Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process
Abstract
1. Introduction
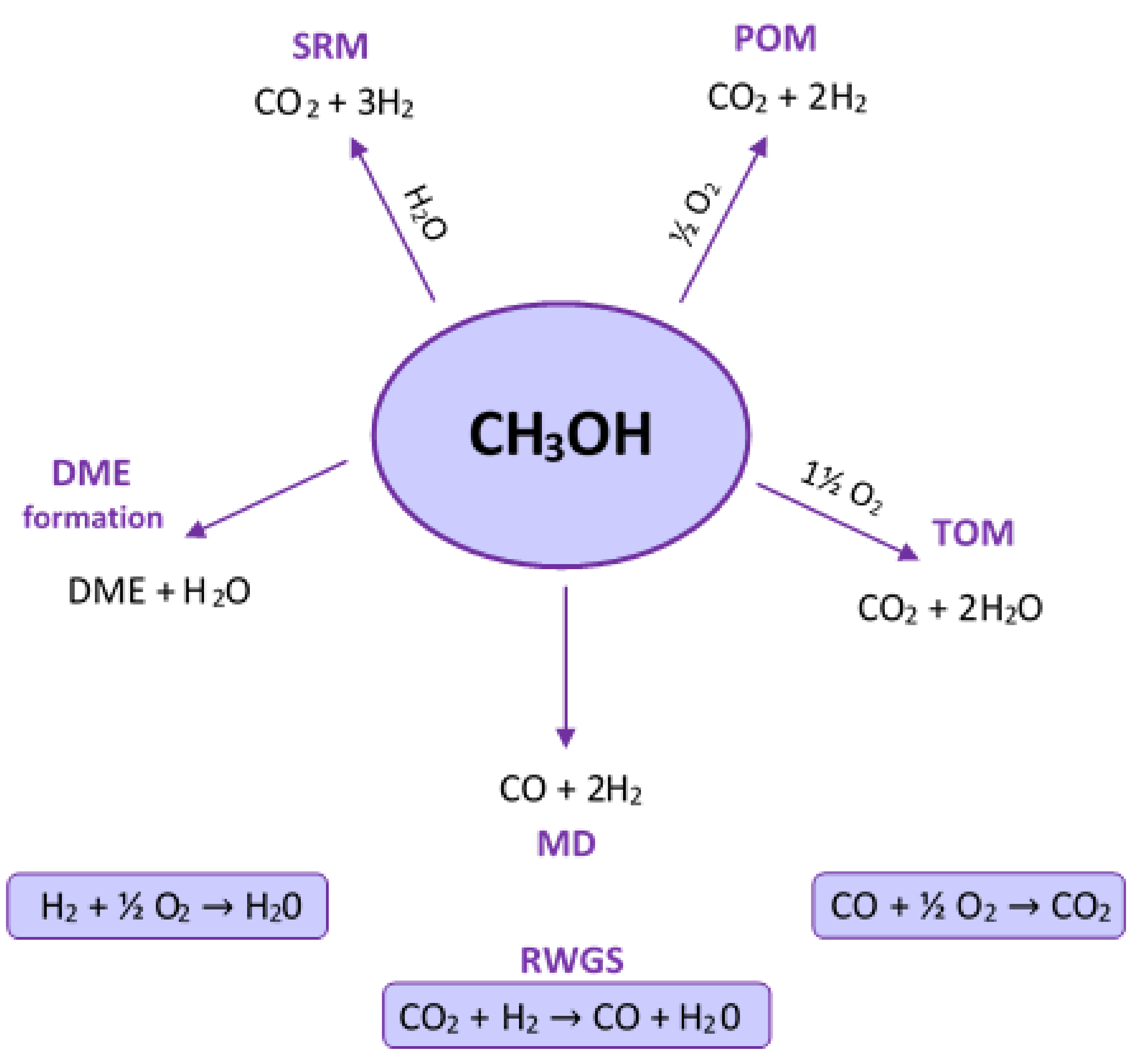
2. Methanol Reforming Reactions
3. Surface Reaction Mechanism and Kinetic Models of the Oxy–Steam Reforming of Methanol Process on Copper-Based System
4. Catalysts Configuration Systems Applied for Hydrogen Production in the Oxy–Steam Reforming of Methanol Process
4.1. The Influence of the Preparation Method on the Catalytic Properties of the Tested Catalytic Systems in the Oxy–Steam Reforming of Methanol Process
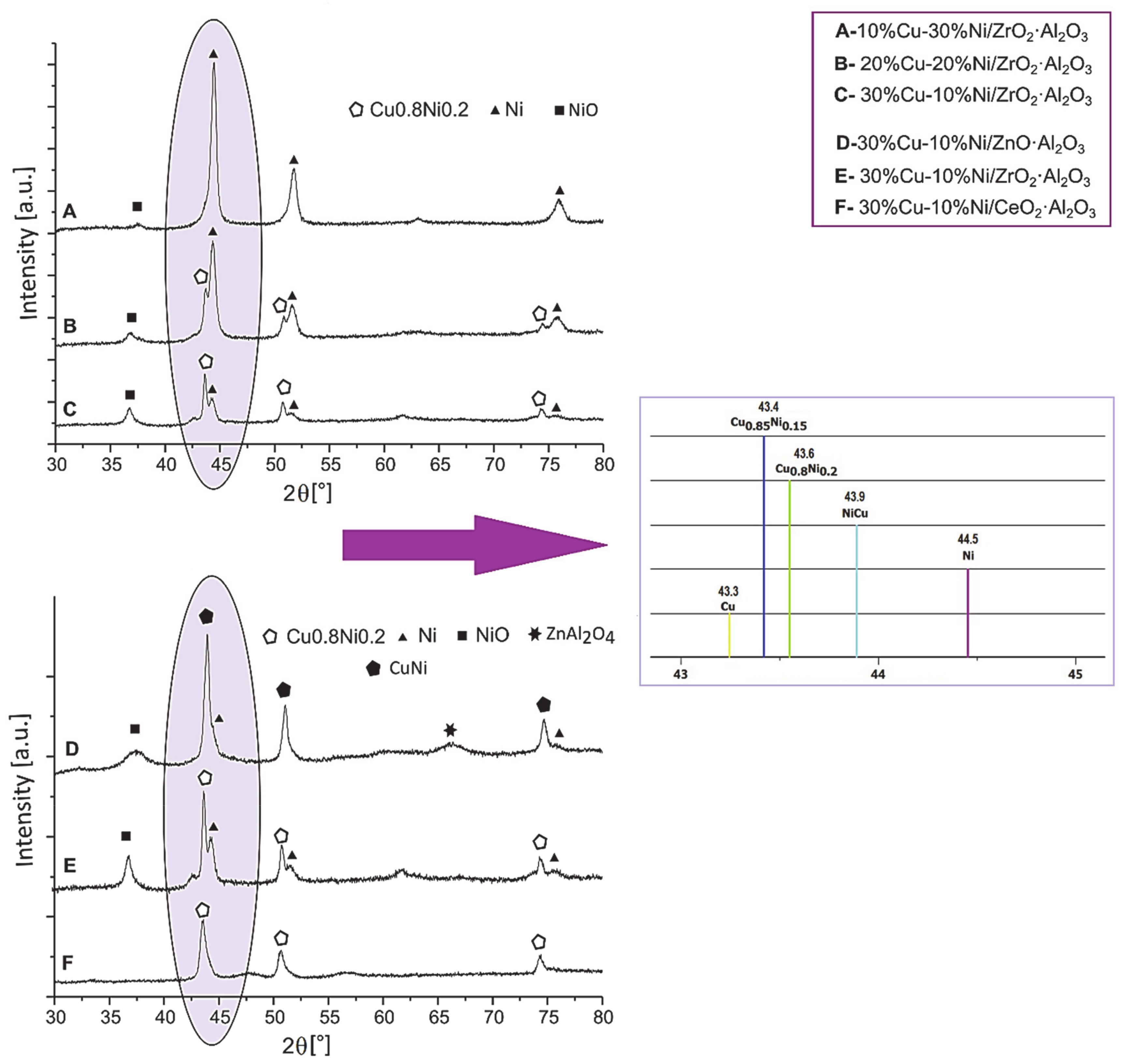
4.2. Effect of the Type of Carrier on the Catalytic Reactivity of the Catalytic Systems Applied in the Oxy–Steam Reforming of Methanol
4.3. Role of Promotors Addition on the Catalytic and Physicochemical Properties of Catalytic Materials Tested in the Oxy–Steam Reforming of Methanol Reaction
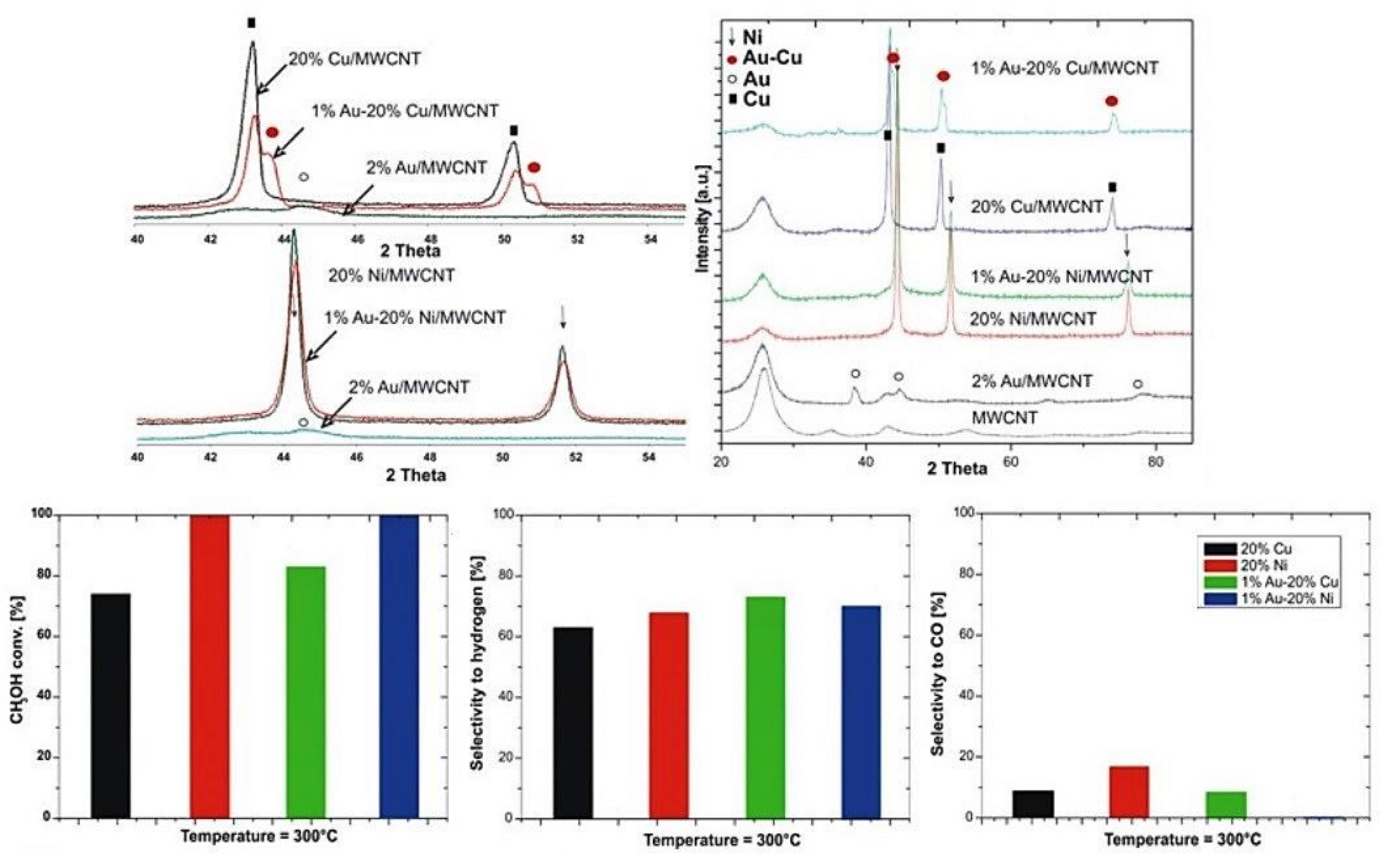
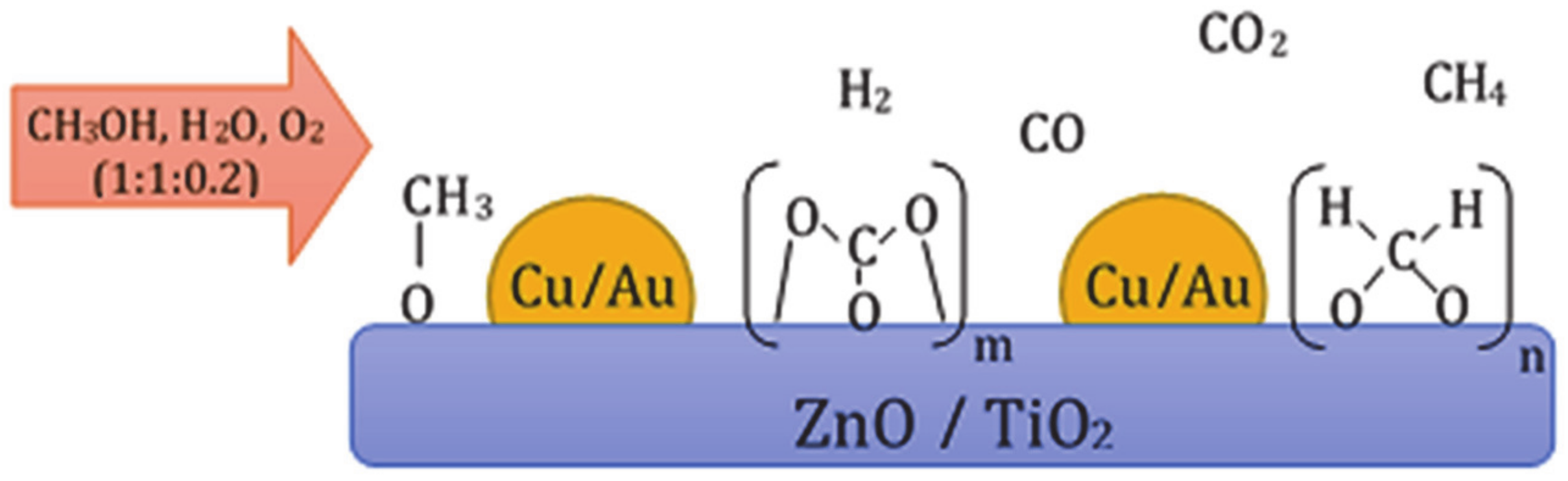
4.4. Role of Active Phase Composition on Reactivity Properties of Catalytic Materials Applied in Oxy–Steam Reforming of Methanol
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATRM | Autothermal reforming of methanol |
| CIP | Co-impregnation method |
| CNTs | Carbon nanotubes |
| CP | Co-precipitation method |
| CRM | Combined (oxy–steam) reforming of methanol |
| DME | Dimethyl ether |
| DMFC | Direct methanol fuel cell |
| DP | Deposition–precipitation method |
| FTIR | Fourier-transform infrared spectroscopy |
| GHSV | Gas hourly space velocity |
| IP | Impregnation method |
| MD | Decomposition of methanol |
| MWCNTs | Multiwalled carbon nanotubes |
| OSR | Oxy–steam-reforming of methanol |
| POM | Partial oxidation of methanol |
| RDS | Rate-determining step |
| RWGS | Reverse water gas shift |
| SBET | Specific surface area |
| SIP | Subsequent impregnation method |
| SMSI | Strong metal-support interaction |
| SRM | Steam reforming of methanol |
| TOM | Total oxidation of methanol |
| UNC | Urea nitrate combustion method |
| W/F | Catalyst weight/volume flow rate ratio |
| WGS | Water-gas shift |
| XANES | X-ray absorption near edge structure |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction spectroscopy |
References
- Salvi, B.L.; Subramanian, K.A.; Panwar, N.L. Alternative fuels for transportation vehicles: A technical review. Renew. Sustain. Energy Rev. 2013, 25, 404–419. [Google Scholar] [CrossRef]
