Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Anode Material
2.2. Single Cell Fabrication and Test
2.3. Post-Test Analysis
3. Results and Discussion
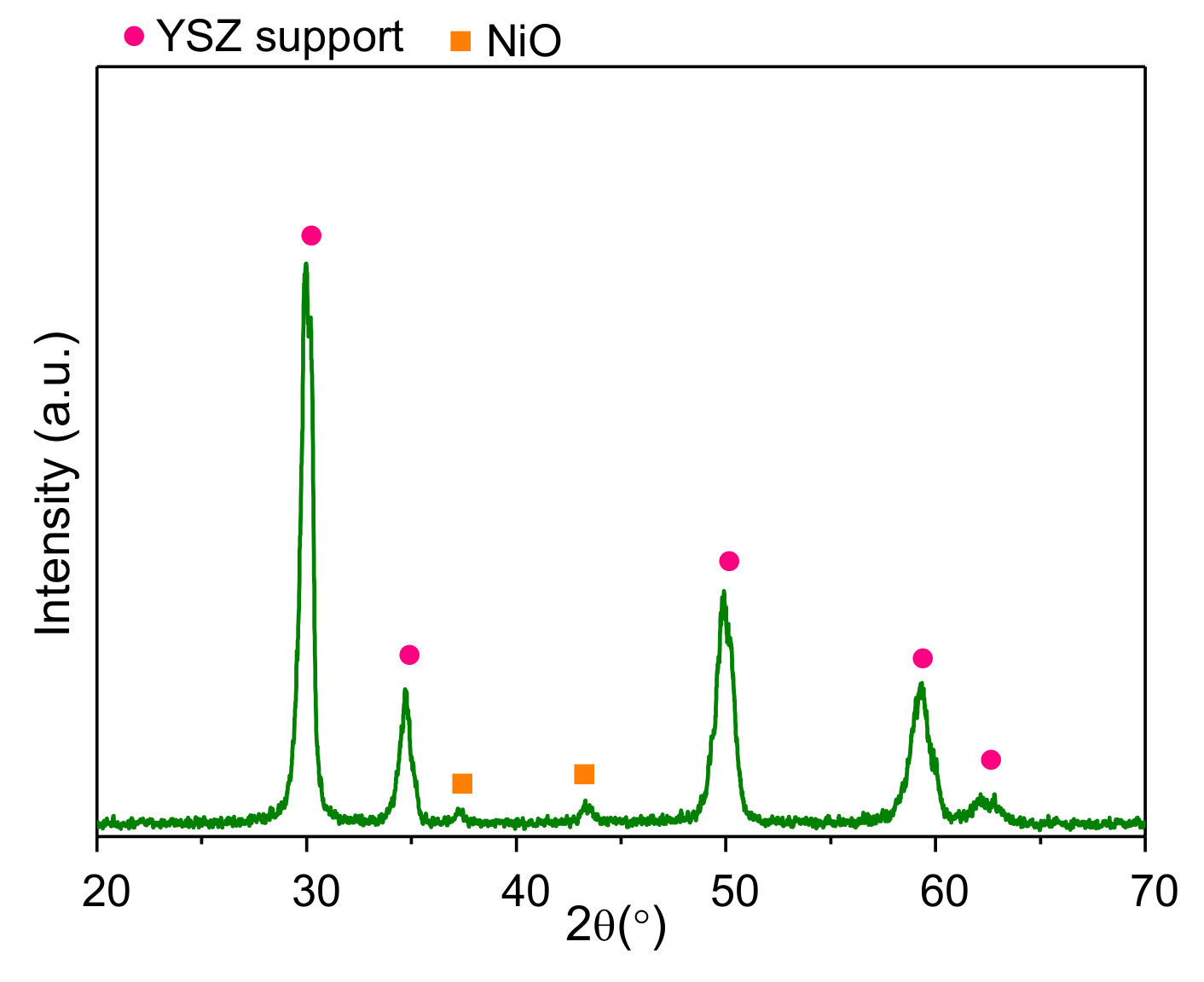
3.1. Structural Characterization of the Anode Material
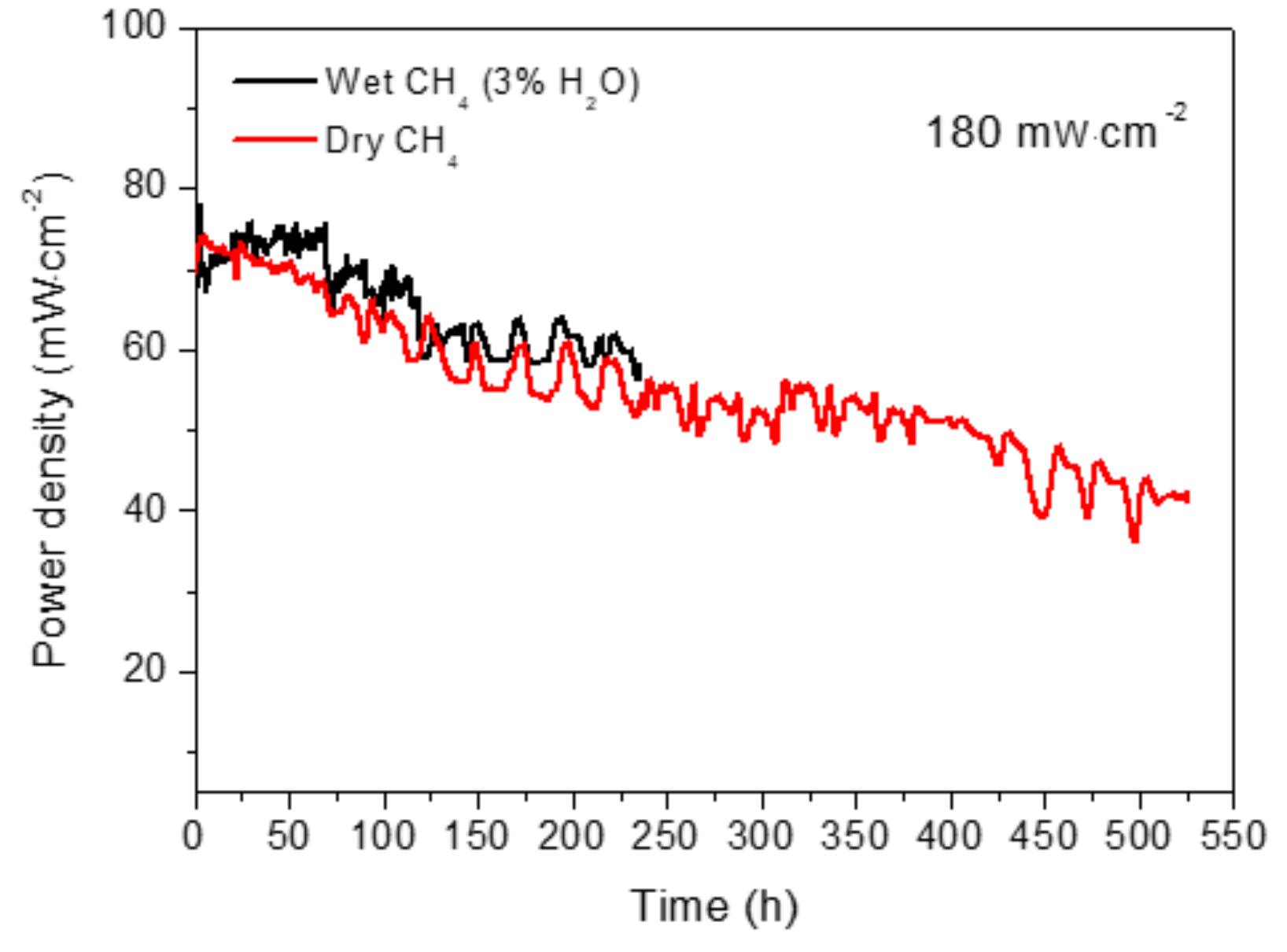
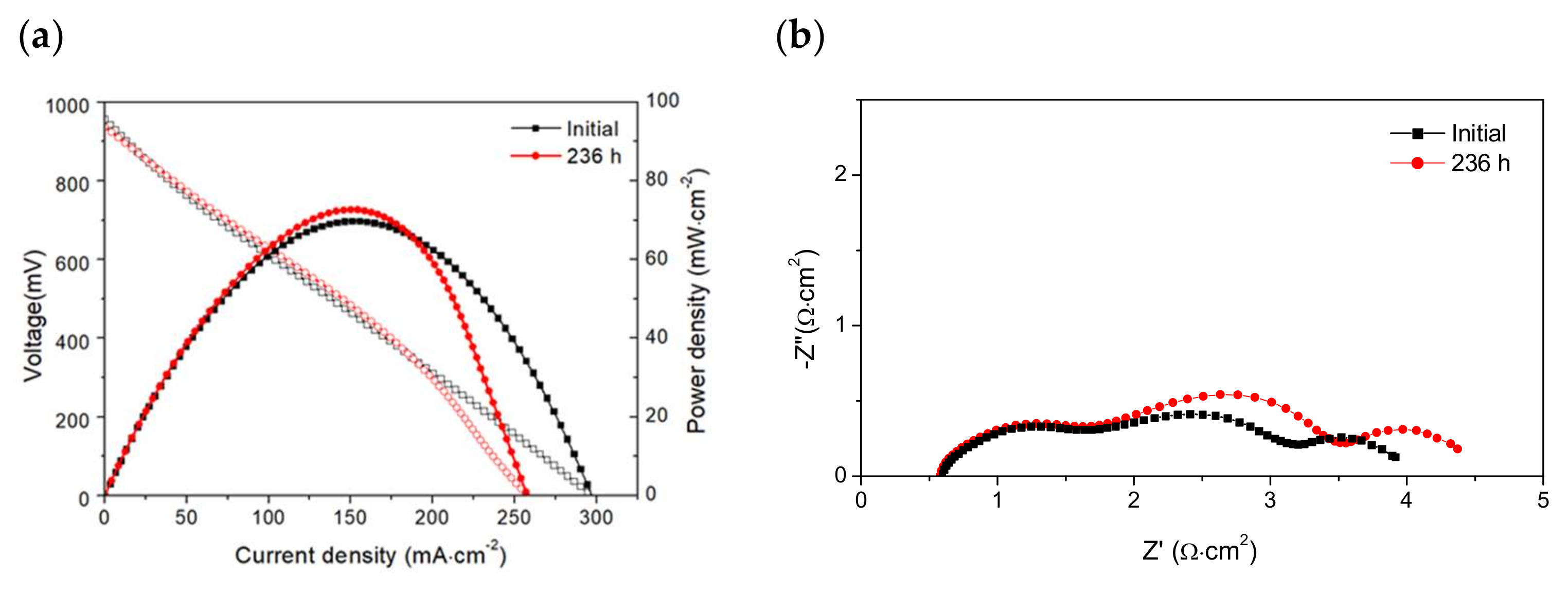
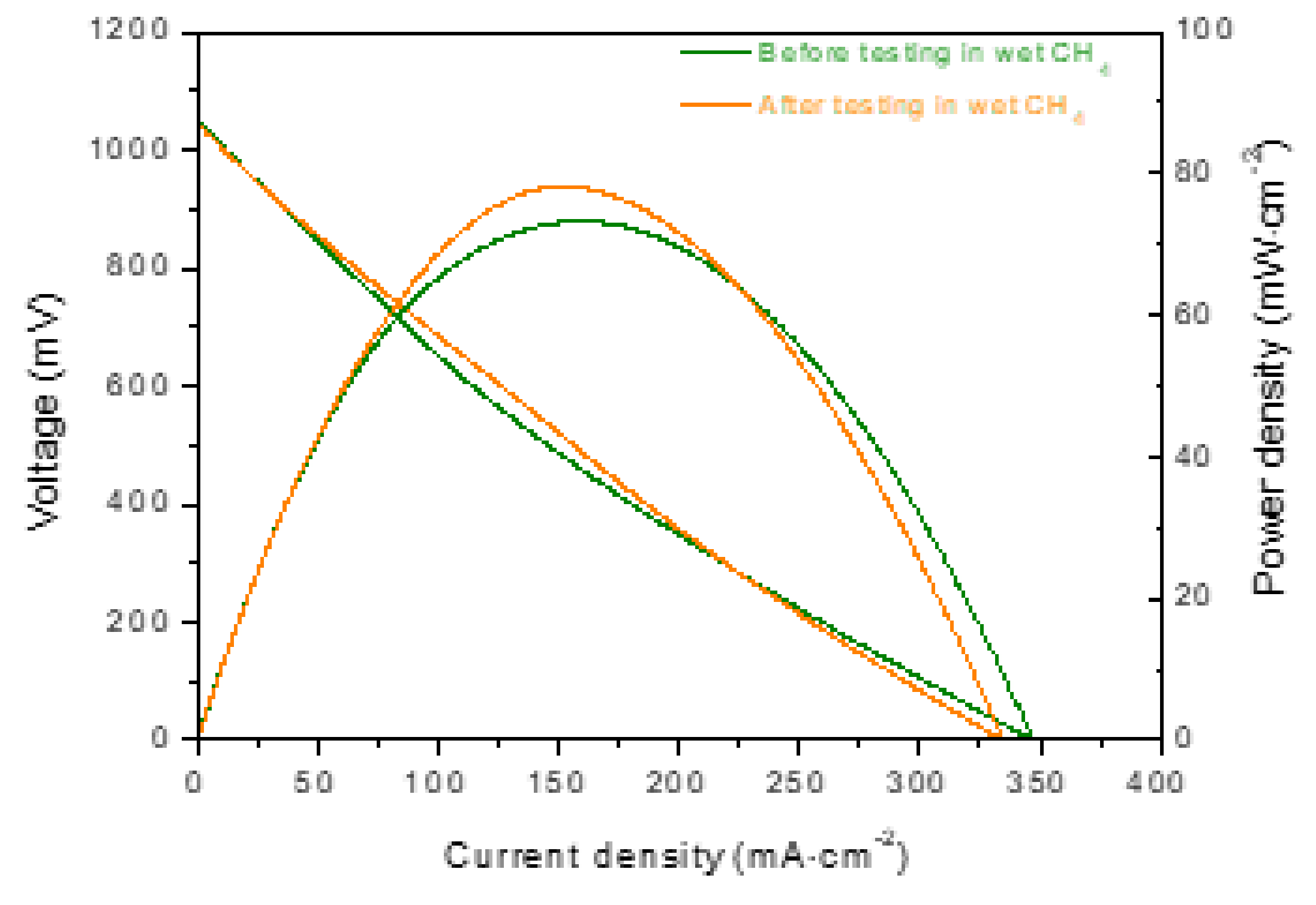
3.2. Electrochemical Performance and Long-Term Stability under Dry and Wet CH4
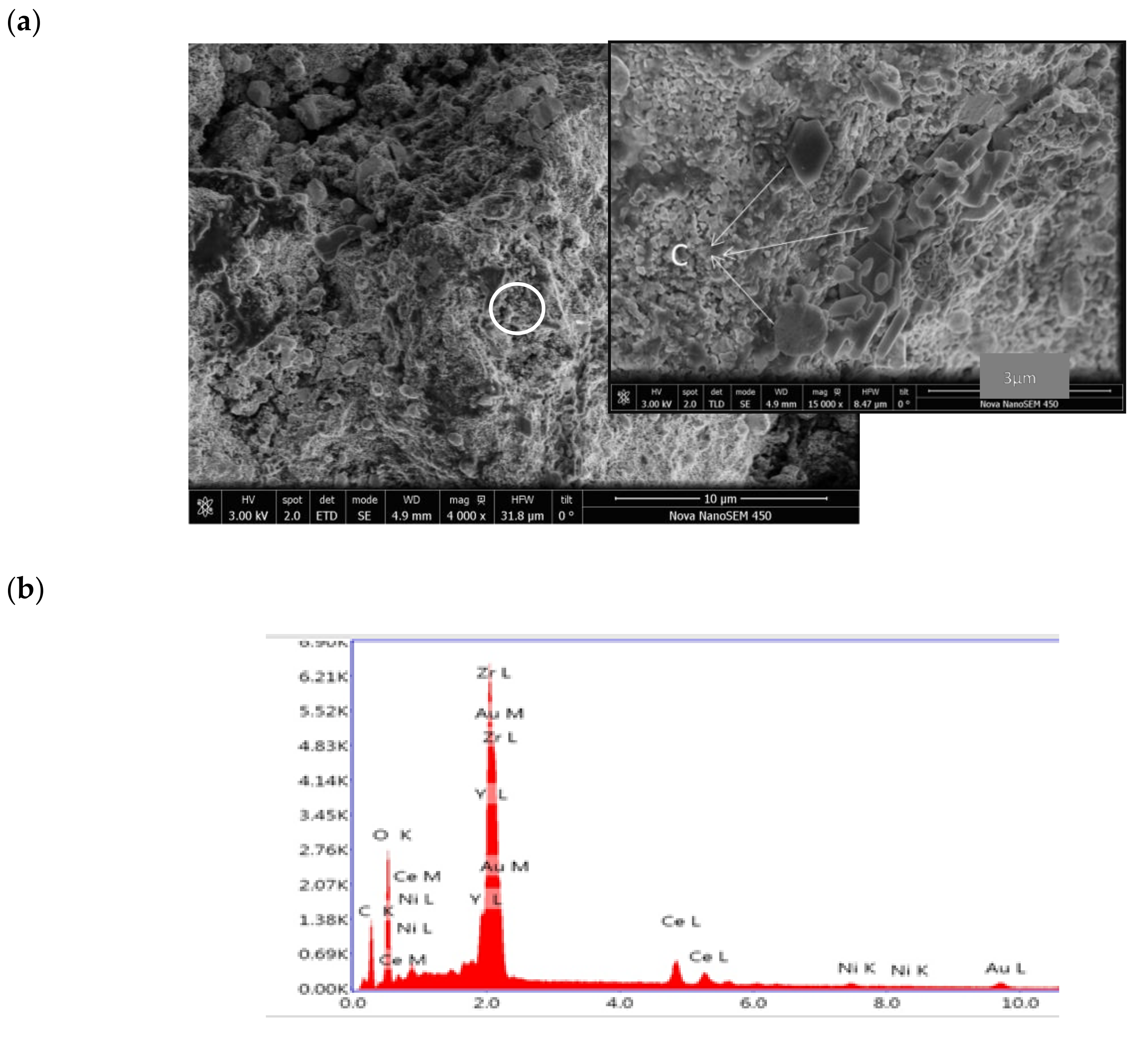
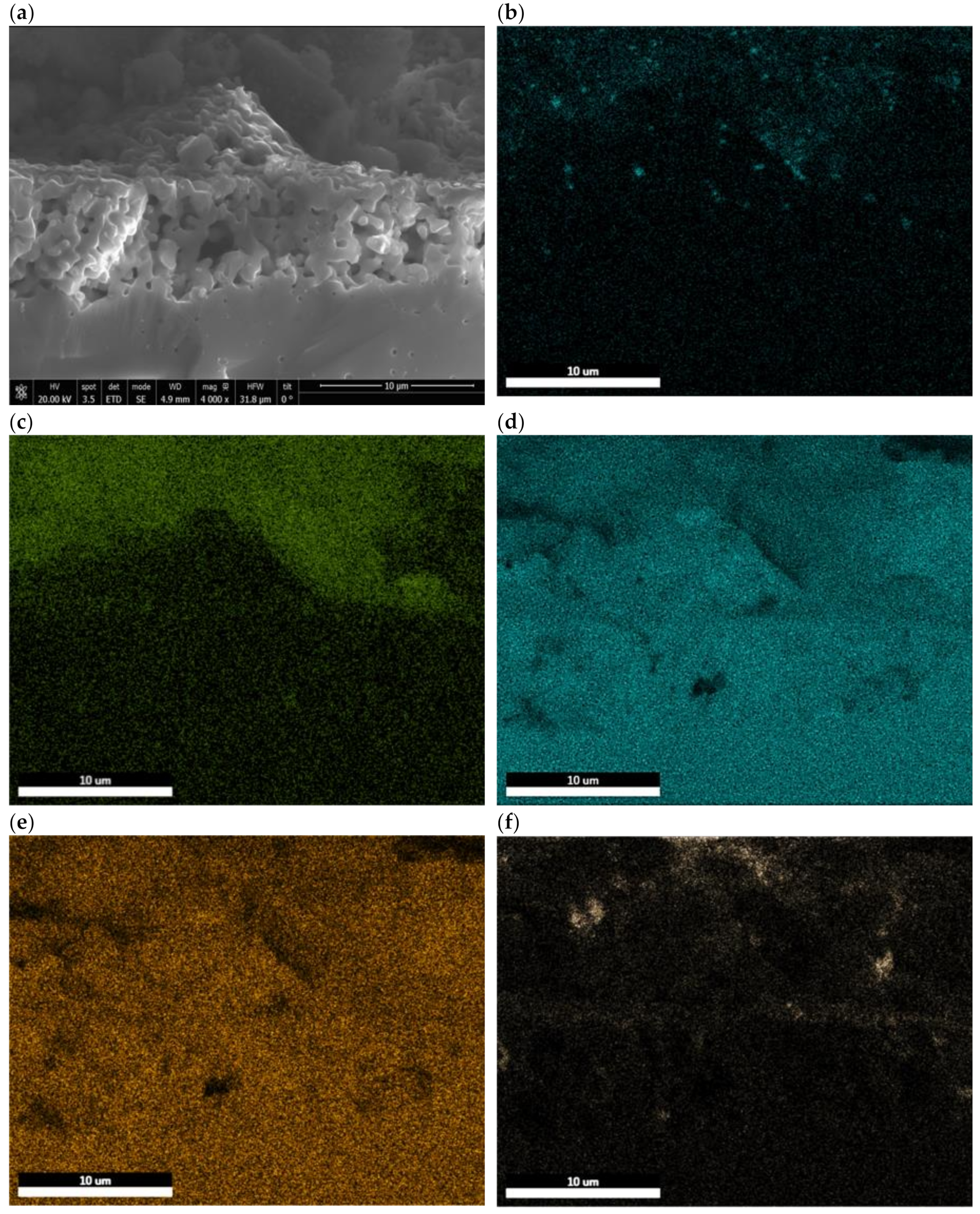
3.3. Post-Test Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, J.; Qiao, J.; Sun, W.; Yang, P.; Li, H.; Wang, Z.; Sun, K. Characteristic and preparation of Ce0.5Zr0.5O, as the anode support for solid oxide fuel cells by phase inversion technology. Int. J. Hydrogen Energy 2015, 40, 12784–12789. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.P.; Tok, A.I.Y.; Luo, L. GDC-impregnated Ni anodes for direct utilization of methane in solid oxide fuel cells. J. Power Sources 2006, 159, 68–72. [Google Scholar] [CrossRef]
- Torabi, A.; Etsell, T.H. Ni Modified WC-based anode materials for direct methane solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, B714–B722. [Google Scholar] [CrossRef]
- Lanzini, A.; Leone, P.; Guera, C.; Smeacetto, F.; Brandon, N.P.; Santarelli, M. Durability of anode supported Solid Oxides Fuel Cells (SOFC) under direct dry-reforming of methane. Chem. Eng. J. 2013, 220, 254–263. