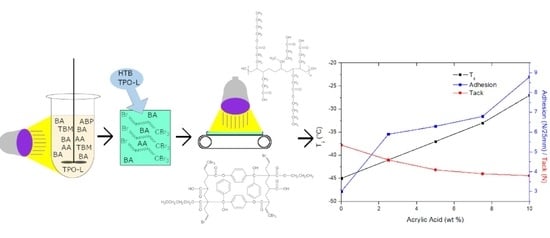
Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
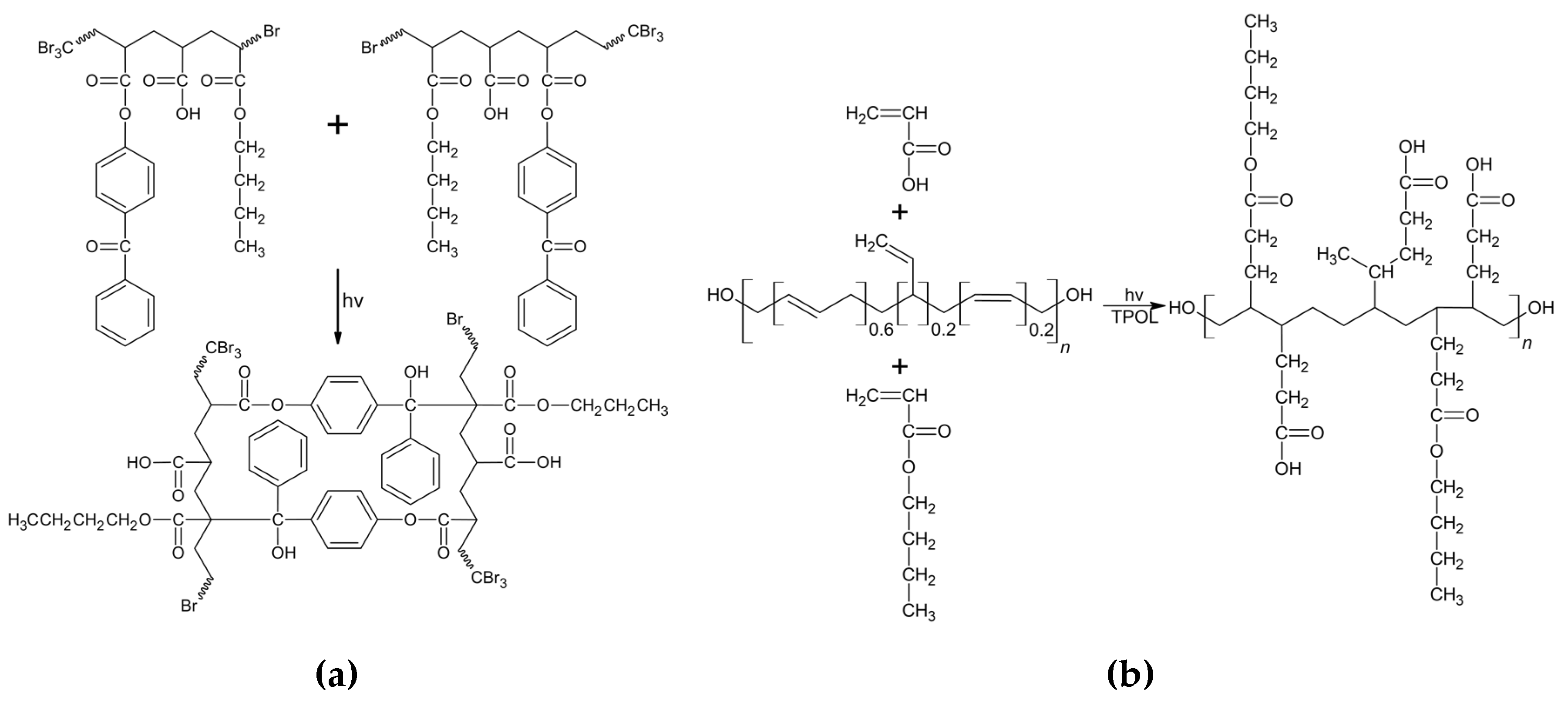
2.2. Synthesis and Characterization of BA/AA/ABP Cotelomers
