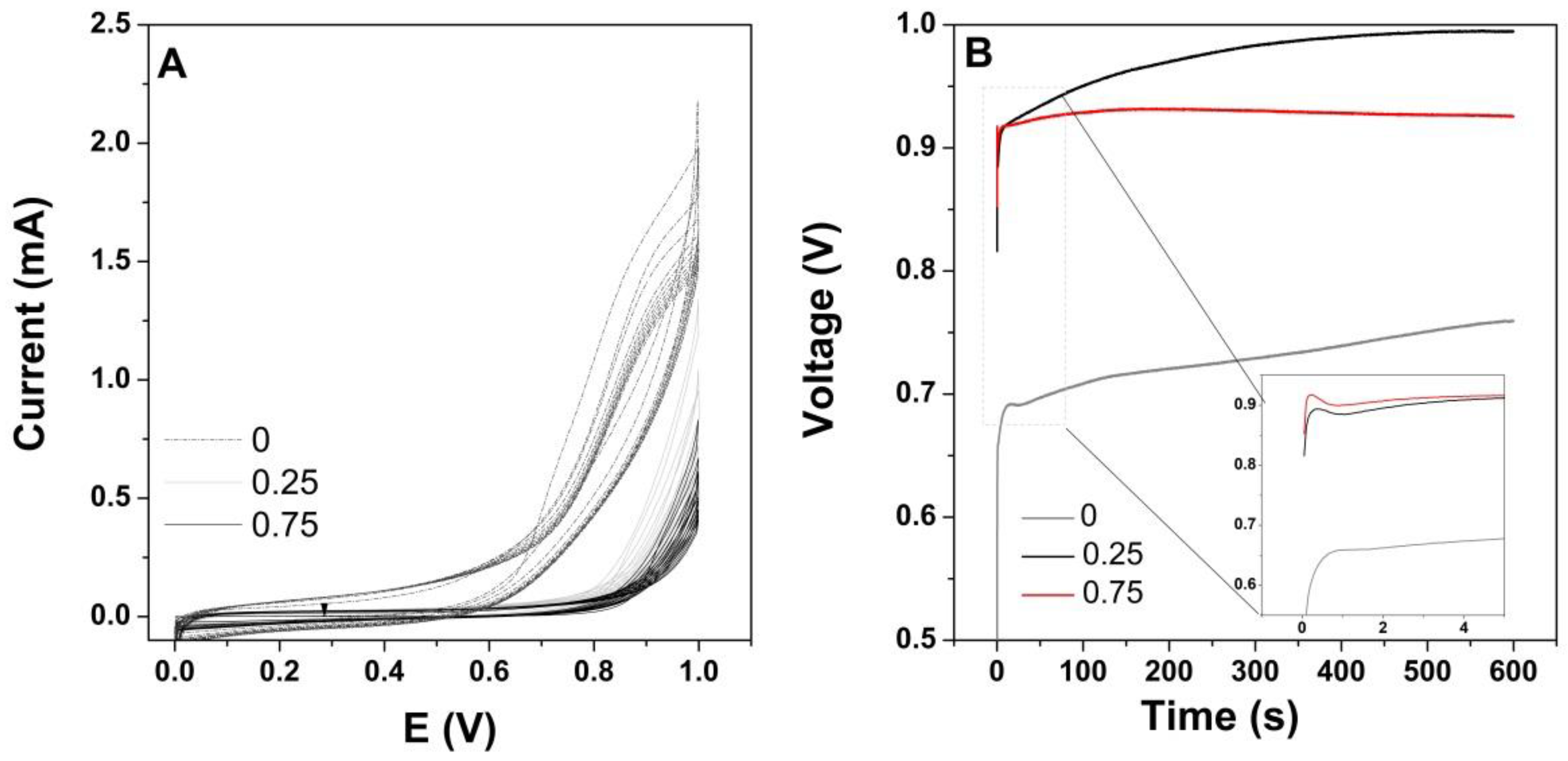
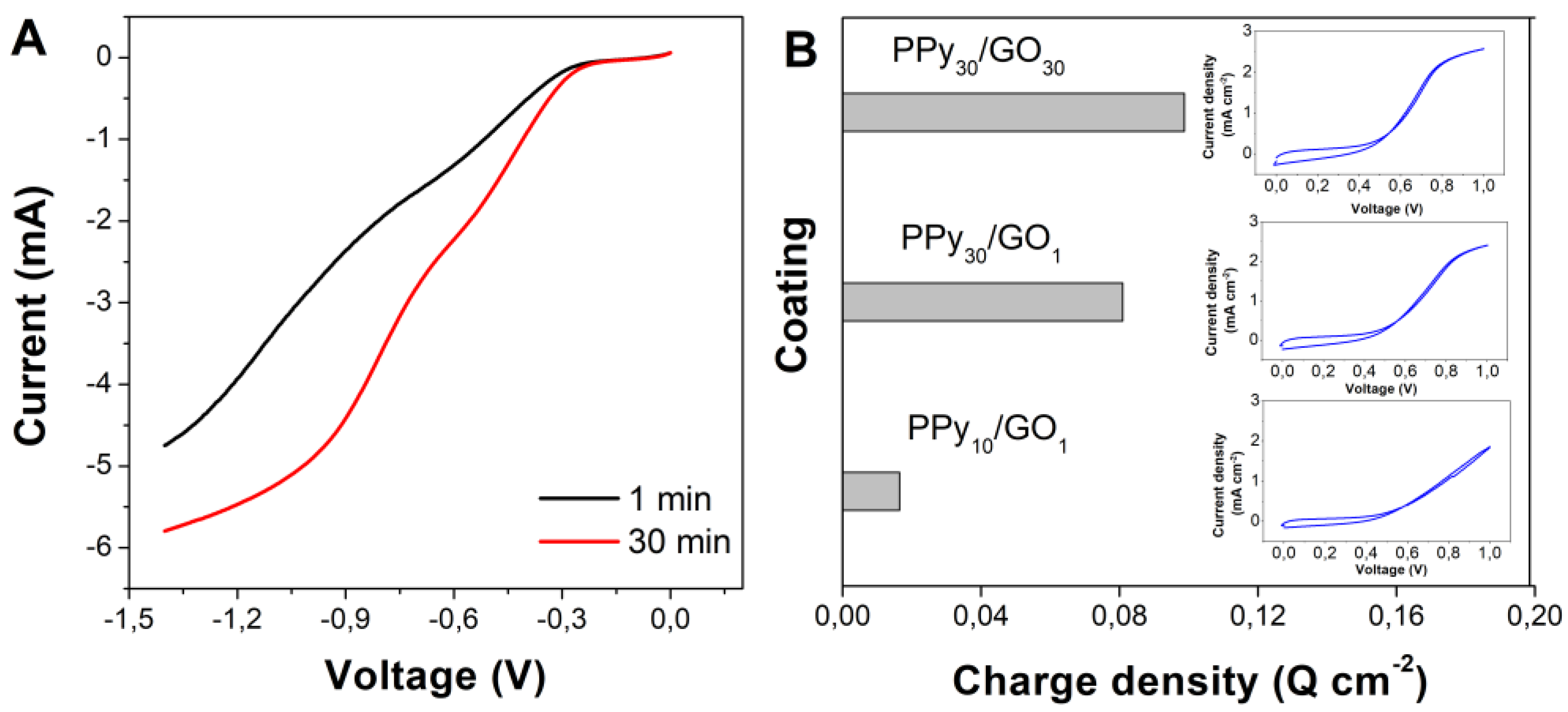
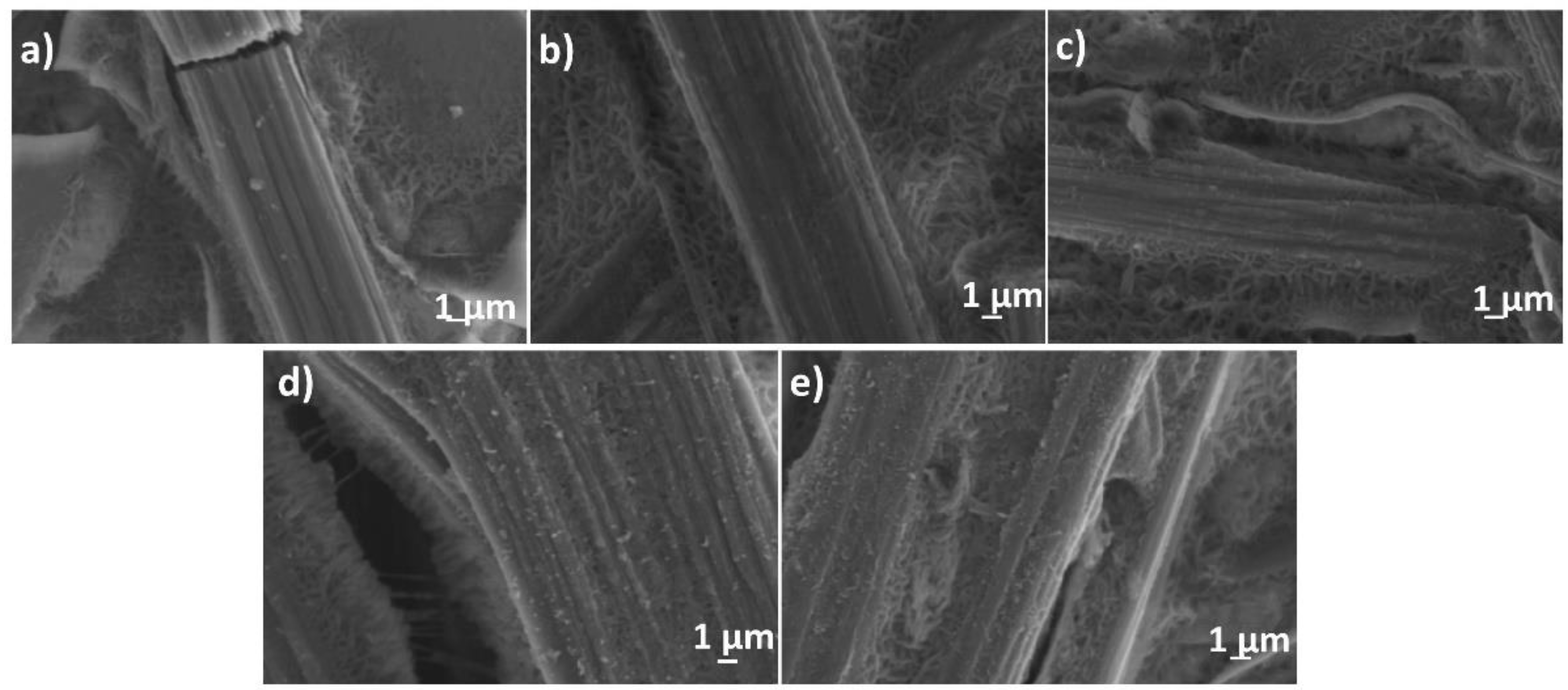
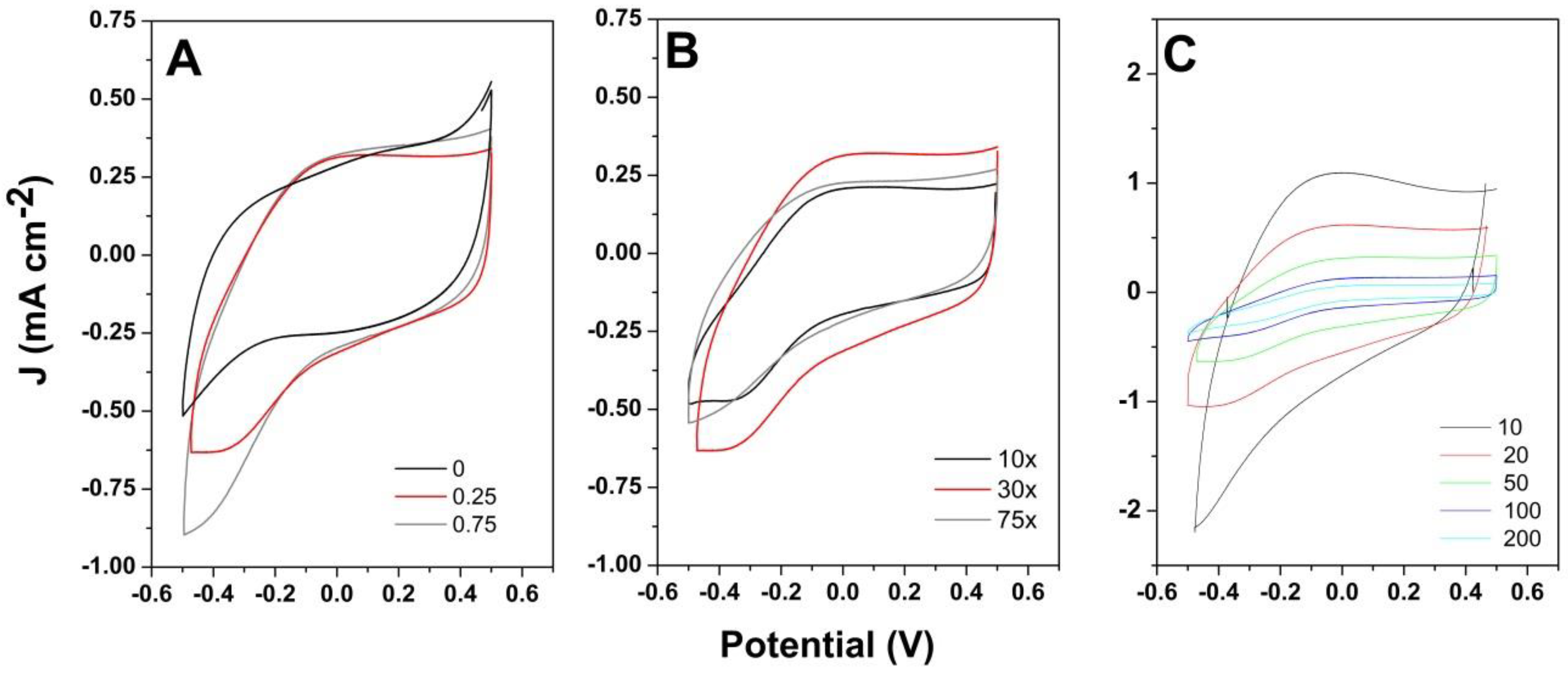
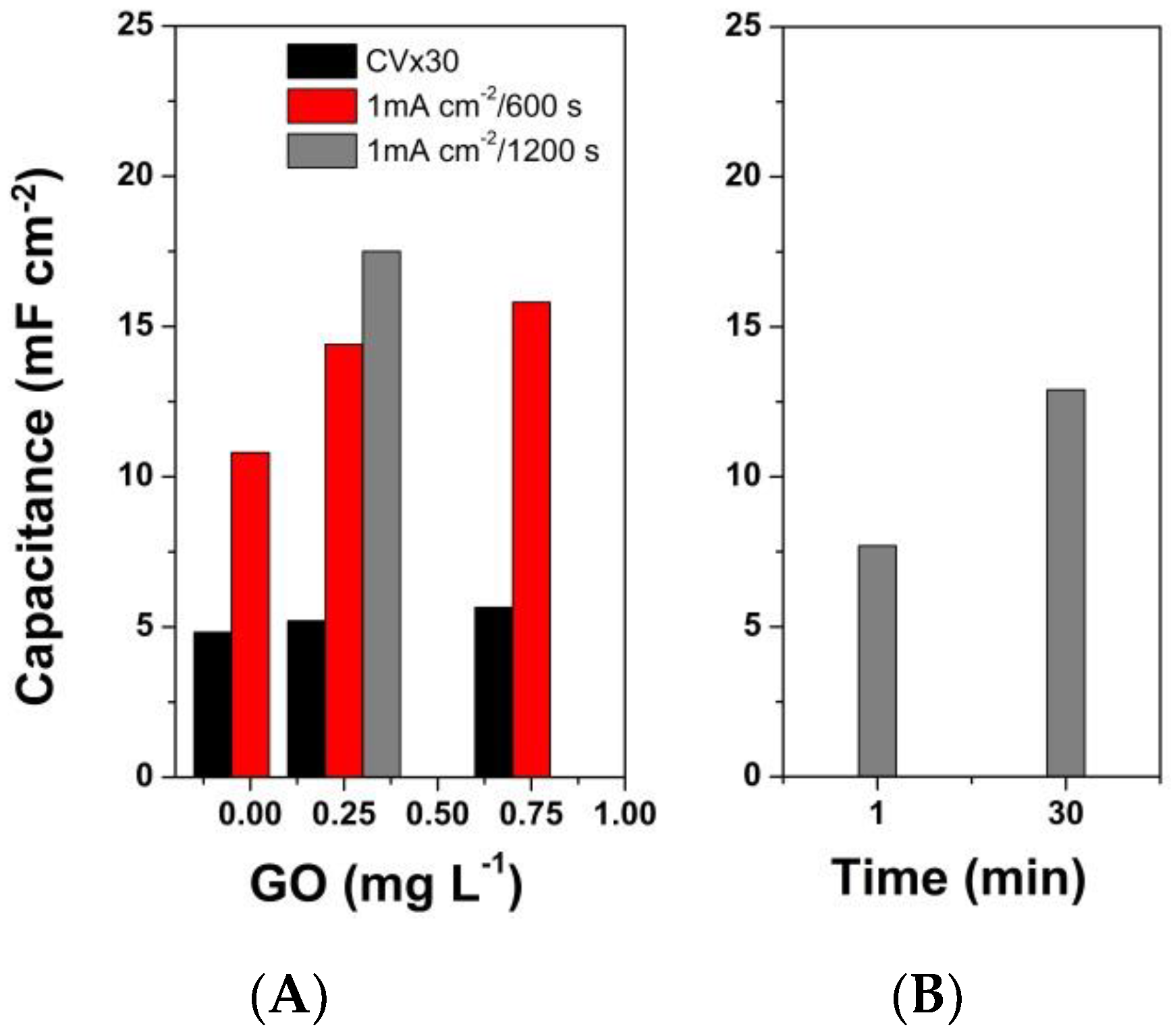
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Ren, L.; Han, W.; Meng, L.; Wei, X.; Qi, X.; Zhong, J. One-pot electrodeposition synthesis of ZnO/graphene composite and its use as binder-free electrode for supercapacitor. Ceram. Int. 2015, 41, 4374–4380. [Google Scholar] [CrossRef]
- Tang, J.; Shen, J.; Li, N.; Ye, M. Facile synthesis of layered MnWO4/reduced graphene oxide for supercapacitor application. J. Alloy. Compd. 2016, 666, 15–22. [Google Scholar] [CrossRef]
- Bashid, H.A.A.; Lim, H.N.; Kamaruzaman, S.; Rashid, S.A.; Yunus, R.; Huang, N.M.; Yin, C.Y.; Rahman, M.M.; Altarawneh, M.; Jiang, Z.T.; et al. Electrodeposition of Polypyrrole and Reduced Graphene Oxide onto Carbon Bundle Fibre as Electrode for Supercapacitor. Nanoscale Res. Lett. 2017, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Fan, R.; Zhao, X.S.; Zou, L. Assembly of Ni-Al layered double hydroxide and graphene electrodes for supercapacitors. Electrochim. Acta 2014, 134, 127–135. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G.; Xiao, Y.; Chang, Y.; Zhai, H.-J. Facile preparation of polypyrrole/graphene oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J. Power Sources 2014, 263, 259–267. [Google Scholar] [CrossRef]
