The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Characterization
2.3. Iodine Adsorption Measurements
2.4. Calculation Method
3. Results and Discussion
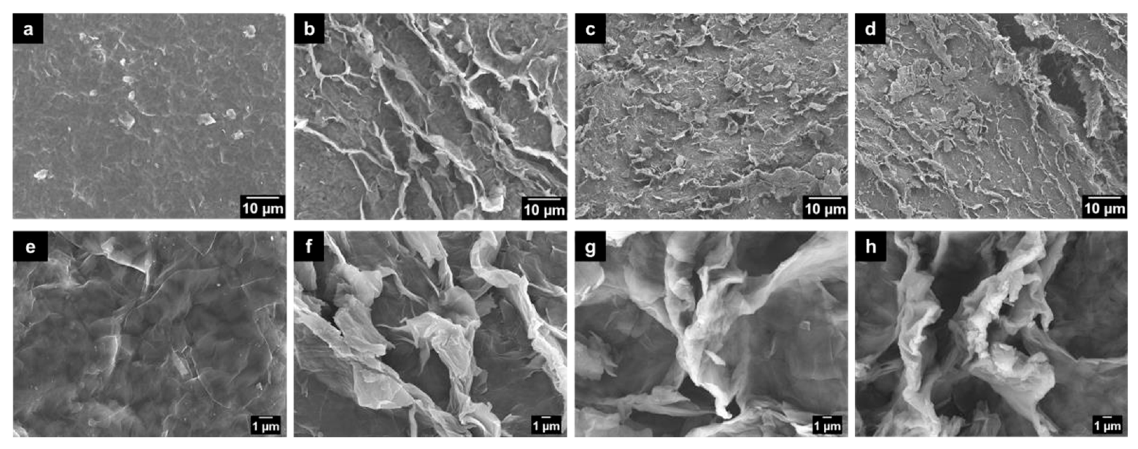
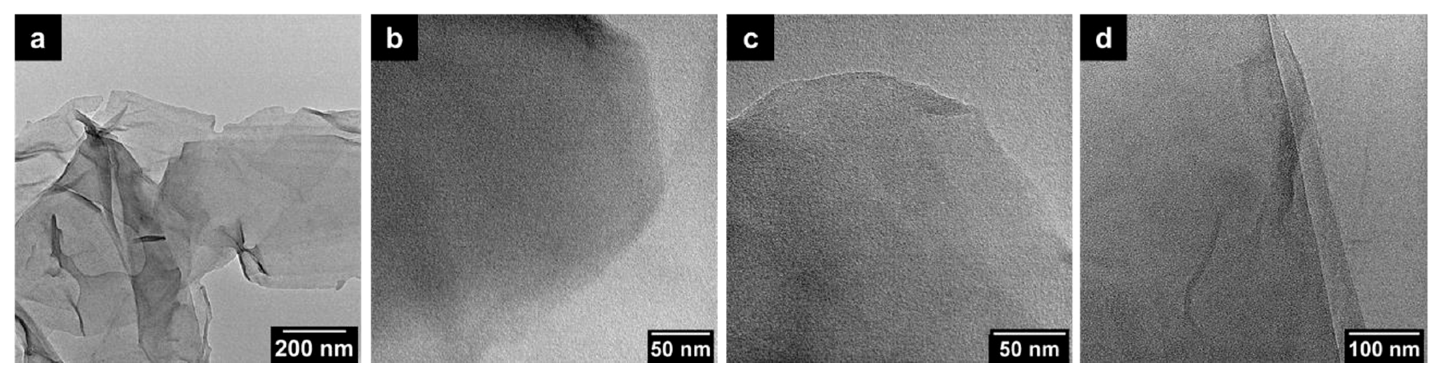
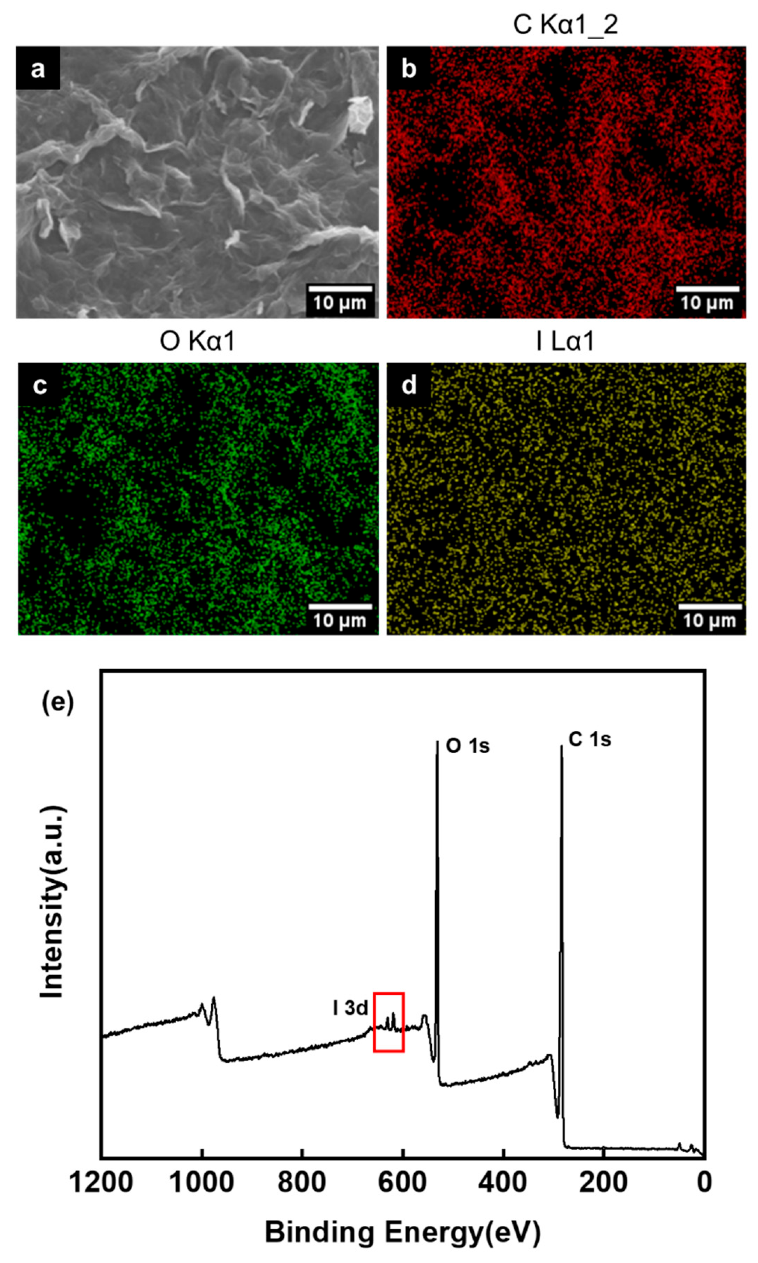
3.1. The Morphology of GO after HNO3 Treatment
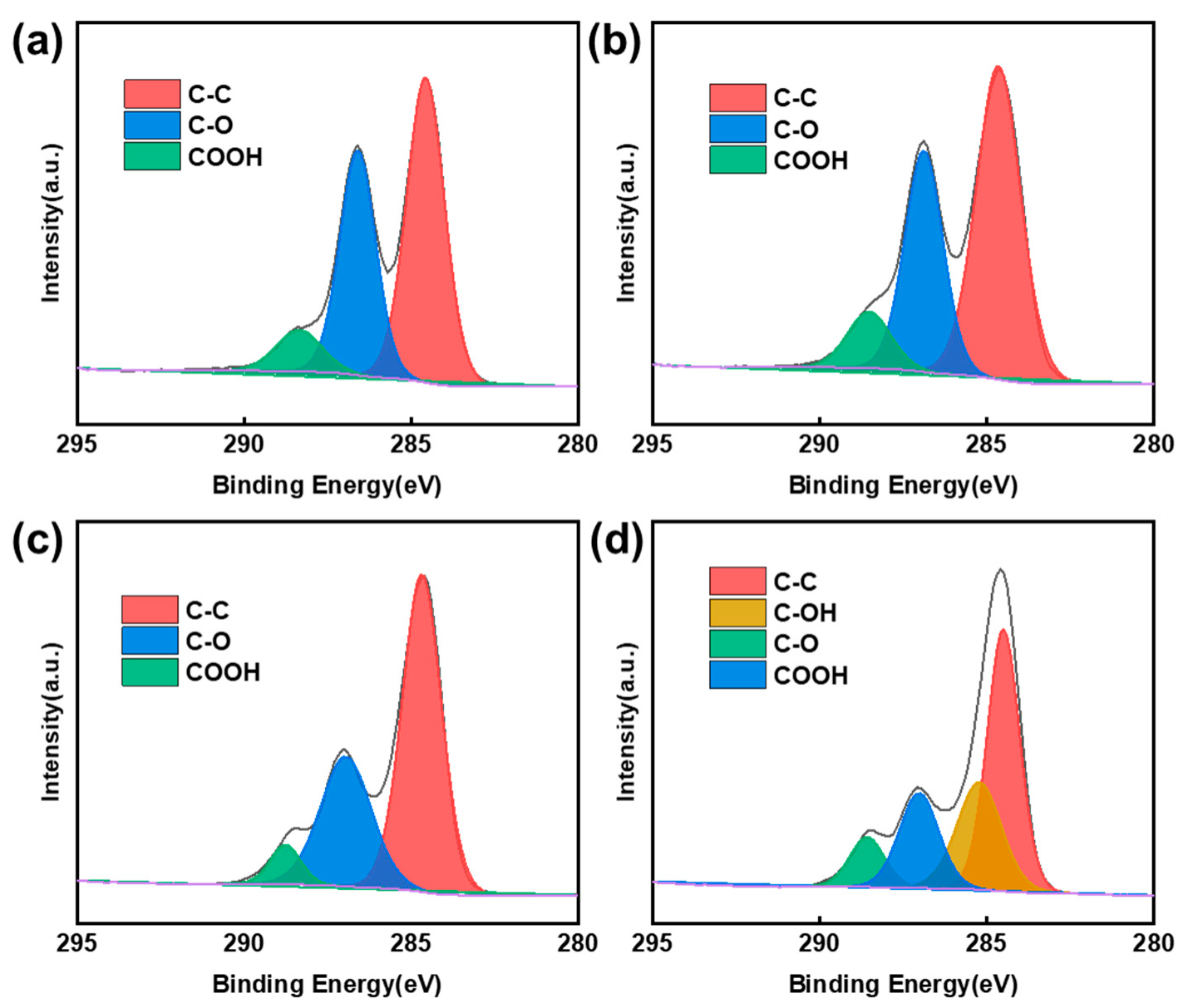
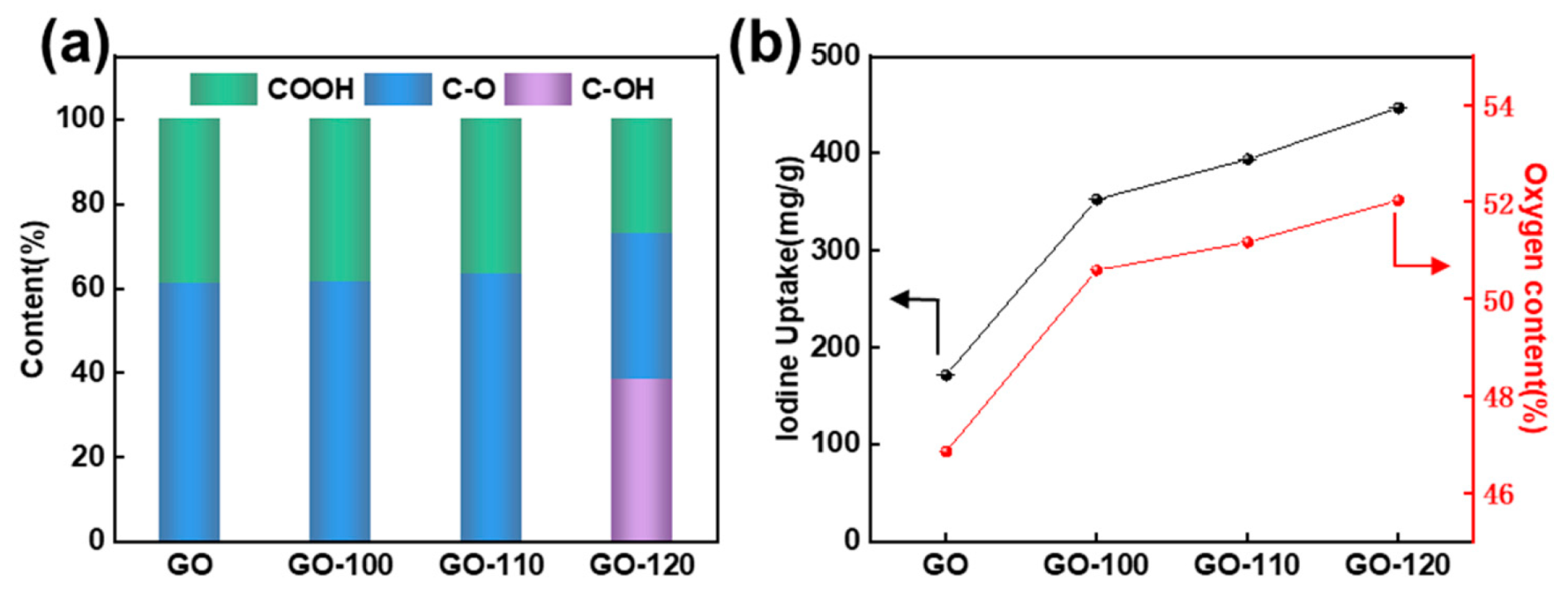
3.2. Oxygen Functional Groups
3.3. The Iodine Adsorption Performance
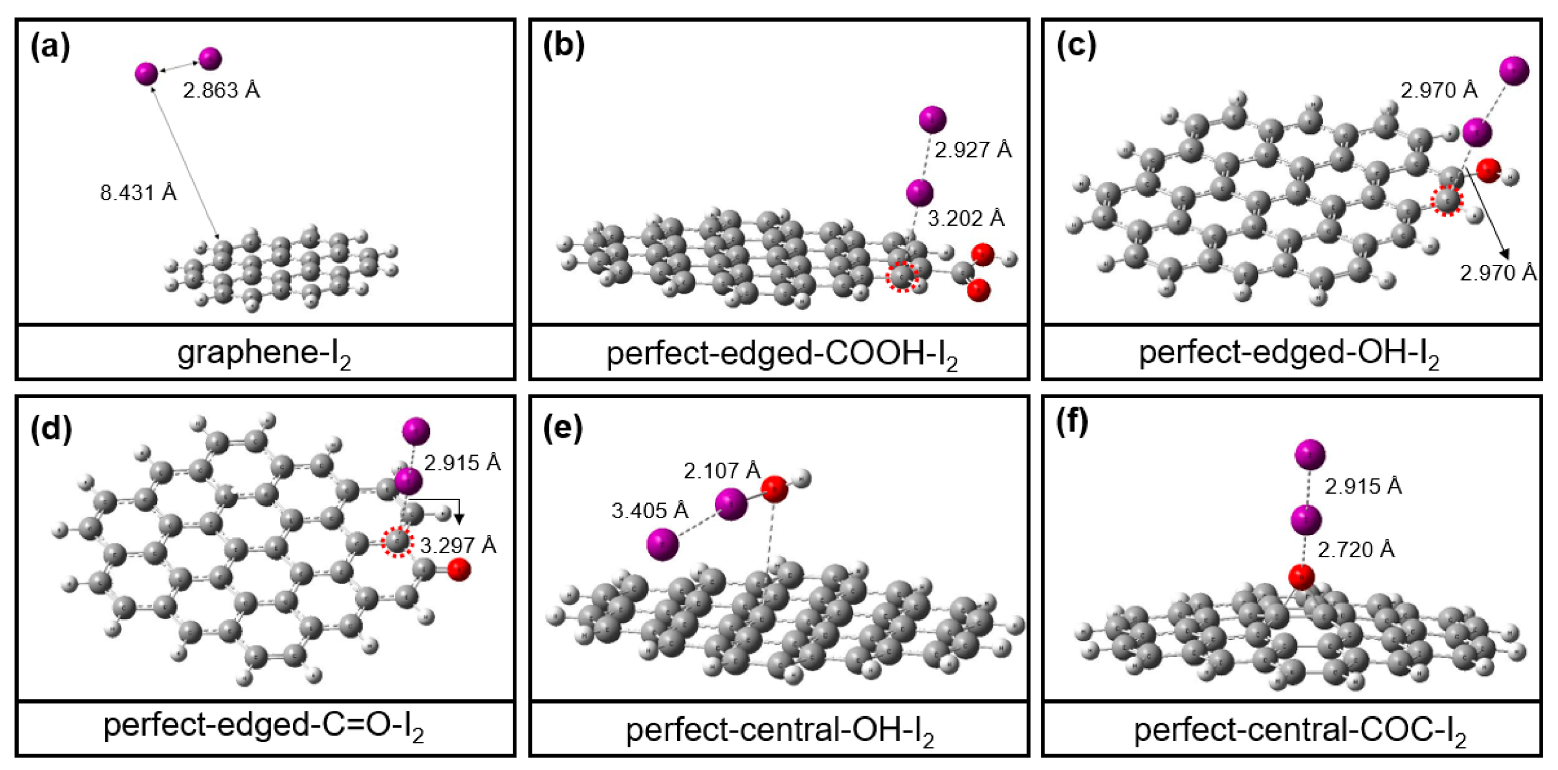
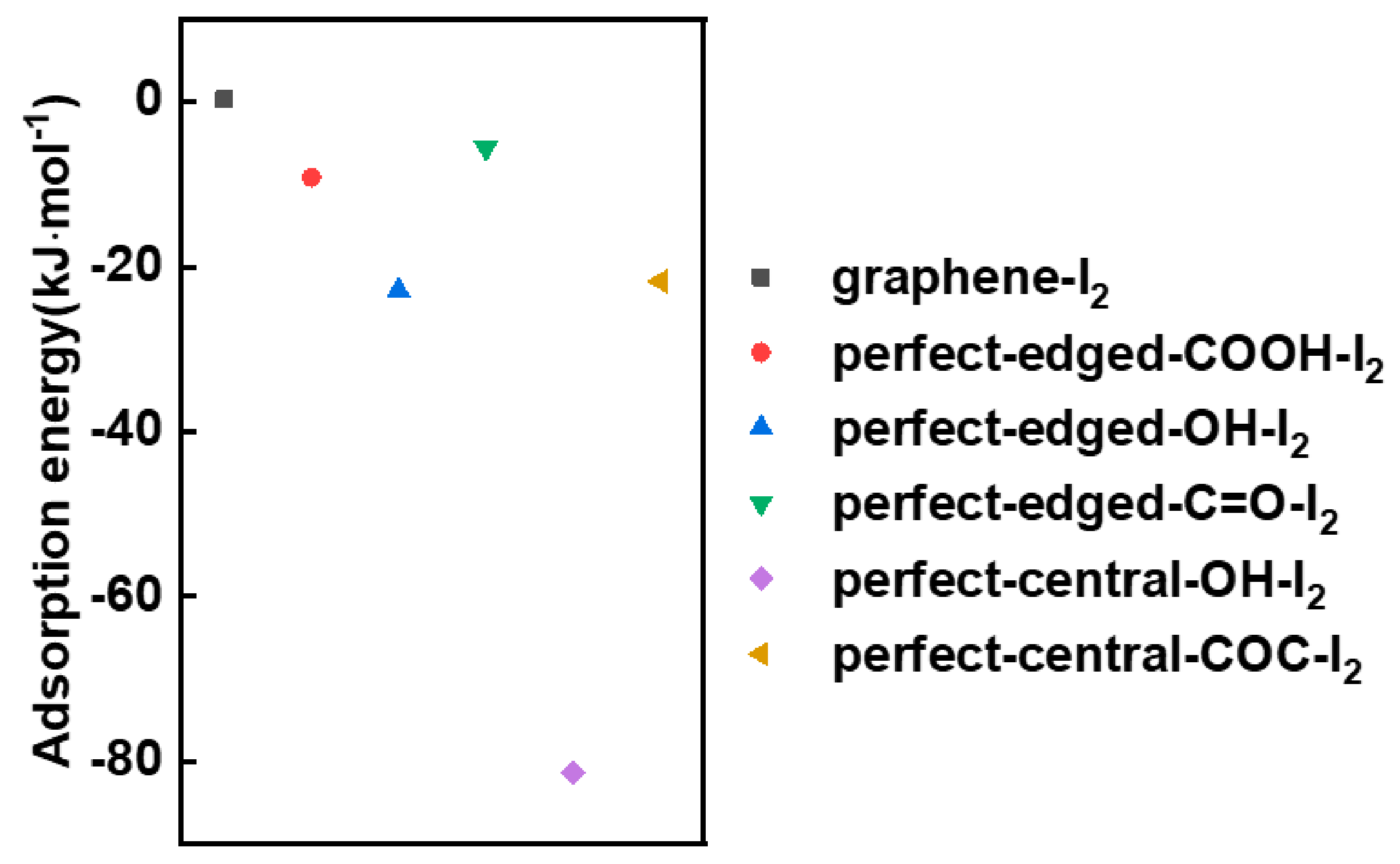
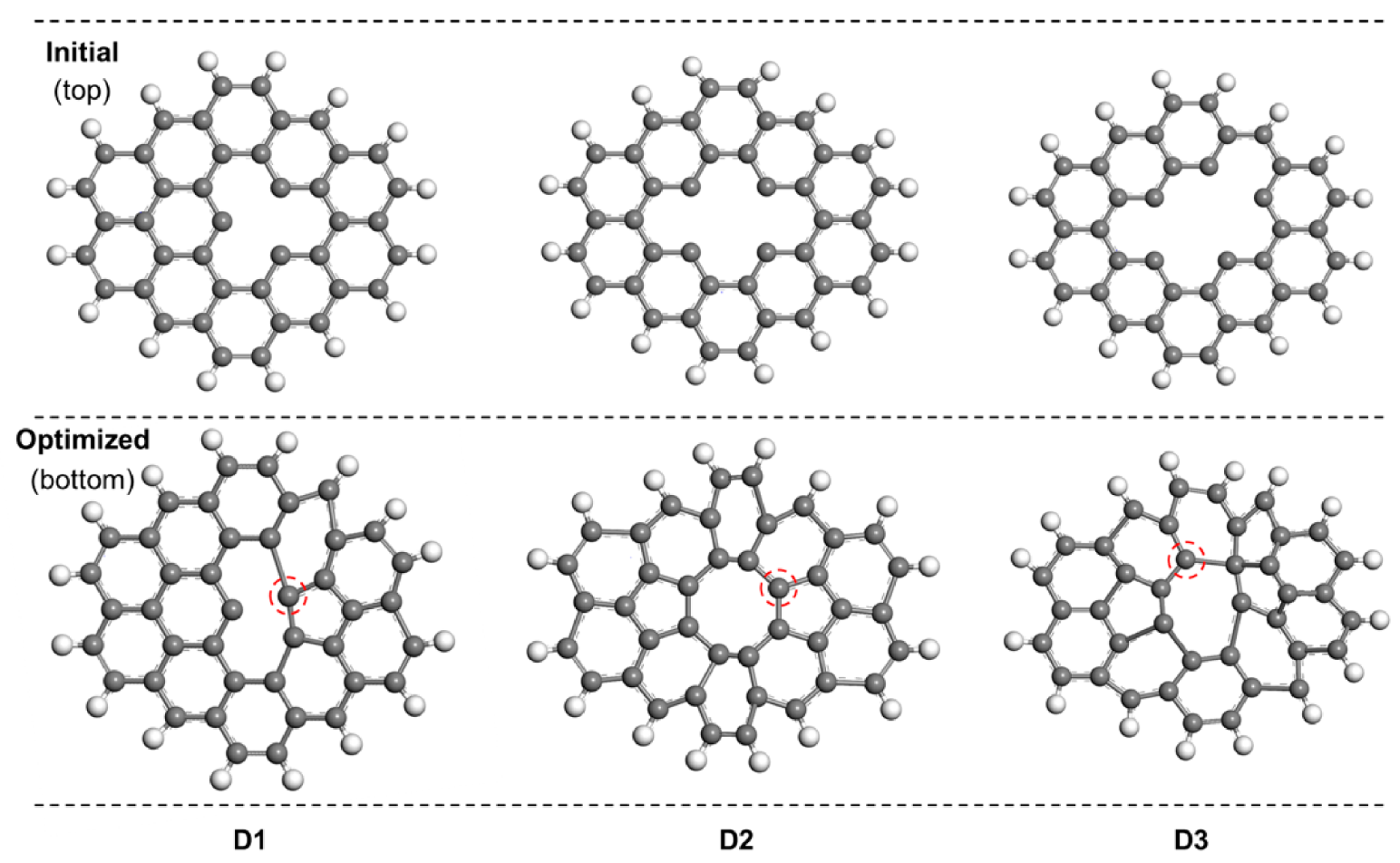
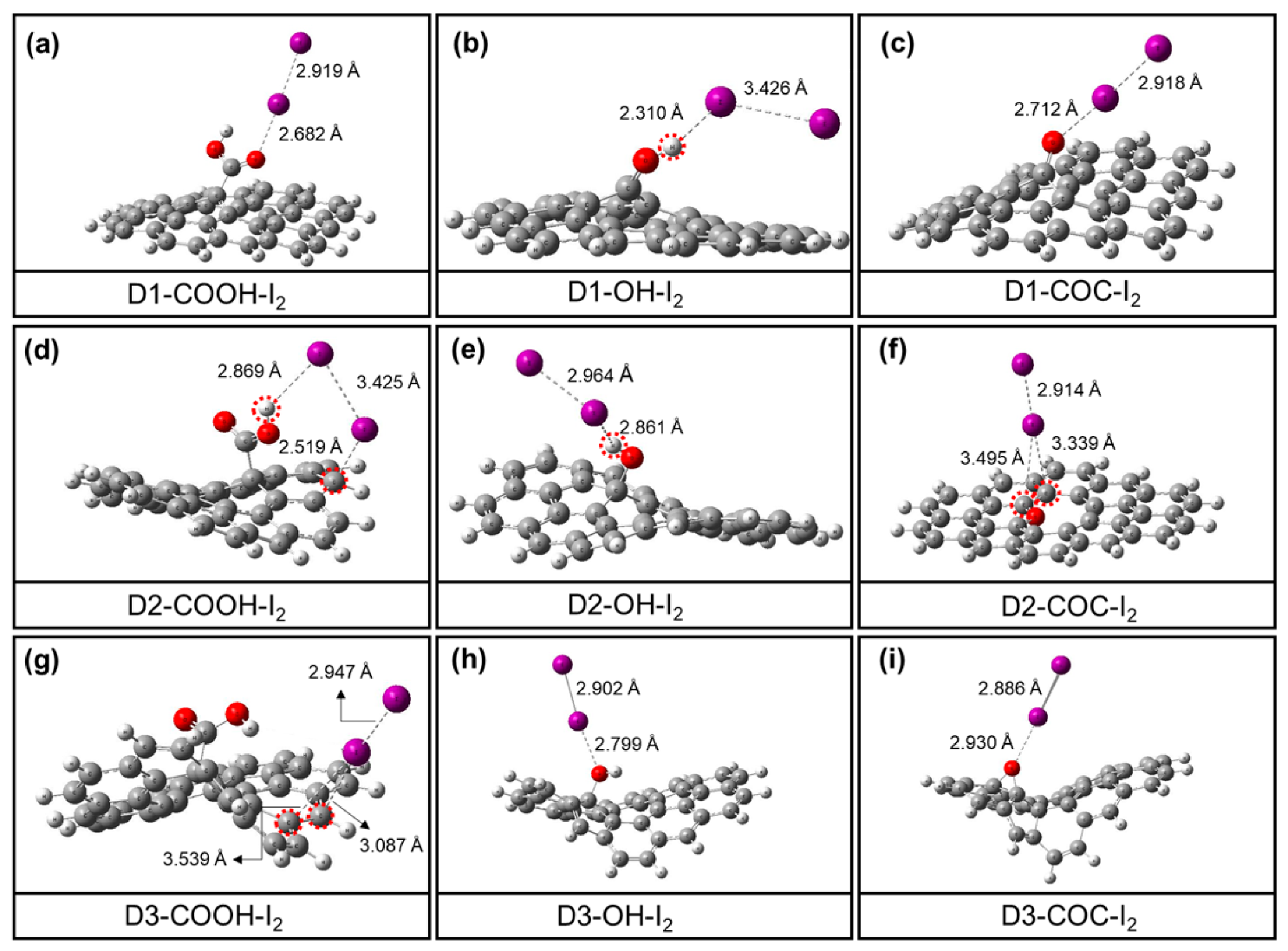
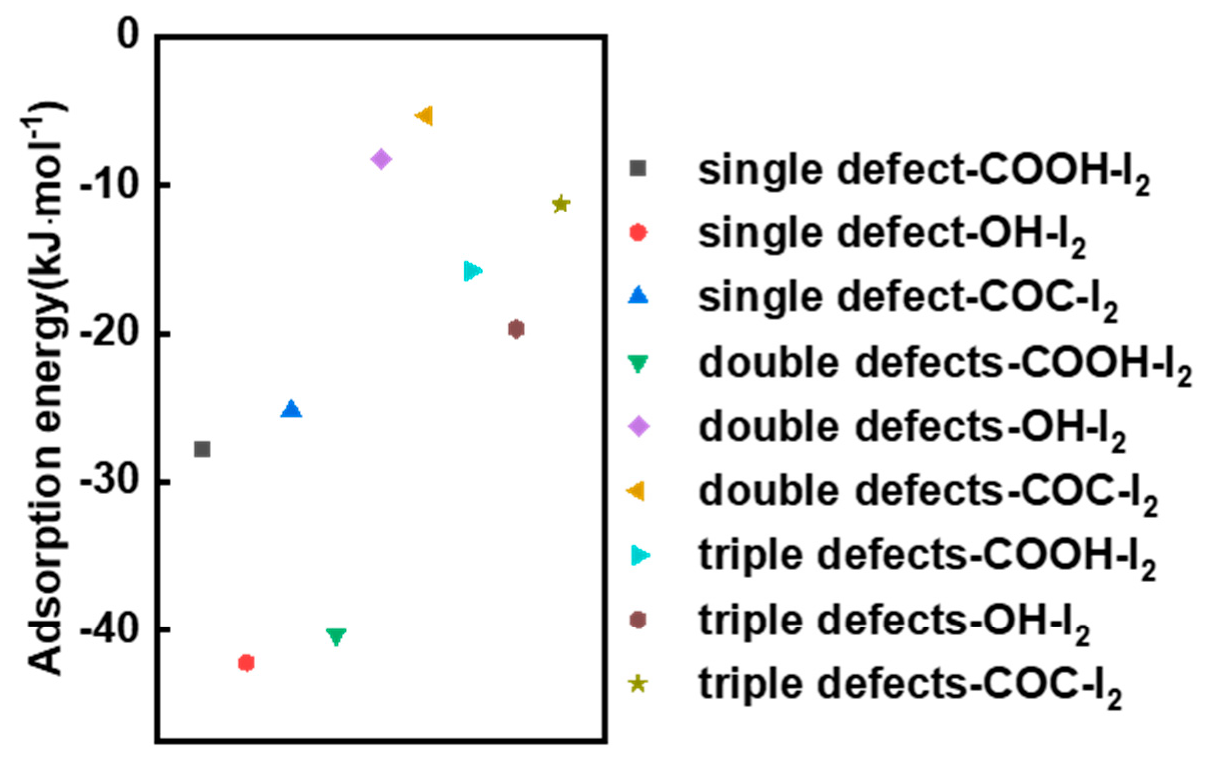
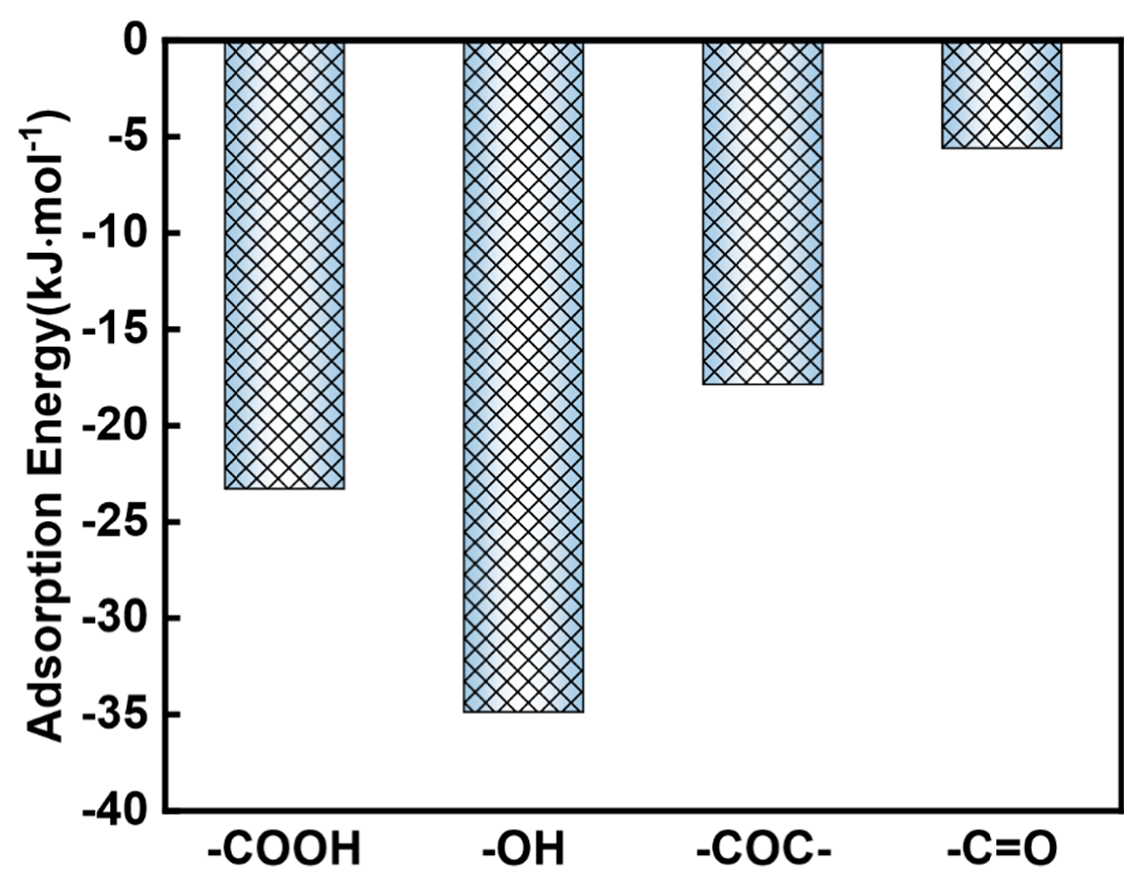