2.3. Preparation and Characterization of Obtained Pressure-Sensitive Adhesives (PSAs)
3. Results
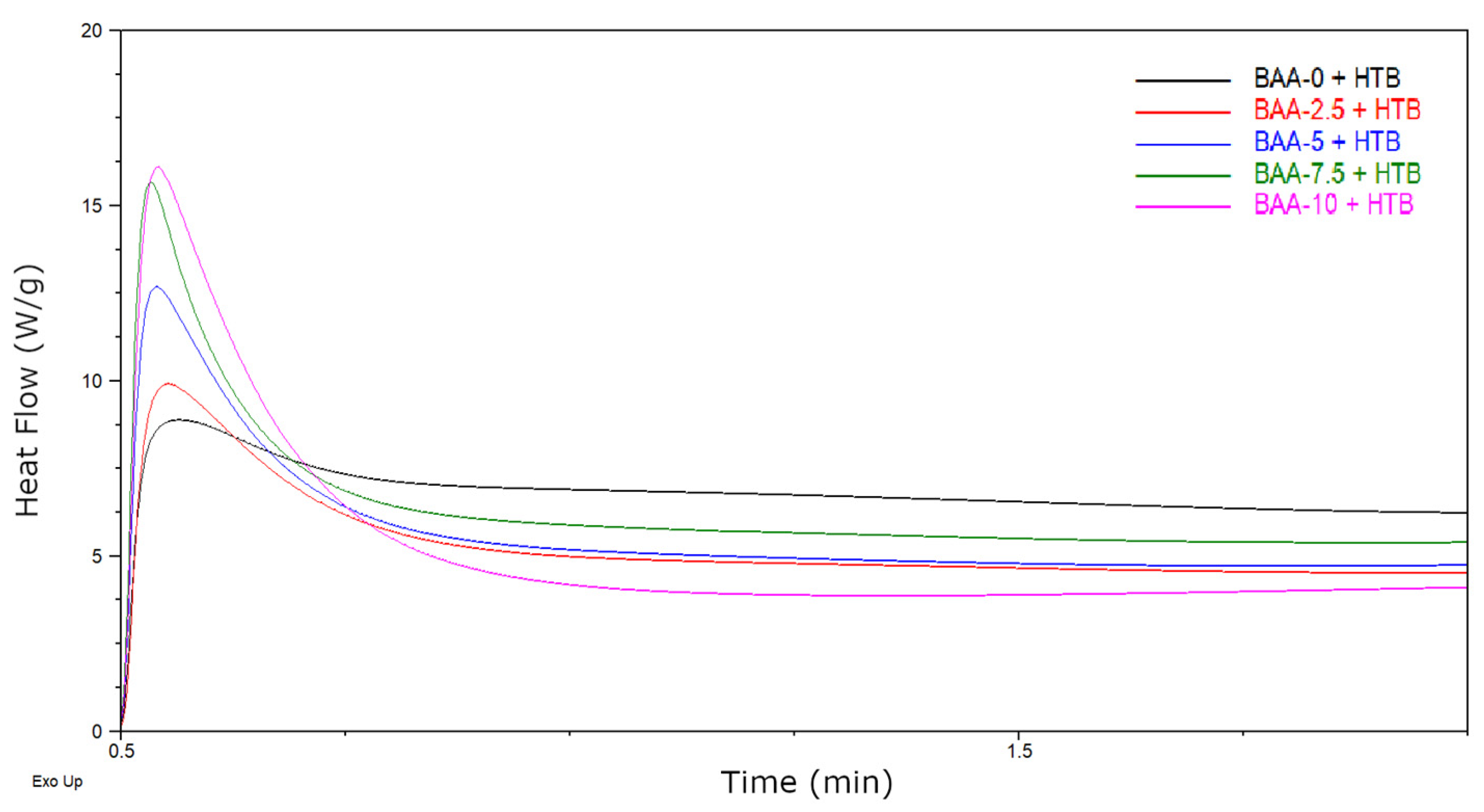
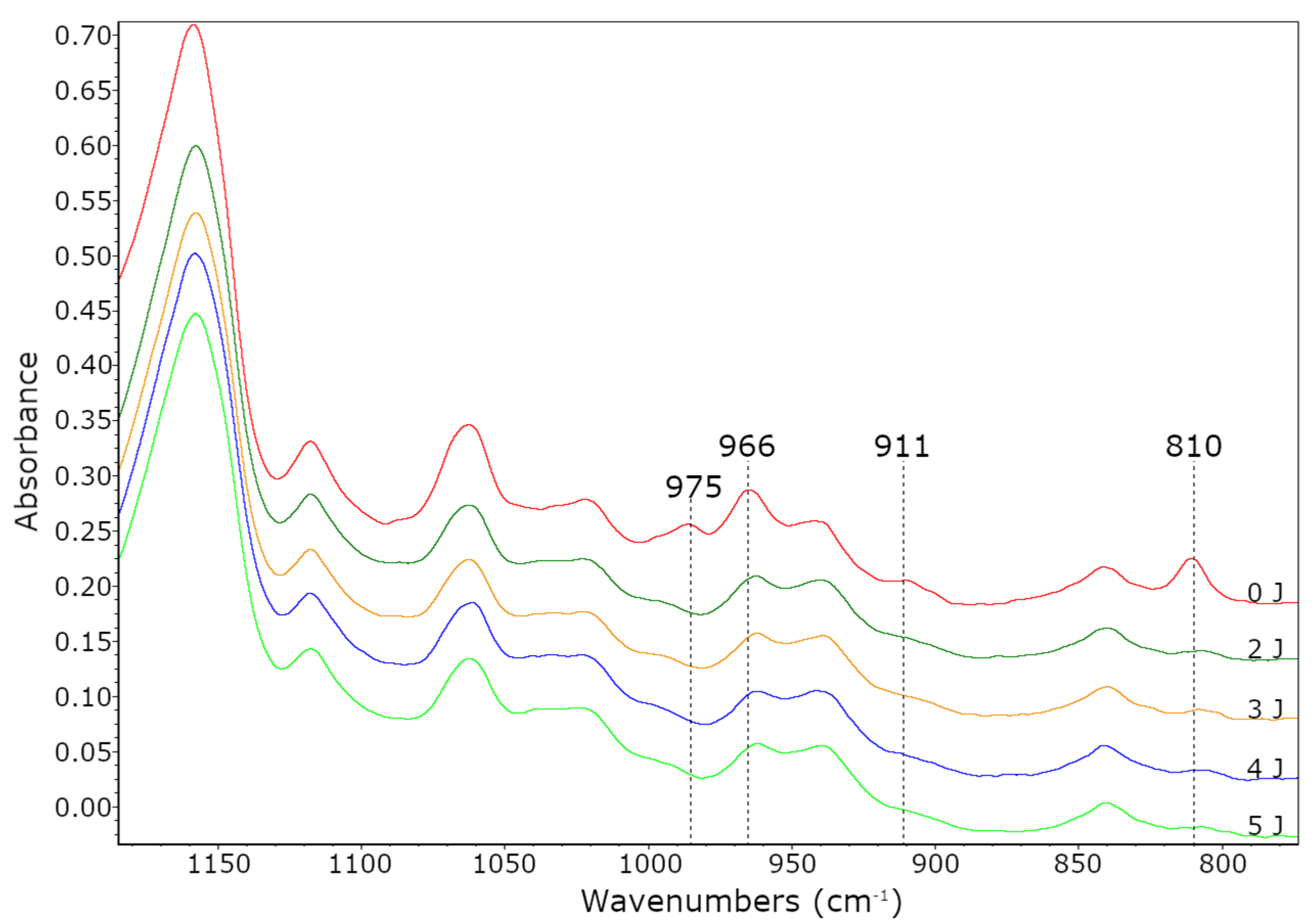
