Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of L/C
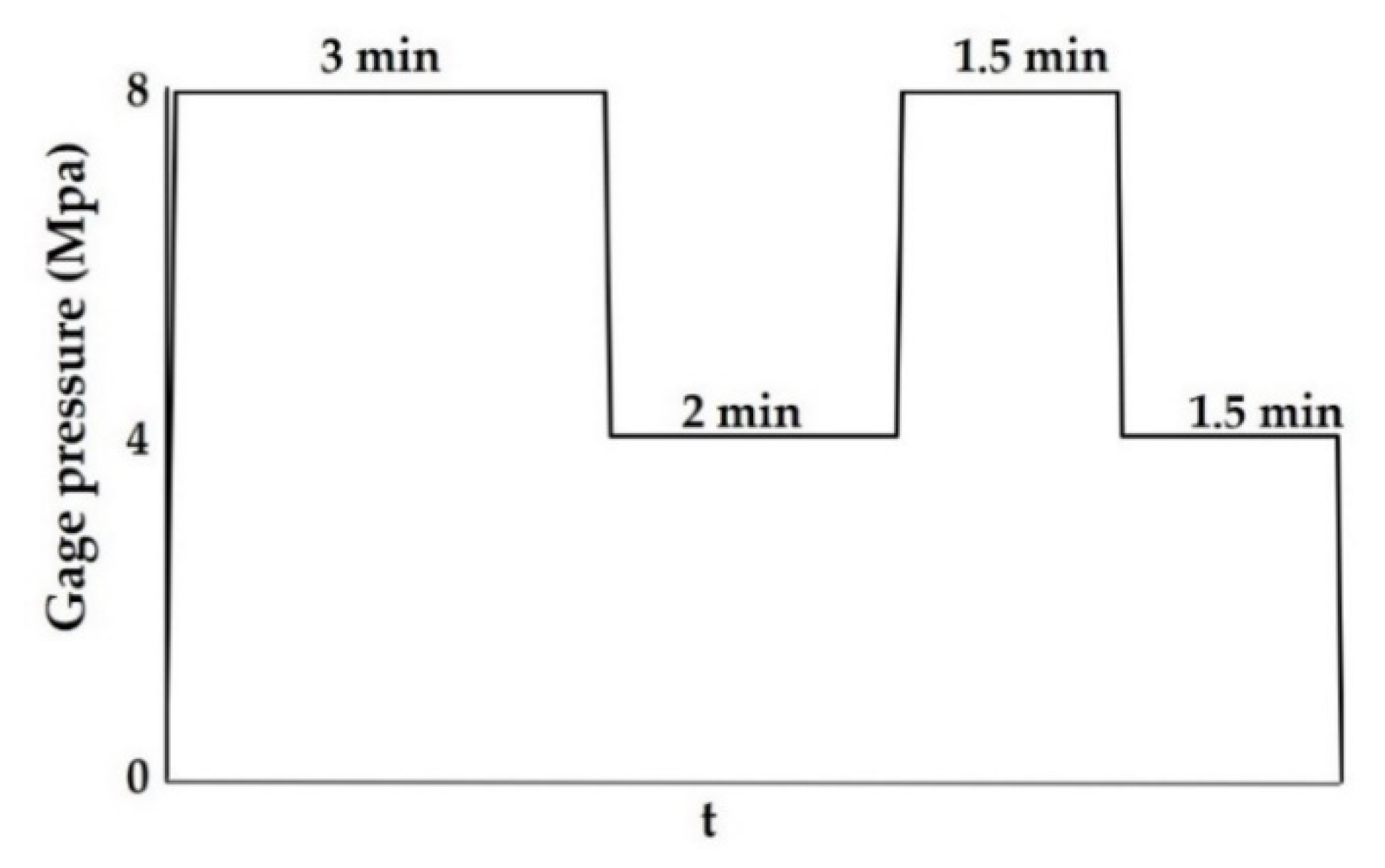
2.3. MDF Preparation
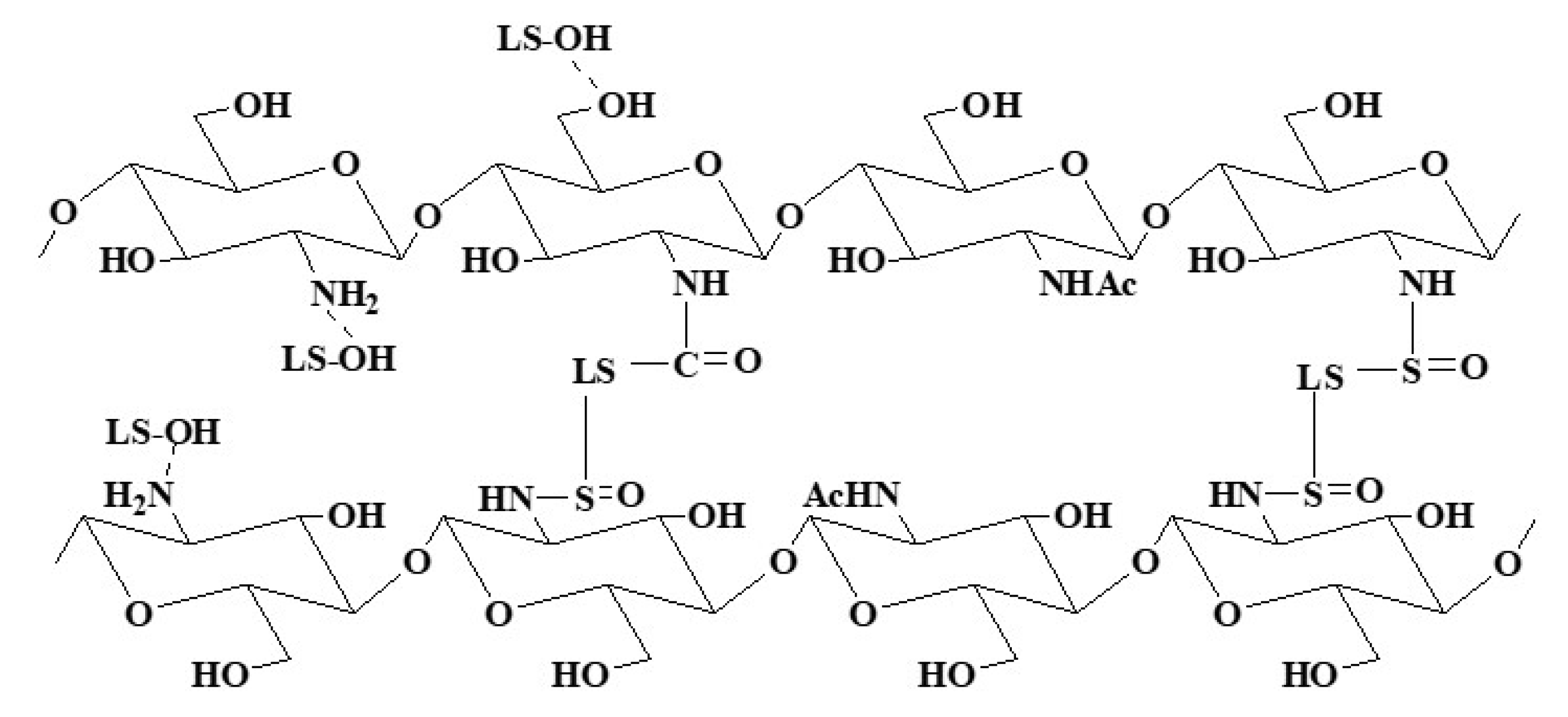
2.4. Synthesis Mechanism Analysis
2.5. Bonding Performance Test
3. Results and Discussion
3.1. FTIR Analysis
3.2. Thermal Analysis
3.3. XRD Analysis
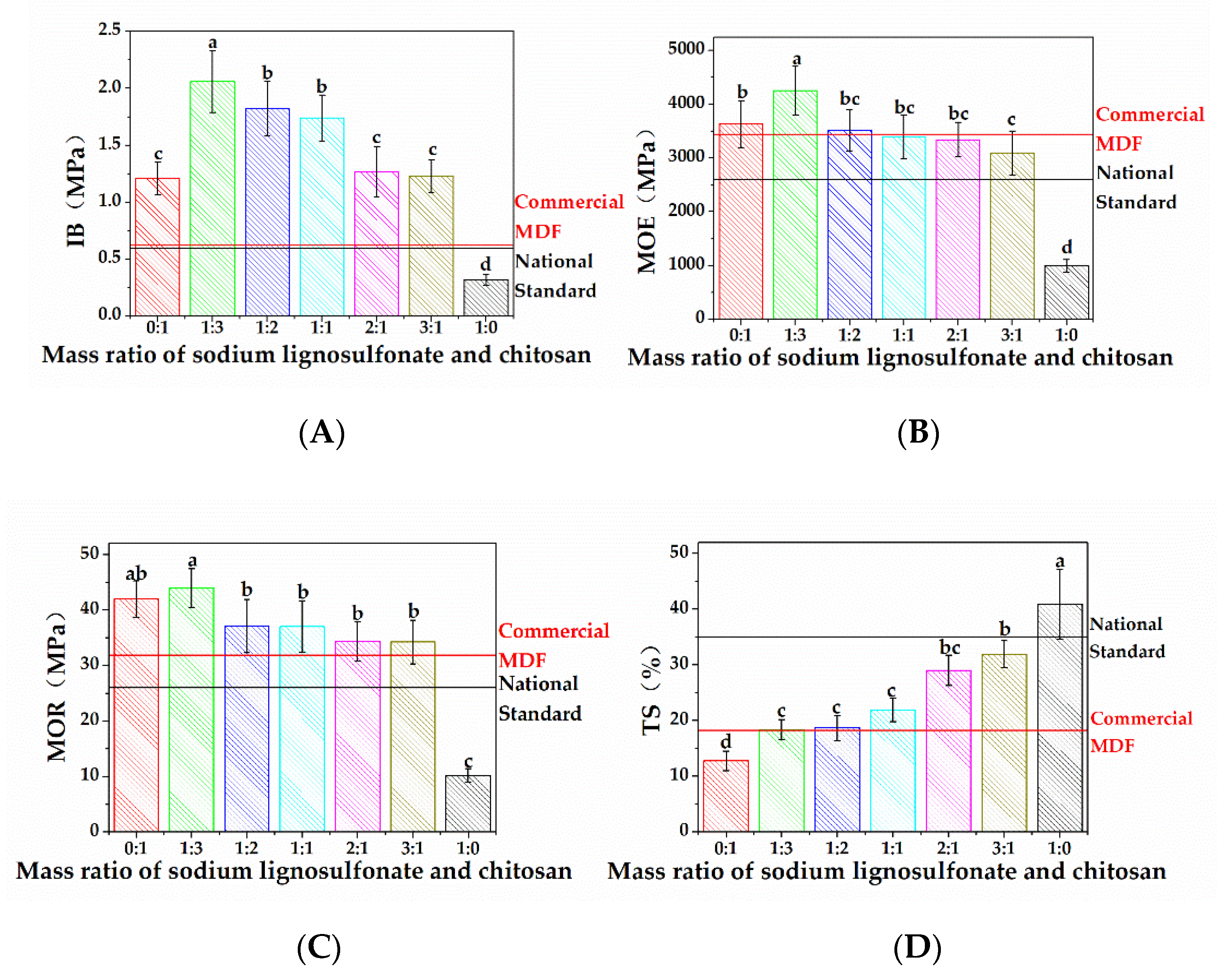
3.4. Bonding Performance Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heinrich, L.A. Future opportunities for bio-based adhesives–advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, V.; Mishra, S.; Varma, I.K.; Mattiason, B. Adhesives and plastics based on soy protein products. Ind. Crop. Prod. 2002, 16, 155–172. [Google Scholar] [CrossRef]
- Imam, S.H.; Gordon, S.H.; Mao, L.; Chen, L. Environmentally friendly wood adhesive from a renewable plant polymer: Characteristics and optimization. Polym. Degrad. Stab. 2001, 73, 529–533. [Google Scholar] [CrossRef]
- Oh, M.; Ma, Q.; Simsek, S.; Bajwa, D.; Jiang, L. Comparative study of zein- and gluten-based wood adhesives containing cellulose nanofibers and crosslinking agent for improved bond strength. Int. J. Adhes. Adhes. 2019, 92, 44–57. [Google Scholar] [CrossRef]
- Moslemi, A.; Koohi, M.Z.; Behzad, T.; Pizzi, A. Addition of cellulose nanofibers extracted from rice straw to urea formaldehyde resin; effect on the adhesive characteristics and medium density fiberboard properties. Int. J. Adhes. Adhes. 2020, 99. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crop. Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Vo, D.-T.; Lee, C.-K. Antimicrobial sponge prepared by hydrophobically modified chitosan for bacteria removal. Carbohydr. Polym. 2018, 187, 1–7. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Pereira, J.; Almeida, M.; Ferra, J.; Paiva, N.; Martins, J.; Magalhães, F.D.; Carvalho, L.H. Low-cost natural binder for particleboards production: Study of manufacture conditions and stability. Int. J. Adhes. Adhes. 2019, 93, 102325. [Google Scholar] [CrossRef]
