Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene
Abstract
1. Introduction
2. Materials and Methods
2.1. Support Preparation
2.2. Catalyst Preparation
2.3. Catalytic Performance Evaluation
2.4. Catalyst Characterization
- D = Average thickness of particle perpendicular to crystal plane (nm);
- K = Scherrer constant;
- γ = X-ray wavelength (0.154 nm);
- B = Full width at half maxima (FWHM);
- θ = Diffraction angle.
3. Results and Discussion
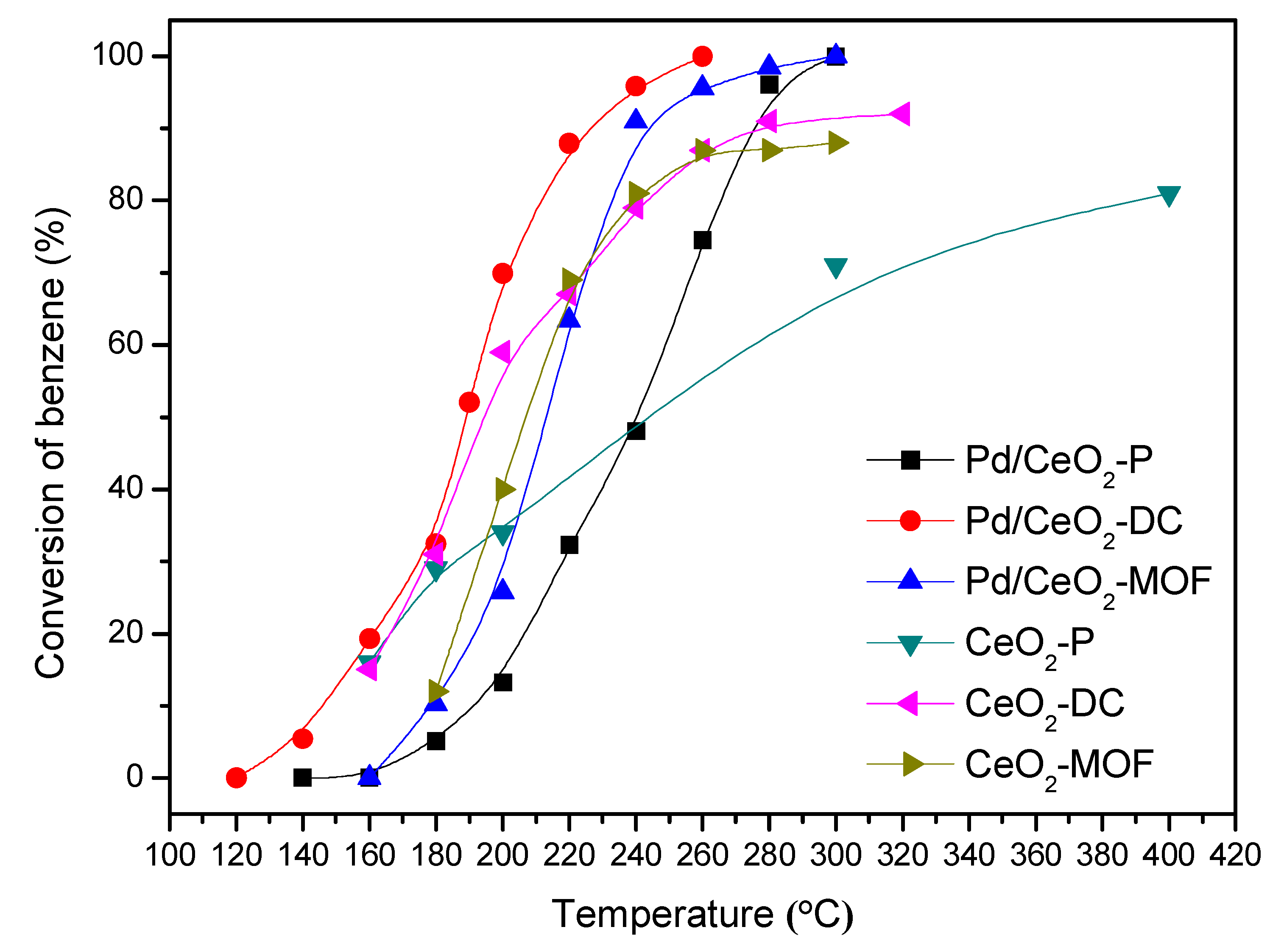
3.1. Catalytic Activity Evaluation
3.2. Durability Test
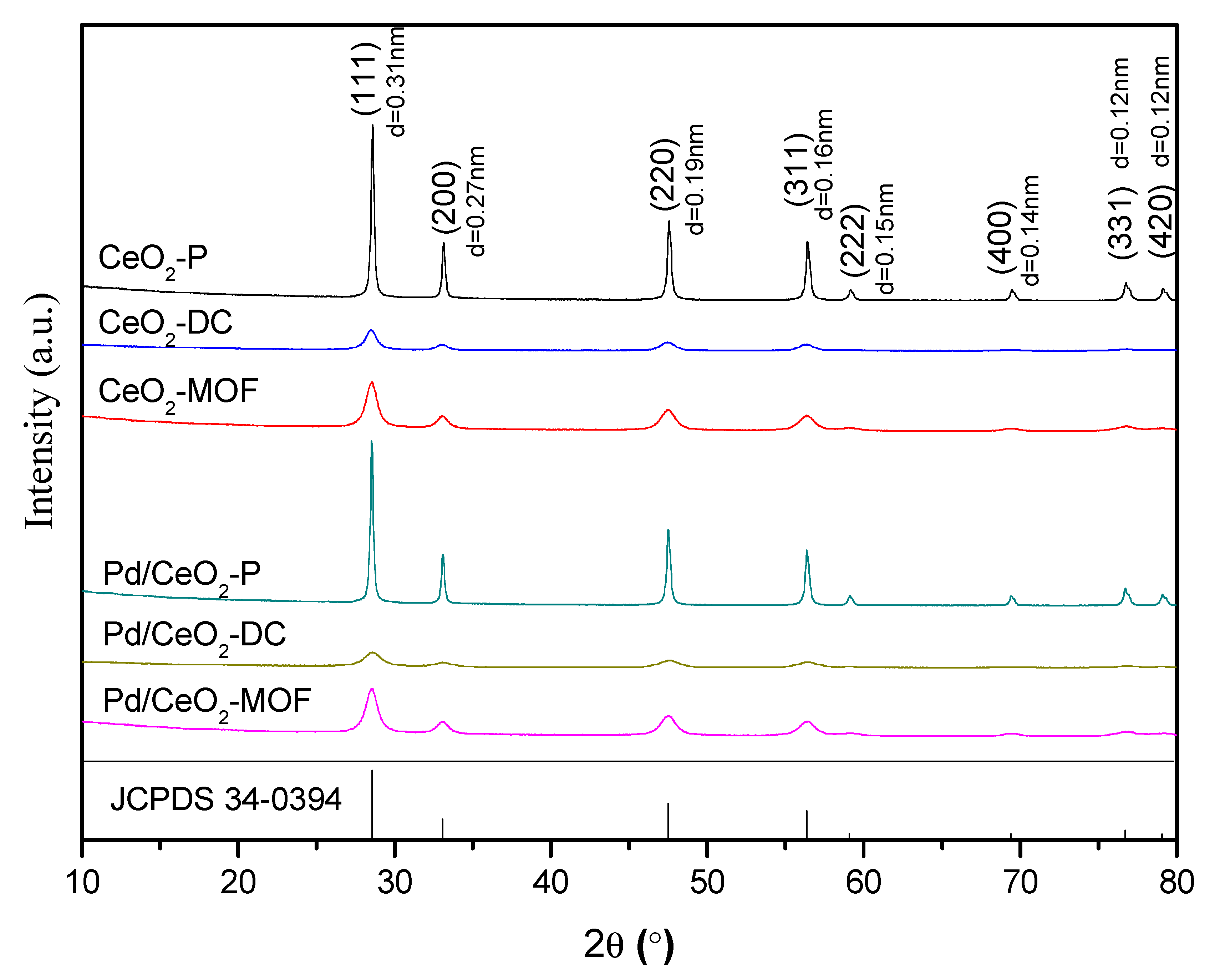
3.3. XRD Analysis
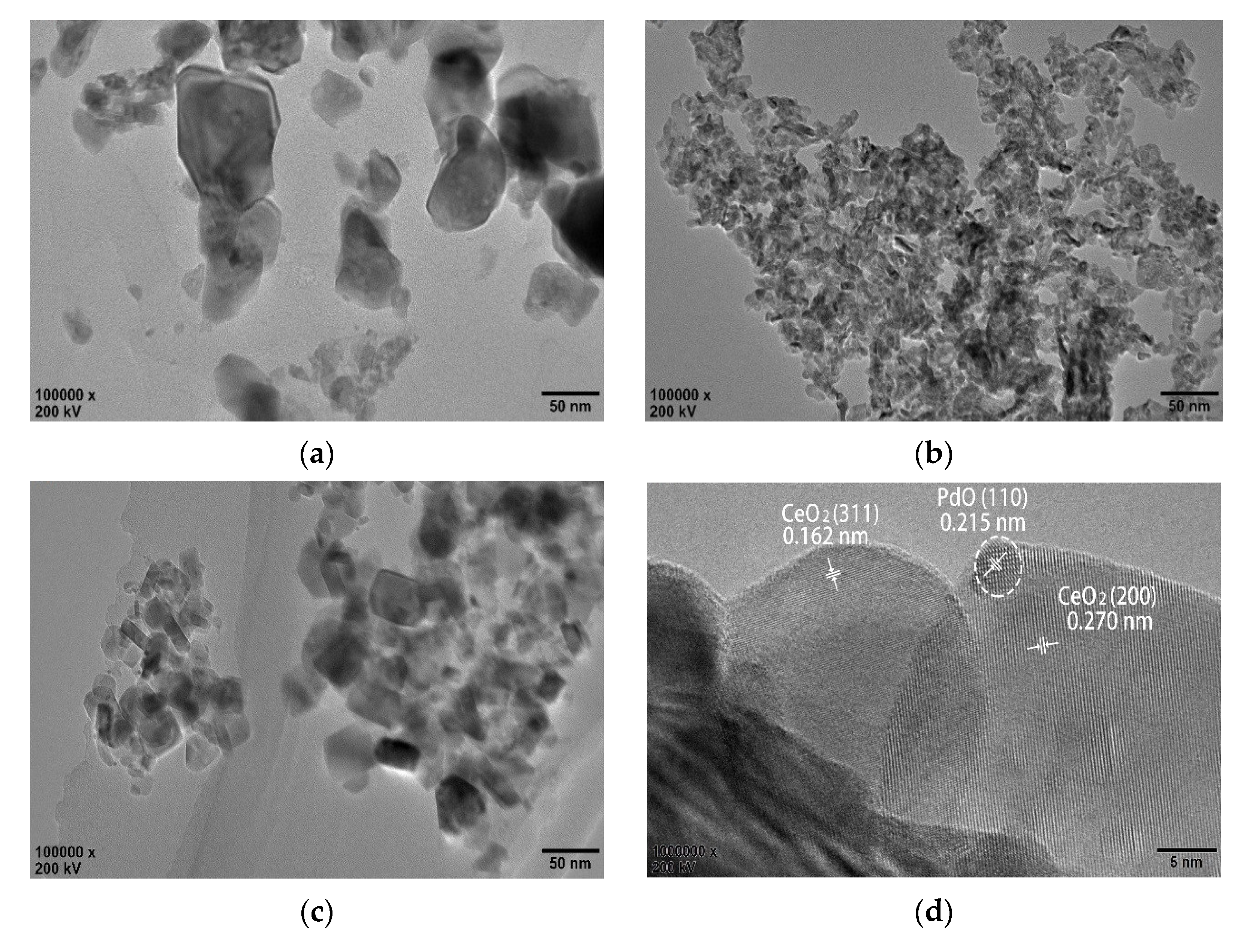
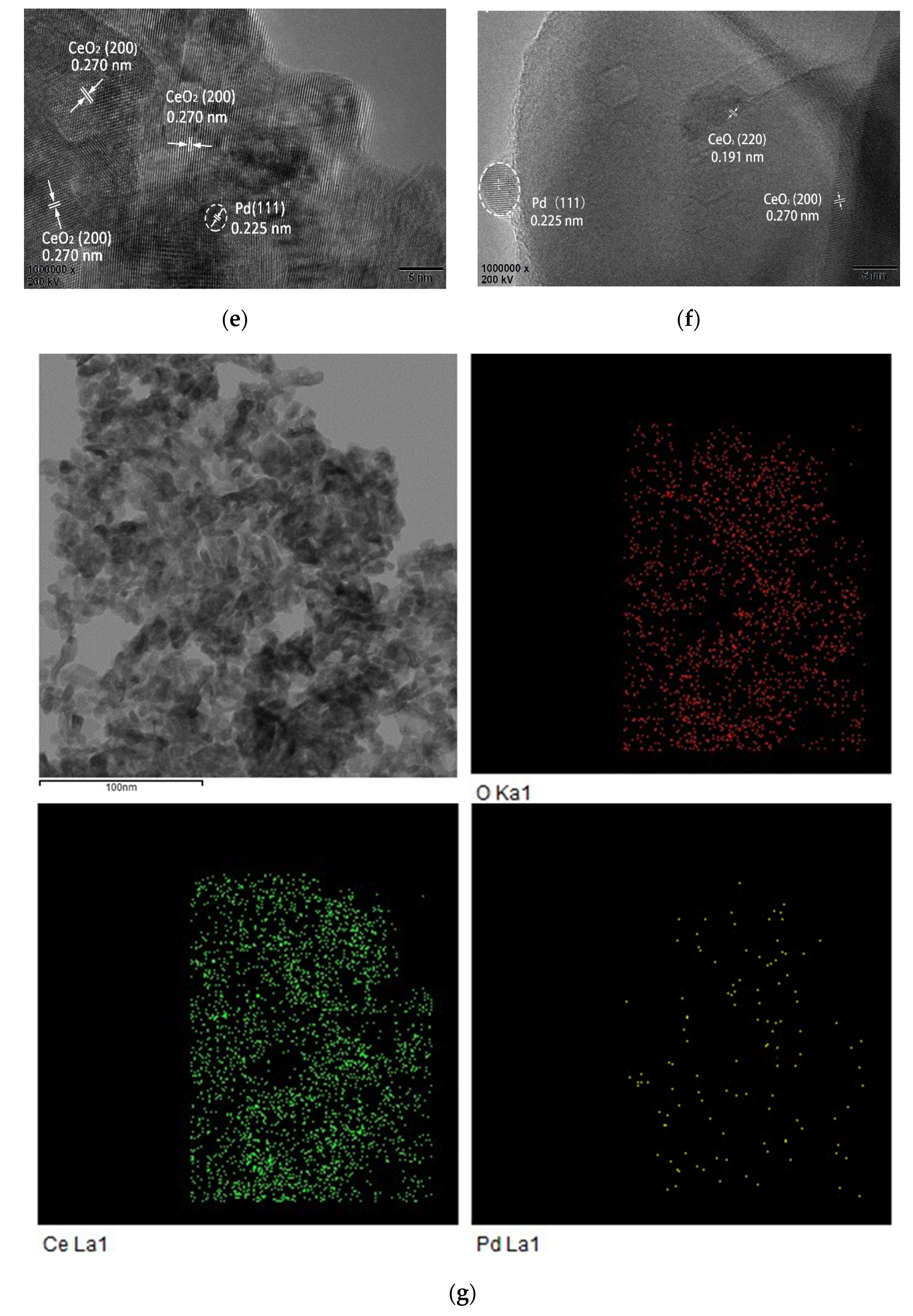
3.4. HRTEM Analysis
3.5. N2 Adsorption/Desorption Analysis
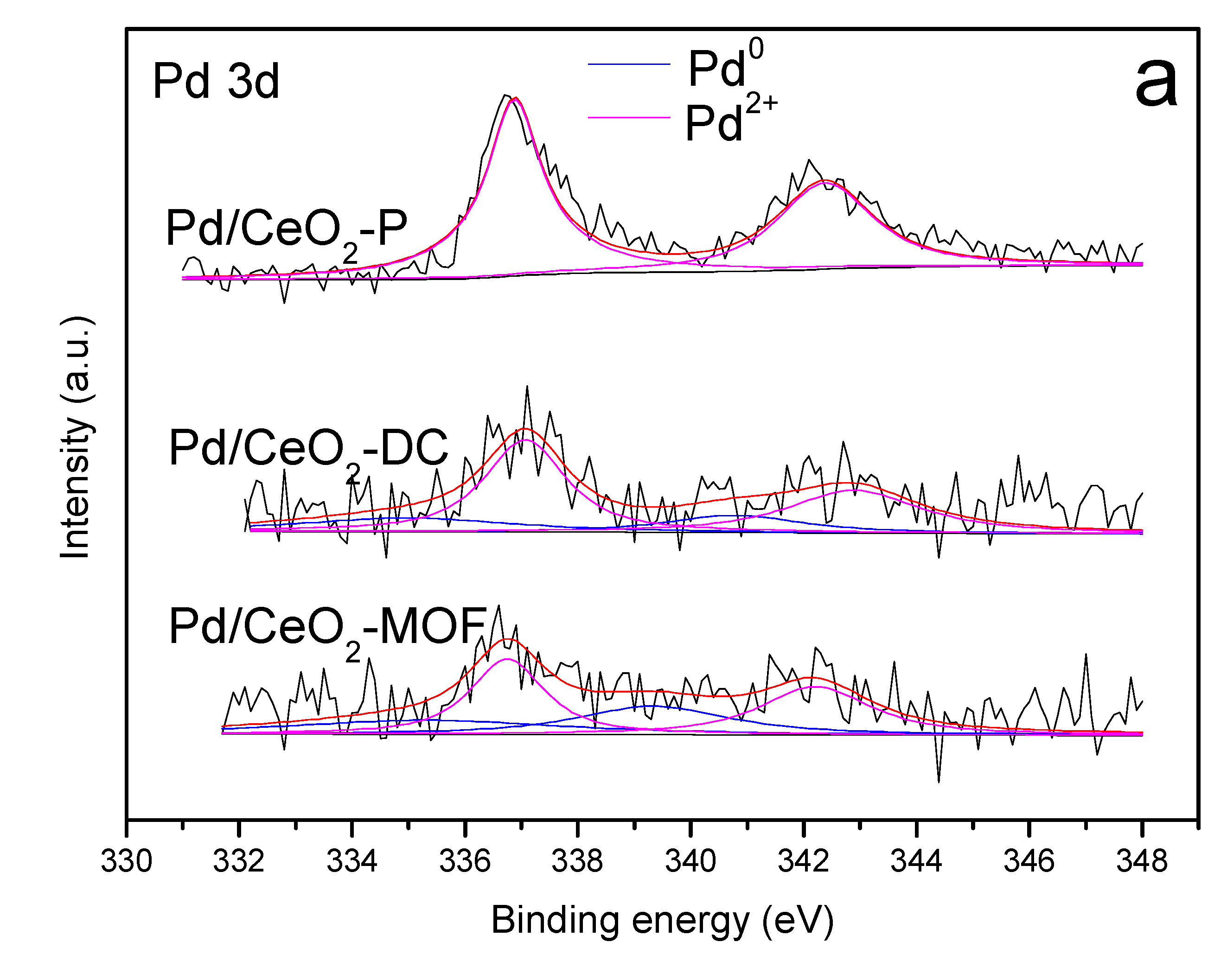
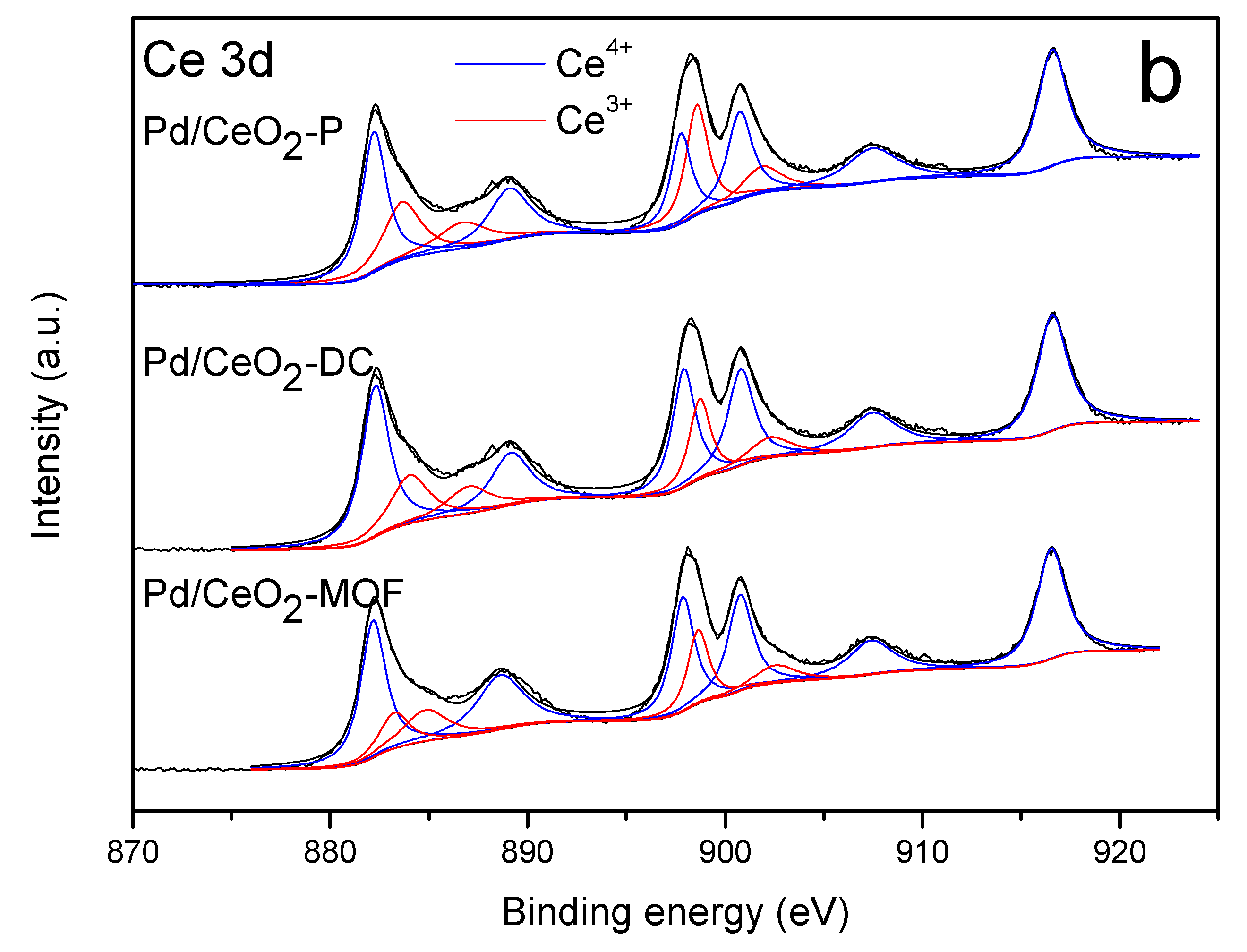
3.6. XPS Analysis
3.7. H2-TPR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oztürk, N.; Ergenekon, P.; Seçkin, G.O.; Bayır, S. Spatial Distribution and Temporal Trends of VOCs in a Highly Industrialized Town in Turkey. Bull. Environ. Contam. Toxicol. 2015, 94, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sofuoğlu, S.C.; Aslan, G.; Inal, F.; Sofuoglu, A. An Assessment of Indoor Air Concentrations and Health Risks of Volatile Organic Compounds in Three Primary Schools. Int. J. Hyg. Environ. Health 2011, 214, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Shim, W.G. Catalytic Combustion of VOCs over