3.1. Properties of BAA Cotelomers
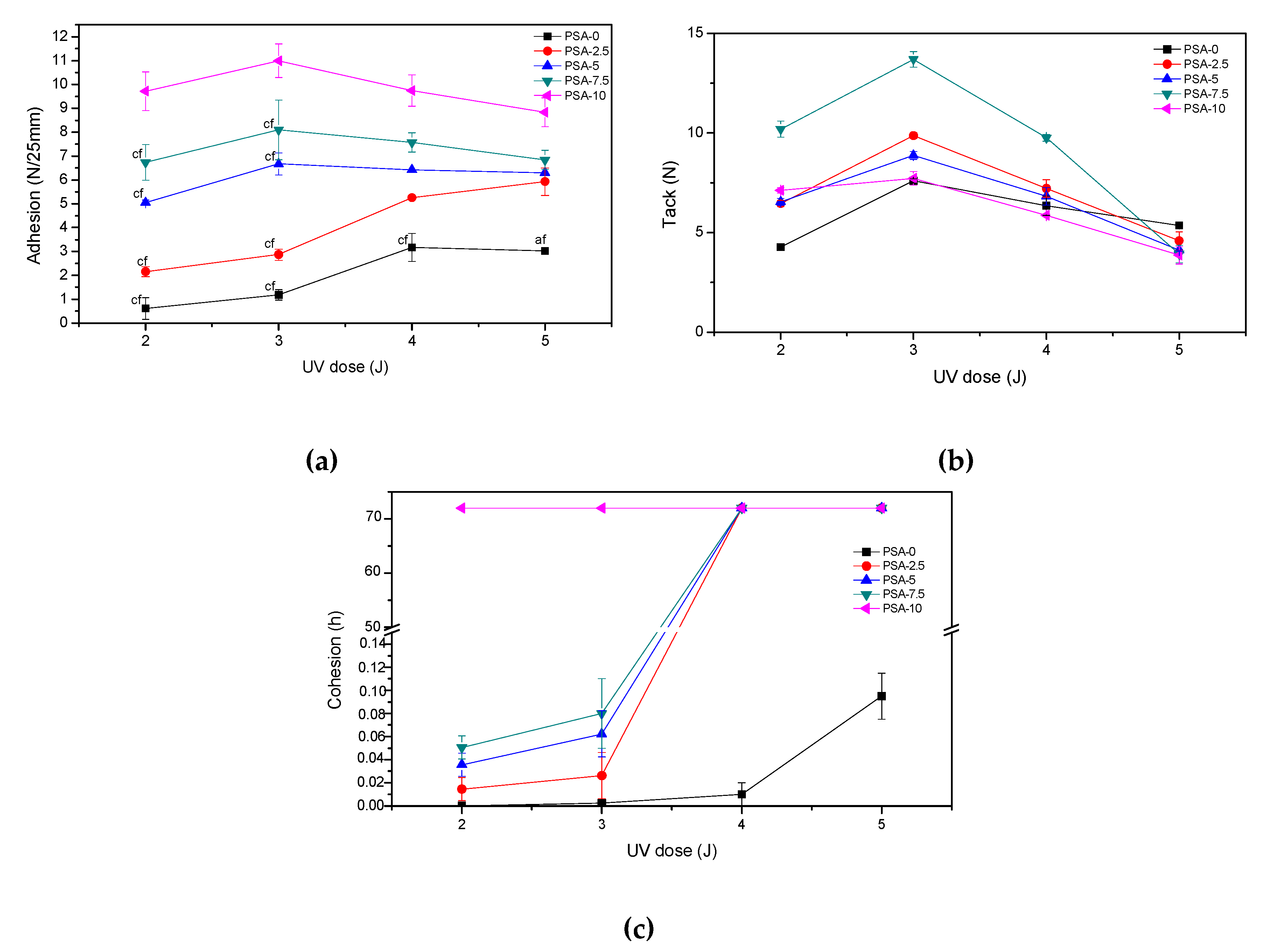
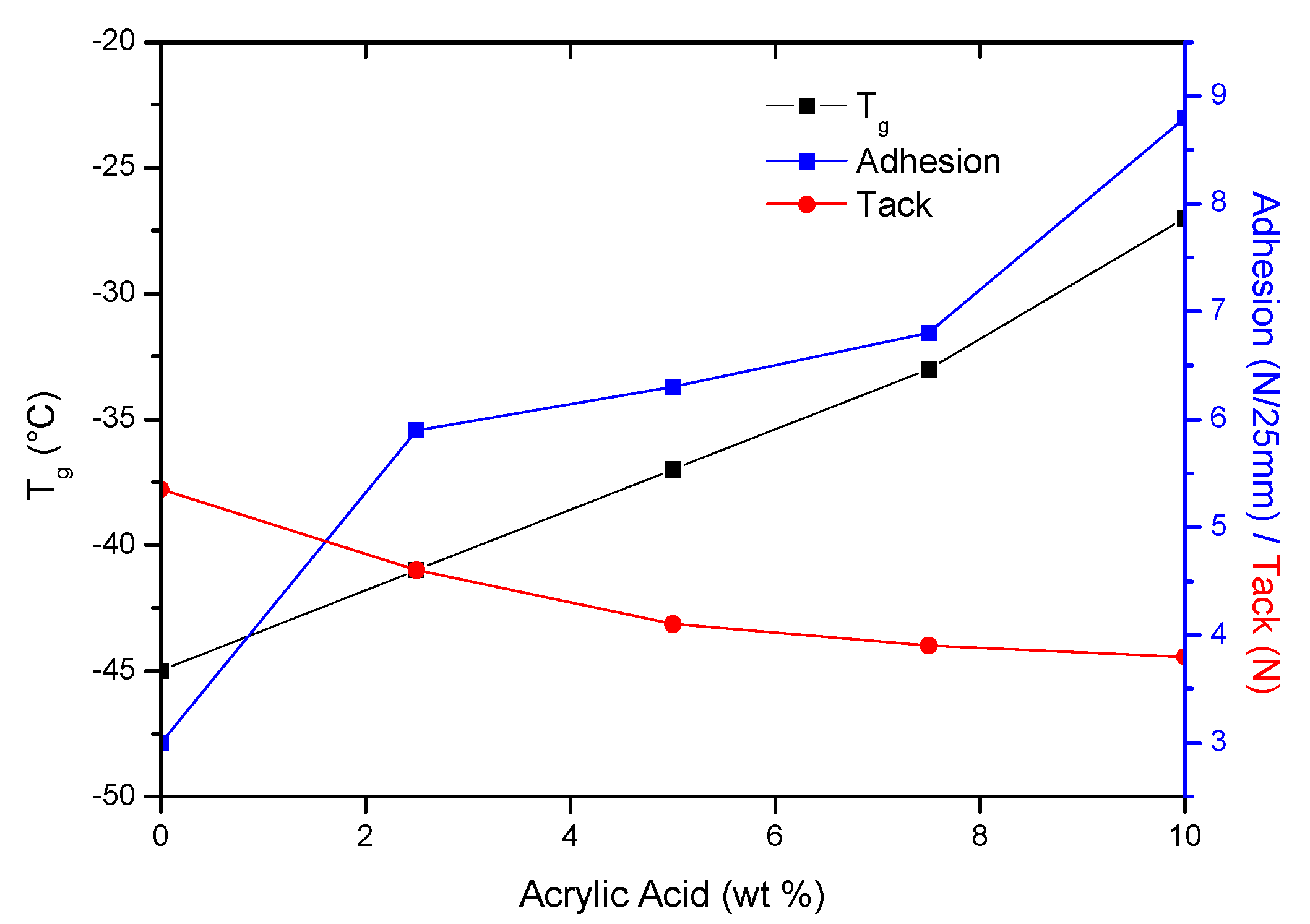
3.2. Properties of PSAs Based on BAA Cotelomers
4. Conclusions
- -
- The rate of the reaction (Rp) increased,
- -
- The conversion of the monomers, solid content in prepolymers and its viscosity values decreased,
- -
- The adhesion and cohesion values increased and tack values decreased; only in PSA-10 with the highest AA content, the highest values of adhesion and cohesion were obtained (and adhesive film were free from failures), regardless of the applied UV dose.