- He, L.-Y.; Qiu, L.-Y. Transport demand, harmful emissions, environment and health co-benefits in China. Energy Policy 2016, 97, 267–275. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P. Highly selective Pd–Cu/ZnAl2O4 catalyst for hydrogen production. Appl. Catal. A Gen. 2014, 479, 26–34. [Google Scholar] [CrossRef]
- Mosinska, M.; Stępińska, N.; Maniukiewicz, W.; Rogowski, J.; Mierczynska-Vasilev, A.; Vasilev, K.; Szynkowska, M.I.; Mierczynski, P. Hydrogen Production on Cu-Ni Catalysts via the Oxy-Steam Reforming of Methanol. Catalysts 2020, 10, 273. [Google Scholar] [CrossRef]
- Alves, H.J.; Bley Junior, C.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Mierczynski, P. Comparative Studies of Bimetallic Ru–Cu, Rh–Cu, Ag–Cu, Ir–Cu Catalysts Supported on ZnO–Al2O3, ZrO2–Al2O3 Systems. Catal. Lett. 2016, 146, 1825–1837. [Google Scholar] [CrossRef]
- Echigo, M.; Tabata, T. A study of CO removal on an activated Ru catalyst for polymer electrolyte fuel cell applications. Appl. Catal. A Gen. 2003, 251, 157–166. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R. Chapter 14—Direct Methanol Fuel Cell. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–397. [Google Scholar]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerol: An update. Energy Convers. Manag. 2009, 50, 2600–2604. [Google Scholar] [CrossRef]
- Agrell, J.; Boutonnet, M.; Melián-Cabrera, I.; Fierro, J. Production of hydrogen from methanol over binary Cu/ZnO catalysts. Appl. Catal. A Gen. 2003, 253, 201–211. [Google Scholar] [CrossRef]
- Alejo, L.; Lago, R.; Peña, M.A.; Fierro, J.L.G. Partial oxidation of methanol to produce hydrogen over CuZn-based catalysts. Appl. Catal. A Gen. 1997, 162, 281–297. [Google Scholar] [CrossRef]
- Azadi, P.; Otomo, J.; Hatano, H.; Oshima, Y.; Farnood, R. Hydrogen production by catalytic near-critical water gasification and steam reforming of glucose. Int. J. Hydrog. Energy 2010, 35, 3406–3414. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.; Kim, S.; Oh, J.; Choi, S.; Bae, M.; Kang, I.; Katikaneni, S.P. Liquid fuel processing for hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 19990–20022. [Google Scholar] [CrossRef]
- Zhao, X.-l.; LÜ, Y.-a.; Liao, W.-p.; Jin, M.-s.; Suo, Z.-h. Hydrogen production from steam reforming of ethylene glycol over supported nickel catalysts. J. Fuel Chem. Technol. 2015, 43, 581–588. [Google Scholar] [CrossRef]