- Pang, H.W.; Zhao, S.J.; Mo, L.T.; Wang, Z.; Zhang, W.; Huang, A.M.; Zhang, S.F.; Li, J.Z. Mussel-inspired bio-based water-resistant soy adhesives with low-cost dopamine analogue-modified silkworm silk Fiber. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Santiago-Medina, F.; Foyer, G.; Pizzi, A.; Caillol, S.; Delmotte, L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int. J. Adhes. Adhes. 2016, 70, 239–248. [Google Scholar] [CrossRef]
- Yelle, D.J. Solution-state NMR analysis of hydroxymethylated resorcinol cured in the presence of crude milled-wood lignin from Acer saccharum. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.Y.; Li, N.B.; Sun, X.Z.S.; Wang, D.H. Adhesion properties of soy protein adhesives enhanced by biomass lignin. Int. J. Adhes. Adhes. 2017, 75, 66–73. [Google Scholar] [CrossRef]
- Hemmila, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium Lignosulfonate Adhesives for Particleboards with pMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95. [Google Scholar] [CrossRef]
- Sohni, S.; Hashim, R.; Nidaullah, H.; Lamaming, J.; Sulaiman, O. Chitosan/nano-lignin based composite as a new sorbent for enhanced removal of dye pollution from aqueous solutions. Int. J. Biol. Macromol. 2019, 132, 1304–1317. [Google Scholar] [CrossRef]
- Jaganathan, G.; Manivannan, K.; Lakshmanan, S.; Sithique, M.A. Fabrication and characterization of Artocarpus heterophyllus waste derived lignin added chitosan biocomposites for wound dressing application. Sustain. Chem. Pharm. 2018, 10, 27–32. [Google Scholar] [CrossRef]