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Zheng, Y.; Wu, T.; Zhao, C.; Zhao, J.; Geng, G.; Zhang, W.; Chen, J.; Zhu, J.; et al. Enhancing coking resistance of Ni/YSZ electrodes: In situ characterization, mechanism research, and surface engineering. Nano Energy 2019, 62, 74–78. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Luo, Y.; Wang, Y.; Cai, N. Carbon deposition on patterned nickel/yttria stabilized zirconia electrodes for solid oxide fuel cell/solid oxide electrolysis cell modes. J. Power Sources 2015, 276, 26–31. [Google Scholar] [CrossRef]
- Shri Prakash, B.S.; Senthil Kumar, S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material on oxide fuel cell. A review. Renew. Sust. Energ. Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. Recent progress in SOFC anodes for direct utilization of hydrocarbons. J. Mater. Chem. 2007, 17, 3071–3077. [Google Scholar] [CrossRef]
- Sum, C.; Stimmming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar]
- Goodenough, J.B.; Huang, Y.H. Alternative anode materials for solid oxide fuel cells. J. Power Sources 2007, 173, 1–10. [Google Scholar] [CrossRef]
- Zimic, M.C.; Nuñez, P.; Ruiz-Morales, J.C.; Lamas, D.G.; Larrondo, S.A. Electro-catalytic performance of 60%NiO/Ce0.9Zr0.1O2 cermets as anodes of intermediate temperature solid oxide fuel cells. J. Power Sources 2013, 238, 87–94. [Google Scholar] [CrossRef]
- Tatsuya Takeguchi, T.; Kani, Y.; Yano, T.; Kikuchi, R.; Eguchi, K.; Tsujimoto, K.; Uchida, Y.; Ueno, A.; Omoshiki, K.; Masanobu, M. Study on steam reforming of CH4 and C2 hydrocarbons and carbon deposition on Ni-YSZ cermets. J. Power Sources 2002, 112, 588–595. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Green, R.G.; Giorgi, J.B. SOFC anodes for direct oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 179, 317–318. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater Sci. 2016, 72, 141–337. [Google Scholar] [CrossRef]