- Profeti, L.P.R.; Ticianelli, E.A.; Assaf, E.M. Production of hydrogen via steam reforming of biofuels on Ni/CeO2–Al2O3 catalysts promoted by noble metals. Int. J. Hydrog. Energy 2009, 34, 5049–5060. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Maniecki, T.P. Bimetallic Au–Cu, Au–Ni catalysts supported on MWCNTs for oxy-steam reforming of methanol. Appl. Catal. B Environ. 2016, 185, 281–294. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Vasilev, K.; Szynkowska, M.I. Novel Rh(Pd)-Cu(Ni) supported catalysts for oxy-steam reforming of methanol. Arab. J. Chem. 2020, 13, 3183–3195. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P.; Vasilev, K. MWCNTs as a catalyst in oxy-steam reforming of methanol. RSC Adv. 2016, 6, 81408–81413. [Google Scholar] [CrossRef]
- Barthos, R.; Solymosi, F. Hydrogen production in the decomposition and steam reforming of methanol on Mo2C/carbon catalysts. J. Catal. 2007, 249, 289–299. [Google Scholar] [CrossRef]
- Wang, J.B.; Li, C.-H.; Huang, T.-J. Study of Partial Oxidative Steam Reforming of Methanol over Cu–ZnO/samaria-doped Ceria Catalyst. Catal. Lett. 2005, 103, 239–247. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Zakrzewski, M.; Dawid, B.; Ciesielski, R.; Maniukiewicz, W.; Maniecki, T. Influence of the Zn–Al binary oxide composition on the physicochemical and catalytic properties of Ni catalysts in the oxy-steam reforming of methanol. React. Kinet. Mech. Catal. 2017, 121, 453–472. [Google Scholar] [CrossRef][Green Version]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, I.M.; Vasilev, K. High Active and Selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 Catalysts for Oxy-Steam Reforming of Methanol. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- Geissler, K.; Newson, E.; Vogel, F.; Truong, T.-B.; Hottinger, P.; Wokaun, A. Autothermal methanol reforming for hydrogen production in fuel cell applications. Phys. Chem. Chem. Phys. 2001, 3, 289–293. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Deshmane, V.G.; Kuila, D. Comparative performance of M-MCM-41 (M: Cu, Co, Ni, Pd, Zn and Sn) catalysts for steam reforming of methanol. J. Mol. Catal. A Chem. 2016, 425, 10–20. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kwon, O.J.; Choi, I.; Kim, J.J. Synergetic effect of combined use of Cu–ZnO–Al2O3 and Pt–Al2O3 for the steam reforming of methanol. Catal. Commun. 2009, 10, 2018–2022. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, C.-T.; Chiang, S.-J.; Liaw, B.-J.; Chen, Y.