- Zou, T.; Sipponen, M.H.; Osterberg, M. Natural Shape-Retaining Microcapsules with Shells Made of Chitosan-Coated Colloidal Lignin Particles. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Xu, J.D.; Niu, Y.S.; Yue, P.P.; Hu, Y.J.; Bian, J.; Li, M.F.; Peng, F.; Sun, R.C. Composite Film Based on Pulping Industry Waste and Chitosan for Food Packaging. Materials 2018, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.D.; Dong, Y.; Yuan, B.N.; Li, B.; Guo, M.H. Influence of glutaraldehyde on the performance of a lignosulfonate/chitosan-based medium density fiberboard adhesive. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Ji, X.D.; Guo, M.H. Preparation and properties of a chitosan-lignin wood adhesive. Int. J. Adhes. Adhes. 2018, 82, 8–13. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, L.; Moszynski, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwinska-Stefanska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of Chitin with Kraft Lignin and Development of New Biosorbents for Removal of Cadmium(II) and Nickel(II) Ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(VI) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.-X.; Lü, Q.-F.; Lin, T.-T. Facile Preparation of Lignosulfonate–Graphene Oxide–Polyaniline Ternary Nanocomposite as an Effective Adsorbent for Pb(II) Ions. ACS Sustain. Chem. Eng. 2014, 2, 1203–1211. [Google Scholar] [CrossRef]
- Vijay, A.; Sathyanarayana, D.N. Ab initio study of the vibrational assignment and force field of thiosemicarbazide-d0 and -d5. Spectrochim. Acta A 1992, 48, 1601–1609. [Google Scholar] [CrossRef]
