Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry
Abstract
1. Introduction: Modern Carbocatalysis and Its Problems
2. Structure and Chemical Composition of Amorphous Carbons BSUs
3. Radical Character of the Amorphics BSUs; Active Sites of the Carbocatalysts
3.1. Graphene Oxyhydrides
3.2. Graphene Oxynitrothiohydrides
3.3. Active Sites of Amorphous Carbocatalysts
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Radovich, L.R. Physicochemical Properties of Carbon Materials: A Brief Overview. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–44. [Google Scholar]
- Rideal, K.; Wright, W.M. Low Temperature Oxidation at Charcoal Surfaces. Part I. The Behaviour of Charcoal in the Absence of Promoters. J. Chem. Soc. Trans. 1925, 127, 1347–1357. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Donet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black. Science and Technology; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 1993. [Google Scholar]
- Francisco, R.R. The Role of Carbon Materials in Heterogeneous Catalysis. Carbon 1998, 36, 159–175. [Google Scholar]
- Radovich, L.R. Chemistry and Physics of Carbon; Marcel Dekker: New York, NY, USA, 2001; Volume 27, pp. 131–136. [Google Scholar]
- Toyoda, M.; Tsumura, T.; Tryba, B.; Mozia, S.; Janus, M.; Morawski, A.W.; Inagaki, M. Carbon Materials in Photocatalysis. In Chemistry and Physics of Carbon; Radovich, L.R., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 171–267. [Google Scholar]
- Serp, P.; Machado, B. (Eds.) Nanostructured Carbon Materials for Catalysis; Royal Society of Chemistry: Croydon, UK, 2015. [Google Scholar]
- Bandosz, T.J. Surface Chemistry of Carbon Materials. Spectroscopic Methods. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 45–92. [Google Scholar]