-Z. Oxidative steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2010, 35, 7675–7683. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsu, C.-C.; Chang, C.-T.; Chen, Y.-P.; Liaw, B.-J.; Chen, Y.-Z. Effect of noble metal on oxidative steam reforming of methanol over CuO/ZnO/Al2O3 catalysts. Int. J. Hydrog. Energy 2012, 37, 11176–11184. [Google Scholar] [CrossRef]
- Clancy, P.; Breen, J.P.; Ross, J.R.H. The preparation and properties of coprecipitated Cu–Zr–Y and Cu–Zr–La catalysts used for the steam reforming of methanol. Catal. Today 2007, 127, 291–294. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, T.H.; Ko, C.H.; Park, H.C.; Song, I.K. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Das, D.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Gayen, A. Methanol steam reforming behavior of copper impregnated over CeO2–ZrO2 derived from a surfactant assisted coprecipitation route. Int. J. Hydrog. Energy 2015, 40, 10463–10479. [Google Scholar] [CrossRef]
- Yong-Feng, L.; Xin-Fa, D.; Wei-Ming, L. Effects of ZrO2-promoter on catalytic performance of CuZnAlO catalysts for production of hydrogen by steam reforming of methanol. Int. J. Hydrog. Energy 2004, 29, 1617–1621. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Govind Menon, P. Activity and characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-alumina for methanol reforming for fuel cell vehicles. Appl. Catal. A Gen. 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Ilinich, O.M.; Liu, Y.; Waterman, E.M.; Farrauto, R.J. Kinetics of Methanol Steam Reforming with a Pd–Zn–Y/CeO2 Catalyst under Realistic Operating Conditions of a Portable Reformer in Fuel Cell Applications. Ind. Eng. Chem. Res. 2013, 52, 638–644. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M.; Minaei, S.; Ajamein, H.; Abdollahifar, M. Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 nanocatalysts via homogeneous precipitation and combustion methods used in methanol steam reforming for fuel cell grade hydrogen production. RSC Adv. 2016, 6, 57199–57209. [Google Scholar] [CrossRef]