a Series of Manganese Oxide Catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Liu, F.F.; Peng, C.; Ng, J.C. BTEX In Vitro Exposure Tool Using Human Lung Cells: Trips and Gains. Chemosphere 2015, 128, 321–326. [Google Scholar] [CrossRef]
- Lim, S.K.; Shin, H.S.; Yoon, K.S.; Kwack, S.J.; Um, Y.M.; Hyeon, J.H.; Kwak, H.M.; Kim, J.Y.; Kim, T.H.; Kim, Y.J.; et al. Risk Assessment of Volatile Organic Compounds Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) in Consumer Products. J. Toxicol. Environ. Health Part A 2014, 77, 1502–1521. [Google Scholar] [CrossRef]
- Liaud, C.; Nguyen, T.N.; Nasreddine, R.; Le Calvé, S. Experimental Performances Study of a Transportable GC-PID and Two Thermo-Desorption Based Methods Coupled to FID and MS Detection to Assess BTEX Exposure at Sub-ppb Level in Air. Talanta 2014, 127, 33–42. [Google Scholar] [CrossRef]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and Adsorption Performance of a Modified Micro-Mesoporous MIL-101(Cr) for VOCs Removal at Ambient Conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Andana, T.; Galletti, C.; Fino, D.; Russo, N. Photocatalytic Abatement of Volatile Organic Compounds by TiO2 Nanoparticles Doped with Either Phosphorous or Zirconium. Materials 2019, 12, 2121. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ying, D.; Wang, Y.; Sun, T.; Jia, J. In Plasma Catalytic Oxidation of Toluene Using Monolith CuO Foam as a Catalyst in a Wedged High Voltage Electrode Dielectric Barrier Discharge Reactor: Influence of Reaction Parameters and Byproduct Control. Int. J. Environ. Res. Public Health 2019, 16, 711. [Google Scholar] [CrossRef]