- Yang, B.J.; Jiang, L.L.; Li, Y.J.; Cai, F.G.; Zhang, Q.Y. Three-dimensional porous biocarbon wrapped by graphene and polypyrrole composite as electrode materials for supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 2568–2572. [Google Scholar] [CrossRef]
- Sidhu, N.K.; Rastogi, A. Bifacial carbon nanofoam-fibrous PEDOT composite supercapacitor in the 3-electrode configuration for electrical energy storage. Synth. Met. 2016, 219, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Zhou, Y.; Ouyang, J.-H.; Jia, D. Facile Co-Electrodeposition Method for High-Performance Supercapacitor Based on Reduced Graphene Oxide/Polypyrrole Composite Film. ACS Appl. Mater. Interfaces 2017, 9, 19831–19842. [Google Scholar] [CrossRef]
- Gan, J.K.; Lim, Y.S.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Graphene/polypyrrole-coated carbon nanofiber core–shell architecture electrode for electrochemical capacitors. RSC Adv. 2015, 5, 12692–12699. [Google Scholar] [CrossRef]
- Márquez-Calles, O.J.; Martínez-Orozco, R.D.; Gallardo-Rivas, N.V.; Mendoza-Martínez, A.M.; Mayén-Mondragón, R.; Páramo-García, U. Fabrication and characterization of Polypyrrole/Reduced Graphene-Oxide films for electrochemical capacitors. Int. J. Electrochem. Sci. 2019, 14, 5200–5210. [Google Scholar] [CrossRef]
- Ding, X.; Xu, T.; Gao, J.; Qi, Y.; Zhang, H.-M.; Qu, L. Dimensional confinement of graphene in a polypyrrole microbowl for sensor applications. J. Mater. Chem. B 2017, 5, 5733–5737. [Google Scholar] [CrossRef]