- Luengnaruemitchai, A.; Pojanavaraphan, C.; Kumyam, A.; Thunyaratchatanon, C.; Gulari, E. Hydrogen production from the oxidative steam reforming of methanol over AuCu nanoparticles supported on Ce1-xZrxO2 in a fixed-bed reactor. Int. J. Hydrog. Energy 2019, 44, 1686–1700. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; He, S.; Han, C.; Wan, G.; Lei, Y.; Chen, R.; Liu, P.; Chen, K.; Zhang, L.; et al. Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports. Int. J. Hydrog. Energy 2017, 42, 3647–3657. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Ma, L.; Gong, B.; Tran, T.; Wainwright, M.S. Cr2O3 promoted skeletal Cu catalysts for the reactions of methanol steam reforming and water gas shift. Catal. Today 2000, 63, 499–505. [Google Scholar] [CrossRef]
- Yao, C.-Z.; Wang, L.-C.; Liu, Y.-M.; Wu, G.-S.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Shen, J.-P.; Song, C. Influence of preparation method on performance of Cu/Zn-based catalysts for low-temperature steam reforming and oxidative steam reforming of methanol for H2 production for fuel cells. Catal. Today 2002, 77, 89–98. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation method in steam reforming of methanol. Appl. Catal. A Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Larrubia Vargas, M.A.; Busca, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Patrono, P.; Pinzari, F.; Ramis, G. An IR study of methanol steam reforming over ex-hydrotalcite Cu–Zn–Al catalysts. J. Mol. Catal. A Chem. 2007, 266, 188–197. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Uematsu, K.; Ayabe, M. Hydrogen production by oxidative methanol reforming on Pd/ZnO. Appl. Catal. A Gen. 2005, 283, 125–135. [Google Scholar] [CrossRef]
- Takahashi, K.; Takezawa, N.; Kobayashi, H. The mechanism of steam reforming of methanol over a copper-silica catalyst. Appl. Catal. 1982, 2, 363–366. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Ramis, G.; Busca, G. Production of hydrogen from oxidative steam reforming of methanol: II. Catalytic activity and reaction mechanism on Cu/ZnO/Al2O3 hydrotalcite-derived catalysts. J. Catal. 2004, 228, 56–65. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Cammarano, C.; Senese, P.; Costantino, U.; Sisani, M. Cu/ZnO/Al2O3 catalysts for oxidative steam reforming of methanol: The role of Cu and the dispersing oxide matrix. Appl. Catal. B Environ. 2007, 77, 46–57. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrog. Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Turco, G.; Donati, I.; Grassi, M.; Marchioli, G.; Lapasin, R.; Paoletti, S. Mechanical Spectroscopy and Relaxometry on Alginate Hydrogels: A Comparative Analysis for Structural Characterization and Network Mesh Size Determination. Biomacromolecules 2011, 12, 1272–1282. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Ramis, G.; Busca, G. Production of hydrogen from oxidative steam reforming of methanol: I. Preparation and characterization of Cu/ZnO/Al2O3 catalysts from a hydrotalcite-like LDH precursor. J. Catal. 2004; 228, 43–55. [Google Scholar] [CrossRef]
