Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide
Abstract
1. Introduction
2. Materials and Methods
3. Results
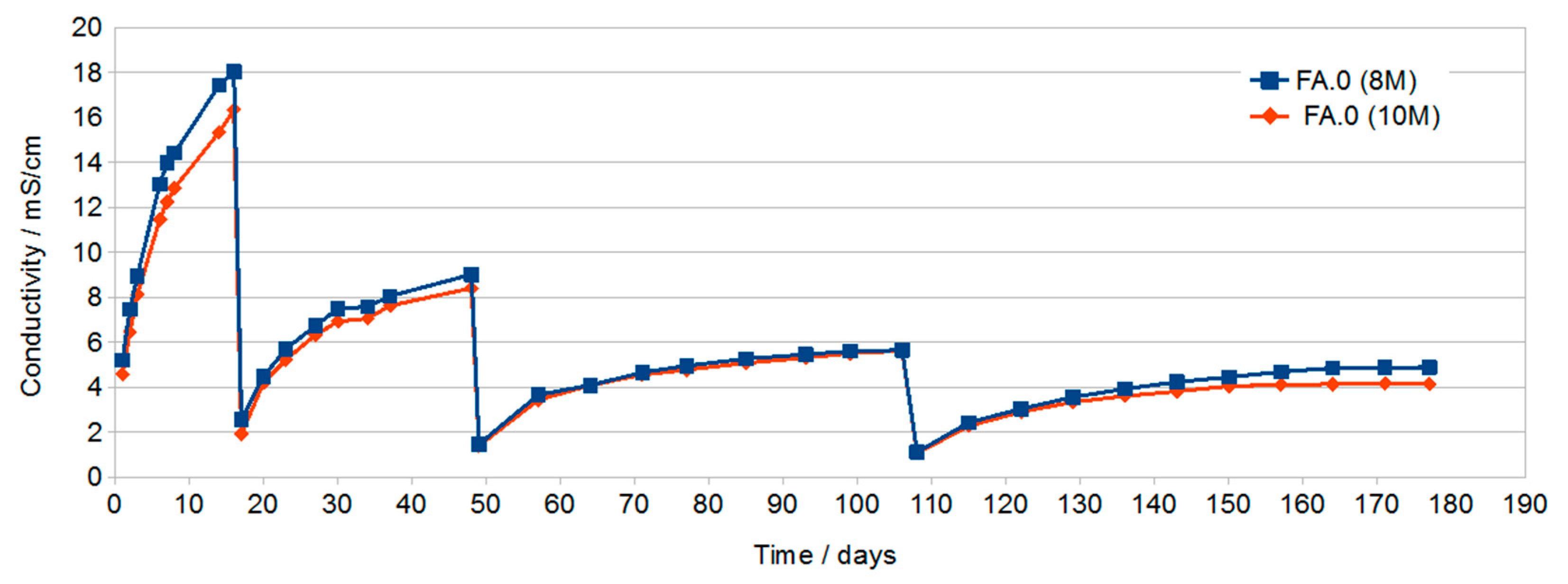
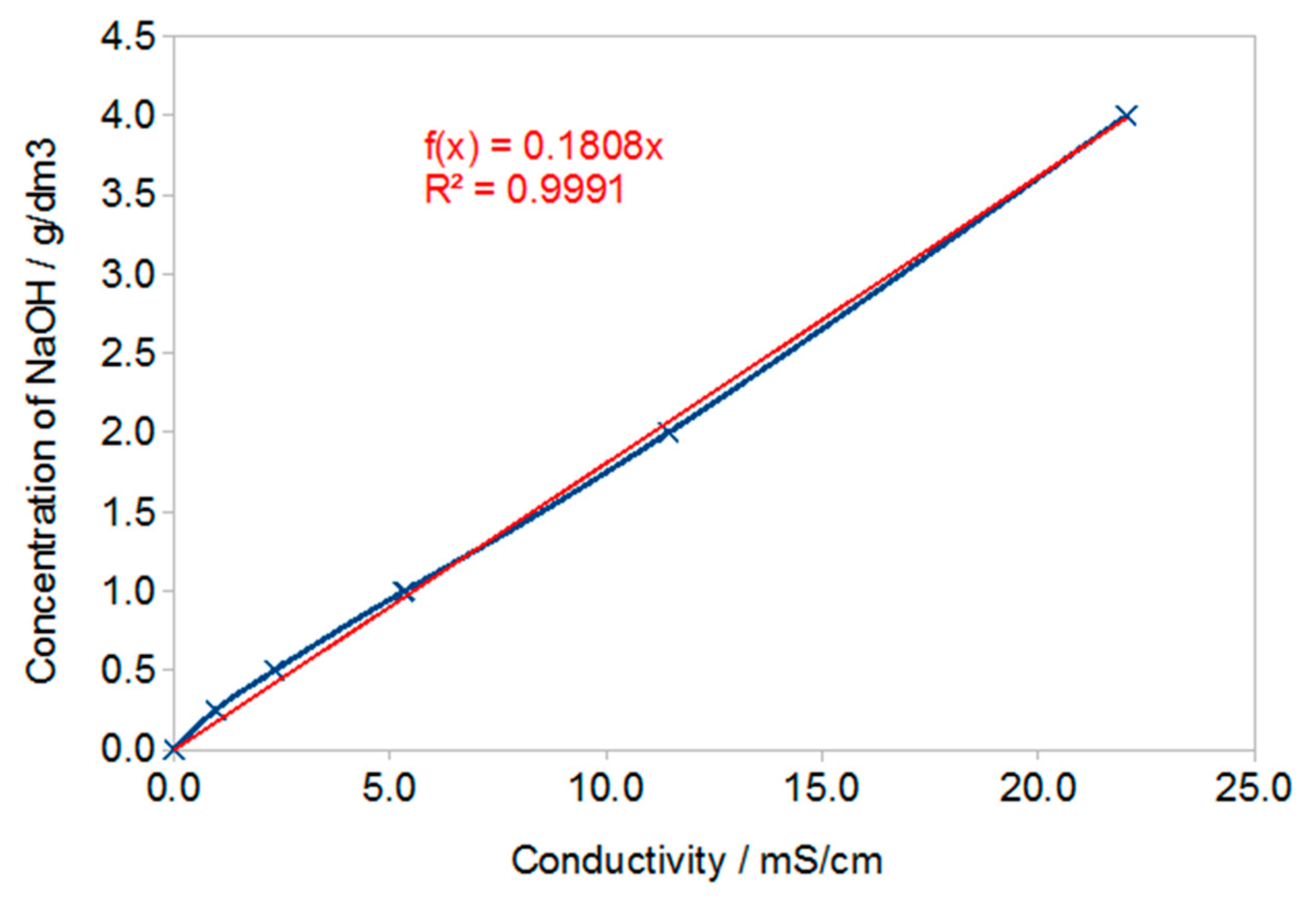
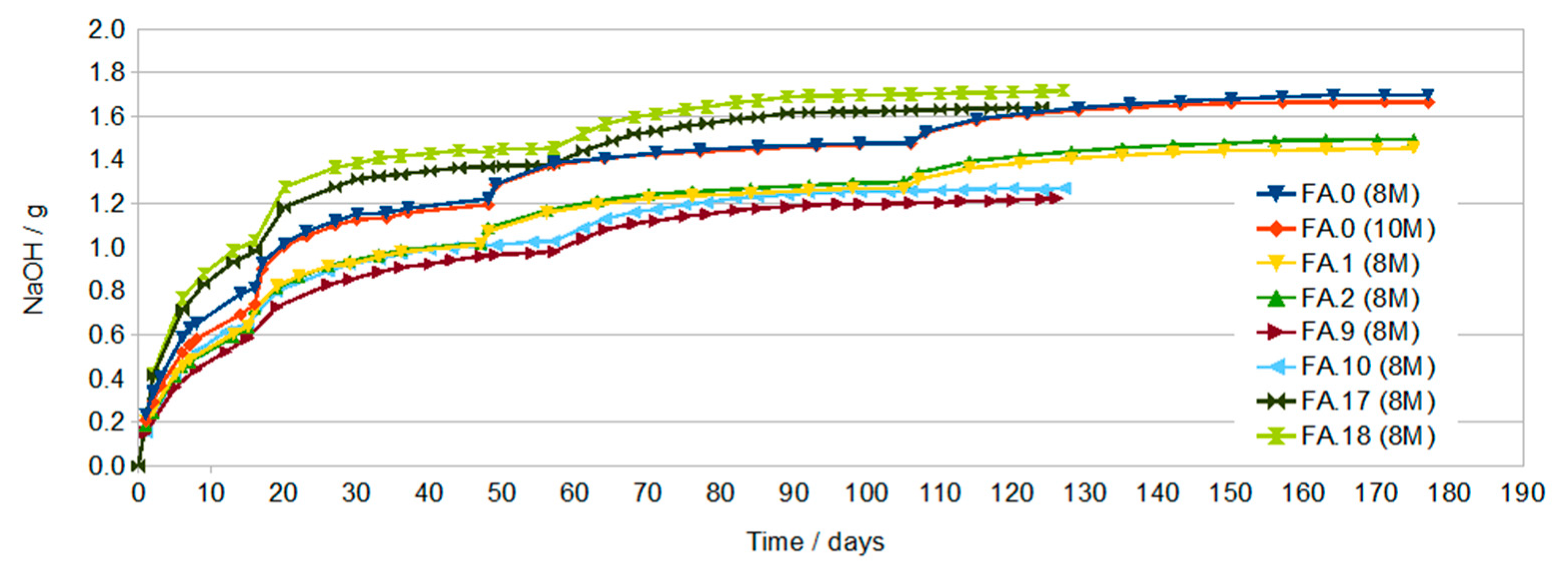
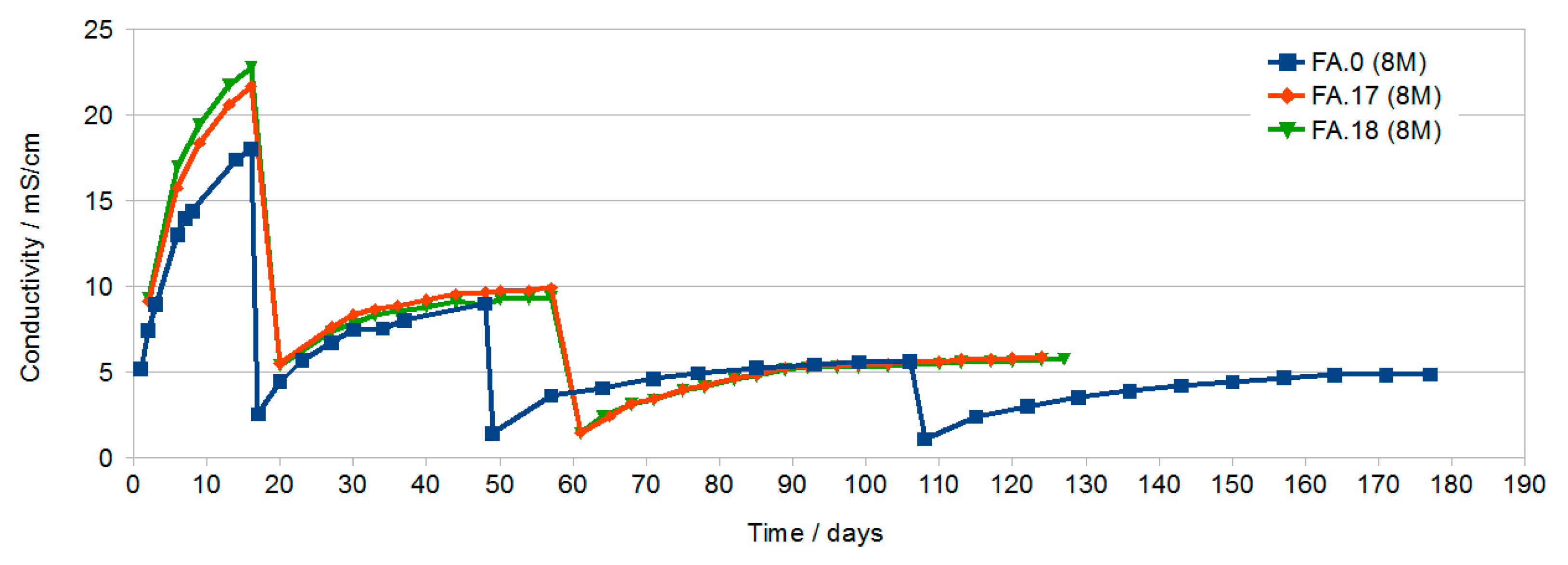
3.1. Results of Conductivity Testing