- Bandosz, T.J. Nanoporous Carbons: Looking Beyond Their Perception as Adsorbents, Catalyst Supports and Supercapacitors. Chem. Rec. 2016, 16, 205–218. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous Carbon: A Versatile Material for Catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Bel’skaya, R.I. Shungites—New Carbon Raw Materials; Karelian Sciences Center: Petrozavodsk, Russia, 1984; pp. 124–138. (In Russian) [Google Scholar]
- Grigoyeva, E.N.; Rozhkova, N.N. Shungite Carbon in Reactions that Model Coal Heat Transformation. Russ. J. Appl. Chem. 2000, 73, 600–605. (In Russian) [Google Scholar]
- Rozhkova, N.N. Role of Fullerene-Like Structures in the Reactivity of Shungite Carbon as Used in New Materials with Advanced Properties. In Perspectives of Fullerene Nanotechnology; Osava, E., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 237–251. [Google Scholar]
- Rozhkova, N.N.; Gorlenko, L.E.; Yemel’yanova, G.I.; Jankowska, A.; Korobov, M.V.; Lunin, V.V.; Osawa, E. The Effect of Ozone on the Structure and Physico-Chemical Properties of Ultradisperse Diamond and Shungite Nanocarbon Elements. Pure Appl. Chem. 2009, 81, 2092–2105. [Google Scholar] [CrossRef]
- Su, D.S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.; Paraknowitsch, J.; Schlögl, R. Metal-Free Heterogeneous Catalysis for Sustainable Chemistry. ChemSusChem 2010, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Bielawski, C.W. Carbocatalysis: Heterogeneous carbons finding utility in synthetic chemistry. Chem. Sci. 2011, 2, 1227–1233. [Google Scholar] [CrossRef]
- Schaetz, A.; Zeltner, M.; Stark, W.J. Carbon Modifications and Surfaces for Catalytic Organic Transformations. ACS Catal. 2012, 2, 1267–1284. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.-J.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J.; et al. Probing the Catalytic Activity of Porous Graphene Oxide and the Origin of This Behavior. Nat. Commun. 