- Reitz, T.L.; Lee, P.L.; Czaplewski, K.F.; Lang, J.C.; Popp, K.E.; Kung, H.H. Time-Resolved XANES Investigation of CuO/ZnO in the Oxidative Methanol Reforming Reaction. J. Catal. 2001, 199, 193–201. [Google Scholar] [CrossRef]
- Mrad, M.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. Cu/Zn-based catalysts for H2 production via steam reforming of methanol. Catal. Today 2011, 176, 88–92. [Google Scholar] [CrossRef]
- Sexton, B.A. Surface vibrations of adsorbed intermediates in the reaction of alcohols with Cu(100). Surf. Sci. 1979, 88, 299–318. [Google Scholar] [CrossRef]
- Wachs, I.E.; Madix, R.J. The selective oxidation of CH3OH to H2CO on a copper(110) catalyst. J. Catal. 1978, 53, 208–227. [Google Scholar] [CrossRef]
- Russell, J.N.; Gates, S.M.; Yates, J.T. Reaction of methanol with Cu(111) and Cu(111) + O(ads). Surf. Sci. 1985, 163, 516–540. [Google Scholar] [CrossRef]
- Chinchen, G.C.; Spencer, M.S.; Waugh, K.C.; Whan, D.A. Promotion of methanol synthesis and the water-gas shift reactions by adsorbed oxygen on supported copper catalysts. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 2193–2212. [Google Scholar] [CrossRef]
- Lwin, Y.; Daud, W.R.W.; Mohamad, A.B.; Yaakob, Z. Hydrogen production from steam–methanol reforming: Thermodynamic analysis. Int. J. Hydrog. Energy 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carrá, S. Kinetics of catalytic steam reforming of methanol in a cstr reactor. Appl. Catal. 1983, 5, 345–358. [Google Scholar] [CrossRef]
- Breen, J.P.; Meunier, F.C.; Ross, J.R.H. Mechanistic aspects of the steam reforming of methanol over a CuO/ZnO/ZrO2/Al2O3 catalyst. Chem. Commun. 1999, 2247–2248. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M. Steam reforming of methanol over a Cu/ZnO/Al2O3 catalyst: a kinetic analysis and strategies for suppression of CO formation. J. Power Sources 2002, 106, 249–257. [Google Scholar] [CrossRef]
- Mastalir, A.; Frank, B.; Szizybalski, A.; Soerijanto, H.; Deshpande, A.; Niederberger, M.; Schomäcker, R.; Schlögl, R.; Ressler, T. Steam reforming of methanol over Cu/ZrO2/CeO2 catalysts: a kinetic study. J. Catal. 2005, 230, 464–475. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. Hydrogen Recovery from Methanol Steam Reforming in a Dense Membrane Reactor: Simulation Study. Ind. Eng. Chem. Res. 2004, 43, 2420–2432. [Google Scholar] [CrossRef]