Author Contributions
Funding
Conflicts of Interest
References
- Doyle, J.; Quinn, R. Adhesives: Types, Mechanics and Applications; Nova Science Publishers: New York, NY, USA, 2011; pp. 47–71. [Google Scholar]
- Satas, D. Handbook of Pressure Sensitive Adhesive Technology; Springer: Warwick, RI, USA, 1999; pp. 346–398. [Google Scholar]
- Brockmann, W.; Geiss, P.; Klingen, J.; Schröder, B. Adhesive Bonding: Materials, Applications and Technology; Wiley-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2009; pp. 39–46. [Google Scholar]
- Ulrich, E. Pressure-Sensitive Adhesive Sheet Materials. U.S. Patent 2,884,126, 4 April 1959. [Google Scholar]
- Troughton, M. Handbook of Plastics Joining; William Andrew Inc.: Norwich, NY, USA, 2009; pp. 145–173. [Google Scholar]
- Márquez, I.; Alarcia, F.; Velasco, J. Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels. Polymers 2020, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Benedek, I.; Feldstein, M. Handbook of Pressure-Sensitive Adhesives and Products, Technology of Pressure-Sensitive Adhesives and Products; CRC Press: New York, NY, USA, 2009; pp. 31–45. [Google Scholar]
- Silva, L.; Öchsner, A.; Adams, R. Handbook of Adhesion Technology; Springer: Berlin, Germany, 2011; pp. 342–372. [Google Scholar]
- Gziut, K.; Kowalczyk, A. Influence of radical photoinitiators on features of polyacrylate syrups and self-adhesives. Polym. Wars. 2020, 65, 268–274. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Lee, S.; Hwang, S. Effect of Chemical Structure of Acrylate Monomer on the Transparent Acrylic Pressure Sensitive Adhesives for Optical Applications. Polym. Korea 2014, 38, 682. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives: Effects of substituent structure of acrylate monomer. Int. J. Adhes. Adhes. 2016, 64, 72–77. [Google Scholar] [CrossRef]
- Beak, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure-sensitive adhesives containing menthyl acrylate. Polym. Bull. 2016, 73, 687–701. [Google Scholar] [CrossRef]
- Beak, S.; Hwang, S. Eco-friendly UV-curable pressure sensitive adhesives containing acryloyl derivatives of monosaccharides and their adhesive performances. Int. J. Adhes. Adhes. 2016, 70, 110–113. [Google Scholar] [CrossRef]
- Baek, S.; Jang, S.; Hwang, S. Construction and adhesion performance of biomass tetrahydro-geraniol-based sustainable/transparent pressure sensitive adhesives. J. Ind. Eng. Chem. 2017, 53, 429–434. [Google Scholar] [CrossRef]
- Kim, J.; Shim, G.; Baek, D.; Back, J.; Jang, S.; Kim, H.; Choi, J.; Yeom, J. UV/UV step-curing of optically clear acrylate adhesives for mobile devices. Express Polym. Lett. 2019, 13, 794–805. [Google Scholar] [CrossRef]
- Seok, W.; Leem, J.; Kang, J.; Kim, Y.; Lee, S.; Song, H. Change of Characterization and Film Morphology Based on Acrylic Pressure Sensitive Adhesives by Hydrophilic Derivative Ratio. Polymers 2020, 12, 1504. [Google Scholar] [CrossRef]
- Zhu, M.; Cao, Z.; Zhou, H.; Xie, Y.; Li, G.; Wang, N.; Liu, Y.; He, L.; Qu, X. Preparation of environmentally friendly acrylic pressure-sensitive adhesives by bulk photopolymerization and their performance. RSC Adv. 2020, 10, 10277. [Google Scholar] [CrossRef]
- Starks, C. Free Radical Telomerization; Academic Press: New York, NY, USA; London, UK, 1974; pp. 1–3. [Google Scholar]
- Peterson, M.; Weber, A. Ethylene-Acetal Reaction Product. U.S. Patent 2,395,292, 1 January 1946. [Google Scholar]
- Andrew, L.; Carver, T. Reactions of Alkali-Metal Atoms with Carbon Tetrabromide. Infrared Spectra and Bonding in the Tribromomethyl Radical and Dibromocarbene in Solid Argon. J. Chem. Phys. 1968, 49, 896. [Google Scholar] [CrossRef]