2012, 3, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P.; Clauss, A.; Fischer, G.O.; Hofmann, U. The Adsorption Behavior of Very Thin Carbon Films. Z. Anorg. Allg. Chem. 1962, 316, 119–127. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, E.A. Technical Graphene (Reduced Graphene Oxide) and Its Natural Analog (Schungite). Tech. Phys. 2016, 61, 1032–1038. [Google Scholar] [CrossRef]
- Mohammadi, O.; Golestanzadeh, M.; Abdouss, M. Recent Advances in Organic Reactions Catalyzed by Graphene Oxide and Sulfonated Graphene as Heterogeneous Nanocatalysts: A Review. New J. Chem. 2017, 41, 11471–11497. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-Based Materials for Catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Su, C.; Loh, K.P. Carbocatalysis: Graphene Oxide and Its Derivatives. Acc. Chem. Res. 2013, 46, 2275–2285. [Google Scholar] [CrossRef]
- Schlögl, R. Carbon in Catalysis. In Advances in Catalysis; Gates, B.C., Jentoft, F.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 56, pp. 103–185. [Google Scholar]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the Development of Advanced Catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Su, D.S.; Wen, G.; Wu, S.; Peng, F.; Schlögl, R. Carbocatalysis in Liquid-Phase Reactions. Angew. Chem. Int. Ed. 2016, 55, 2–31. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Carbon-Based Metal-Free Catalysts for Electrocatalysis Beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with Two-Dimensional Materials and Their Heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; García, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yao, Z.; Wang, X. Graphene-Based Nanomaterials for Catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Alvaro, M.; Garcia, H. General Aspects in the Use of Graphenes in Catalysis. Mater. Horiz. 