- Morillo, A.; Freund, A.; Merten, C. Concept and Design of a Novel Compact Reactor for Autothermal Steam Reforming with Integrated Evaporation and CO Cleanup. Ind. Eng. Chem. Res. 2004, 43, 4624–4634. [Google Scholar] [CrossRef]
- Jiang, C.J.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Kinetic study of steam reforming of methanol over copper-based catalysts. Appl. Catal. A Gen. 1993, 93, 245–255. [Google Scholar] [CrossRef]
- Breen, J.P.; Ross, J.R.H. Methanol reforming for fuel-cell applications: development of zirconia-containing Cu–Zn–Al catalysts. Catal. Today 1999, 51, 521–533. [Google Scholar] [CrossRef]
- Iwasa, N.; Kudo, S.; Takahashi, H.; Masuda, S.; Takezawa, N. Highly selective supported Pd catalysts for steam reforming of methanol. Catal. Lett. 1993, 19, 211–216. [Google Scholar] [CrossRef]
- Amiri, T.Y.; Moghaddas, J. Reaction parameters influence on the catalytic performance of copper-silica aerogel in the methanol steam reforming. J. Fuel Chem. Technol. 2016, 44, 84–90. [Google Scholar] [CrossRef]
- Murcia-Mascarós, S.; Navarro, R.M.; Gómez-Sainero, L.; Costantino, U.; Nocchetti, M.; Fierro, J.L.G. Oxidative Methanol Reforming Reactions on CuZnAl Catalysts Derived from Hydrotalcite-like Precursors. J. Catal. 2001, 198, 338–347. [Google Scholar] [CrossRef]
- Rabe, S.; Vogel, F. A thermogravimetric study of the partial oxidation of methanol for hydrogen production over a Cu/ZnO/Al2O3 catalyst. Appl. Catal. B Environ. 2008, 84, 827–834. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Kinetic modeling of oxidative steam reforming of methanol over Cu/ZnO/CeO2/Al2O3 catalyst. Appl. Catal. A Gen. 2009, 356, 189–200. [Google Scholar] [CrossRef]
- Patel, S.; Pant, K.K. Hydrogen production by oxidative steam reforming of methanol using ceria promoted copper–alumina catalysts. Fuel Process. Technol. 2007, 88, 825–832. [Google Scholar] [CrossRef]
- Horny, C.; Renken, A.; Kiwi-Minsker, L. Compact string reactor for autothermal hydrogen production. Catal. Today 2007, 120, 45–53. [Google Scholar] [CrossRef]
- Horng, R.-F.; Chou, H.-M.; Lee, C.-H.; Tsai, H.-T. Characteristics of hydrogen produced by partial oxidation and auto-thermal reforming in a small methanol reformer. J. Power Sources 2006, 161, 1225–1233. [Google Scholar] [CrossRef]