- Gordon, R.; Loftus, R. Telomerization. In Encyclopedia Polymer Science Technology; Kirk, R.E., Othmer, D.F., Eds.; Wiley: New York, NY, USA, 1989; p. 533. [Google Scholar]
- Boutevin, B. From telomerization to living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3235–3243. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B. Topics in Current Chemistry; Springer: Heidelberg, Germany, 1997; pp. 165–233. [Google Scholar]
- Barson, C.; Luxton, A.; Robb, J. Telomerization of methyl methacrylate with bromotrichloromethane. J. Chem. Soc. Faraday Trans. 1972, 68, 1666–1675. [Google Scholar] [CrossRef]
- Harashima, S.; Matsumoto, T. Synthesis of Telechelic Acrylamide Oligomers by Photo-assisted Living Radical Telomerization in Water Using Disulfide Iniferters. J. Photopolym. Sci. Tech. 2013, 26, 733–737. [Google Scholar] [CrossRef][Green Version]
- Bolshakov, A.; Kuzina, S.; Kiryukhin, D. Tetrafluoroethylene Telomerization Initiated by Benzoyl Peroxide. Russ. J. Phys. Chem. 2017, 91, 482–489. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B.; Guida-Pietrasanta, F.; Rousseau, A. Fluorinated Oligomers, Telomers and (Co)polymers: Synthesis and Applications. J. Fluor. Chem. 2001, 107, 397–409. [Google Scholar] [CrossRef]
- Balagué, J.; Améduri, B.; Boutevin, B.; Caporiccio, G. Synthèse de télomères fluorés. Partie III. Télomérisation de l’hexafluoropropène avec des iodures de perfluoroalkyle. J. Fluor. Chem. 1995, 74, 49–58. [Google Scholar] [CrossRef]
- Loubat, C.; Boutevin, B. Synthesis of poly(methylmethacrylate) macromonomers prepared by radical chain transfer reaction H NMR study of macromonomer end-groups. Polym. Bull. 2000, 44, 569–576. [Google Scholar] [CrossRef]
- Loubat, C.; Boutevin, B. Telomerization of acrylic acid with mercaptans: Part 2. Kinetics of the synthesis of star-shaped macromolecules of acrylic acid. Polym. Int. 2001, 50, 375–380. [Google Scholar] [CrossRef]
- Boyer, C.; Boutevin, G.; Robin, J.; Boutevin, B. Synthesis of macromonomers of acrylic acid by telomerization: Application to the synthesis of polystyrene-g-poly(acrylic acid) copolymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 395–415. [Google Scholar] [CrossRef]
- Mezhuev, Y.; Sizova, O.; Korshak, Y.; Luss, A.; Plyushchii, I.; Svistunova, A.; Stratidakis, A. Kinetics of radical telomerization of acrylic acid in the presence of 1-octadecanethiol. Pure Appl. Chem. 2018, 90, 1743–1754. [Google Scholar] [CrossRef]
- Yoshimura, T.; Hontake, K.; Shosenji, H.; Esumi, K. Preparation and Surface-Active Properties of N-Alkyl Maleamic Acid Telomer Type Surfactants Having Several Hydrocarbon Chains. J. Oleo Sci. 2001, 50, 103–108. [Google Scholar] [CrossRef][Green Version]
- Boutevin, B.; Parisi, J.; Vaneeckhoutt, P. Easy use of Lewis and Mayo’s rule to determine monomer conversion-rates in cotelomerization. Polym. Bull. 1992, 28, 703–707. [Google Scholar] [CrossRef]
- Rotta, J.; Pham, P.D.; Lapinte, V.; Borsali, R. Synthesis of Amphiphilic Polymers Based on Fatty Acids and Glycerol-Derived Monomers—A Study of Their Self-Assembly in Water. Macromol. Chem. Phys. 2014, 215, 131–139. [Google Scholar] [CrossRef]
- Li, G.; Yang, P.; Gao, Z.; Zhu, Y. Synthesis and micellar behavior of poly(acrylic acid-b-styrene) block copolymers. Colloid Polym. Sci. 2012, 290, 1825–1831. [Google Scholar] [CrossRef]
- Ma, M.; Lopez, G.; Ameduri, B.; Takahara, A. Fluoropolymer Nanoparticles Prepared Using Trifluoropropene Telomer Based Fluorosurfactants. Langmuir 2020, 36, 1754–1760. [Google Scholar] [CrossRef]
- Prorokova, N.; Kumeeva, T.; Kiryukhin, D.; Kichigina, G.; Kushch, P. Coatings based on tetrafluoroethylene telomeres synthesized in trimethylchlorosilane for obtaining highly hydrophobic polyester fabrics. Prog. Org. Coat. 2020, 139, 105485. [Google Scholar] [CrossRef]