- Song, S.; Fuentes, R.O.; Baker, R.T. Nanoparticulate ceria-zirconia anode materials for intermediate temperature solid oxide fuel cells using hydrocarbon fuels. J. Mater. Chem. 2010, 20, 9760–9769. [Google Scholar] [CrossRef]
- He, H.; Vohs, J.M.; Gorte, R.J. Effect of Synthesis Conditions on the Performance of Cu-CeO2 -YSZ Anodes in SOFCs. J. Electrochem. Soc. 2003, 150, A1470. [Google Scholar] [CrossRef]
- Boaro, M.; Pappacena, A.; Abate, C.; Ferluga, M.; Llorca, J.; Trovarelli, A. Effect of redox treatments on Ce0.50Zr0.5O2 based solid oxide fuel cell anodes. J. Power Sources 2014, 270, 79–91. [Google Scholar] [CrossRef]
- Yeste, M.P.; Hernández-Garrido, J.C.; Arias, D.C.; Blanco, G.; Rodríguez-Izquierdo, J.M.; Pintado, J.M.; Bernal, S.; Pérez-Omil, J.A.; Calvino, J.J. Rational design of nanostructured, noble metal free, ceria–zirconia catalysts with outstanding low temperature oxygen storage capacity. J. Mater. Chem. A. 2013, 1, 4836–4844. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Calvino, J.J.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Arias, D.C.; Pérez-Omil, J.A.; Hernández-Garrido, J.C.; González-Leal, J.M.; Cauqui, M.A.; Yeste, M.P. Highly stable ceria-zirconia-yttria supported Ni catalysts for syngas production by CO2 reforming of methane. Appl. Surf. Sci. 2017, 426, 864–873. [Google Scholar] [CrossRef]