- Besharat, S.F.; Manteghian, M.; Abdollahi, M. Study of Polypyrrole/Graphene Oxide Nanocomposite Structural and Morphological Changes Including Porosity. Polym. Sci. Ser. B 2018, 60, 664–674. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Razali, N.S.M.; Lim, P.T.; Kulandaivalu, S.; Sulaiman, Y. One-step potentiostatic electrodeposition of polypyrrole/graphene oxide/multi-walled carbon nanotubes ternary nanocomposite for supercapacitor. Mater. Chem. Phys. 2018, 219, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, D.; Ma, W.; Yang, T.; Zhang, Y.; Zhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407. [Google Scholar] [CrossRef]
- Cao, J.; Walsh, F.C.; Ouyang, J.-H.; Wang, Y.; Chen, J.; Li, X.; Jia, D.; Zhou, Y. Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14445–14457. [Google Scholar] [CrossRef]
- Tiwari, A.; Syväjärvi, M. Graphene Materials Fundamentals and Emerging Applications; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liu, Y.; Xu, K.; Zhang, X.; Qi, C.; Lv, Q.; Feng, H. Electrochemical codeposition of graphene/polypyrrole composites on carbon paper for electrochemical capacitors. Curr. Appl. Phys. 2016, 16, 520–526. [Google Scholar] [CrossRef]
- Haque, M.; Wong, D.K.Y. Improved dye entrapment–liberation performance at electrochemically synthesised polypyrrole–reduced graphene oxide nanocomposite films. J. Appl. Electrochem. 2017, 47, 777–788. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Silke, M.; Qiu, W.; Xu, M.; Borghs, G.; Chen, H. Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sens. Actuators B Chem. 2011, 158, 176–184. [Google Scholar] [CrossRef]