2018, 5, 363–378. [Google Scholar]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N. (Eds.) Metal-Free Functionalized Carbons in Catalysis: Synthesis, Characterization and Applications; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Hu, C.; Qu, J.; Xiao, Y.; Zhao, S.; Chen, H.; Dai, L. Carbon Nanomaterials for Energy and Biorelated Catalysis: Recent. Advances and Looking Forward. ACS Cent. Sci. 2019, 5, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yu, L.; Pan, X.; Wang, S.; Chen, X.; Hu, P.; Sunb, L.; Bao, X. Size Effect of Graphene on Electrocatalytic Activation of Oxygen. Chem. Commun. 2011, 47, 10016–10018. [Google Scholar] [CrossRef]
- Sheka, E.F.; Rozhkova, N.N. Shungite as the Natural Pantry o Nanoscale Reduced Graphene Oxide. Int. J. Smart Nanomater. 2014, 1, 1–16. [Google Scholar] [CrossRef]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F.; Nelson, D.K.; Starukhin, A.N. Fractals of Graphene Quantum Dots in Photoluminescence of Shungite. J. Exp. Theor. Phys. 2014, 118, 838–850. [Google Scholar] [CrossRef][Green Version]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F.; Nelson, D.K.; Starukhin, A.N.; Goryunov, A.S. Spectral Properties of Shungite Quantum Dots. Nanosyst. Phys. Chem. Math. 2014, 5, 217–233. [Google Scholar]
- Sheka, E.F.; Natkaniec, I.; Rozhkova, N.N.; Holderna-Natkaniec, K. Neutron Scattering Study of Reduced Graphene Oxide of Natural Origin. JETP Lett. 2014, 118, 735–746. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Isaenko, S.I.; Prikhodko, A.S.; Borgardt, N.I.; Suvorova, E.I. Raman Spectroscopic Study of Natural Nanostructured Carbon Materials: Shungite vs. Anthraxolite. Eur. J. Mineral. 2016, 28, 545–554. [Google Scholar] [CrossRef]