- Escudero, M.J.; Gómez de Parada, I.; Fuerte, A.; Serrano, J.L. Analysis of the electrochemical performance of MoNi-CeO2 cermet as anode material for solid oxide fuel cell. Part I. H2, CH4 and H2/CH4 mixtures as fuels. J. Power Sources 2014, 253, 64–73. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, L.; An, W.; Zhou, W.; Sha, Y.; Bai, J.; Li, D.D. Controlled deposition and utilization of carbon on Ni-YSZ anodes of SOFCs operating on dry methane. Energy 2006, 113, 432–443. [Google Scholar] [CrossRef]
- Hornes, A.; Escudero, M.J.; Daza, L.; Martínez-Arias, A. Electrochemical performance of a solid oxide fuel cell with an anode based on Cu-Ni/CeO2 for methane direct oxidation. J. Power Sources 2014, 249, 520–526. [Google Scholar] [CrossRef]
- You, H.; Abuliti, A.; Ding, X.; Zhou, Y. Reactions of low and middle concentration dry methane over Ni/YSZ anode of solid oxide fuel cell. J. Power Sources 2007, 165, 722–727. [Google Scholar] [CrossRef]
- Kendall, K.; Finnerty, C.M.; Saunders, G.; Chung, J.T. Effects of dilution on methane entering an SOFC anode. J. Power Sources 2002, 106, 323–327. [Google Scholar] [CrossRef]
- Buccheri, M.A.; Singh, A.; Hill, J.M. Anode- versus electrolyte-supported Ni-YSZ/YSZ/Pt SOFCs: Effect of cell design on OCV, performance and carbon formation for the direct utilization of dry methane. J. Power Sources 2011, 196, 968–976. [Google Scholar] [CrossRef]
- Eguchi, K.; Kojo, H.; Takeguchi, T.; Kikuchi, R.; Sasaki, K. Fuel flexibility in power generation by solid oxide fuel cells. Solid State Ionic 2002, 152, 411–416. [Google Scholar] [CrossRef]