- Rusi, S.R. Effects of Electrodeposition Mode and Deposition Cycle on the Electrochemical Performance of MnO2-NiO Composite Electrodes for High-Energy-Density Supercapacitors. PLOS ONE 2016, 11, e0154566. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yi, G.; Hou, Y.; Cheng, H.; Luo, X.; Pavlostathis, S.G.; Luo, S.; Wang, A. Building electrode with three-dimensional macroporous interface from biocompatible polypyrrole and conductive graphene nanosheets to achieve highly efficient microbial electrocatalysis. Biosens. Bioelectron. 2019, 141, 111444. [Google Scholar] [CrossRef]
- Saha, P.; Pyne, D.K.; Ghosh, S.; Banerjee, S.; Das, S.; Ghosh, S.; Dutta, P.; Halder, A. Effect of an anionic surfactant (SDS) on the photoluminescence of graphene oxide (GO) in acidic and alkaline medium. RSC Adv. 2018, 8, 584–595. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zhai, J.; Wen, D.; Dong, S. Graphene oxide/polypyrrole nanocomposites: One-step electrochemical doping, coating and synergistic effect for energy storage. J. Mater. Chem. 2012, 22, 6300. [Google Scholar] [CrossRef]
- Feng, X.; Li, R.; Yan, Z.; Liu, X.; Chen, R.; Ma, Y.; Li, X.; Fan, Q.; Huang, W. Preparation of Graphene/Polypyrrole Composite Film via Electrodeposition for Supercapacitors. IEEE Trans. Nanotechnol. 2012, 11, 1080–1086. [Google Scholar] [CrossRef]