- Shamskar, K.R.; Rashidi, A.; Azar, P.A.; Yousefi, M.; Baniyaghoob, S. Synthesis of Graphene by in situ Catalytic Chemical Vapor Deposition of Reed as a Carbon Source for VOC Adsorption. Environ. Sci. Pollut. Res. 2019, 26, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Li, J.-J.; Yu, E.-Q.; Cai, S.-C.; Chen, X.; Chen, J.; Jia, H.; Xu, Y.-J. Noble Metal Free, CeO2/LaMnO3 Hybrid Achieving Efficient Photo-Thermal Catalytic Decomposition of Volatile Organic Compounds under IR Light. Appl. Catal. B Environ. 2019, 240, 141–152. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Jiang, Z.; Shangguan, W.F. Low-Temperature Catalysis for VOCs Removal in Technology and Application: A State-of-the-Art Review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Alifanti, M.; Florea, M.; Parvulescu, V.I. Ceria-Based Oxides as Supports for LaCoO3 Perovskite; Catalysts for Total Oxidation of VOC. Appl. Catal. B Environ. 2007, 70, 400–405. [Google Scholar] [CrossRef]
- Silva, B.; Figueiredo, H.; Santos, V.P.; Pereira, M.F.; Figueiredo, J.L.; Lewandowska, A.E.; Bañares, M.A.; Neves, I.C.; Tavares, T. Reutilization of Cr-Y Zeolite Obtained by Biosorption in the Catalytic Oxidation of Volatile Organic Compounds. J. Hazard. Mater. 2011, 192, 545–553. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Ivanova, L.; Rosenholm, J.; Linden, M. Cobalt Oxide Species Supported on SBA-15, KIT-5 and KIT-6 Mesoporous Silicas for Ethyl Acetate Total Oxidation. Appl. Catal. B Environ. 2009, 89, 365–374. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.H.; Wang, L.N.; Chu, J.L.; Qu, J.K.; Hao, Z.P.; Qi, T. Catalytic Combustion of Benzene on the Pd/Nanosize Al-HMS. Microporous Mesoporous Mater. 2011, 138, 215–220. [Google Scholar] [CrossRef]
- Zhao, S.; Li, K.; Jiang, S.; Lib, J. Pd–Co Based Spinel Oxides Derived from Pd Nanoparticles Immobilized on Layered Double Hydroxides for Toluene Combustion. Appl. Catal. B Environ. 2016, 181, 236–248. [Google Scholar] [CrossRef]
- Huang, Q.; Zuo, S.; Zhou, R. Catalytic Performance of Pillared Interlayered Clays (PILCs) Supported CrCe Catalysts for Deep Oxidation of Nitrogen-Containing VOCs. Appl. Catal. B Environ. 2010, 95, 327–334. [Google Scholar] [CrossRef]
- Deng, W.; Tang, Q.; Huang, S.; Zhang, L.; Jia, Z.; Guo, L. Low Temperature Catalytic Combustion of Chlorobenzene over Cobalt Based Mixed Oxides Derived from Layered Double Hydroxides. Appl. Catal. B Environ. 2020, 278, 119336. [Google Scholar] [CrossRef]
- Huang, H.; Gu, Y.F.; Zhao, J.; Wang, X.Y. Catalytic Combustion of Chlorobenzene over VOx/CeO2 Catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Guo, S.; Wang, Y.; Han, X.; Bai, Y. Efficient Oxidation of O-Xylene over CeO2 Catalyst Prepared from a Ce-MOF Template: The Promotion of K+ Embedding Substitution. Mol. Catal. 2017, 436, 120–127. [Google Scholar] [CrossRef]