- Gorte, R.J.; Park, S.; Vohs, J.M.; Wang, C. Anodes for direct oxidation of dry hydrocarbons in a solid-oxide fuel cell. Adv. Mater. 2000, 12, 1465–1469. [Google Scholar] [CrossRef]
- Sasaki, K.; Teaoka, Y. Equilibria in fuel cell gases I. Equilibrium compositions and reforming conditions. J. Electrochem. Soc. 2003, 150, A878–A884. [Google Scholar] [CrossRef]
- You, H.; Gao, H.; Gen, G.; Abudula, A.; Ding, X. The conversion among reactions at Ni-based anodes in solid oxide fuel cells with low concentrations of dry methane. J. Power Sources 2011, 196, 2779–2784. [Google Scholar] [CrossRef]
- Koh, J.H.; Kang, B.S.; Lim, H.C.; Yoo, Y.S. Thermodynamic analysis of carbon deposition and electrochemical oxidation of methane for SOFC anodes. Electrochem. Solid-State Lett. 2001, 4, A12–A15. [Google Scholar] [CrossRef]
- Bucheri, M.A.; Hill, J.M. Methane electrochemical oxidation pathway over a Ni/YSZ and La0.3Sr0.7TiO3 bi-layer SOFC anode. J. Electrochem. Soc. 2012, 159, B361–B367. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Jiang, S.O. Potential oscillation of methane oxidation reaction on (La0.75Sr0.25)(Cr0.5Mn0.5)O3 electrodes of solid oxide fuel cells. J. Power Sources 2008, 178, 92–96. [Google Scholar] [CrossRef]
- Escudero, M.J.; Aguadero, A.; Alonso, J.A.; Daza, L. A kinetic study of oxygen reduction reaction on La2NiO4 cathodes by means of impedance spectroscopy. J. Electroanal. Chem. 2007, 611, 107–116. [Google Scholar] [CrossRef]