3.4. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoshida, N.; Kanda, J. Tracking the Fukushima radionuclides. Science 2012, 336, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, K.; Kim, K.H.; Pournara, A.; Deep, A. Towards high-efficiency sorptive capture of radionuclides in solution and gas. Prog. Mater. Sci. 2018, 94, 1–67. [Google Scholar] [CrossRef]
- Kintisch, E. Congress tells DOE to take fresh look at recycling spent reactor fuel. Science 2005, 310, 1406. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.C.; Von Hippel, F.N. Nuclear waste management in the United States—Starting over. Science 2009, 325, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, N.R.; Garn, T.G.; Greenhalgh, M.R.; Law, J.D.; Jubin, R.; Strachan, D.M.; Thallapally, P.K. Radioactive iodine and krypton control for nuclear fuel reprocessing facilities. Sci. Technol. Nucl. Install. 2013, 2013, 702496. [Google Scholar] [CrossRef]
- Mushkacheva, G.; Rabinovich, E.; Privalov, V.; Povolotskaya, S.; Shorokhova, V.; Sokolova, S.; Turdakova, V.; Ryzhova, E.; Hall, P.; Schneider, A.B.; et al. Thyroid abnormalities associated with protracted childhood exposure to 131I from atmospheric emissions from the Mayak weapons facility in Russia. Radiat. Res. 2006, 166, 715–722. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide aerogels as sorbents for radioactive iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O-Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2014, 284, 171–181. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Malliakas, C.D.; Sarma, D.; Armatas, G.S.; Wu, J.; Kanatzidis, M.G. Ion-Exchangeable Molybdenum Sulfide Porous Chalcogel: Gas Adsorption and Capture of Iodine and Mercury. J. Am. Chem. Soc. 2015, 137, 13943–13948. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, H.; Li, Y.; Yang, G.; Wang, Y.; Yao, H. Excellent performance of porous carbon from urea-assisted hydrochar of orange peel for toluene and iodine adsorption. Chem. Eng. J. 2020, 382, 122997. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Docao, S.; Hwang, I.C.; Song, M.K.; Choi, D.Y.; Moon, D.; Oleynikov, P.; Yoon, K.B. Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energy Environ. Sci. 2016, 9, 1050–1062. [Google Scholar] [CrossRef]
- Pei, C.; Ben, T.; Xu, S.; Qiu, S. Ultrahigh iodine adsorption in porous organic frameworks. J. Mater. Chem. A 2014, 2, 7179–7187. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, 1801991. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ge, R.; Song, X.; Xing, X.; Jiang, Q.; Lu, H.; Hao, C.; Guo, X.; Gao, Y.; et al. A 3D Covalent Organic Framework with Exceptionally High Iodine Capture Capability. Chem. Eur. J. 2018, 24, 585–589. [Google Scholar] [CrossRef]
- Li, B.; Dong, X.; Wang, H.; Ma, D.; Tan, K.; Jensen, S.; Deibert, B.J.; Butler, J.; Cure, J.; Shi, Z.; et al. Capture of organic iodides from nuclear waste by metal-organic framework-based molecular traps. Nat. Commun. 2017, 8, 485. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhang, W.; Zhu, Y.; Wang, D.G.; Yu, G.; Kuang, G.C. Nitrogen-rich porous polyaminal network as a platform for iodine adsorption through physical and chemical interaction. J. Appl. Polym. Sci. 2018, 135, 6–11. [Google Scholar] [CrossRef]
- Hijazi, A.; Azambre, B.; Finqueneisel, G.; Vibert, F.; Blin, J.L. High iodine adsorption by polyethyleneimine impregnated nanosilica sorbents. Microporous Mesoporous Mater. 2019, 288, 109586. [Google Scholar] [CrossRef]