- Jabłońska, M.; Król, A.; Kukulska-Zając, E.; Tarach, K.; Girman, V.; Chmielarz, L.; Góra-Marek, K. Zeolites Y Modified with Palladium as Effective Catalysts for Low-Temperature Methanol Incineration. Appl. Catal. B Environ. 2015, 166, 353–365. [Google Scholar] [CrossRef]
- Li, P.; He, C.; Cheng, J.; Ma, C.Y.; Dou, B.J.; Hao, Z. Catalytic Oxidation of Toluene over Pd/Co3AlO Catalysts Derived from Hydrotalcite-Like Compounds: Effects of Preparation Methods. Appl. Catal. B Environ. 2011, 101, 570–579. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Song, F.; Zhang, S.; Zhong, Q. The Effect of CuO Loading on Different Method Prepared CeO2 Catalyst for Toluene Oxidation. Sci. Total. Environ. 2020, 712, 135635. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Soomro, A.; Ma, S.; Zhu, M.; Hua, X.; Xiang, W. Investigation of Synergistic Effects and High Performance of La-Co Composite Oxides for Toluene Catalytic Oxidation at Low Temperature. Environ. Sci. Pollut. Res. Int. 2019, 26, 12123–12135. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Xiang, W. Oxygen Vacancy Induced Performance Enhancement of Toluene Catalytic Oxidation Using LaFeO3 Perovskite Oxides. Chem. Eng. J. 2020, 387, 124101. [Google Scholar] [CrossRef]