- Natkaniec, I.; Sheka, E.F.; Druzbicki, K.; Hołderna-Natkaniec, K.; Gubin, S.P.; Buslaeva, E.Y.; Tkachev, S.V. Computationally Supported Neutron Scattering Study of Parent and Chemically Reduced Graphene Oxide. J. Phys. Chem. C 2015, 119, 18650–18662. [Google Scholar] [CrossRef]
- Sheka, E.F.; Natkaniec, I.; Rozhkova, N.N.; Buslaeva, E.Y.; Tkachev, S.V.; Gubin, S.P.; Mel’nikov, V.P. Parent and Reduced Graphene Oxide of Different Origin in Light of Neutron Scattering. Nanosyst. Phys. Chem. Math. 2016, 7, 71–80. [Google Scholar] [CrossRef][Green Version]
- Sheka, E.F.; Natkaniec, I. Neutron Scattering of Parent and Reduced Graphene Oxide. Rev. Adv. Mater. Sci. 2017, 49, 1–27. [Google Scholar]
- Sheka, E.F.; Hołderna-Natkaniec, K.; Natkaniec, I.; Krawczyk, J.X.; Golubev, Y.A.; Rozhkova, N.N.; Kim, V.V.; Popova, N.A.; Popova, V.A. Computationally Supported Neutron Scattering Study of Natural and Synthetic Amorphous Carbons. J. Phys. Chem. C 2019, 123, 15841–15850. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Rozhkova, N.N.; Kabachkov, E.N.; Shul’ga, Y.M.; Natkaniec-Hołderna, K.; Natkaniec, I.; Antonets, I.V.; Makeev, B.A.; Popova, N.A.; Popova, V.A. sp2 Amorphous Carbons in View of Multianalytical Consideration: Normal, Expected and New. J. Non-Cryst. Solids 2019, 524, 119608. [Google Scholar] [CrossRef]
- Sheka, E.F.; Natkaniec, I.; Ipatova, E.U.; Golubev, Y.A.; Kabachkov, E.N.; Popova, V.A. XPS Supported INS and DRIFT Vbrational Spectroscopy of sp2 Amorphous Carbons. arXiv, 2019; arXiv:1911.07600. [Google Scholar]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, W.T.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S. Analysis of the Structure and Chemical Properties of Some Commercial Carbon Nanostructures. Carbon 2009, 47, 1779. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.