- Yao, C.; Li, G.; Wang, J.; Xu, Y.; Chang, L. Template-free synthesis of porous carbon from triazine based polymers and their use in iodine adsorption and CO2 capture. Sci. Rep. 2018, 8, 1867. [Google Scholar] [CrossRef]
- Guo, B.; Li, F.; Wang, C.; Zhang, L.; Sun, D. A rare (3,12)-connected zirconium metal-organic framework with efficient iodine adsorption capacity and pH sensing. J. Mater. Chem. A 2019, 7, 13173–13179. [Google Scholar] [CrossRef]
- Deng, L.Y.; Xu, G.R.; Li, G.B. Surface properties and adsorption characteristics to methylene blue and iodine of adsorbents from sludge. Water Sci. Technol. 2010, 62, 1705–1712. [Google Scholar] [CrossRef]
- Zyryanov, S.S.; Kruzhalov, A.V.; Neshov, F.G.; Ryabukhin, O.V.; Kuznetsov, M.V. Investigation of a stainless-steel surface irradiated with protons in an iodine medium. J. Surf. Investig. 2013, 7, 322–327. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.L.; Deng, J.; He, C.H.; Ding, S.J.; Shi, W.Q. Adsorption of radioiodine on Cu2O surfaces: A first-principles density functional study. Acta Phys. Chim. Sin. 2016, 32, 2264–2270. [Google Scholar] [CrossRef]
- Banerjee, D.; Chen, X.; Lobanov, S.S.; Plonka, A.M.; Chan, X.; Daly, J.A.; Kim, T.; Thallapally, P.K.; Parise, J.B. Iodine Adsorption in Metal Organic Frameworks in the Presence of Humidity. ACS Appl. Mater. Interfaces 2018, 10, 10622–10626. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Gao, Y.; Wang, S.; Lv, Y.; Kobayashi, H.; Wang, J. Exceptional iodine uptake in intrinsic oxygen-rich microporous carbons. (unpublished work).