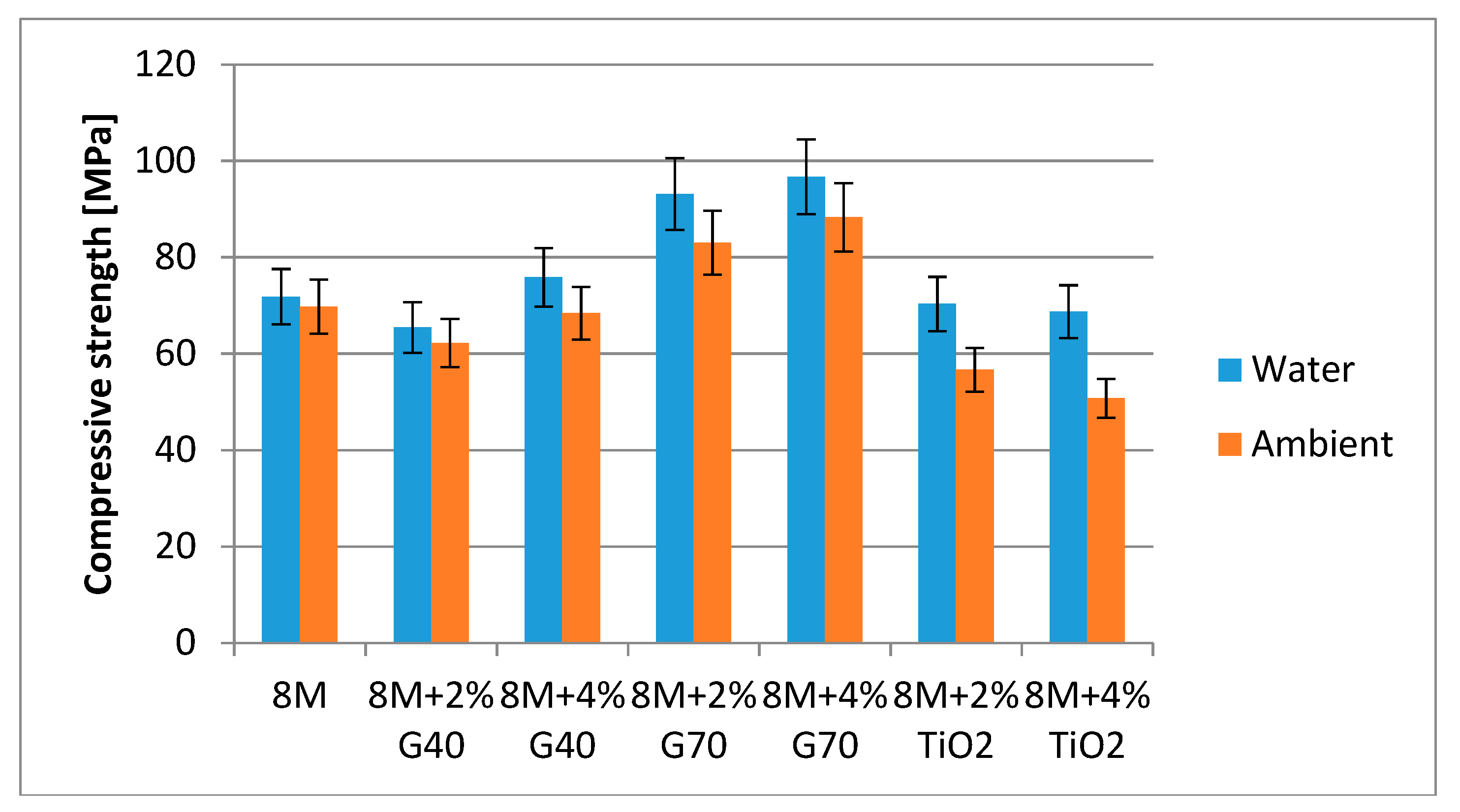
3.2. Results of Compressive Strength
4. Conclusions
- The addition of 2% and 4% G70 significantly increases the compressive strength of geopolymers, both cured in ambient conditions and in water.
- The highest compressive strengths were obtained for geopolymers based on fly ash activated with 8 M NaOH solution and 4% G70 and cured in water—approx. 100 MPa.
- The addition of TiO2 reduced the compressive strength of geopolymer samples.
- Studies on the tendency to efflorescence have shown that special additives in the form of G40, G70, and TiO2 do not effectively protect geopolymers against efflorescence. The observations show that they only delay the formation time and the amount of arising efflorescence on the surface of the samples.
- Curing geopolymer samples in water conditions is an effective method of preventing efflorescence on their surfaces.
- Conductivity testing of solutions showed very low conductivity (approx. 0.5 mS/cm) for samples cured in water.