- Yan, S.; Deng, J.; Zhao, M.; Cheng, T.; Wang, J.; Jiao, Y.; Chen, Y. Preparation of Ce0.5Zr0.5O2-Al2O3 with High-Temperature Sintering Resistance and Its Supported Pd-Only Three-Way Catalyst. J. Mater. Sci. 2019, 54, 2796–2813. [Google Scholar] [CrossRef]
- Lei, Y.; Li, W.; Liu, Q.; Lin, Q.; Zheng, X.; Huang, Q.; Guan, S.; Wang, X.; Wang, C.; Li, F. Typical Crystal Face Effects of Different Morphology Ceria on the Activity of Pd/CeO2 Catalysts for Lean Methane Combustion. Fuel 2018, 233, 10–20. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In Situ Pyrolysis of Ce-MOF to Prepare CeO2 Catalyst with Obviously Improved Catalytic Performance for Toluene Combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Li, X.; Zhuang, G.; Wang, K.; Zhuang, G.; Sun, D.; Zheng, Y.; Li, Q. Catalytic Benzene Oxidation by Biogenic Pd Nanoparticles over 3D-Ordered Mesoporous CeO2. Chem. Eng. J. 2019, 362, 41–52. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and Physicochemical Characterizations of Nanostructured Pt/Al2O3-CeO2 Catalysts for Total Oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Chen, Z.; Zuo, S. Mechanism of CeO2 Synthesized by Thermal Decomposition of Ce-MOF and Its Performance of Benzene Catalytic Combustion. J. Rare Earths 2020. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Yang, P.; Cheng, Z.; Li, J.; Zuo, S. Ce-modified Mesoporous γ-Al2O3 Supported Pd-Pt Nanoparticle Catalysts and Their Structure-Function Relationship in Complete Benzene Oxidation. Chem. Eng. J. 2019, 356, 255–261. [Google Scholar] [CrossRef]