-B. Surface Groups on Carbon Blacks. In Carbon Black. Science and Technology; Donet, J.-B., Bansal, R.C., Wang, M.-J., Eds.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 1993; pp. 152–181. [Google Scholar]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; García, H. Active Sites on Graphene-Based Materials as Metal-Free Catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef]
- Jiang, D.-E.; Chen, Z. (Eds.) Graphene Chemistry: Theoretical Perspectives; Wiley: Chichester, UK, 2013. [Google Scholar]
- Sheka, E.F. Spin Chemical Physics of Graphene; Pan Stanford: Singapore, 2018. [Google Scholar]
- Sheka, E.F.; Popova, N.A.; Popova, V.A. Physics and Chemistry of Graphene. Emergentness, Magnetism, Mechanophysics, and Mechanochemistry. Phys.-Uspekhi 2018, 61, 645–691. [Google Scholar] [CrossRef]
- Boukhvalov, D.V.; Dreyer, D.R.; Bielawski, C.W.; Soon, Y.-W. A Computational Investigation of the Catalytic Properties of Graphene Oxide: Exploring Mechanisms by Using DFT Methods. ChemCatChem 2012, 4, 1844–1849. [Google Scholar] [CrossRef]
- Boukhvalov, D.W. DFT Modeling of the Covalent Functionalization of Graphene: From Ideal to Realistic Models. RSC Adv. 2013, 3, 7150–7159. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalón, S.; Primo, A.; Moral, M.; Fernández Sanz, J.; Álvaro, M.; García, H. Graphenes as Efficient Metal-Free Fenton Catalysts. Chem. Eur. J. 2015, 21, 11966–11971. [Google Scholar] [CrossRef]
- Sheka, E.F. Spin Chemistry of sp2 Nanocarbons. arXiv, 2019; arXiv:1910.06402. [Google Scholar]
- Hellman, A.; Baerends, E.J.; Biczysko, M.; Bligaard, T.; Christensen, C.H.; Clary, D.C.; Dahl, S.; van Harrevelt, R.; Honkala, K.; Jonsson, H.; et al. Predicting Catalysis: Understanding Ammonia Synthesis From First-Principles Calculations. J. Phys. Chem. B 2006, 110, 17719–17735. [Google Scholar] [CrossRef]