- Zahreddine, W.; Karamé, I.; Pinel, C.; Djakovitch, L.; Rataboul, F. First study on telomerization of chitosan and guar hemicellulose with butadiene: Influence of reaction parameters on the substitution degree of the biopolymers. Mol. Catal. 2020, 483, 110706. [Google Scholar] [CrossRef]
- Klinkenberg, J.; Lawry, K. Sterically Encumbered and Poorly Electron-Donating Oxaphosphaadamantane Ligands for the Pd-Catalyzed Telomerization of Butadiene with Methanol. Org. Process Res. Dev. 2019, 23, 1654–1658. [Google Scholar] [CrossRef]
- Kabatc, J. The influence of a radical structure on the kinetics of photopolymerization. J. Polym. Sci. Part A: Polym. Chem. 2017, 55, 1575–1589. [Google Scholar] [CrossRef]
- Whitfield, R.; Anastasaki, A.; Nikolaou, V.; Jones, G.; Engelis, N.; Discekici, E.; Fleischmann, C.; Willenbacher, J.; Hawker, C.; Haddleton, D. Universal Conditions for the Controlled Polymerization of Acrylates, Methacrylates, and Styrene via Cu(0)-RDRP. J. Am. Chem. Soc. 2017, 139, 1003–1010. [Google Scholar] [CrossRef]
- Wu, B.; Zhong, Q.; Xu, Z.; Wan, L. Effects of molecular weight distribution on the self-assembly of end-functionalized polystyrenes. Polym. Chem. 2017, 8, 4290–4298. [Google Scholar] [CrossRef]
- Rusu, M.; Block, C.; Assche, G.; Mele, B. Influence of temperature and UV intensity on photo-polymerization reaction studied by photo-DSC. J. Therm. Anal. Calorim. 2012, 110, 287–294. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Czech, Z.; Loclair, H. Investigations of UV-crosslinkable water-soluble acrylic pressure-sensitive adhesives. Polym. Wars. 2005, 50, 64–68. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Kabatc, J.; Świderska, L. UV-crosslinkable acrylic pressure-sensitive adhesives for industrial application. Polym. Bull. 2012, 69, 71–80. [Google Scholar] [CrossRef]
- Yang, J.; Keijsers, J.; Heek, M.; Stuiver, A.; Cohen, M.; Kamperman, S.; Kamperman, M. The effect of molecular composition and crosslinking on adhesion of a bio-inspired adhesive. Polym. Chem. 2015, 6, 3121. [Google Scholar] [CrossRef]
- Cantor, A. Glass transition temperatures of hydrocarbon blends: Adhesives measured by differential scanning calorimetry and dynamic mechanical analysis. J. Appl. Polym. Sci. 2000, 77, 826–832. [Google Scholar] [CrossRef]



| Cotelomers Acronym | Monomers (wt %) | Telogen (phr) | Photoinitiator (phr) | ||
|---|---|---|---|---|---|
| BA | AA | ABP | |||
| BAA-0 | 99 | 0 | 1 | 2.5 | 0.1 |
| BAA-2.5 | 96.5 | 2.5 | 1 | 2.5 | 0.1 |
| BAA-5 | 94 | 5 | 1 | 2.5 | 0.1 |
| BAA-7.5 | 91.5 | 7.5 | 1 | 2.5 | 0.1 |
| BAA-10 | 89 | 10 | 1 | 2.5 | 0.1 |
| Cotelomers Acronym | η (Pa∙s) | SC (%) | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|---|---|
| BAA-0 | 47.6 | 91.4 | 13,470 | 20,290 | 1.51 |
| BAA-2.5 | 29.0 | 87.2 | 12,210 | 18,630 | 1.53 |
| BAA-5 | 19.6 | 82.6 | 12,870 | 19,590 | 1.52 |
| BAA-7.5 | 14.5 | 76.3 | 14,115 | 21,710 | 1.54 |
| BAA-10 | 9.1 | 70.3 | 13,810 | 21,415 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Gziut, K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2020, 13, 5661. https://doi.org/10.3390/ma13245661
Kowalczyk A, Weisbrodt M, Schmidt B, Gziut K. Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials. 2020; 13(24):5661. https://doi.org/10.3390/ma13245661
Chicago/Turabian StyleKowalczyk, Agnieszka, Mateusz Weisbrodt, Beata Schmidt, and Konrad Gziut. 2020. "Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives" Materials 13, no. 24: 5661. https://doi.org/10.3390/ma13245661
APA StyleKowalczyk, A., Weisbrodt, M., Schmidt, B., & Gziut, K. (2020). Influence of Acrylic Acid on Kinetics of UV-Induced Cotelomerization Process and Properties of Obtained Pressure-Sensitive Adhesives. Materials, 13(24), 5661. https://doi.org/10.3390/ma13245661