- Ye, Y.; He, T.; Li, Y.; Tang, E.H.; Reitz, T.L.; Jiang, S.P. Pd-Promoted La0.75Sr0.25Cr0.5Mn0.5O3/YSZ composite anodes for direct utilization of methane in SOFCs. J. Electrochem. Soc. 2008, 155, B811–B818. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X.J.; Chan, S.H.; Kwok, J.T.; Khor, K.A. (La0.75Sr0.25)(Cr0.5Mn0.5)O3/YSZ composite anodes for methane oxidation reaction in solid oxide fuel cells. Solid State Ionics 2006, 177, 149–157. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Brown, M.; Primdahl, S.; Morgersen, M. Structure/performance relations for Ni/yttria-stabilized zirconia anodes for solid oxide fuel cells. J. Electrochem. Soc. 2000, 147, 475–485. [Google Scholar] [CrossRef]
- Mauvy, F.; Lalanne, C.; Bassat, J.M.; Grenier, J.C.; Zhao, H.; Huo, L.; Stevens, P. Electrode properties of Ln2NiO4 + δ (Ln = La, Nd, Pr): AC impedance and DC polarization studies. J. Electrochem. Soc. 2006, 153, A1547–A1553. [Google Scholar] [CrossRef]
- Vernoux, P.; Guillodo, M.; Fouletier, J.; Hammou, A. Alternative anode material for gradual methane reforming in solid oxide fuel cells. Solid State Ionics 2000, 135, 425–431. [Google Scholar] [CrossRef]
- Fu, Q.X.; Stöver, D. La0.4Sr0.6Ti1-xMnxO3-δ perovskites as anode materials for solid oxide fuel cells. J. Electrochem. Soc. 2006, 153, D74–D83. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Investigation of the mixed conducting oxide ScYZT as a potential SOFC anode material. J. Electrochem. Soc. 2004, 151, A497–A503. [Google Scholar] [CrossRef]
- Chen, M.; Kim, B.H.; Xu, Q.; Ahn, B.G.; Huang, D.P. Effect of Ni content on the microstructure and electrochemical properties of Ni–SDC anodes for IT-SOFC. Solid State Ionics 2010, 181, 1119–1124. [Google Scholar] [CrossRef]