- Xu, L.-L.; Guo, M.-X.; Liu, S.; Bian, S.-W. Graphene/cotton composite fabrics as flexible electrode materials for electrochemical capacitors. RSC Adv. 2015, 5, 25244–25249. [Google Scholar] [CrossRef]
- Nethravathi, C.; Nisha, T.; Ravishankar, N.; Shivakumara, C.; Rajamathi, M. Graphene–nanocrystalline metal sulphide composites produced by a one-pot reaction starting from graphite oxide. Carbon 2009, 47, 2054–2059. [Google Scholar] [CrossRef]
- Yun, H.; Kim, J.D.; Choi, H.C.; Lee, C.W. Antibacterial Activity of CNT-Ag and GO-Ag Nanocomposites Against Gram-negative and Gram-positive Bacteria. Bull. Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Pei, B.; Zhao, X.; Dryfe, R.A. Highly porous graphene on carbon cloth as advanced electrodes for flexible all-solid-state supercapacitors. Nano Energy 2013, 2, 530–536. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Etacheri, V.; Yourey, J.E.; Bartlett, B.M. Chemically bonded TiO2–bronze nanosheet/reduced graphene oxide hybrid for high-power lithium ion batteries. ACS Nano 2014, 8, 1491–1499. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Du, T.; Wang, X.; Chen, W. Transformation of graphene oxide by chlorination and chloramination: Implications for environmental transport and fate. Water Res. 2016, 103, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, G.; Xiao, Y.; Li, Y.; Song, H.; Zhang, Y. Polypyrrole/graphene oxide deposited on two metalized surfaces of porous polypropylene films as all-in-one flexible supercapacitors. Electrochim. Acta 2018, 270, 490–500. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, M.; Chen, Z.; Liu, X.-X. Hierarchical Co3O4@PPy core-shell composite nanowires for supercapacitors with enhanced electrochemical performance. Mater. Res. Bull. 2017, 96, 463–470. [Google Scholar] [CrossRef]
- Pruna, A.; Shao, Q.; Kamruzzaman, M.; Zapien, J.A.; Ruotolo, A. Enhanced electrochemical performance of ZnO nanorod core/polypyrrole shell arrays by graphene oxide. Electrochim. Acta 2016, 187, 517–524. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J. Alloy. Compd. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Wolfart, F.; Dubal, D.P.; Vidotti, M.; Holze, R.; Gómez-Romero, P. Electrochemical supercapacitive properties of polypyrrole thin films: Influence of the electropolymerization methods. J Solid State Electrochem. 2016, 20, 901–910. [Google Scholar] [CrossRef]
- Beck, F.; Oberst, M. Electrodeposition and cycling of polypyrrole. Makromol. Chemie. Macromol. Symp. 1987, 8, 97–125. [Google Scholar] [CrossRef]
- Martha, S.K.; Dudney, N.J.; Kiggans, J.O.; Nanda, J. Electrochemical Stability of Carbon Fibers Compared to Aluminum as Current Collectors for Lithium-Ion Batteries. J. Electrochem. Soc. 2012, 159, A1652–A1658. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Kamboj, N.; Das, M.; Dey, R.S. All-porous heterostructure of reduced graphene oxide–polypyrrole–nanoporous gold for a planar flexible supercapacitor showing outstanding volumetric capacitance and energy density. J. Mater. Chem. A 2018, 6, 22858–22869. [Google Scholar] [CrossRef]