- Rustam, L.; Jeremias, F.; Henninger, S.K.; Wolff, T.; Munz, G.M. Tuning of adsorbent properties—Oxidative hydrophilization of activated carbon monoliths for heat storage applications. Energy Build. 2019, 196, 206–213. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Zhai, T.L.; Wang, Z.; Cheng, G.; Ma, H.; Zhang, Q.P.; Zhao, Y.H.; Tan, B.; Zhang, C. Hyperporous Carbon from Triptycene-Based Hypercrosslinked Polymer for Iodine Capture. Adv. Mater. Interfaces 2019, 6, 1900249. [Google Scholar] [CrossRef]
- Wang, X.; Kholmanov, I.; Chou, H.; Ruoff, R.S. Simultaneous Electrochemical Reduction and Delamination of Graphene Oxide Films. ACS Nano 2015, 9, 8737–8743. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction mechanism of uranium (VI) with three-dimensional graphene oxide-chitosan composite: Insights from batch experiments, IR, XPS, and EXAFS spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, T.J.; Wang, Y.; Liu, J.H.; Li, L.N.; Chen, X.; Huang, X.J. Enhanced electrochemical sensing arsenic(III) with excellent anti-interference using amino-functionalized graphene oxide decorated gold microelectrode: XPS and XANES evidence. Sens. Actuators B Chem. 2017, 245, 230–237. [Google Scholar] [CrossRef]
- Pathak, A.K.; Borah, M.; Gupta, A.; Yokozeki, T.; Dhakate, S.R. Improved mechanical properties of carbon fiber/graphene oxide-epoxy hybrid composites. Compos. Sci. Technol. 2016, 135, 28–38. [Google Scholar] [CrossRef]
- An, S.; Zhu, X.; He, Y.; Yang, L.; Wang, H.; Jin, S.; Hu, J.; Liu, H. Porosity Modulation in Two-Dimensional Covalent Organic Frameworks Leads to Enhanced Iodine Adsorption Performance. Ind. Eng. Chem. Res. 2019, 58, 10495–10502. [Google Scholar] [CrossRef]
- Yang, F.; Ma, X.; Cai, W.B.; Song, P.; Xu, W. Nature of Oxygen-Containing Groups on Carbon for High-Efficiency Electrocatalytic CO2 Reduction Reaction. J. Am. Chem. Soc. 2019, 141, 20451–20459. [Google Scholar] [CrossRef]
- Thomas, H.R.; Day, S.P.; Woodruff, W.E.; Vallés, C.; Young, R.J.; Kinloch, I.A.; Morley, G.W.; Hanna, J.V.; Wilson, N.R.; Rourke, J.P. Deoxygenation of graphene oxide: Reduction or cleaning? Chem. Mater. 2013, 25, 3580–3588. [Google Scholar] [CrossRef]
| Sample | C (wt.%) | O (wt.%) | C/O 1 |
|---|---|---|---|
| GO | 53.14 | 46.86 | 1.51 |
| GO-100 | 49.40 | 50.60 | 1.30 |
| GO-110 | 48.82 | 51.18 | 1.27 |
| GO-120 | 47.97 | 52.04 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Gao, Y.; Xu, Z.; Wang, S.; Kobayashi, H.; Wang, J. The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine. Materials 2020, 13, 5770. https://doi.org/10.3390/ma13245770
Zhang Q, Gao Y, Xu Z, Wang S, Kobayashi H, Wang J. The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine. Materials. 2020; 13(24):5770. https://doi.org/10.3390/ma13245770
Chicago/Turabian StyleZhang, Qian, Yangyang Gao, Zhanglian Xu, Sheng Wang, Hisayoshi Kobayashi, and Jie Wang. 2020. "The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine" Materials 13, no. 24: 5770. https://doi.org/10.3390/ma13245770
APA StyleZhang, Q., Gao, Y., Xu, Z., Wang, S., Kobayashi, H., & Wang, J. (2020). The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine. Materials, 13(24), 5770. https://doi.org/10.3390/ma13245770