- Huang, B.; Fe, X.F.; Wang, S.R.; Nie, H.W.; Shi, J.; Hu, Q.; Qian, J.Q.; Sun, X.F.; Wen, T.L. Performance of Ni/ScSZ cermet anode modified by coating with Gd0.2Ce0.8O2 for an SOFC running on methane fuel. J. Power Sources 2006, 162, 1172–1181. [Google Scholar] [CrossRef]
- McIntosh, S.; Vohs, J.M.; Gorte, R.J. Role of hydrocarbon deposits in the enhanced performance of direct-oxidation SOFCs. J. Electrochem. Soc. 2003, 150, A470–A476. [Google Scholar] [CrossRef]
- Myung, J.H.; Ko, H.J.; Lee, J.J.; Lee, J.H.; Hyun, S.H. Synthesis and characterization of NiO/GDC–GDC dual nano-composite powders for high-performance methane fueled solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 11351–11359. [Google Scholar] [CrossRef]
- Kim, H.; Lu, C.; Worrell, W.L.; Vohs, J.M.; Gorte, R.J. Cu-Ni cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247–A250. [Google Scholar] [CrossRef]
- Wang, Z.; Weng, W.; Cheng, K.; Du, P.; Shen, G.; Han, G. Catalytic modification of Ni–Sm-doped ceria anodes with copperfor direct utilization of dry methane in low-temperature solid oxide fuel cell. J. Power Sources 2008, 179, 541–546. [Google Scholar] [CrossRef]
- Klein, J.M.; Hénault, M.; Roux, C.; Bultel, Y.; Georgdes, S. Direct methane solid oxide fuel cell working by gradual internal steam reforming: Analysis of operation. J. Power Sources 2009, 193, 331–337. [Google Scholar] [CrossRef]
- Singh, A.; Hill, J.M. Carbon tolerance, electrochemical performance and stability of solid oxide fuel cells with Ni/yttria stabilized zirconia anodes impregnated with Sn and operated methane. J. Power Sources 2012, 214, 185–194. [Google Scholar] [CrossRef]