- Khavryuchenko, V.D.; Khavryuchenko, O.V.; Lisnyak, V.V. Effect of Spin Catalysis in H2S Oxidation: A Quantum Chemical Insight. Catal. Commun. 2010, 11, 340–345. [Google Scholar] [CrossRef]
- Piccini, G.M.; Mendels, D.; Parrinello, M. Metadynamics with Discriminants: A Tool for Understanding Chemistry. J. Chem. Theory Comput. 2018, 14, 5040–5044. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Tropin, T.V.; Aksenov, V.L.; Rosta, L.; Garamus, V.M.; Rozhkova, N.N. Pore Structures in Shungites as Revealed by Small-Angle Neutron Scattering. Carbon 2006, 44, 954–961. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Choi, S.K.; Lu, Y. Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef]
- Filippov, M.M. Anthraxolites; VNIGRI: St. Petersburg, Russia, 2013. (In Russian) [Google Scholar]
- SIGMA-ALDRICH. Synthesis of Mesoporous Materials. Mater. Matters 2008, 3, 17–18. [Google Scholar]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of Heat-Treated Graphite Oxide by X-Ray Photoelectron Spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Sheka, E.F. Stretching and Breaking of Chemical Bonds, Correlation of Electrons, and Radical Properties of Covalent Species. Adv. Quant. Chem. 2015, 70, 111–161. [Google Scholar]
- Carotenuto, G.; Romeo, V.; De Nicola, S.; Nicolais, L. Graphite Nanoplatelet Chemical Cross-Linking by Elemental Sulfur. Nanoscale Res. Lett. 2013, 8, 94. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorkiy, P.M. Van der Waals Radii of Atoms. Zh. Strukt. Khimii 1974, 15, 118–122. [Google Scholar]
- Hicks, R.G. (Ed.) Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Wiley: Chichester, UK, 2010. [Google Scholar]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-Stabilized Hydrocarbonradical Chain Reactions May Explain Soot Inception and Growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef]