- Song, C.; Yun, J.; Keum, K.; Jeong, Y.R.; Park, H.; Lee, H.; Lee, G.; Oh, S.Y.; Ha, J.S. High performance wire-type supercapacitor with Ppy/CNT-ionic liquid/AuNP/carbon fiber electrode and ionic liquid based electrolyte. Carbon 2019, 144, 639–648. [Google Scholar] [CrossRef]
- Zhou, H.; Zhai, H.-J. A highly flexible solid-state supercapacitor based on the carbon nanotube doped graphene oxide/polypyrrole composites with superior electrochemical performances. Org. Electron. 2016, 37, 197–206. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruna, A.I.; Rosas-Laverde, N.M.; Busquets Mataix, D. Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films. Materials 2020, 13, 624. https://doi.org/10.3390/ma13030624
Pruna AI, Rosas-Laverde NM, Busquets Mataix D. Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films. Materials. 2020; 13(3):624. https://doi.org/10.3390/ma13030624
Chicago/Turabian StylePruna, Alina Iuliana, Nelly Ma. Rosas-Laverde, and David Busquets Mataix. 2020. "Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films" Materials 13, no. 3: 624. https://doi.org/10.3390/ma13030624
APA StylePruna, A. I., Rosas-Laverde, N. M., & Busquets Mataix, D. (2020). Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films. Materials, 13(3), 624. https://doi.org/10.3390/ma13030624