Author Contributions
Funding
Conflicts of Interest
References
- Bensted, J. Efflorescence—Prevention is better than cure. Concrete 2000, 34, 40–41. [Google Scholar]
- Bortnovsky, O.; Dědeček, J.; Tvarůžkova, Z.; Sobalik, Z.; Šubrt, J. Metal ions as probes for characterization of geopolymer materials. J. Am. Ceram. Soc. 2008, 91, 3052–3057. [Google Scholar] [CrossRef]
- Cloos, P.; Léonard, A.J.; Moreau, J.P.; Herbillon, A.; Fripiat, J.J. Structural organization in amorphous silico-aluminas. Clays Clay Miner. 1969, 17, 279–287. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Institut Geopolymere: Saint-Quentin, France, 2008. [Google Scholar]
- Deichnel, T. Efflorescence—Origins, causes, counter-measures. BFT Mag. 1982, 48, 590–597. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Myškova, L.; Šmilauer, V.; Alberovska, L.; Vinšova, L. Aluminosilicate polymers—Influence of elevated temperatures, efflorescence. Ceram. Silik. 2009, 53, 276–282. [Google Scholar]
- Kresse, P. Efflorescence-mechanism of occurrence and possibilities of prevention. BFT Mag. 1987, 53, 160–168. [Google Scholar]
- Kresse, P. Coloured concrete and its enemy: Efflorescence. Chem. Ind. 1989, 4, 93–95. [Google Scholar]
- Kresse, P. Efflorescence and its prevention. BFT Mag. 1991, 57, 84–88. [Google Scholar]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Neville, A. Efflorescence—Surface blemish or internal problem? Part 1: The knowledge. Concr. Int. 2002, 24, 86–90. [Google Scholar]
- Duxson, P.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. Effects of alkali cations on aluminum incorporation in geopolymeric gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Kani, E.N.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Comp. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Šmilauer, V.; Bittnar, Z. Material and structural characterization of alkali activated low-calcium brown coal fly ash. J. Hazard. Mater. 2009, 168, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Łach, M.; Hebdowska-Krupa, M.; Komar, N. Strength and leachability of geopolymers with the addition of municipal solid waste ashes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012010. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Zhang, T.-T.; Jia, S.B.; Liu, J.; Quan, X.-H.; Zheng, W. Mechanical properties and leaching characteristics of geopolymer-solidified/stabilized lead-contaminated soil. Adv. Civ. Eng. 2019, 2019, 6015769. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Korniejenko, K.; Łach, M.; Mikuła, J.; Krzywda, J. Effect of coffee grounds addition on efflorescence in fly ash-based geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012035. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Xie, J.; Chen, W.; Wang, J.; Fang, C.; Zhang, B.; Liu, F. Coupling effects of recycled aggregate and GGBS/metakaolin on physicochemical properties of geopolymer concrete. Constr. Build. Mater. 2019, 226, 345–359. [Google Scholar] [CrossRef]



| Oxide Composition (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | |
| Fly ash | 55.9 | 1.09 | 5.92 | 23.49 | 2.72 | 2.61 | 3.55 | 0.59 |
| Additions | Al2O3 | CaO | SiO2 | Fe2O3 | Main Phase | Accompanying Phase |
|---|---|---|---|---|---|---|
| G40 | >41% | >35.5% | <4% | <15% | CA | C4AF, C12A7, C2AS |
| G70 | >69% | >28% | <0.5% | <0.3% | CA, CA2 | C12A7, αA |
| Description of Samples | Aqueous NaOH + Aqueous Sodium Silicate (Water Glass) (mL) | Fly Ash (g) | Sand (g) | Additives (g) |
|---|---|---|---|---|
| FA.0 (8 M) | 120 (mL)+ 240 (mL) | 1000 | 1000 | − |
| FA.0 (10 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | − |
| FA.1 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 20 g G40 |
| FA.2 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 40 g G40 |
| FA.9 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 20g G70 |
| FA.10 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 40g G70 |
| FA.17 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 20g TiO2 |
| FA.18 (8 M) | 120 (mL) + 240 (mL) | 1000 | 1000 | 40g TiO2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łach, M.; Korniejenko, K.; Walter, J.; Stefańska, A.; Mikuła, J. Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials 2020, 13, 495. https://doi.org/10.3390/ma13030495
Łach M, Korniejenko K, Walter J, Stefańska A, Mikuła J. Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials. 2020; 13(3):495. https://doi.org/10.3390/ma13030495
Chicago/Turabian StyleŁach, Michał, Kinga Korniejenko, Janusz Walter, Anna Stefańska, and Janusz Mikuła. 2020. "Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide" Materials 13, no. 3: 495. https://doi.org/10.3390/ma13030495
APA StyleŁach, M., Korniejenko, K., Walter, J., Stefańska, A., & Mikuła, J. (2020). Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials, 13(3), 495. https://doi.org/10.3390/ma13030495