- Schmal, M.; Aranda, D.A.G.; Noronha, F.B.; Guimaraes, A.L.; Monteiro, R.D.S. Oxidation and Reduction Effects of Propane-Oxygen on Pd-Chlorine/Alumina Catalysts. Catal. Lett. 2000, 64, 163–169. [Google Scholar] [CrossRef]
- Saikia, H.; Hazarika, K.K.; Chutia, B.; Choudhury, B.; Bharali, P. A Simple Chemical Route toward High Surface Area CeO2 Nanoparticles Displaying Remarkable Radical Scavenging Activity. ChemistrySelect 2017, 2, 3369–3375. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 2. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Shim, W.-G.; Lee, J.-W.; Kim, S.C. Analysis of Catalytic Oxidation of Aromatic Hydrocarbons over Supported Palladium Catalyst with Different Pretreatments Based on Heterogeneous Adsorption Properties. Appl. Catal. B Environ. 2008, 84, 133–141. [Google Scholar] [CrossRef]
- Okumura, K.; Kobayashi, T.; Tanaka, H.; Niwa, M. Toluene Combustion over Palladium Supported on Various Metal Oxide Supports. Appl. Catal. B Environ. 2003, 44, 325–331. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS Investigation of Cerium Oxides and Mixed Cerium Oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]

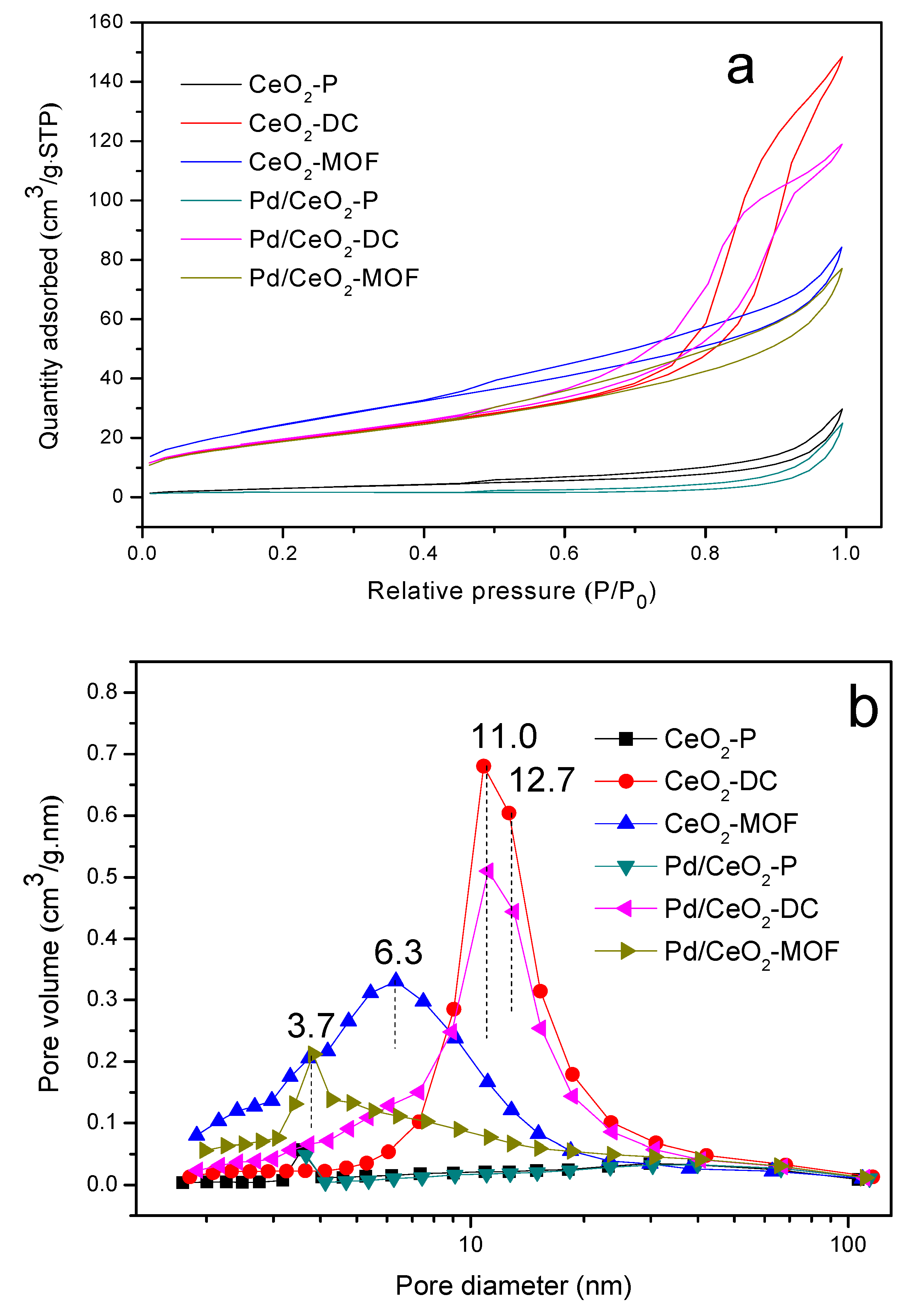

| Samples | SBETa (m2/g) | Amesb (m2/g) | VPc (cm3/g) | Vmicd (cm3/g) |
|---|---|---|---|---|
| CeO2-P | 10.7 | 10.7 | 0.044 | - |
| CeO2-DC | 66.3 | 66.3 | 0.23 | - |
| CeO2-MOF | 90.5 | 90.5 | 0.14 | - |