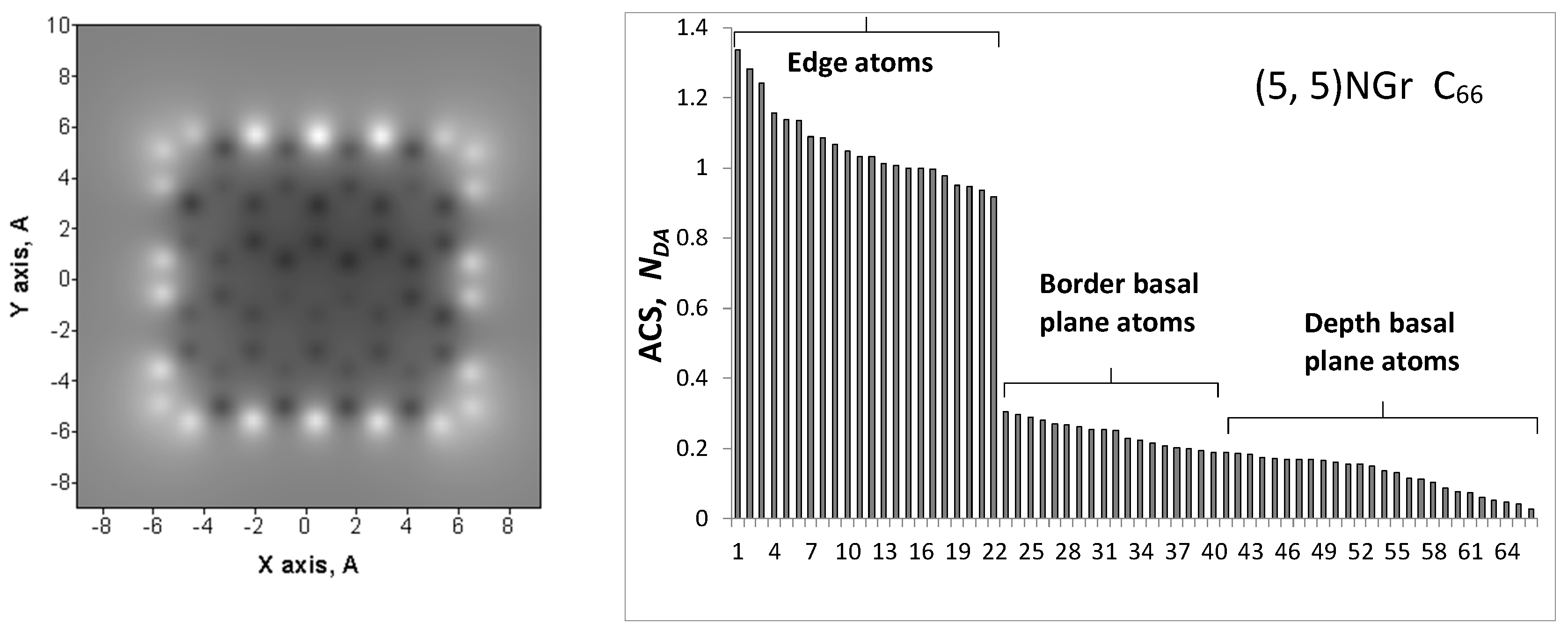

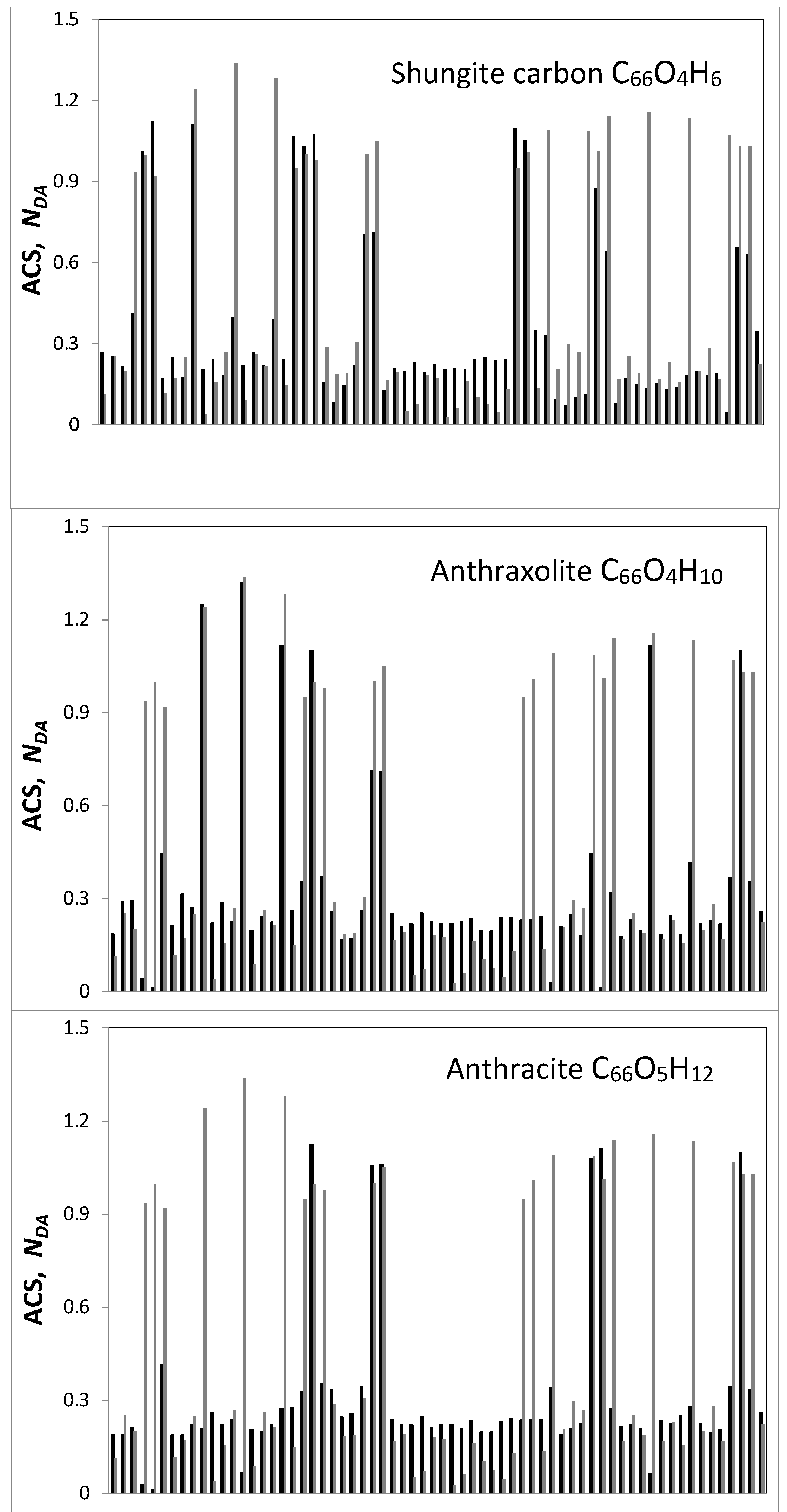
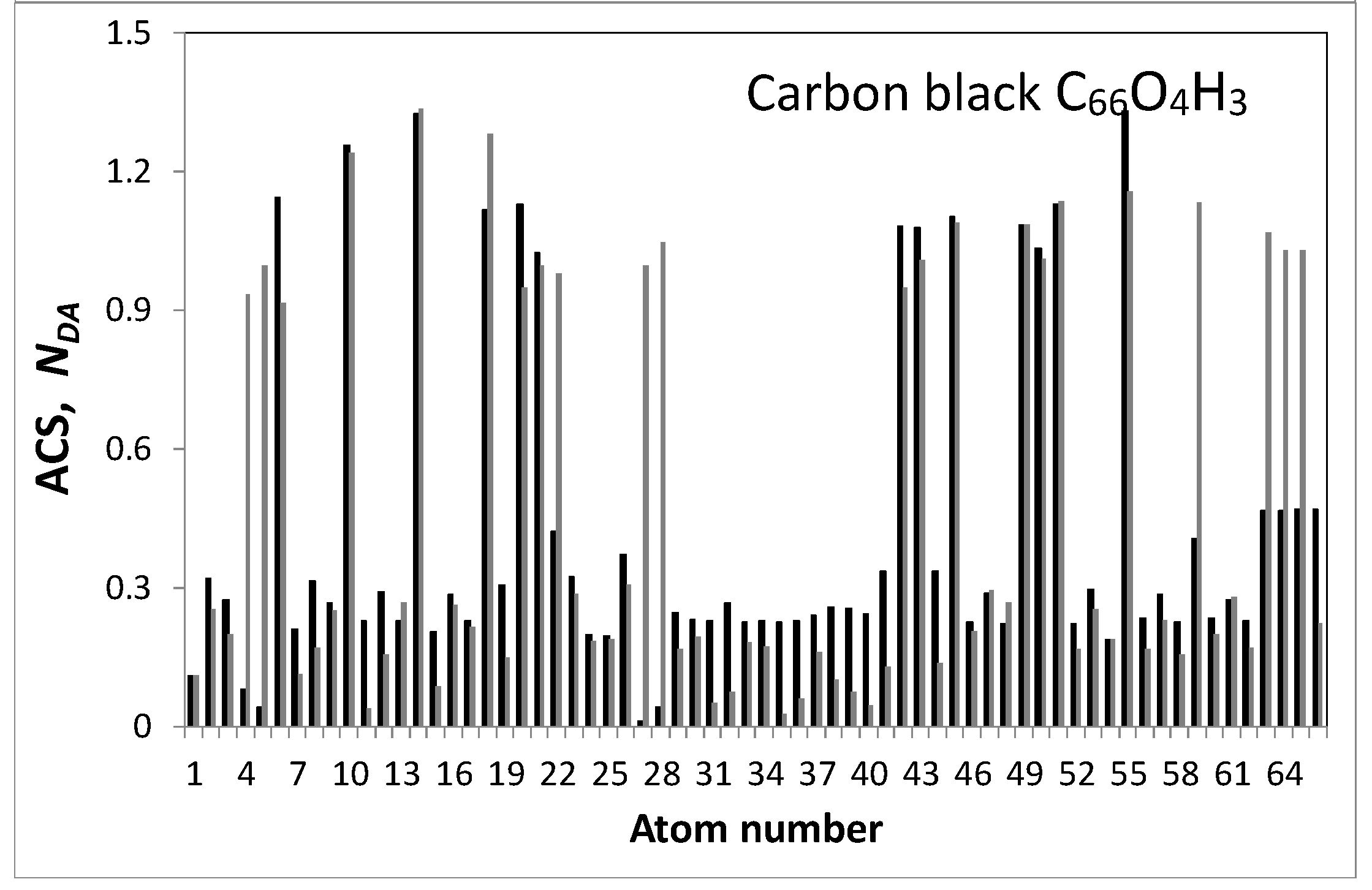
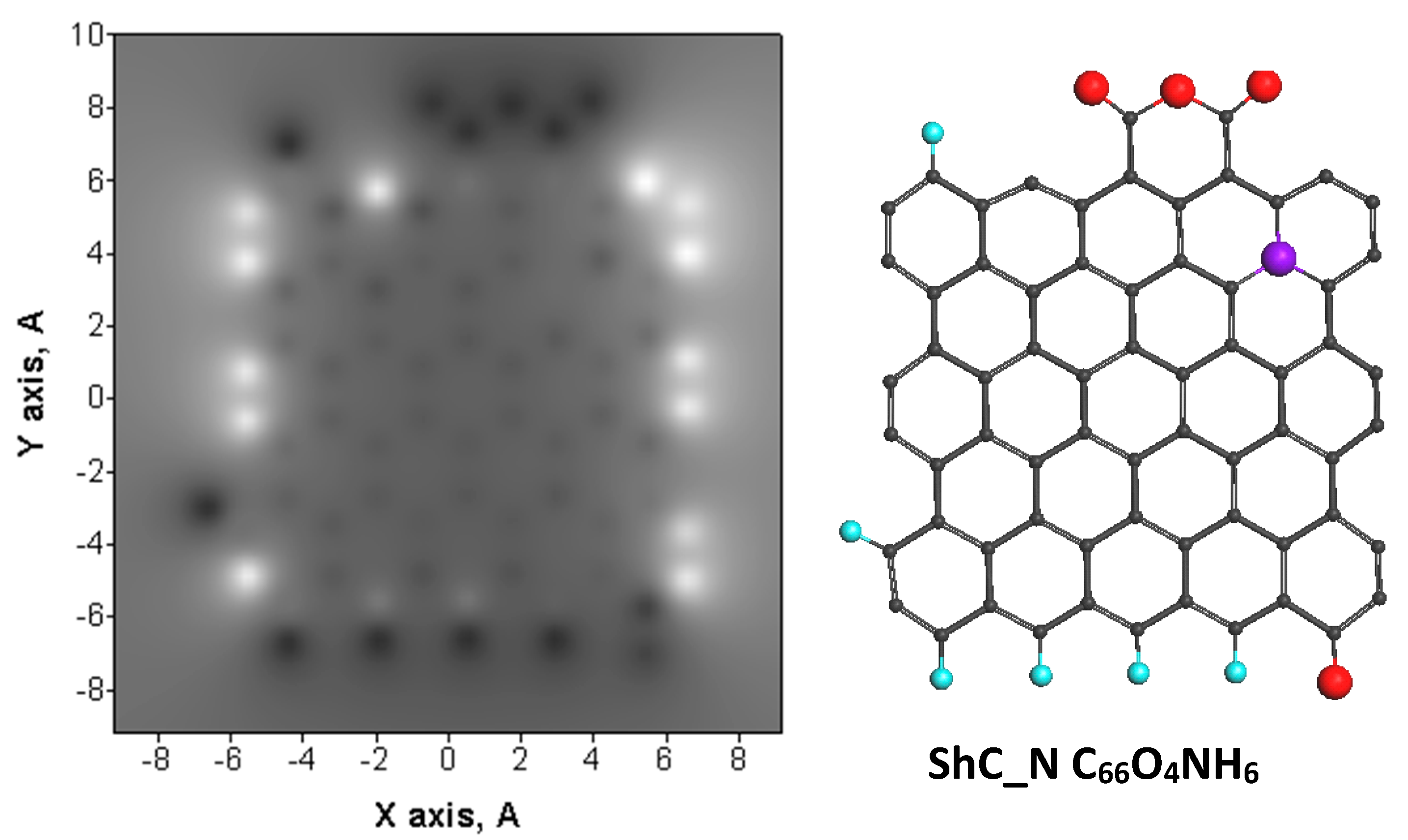

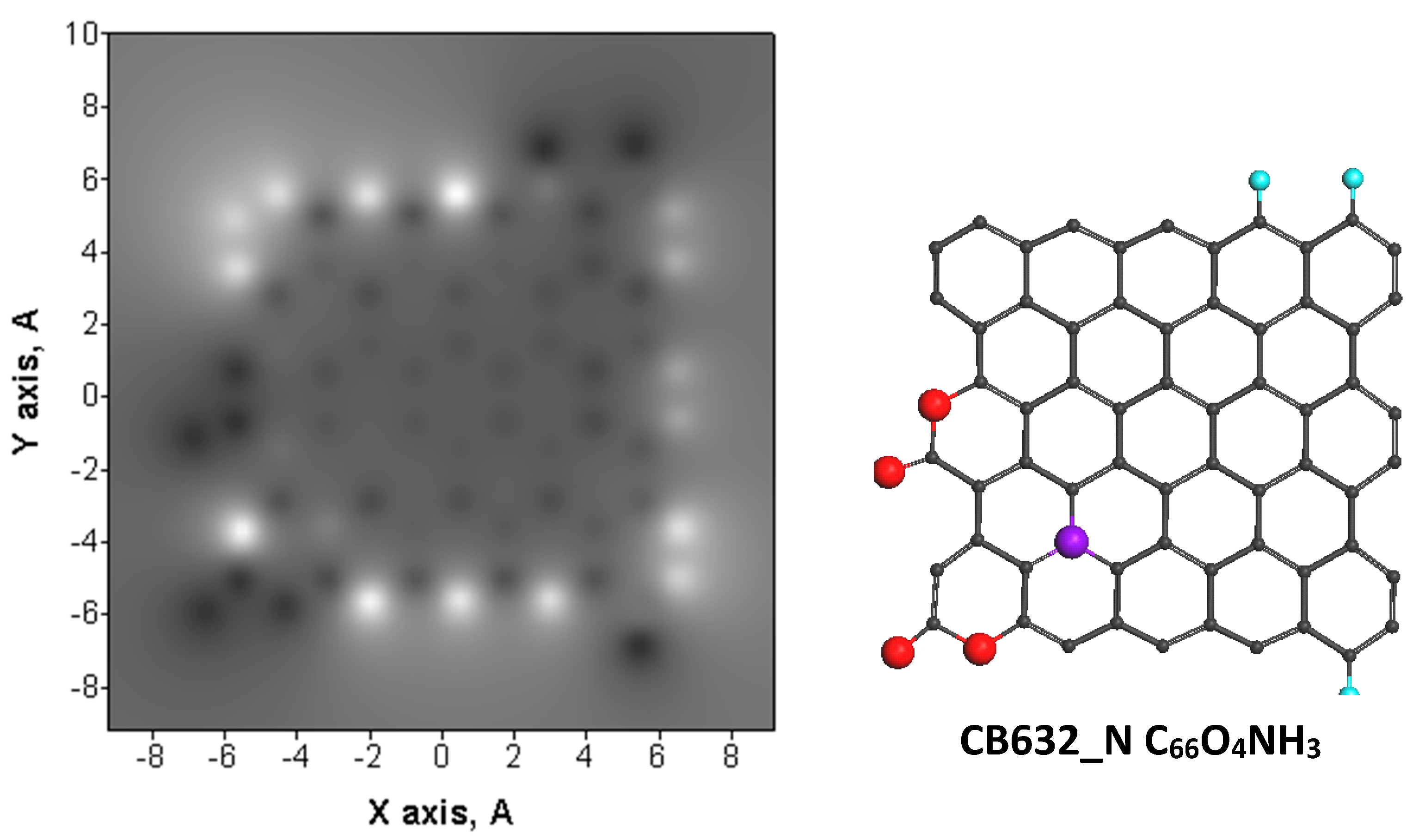

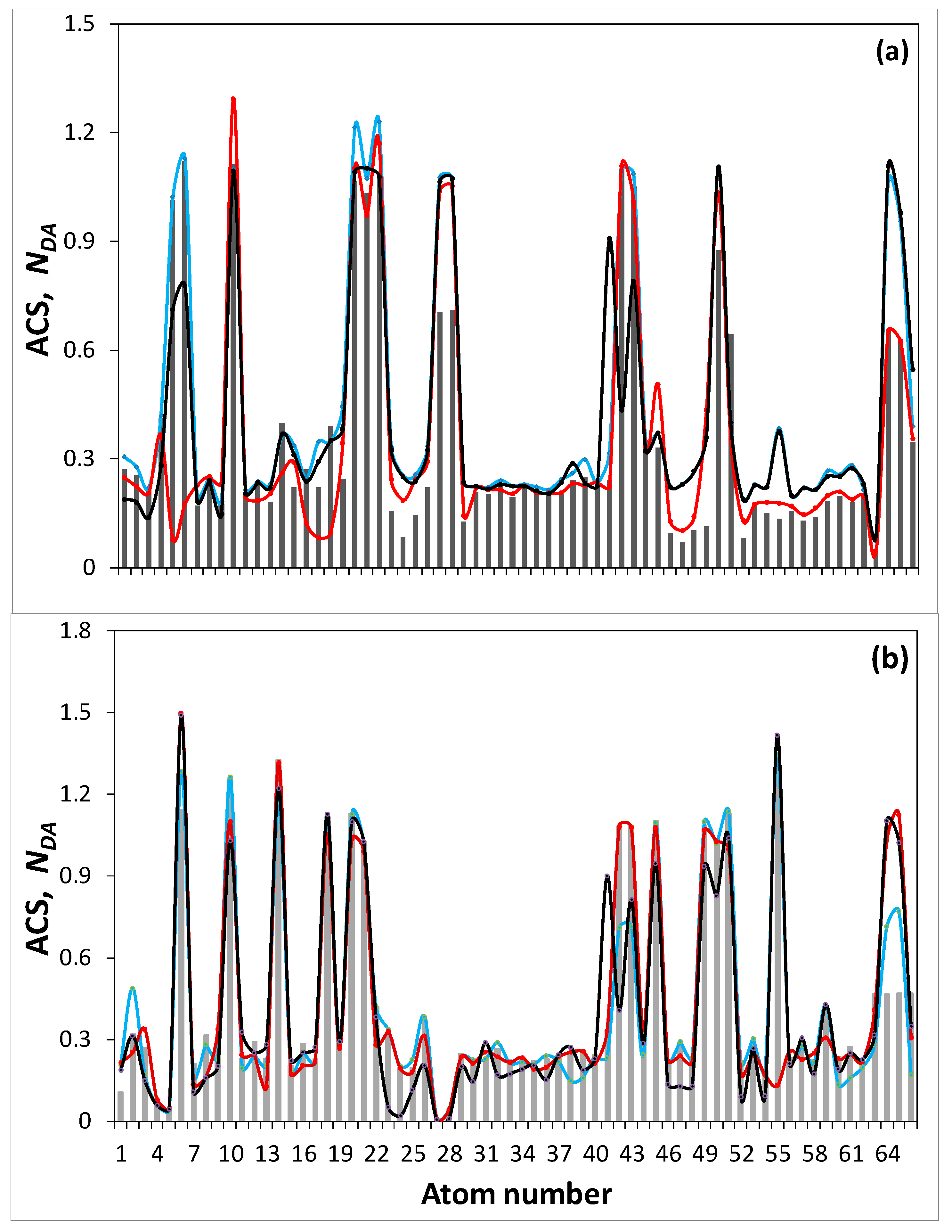
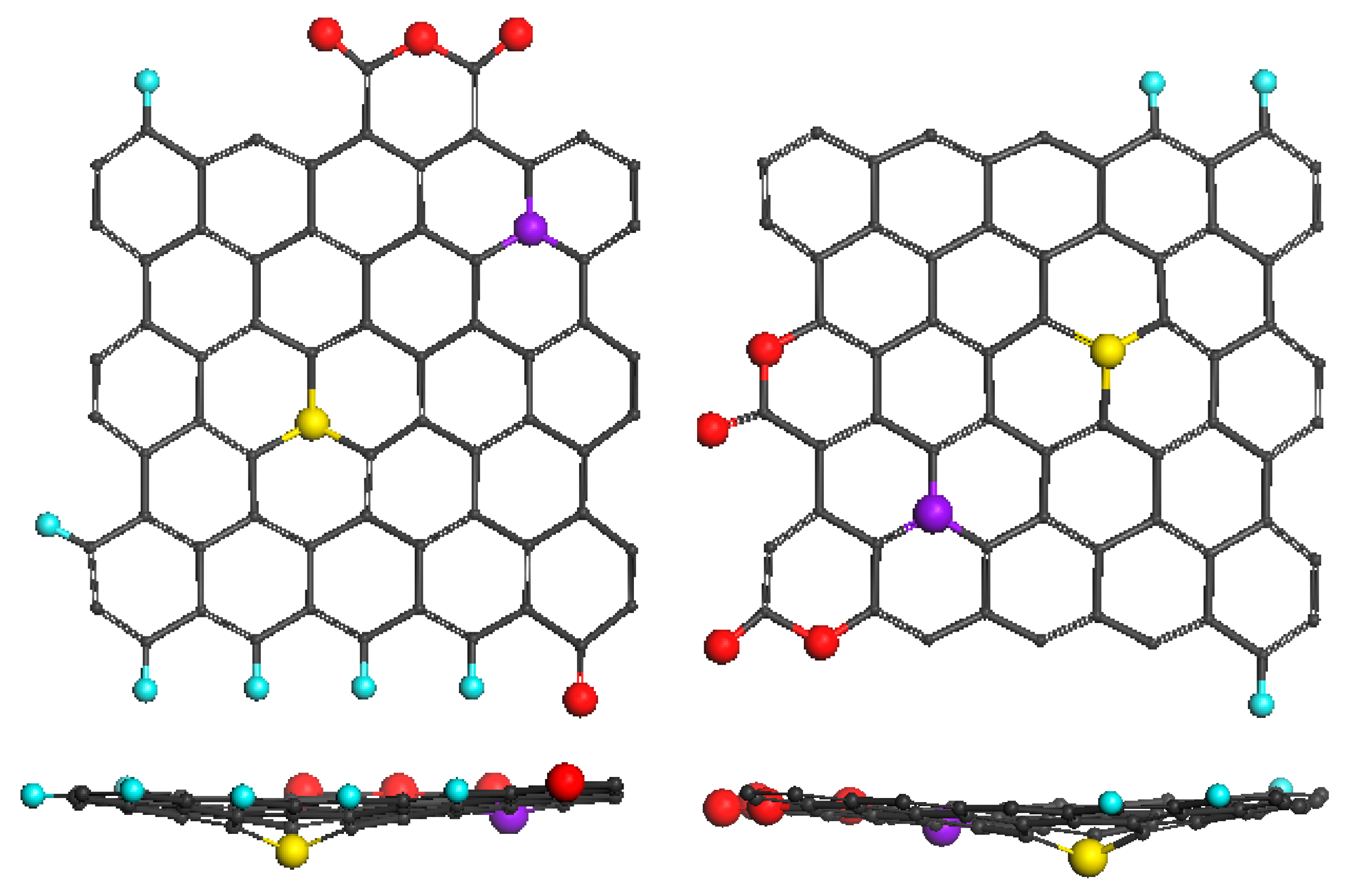
| Samples | d (Å) | Number of BSU Layers | ||
|---|---|---|---|---|
| Graphite | 3.35 | >20 2 | ~100 | >20 |
| ShC | 3.47(n); 3.48(X) | 2.5(n); 2.0(X) | 7(n); 5–6(X) | 2.1(X) |
| AnthX | 3.47(n); 3.47(X) | 2.5(n); 1.9(X) | 7(n); 5–6(X) | 1.6(X) |
| AnthC 3 | 3.50(X) | 2.2(X) | 5–6(X) | 2.1(X) |
| CB632 | 3.57(n); 3.58(X) | 2.2(n); 1.6(X) | 6(n); 4–5(X) | 1.4(X) |
| Samples | Elemental Analysis, wt% | XPS Analysis, at% | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | C | O | Minor Impurities | |
| ShC | 94.44 | 0.63 | 0.88 | 4.28 | 1.11 | 92.05 | 6.73 | S—0.92; Si—0.20; N—0.10 |
| AnthX | 94.01 | 1.11 | 0.86 | 2.66 | 1.36 | 92.83 | 6.00 | S—0.85; Si—0.25; N—0.07 |
| AnthC 1 | 90.53 | 1.43 | 0.74 | 6.44 | 0.89 | 92.94 | 6.61 | Cl—0.11; S: 0.34 |
| CB632 | 97.94 | 0.32 | 0.04 | 1.66 | 0.68 | 93.32 | 6.02 | Si—0.66 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheka, E.F. Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry. Materials 2020, 13, 565. https://doi.org/10.3390/ma13030565
Sheka EF. Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry. Materials. 2020; 13(3):565. https://doi.org/10.3390/ma13030565
Chicago/Turabian StyleSheka, Elena F. 2020. "Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry" Materials 13, no. 3: 565. https://doi.org/10.3390/ma13030565
APA StyleSheka, E. F. (2020). Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry. Materials, 13(3), 565. https://doi.org/10.3390/ma13030565