- Sengupta, P.K.; Krimm, S. Vibrational analysis of peptides, polypeptides, and proteins. XXXII. α-Poly(L-glutamic acid). Biopolymers 1985, 24, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Sarojini, K.; Krishnan, H.; Kanakam, C.C.; Muthu, S. Synthesis, structural, spectroscopic studies, NBO analysis, NLO and HOMO–LUMO of 4-methyl-N-(3-nitrophenyl)benzene sulfonamide with experimental and theoretical approaches. Spectrochim. Acta A 2013, 108, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.M.; Chakraborti, A.K.; Bansal, A.K. FTIR Studies on Differential Intermolecular Association in Crystalline and Amorphous States of Structurally Related Non-Steroidal Anti-Inflammatory Drugs. Mol. Pharmaceut. 2008, 5, 937–945. [Google Scholar] [CrossRef]
- Álvarez, R.M.S.; Cutín, E.H.; Mack, H.-G.; Romano, R.M.; Della Védova, C.O. Vibrational Spectra of Trifluoromethanesulfonyl Isocyanate, CF3SO2NCO. J. Raman Spectrosc. 1997, 28, 277–281. [Google Scholar] [CrossRef]
- Lü, Q.-F.; He, Z.-W.; Zhang, J.-Y.; Lin, Q. Preparation and properties of nitrogen-containing hollow carbon nanospheres by pyrolysis of polyaniline–lignosulfonate composites. J. Anal. Appl. Pyrolysis 2011, 92, 152–157. [Google Scholar] [CrossRef]
- Ye, D.-z.; Jiang, X.-c.; Xia, C.; Liu, L.; Zhang, X. Graft polymers of eucalyptus lignosulfonate calcium with acrylic acid: Synthesis and characterization. Carbohydr. Polym. 2012, 89, 876–882. [Google Scholar] [CrossRef]
- Pereira, L.A.; da Silva Reis, L.; Batista, F.A.; Mendes, A.N.; Osajima, J.A.; Silva-Filho, E.C. Biological properties of chitosan derivatives associated with the ceftazidime drug. Carbohydr. Polym. 2019, 222, 115002. [Google Scholar] [CrossRef]
- Hasegawa, M.; Isogai, A.; Onabe, F. Preparation of low-molecular-weight chitosan using phosphoric acid. Carbohydr. Polym. 1993, 20, 279–283. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Rasheed, P.A.; Gomez, T.; Pasha, M.; Mansour, S.A.; Lee, O.-S.; Mahmoud, K.A. One-step synthesis of an antimicrobial framework based on covalently cross-linked chitosan/lignosulfonate (CS@LS) nanospheres. Green Chem. 2020, 22, 678–687. [Google Scholar] [CrossRef]