| Pd/CeO2-P | 7.7 | 5.9 | 0.039 | 0.00074 |
| Pd/CeO2-DC | 80.4 | 77.3 | 0.21 | 0.00059 |
| Pd/CeO2-MOF | 73.8 | 73.8 | 0.13 | - |
| Catalyst | Pd 3d (eV) | Ce 3d (eV) | Pd2+/(Pd2+ + Pd0) (%) | Ce3+/(Ce3+ + Ce4+) (%) | |||
|---|---|---|---|---|---|---|---|
| Pd0 | Pd2+ | Ce3+ | Ce4+ | ||||
| Pd/CeO2-P | 3d3/2 | - | 342.4 | 883.7, 887.3 | 882.2, 889.3, 897.8 | 100 | 18.1 |
| 3d5/2 | - | 336.9 | 898.6, 901.9 | 900.7, 907.5, 916.6 | |||
| Pd/CeO2-DC | 3d3/2 | 340.8 | 342.9 | 884.0, 887.0 | 882.3, 889.2, 897.9 | 71.1 | 24.8 |
| 3d5/2 | 335 | 337.1 | 898.7, 902.2 | 900.8, 907.4, 916.5 | |||
| Pd/CeO2-MOF | 3d3/2 | 339.3 | 342.2 | 883.3, 884.8 | 882.3, 888.7, 897.9 | 57.1 | 23.1 |
| 3d5/2 | 335.3 | 336.8 | 898.7, 902.4 | 900.8, 907.4, 916.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, Z.; Zheng, J.; Zuo, S. Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene. Materials 2020, 13, 5768. https://doi.org/10.3390/ma13245768
Wang Z, Chen Z, Zheng J, Zuo S. Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene. Materials. 2020; 13(24):5768. https://doi.org/10.3390/ma13245768
Chicago/Turabian StyleWang, Zhuo, Zhu Chen, Jie Zheng, and Shufeng Zuo. 2020. "Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene" Materials 13, no. 24: 5768. https://doi.org/10.3390/ma13245768
APA StyleWang, Z., Chen, Z., Zheng, J., & Zuo, S. (2020). Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene. Materials, 13(24), 5768. https://doi.org/10.3390/ma13245768