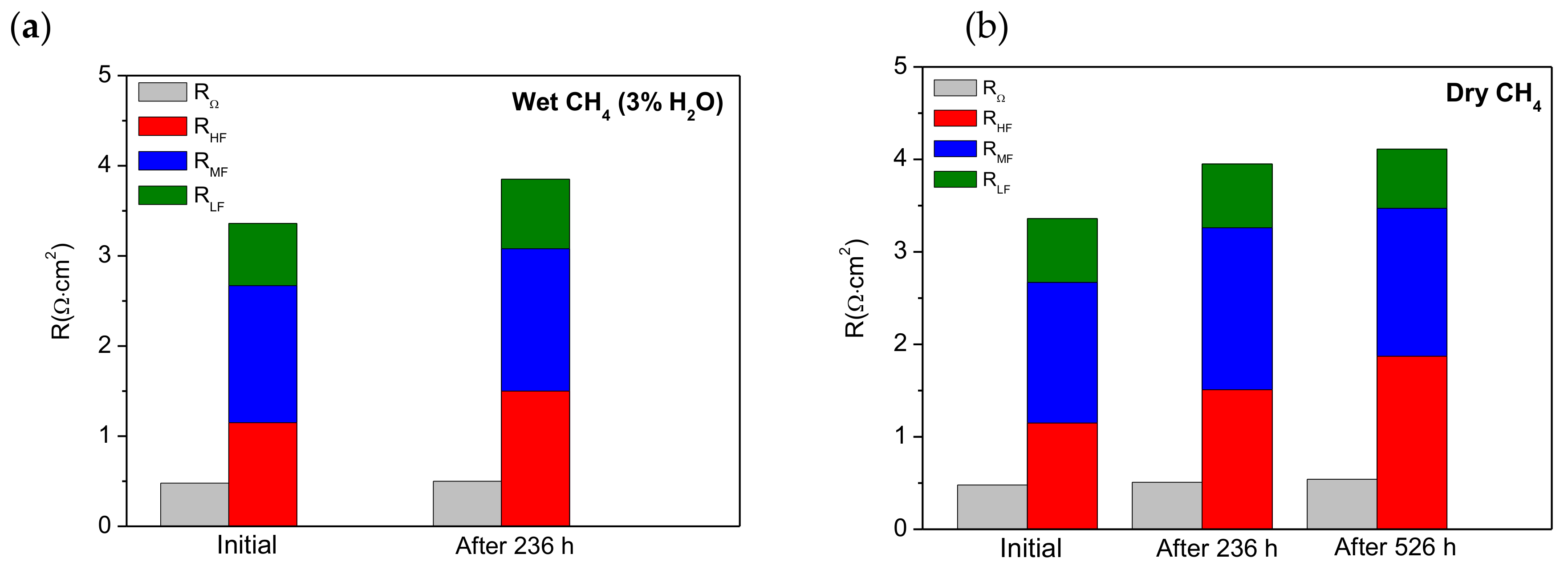
| Operating Time (h) | RΩ (Ω·cm2) | High Frequency Arc | Medium Frequency Arc | Low Frequency Arc | |||
|---|---|---|---|---|---|---|---|
| RHF (Ω·cm2) | CHF (F·cm–2) | RMF (Ω·cm2) | CMF (F·cm–2) | RLF (Ω·cm2) | CLF (F·cm–2) | ||
| Initial | 0.89 | 3.38 | 7 × 10–6 | 1.86 | 5.7 10–3 | 0.58 | 5.2 |
| 24 | 0.79 | 2.38 | 9 10–6 | 1.81 | 6.3 10–3 | 0.55 | 6.1 |
| 48 | 0.76 | 2.34 | 11 10–6 | 1.67 | 6.7 10–3 | 0.58 | 5.2 |
| 72 | 0.67 | 1.94 | 15 10–6 | 1.52 | 7.5 10–3 | 0.59 | 5.4 |
| 96 | 0.59 | 1.67 | 24 10–6 | 1.51 | 8.5 10–3 | 0.64 | 5.2 |
| 120 | 0.54 | 1.43 | 34 10–6 | 1.28 | 11.2 10–3 | 0.70 | 4.6 |
| 144 | 0.52 | 1.54 | 34 10–6 | 1.41 | 10.9 10–3 | 0.65 | 5.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero, M.J.; Yeste, M.P.; Cauqui, M.Á.; Muñoz, M.Á. Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode. Materials 2020, 13, 599. https://doi.org/10.3390/ma13030599
Escudero MJ, Yeste MP, Cauqui MÁ, Muñoz MÁ. Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode. Materials. 2020; 13(3):599. https://doi.org/10.3390/ma13030599
Chicago/Turabian StyleEscudero, María José, María Pilar Yeste, Miguel Ángel Cauqui, and Miguel Ángel Muñoz. 2020. "Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode" Materials 13, no. 3: 599. https://doi.org/10.3390/ma13030599
APA StyleEscudero, M. J., Yeste, M. P., Cauqui, M. Á., & Muñoz, M. Á. (2020). Performance of a Direct Methane Solid Oxide Fuel Cell Using Nickel-Ceria-Yttria Stabilized Zirconia as the Anode. Materials, 13(3), 599. https://doi.org/10.3390/ma13030599