- Ji, X.D.; Dong, Y.; Yu, R.D.; Du, W.; Gu, X.; Guo, M.H. Simple production of medium density fiberboards (MDF) reinforced with chitosan. Holzforschung 2018, 72, 275–281. [Google Scholar] [CrossRef]



| Sample | Stage A | Stage B | Stage C | Residual Amount after 800 °C (%) | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Weight Loss (%) | Temperature (°C) | Weight Loss (%) | Temperature (°C) | Weight Loss (%) | ||
| a | 128 | 0.70 | 228 | 8.16 | 392 | 16.73 | 42.62 |
| b | 162 | 2.77 | 247 | 21.69 | 386 | 11.35 | 33.22 |
| c | 158 | 4.67 | 248 | 23.02 | 413 | 10.46 | 25.67 |
| d | 149 | 8.37 | 252 | 26.09 | 413 | 10.25 | 18.33 |
| e | 154 | 10.84 | 259 | 27.49 | 414 | 9.45 | 30.55 |
| f | 152 | 12.07 | 261 | 28.65 | 435 | 9.31 | 24.73 |
| g | 286 | 66.27 | - | - | - | - | 31.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Guo, M.; Zhu, L.; Du, W.; Wang, H. Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism. Materials 2020, 13, 5697. https://doi.org/10.3390/ma13245697
Ji X, Guo M, Zhu L, Du W, Wang H. Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism. Materials. 2020; 13(24):5697. https://doi.org/10.3390/ma13245697
Chicago/Turabian StyleJi, Xiaodi, Minghui Guo, Li Zhu, Wenxin Du, and Hongbin Wang. 2020. "Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism" Materials 13, no. 24: 5697. https://doi.org/10.3390/ma13245697
APA StyleJi, X., Guo, M., Zhu, L., Du, W., & Wang, H. (2020). Synthesis Mechanism of an Environment-Friendly Sodium Lignosulfonate/Chitosan Medium-Density Fiberboard Adhesive and Response of Bonding Performance to Synthesis Mechanism. Materials, 13(24), 5697. https://doi.org/10.3390/ma13245697