- Riva, A.; Trifirò, F.; Vaccari, A.; Mintchev, L.; Busca, G. Structure and reactivity of zinc–chromium mixed oxides. Part 2.—Study of the surface reactivity by temperature-programmed desorption of methanol. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 1423–1435. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3. Part 1: the reaction network. Appl. Catal. A Gen. 1999, 179, 21–29. [Google Scholar] [CrossRef]
- Shan, W.; Feng, Z.; Li, Z.; Zhang, J.; Shen, W.; Li, C. Oxidative steam reforming of methanol on Ce0.9Cu0.1OY catalysts prepared by deposition–precipitation, coprecipitation, and complexation–combustion methods. J. Catal. 2004, 228, 206–217. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Production of hydrogen via combined steam reforming of methanol over CuO–CeO2 catalysts. Catal. Commun. 2004, 5, 231–235. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takehira, K. Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: Steam reforming and oxidative steam reforming. J. Mol. Catal. A Chem. 2007, 268, 185–194. [Google Scholar] [CrossRef]
- Ritzkopf, I.; Vukojević, S.; Weidenthaler, C.; Grunwaldt, J.-D.; Schüth, F. Decreased CO production in methanol steam reforming over Cu/ZrO2 catalysts prepared by the microemulsion technique. Appl. Catal. A Gen. 2006, 302, 215–223. [Google Scholar] [CrossRef]
- Wang, C.-T.; Willey, R.J. Fine particle iron oxide based aerogels for the partial oxidation of methanol. Catal. Today 1999, 52, 83–89. [Google Scholar] [CrossRef]
- Matsumura, Y.; Tanaka, K.; Tode, N.; Yazawa, T.; Haruta, M. Catalytic methanol decomposition to carbon monoxide and hydrogen over nickel supported on silica. J. Mol. Catal. A Chem. 2000, 152, 157–165. [Google Scholar] [CrossRef]
- Dinka, P.; Mukasyan, A. Solution combustion synthesis of nano materials. In Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show-NSTI Nanotech 2006 Technical Proceedings, Boston, MA, USA, 7–11 May 2006; pp. 456–459. [Google Scholar]
- Liu, S.; Takahashi, K.; Ayabe, M. Hydrogen production by oxidative methanol reforming on Pd/ZnO catalyst: effects of Pd loading. Catal. Today 2003, 87, 247–253. [Google Scholar] [CrossRef]
- Haynes, D.J.; Shekhawat, D.; Spivey, J.J.; Berry, D.A. Chapter 6—Oxidative Steam Reforming. In Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2011; pp. 129–190. [Google Scholar]
- Pijolat, M.; Prin, M.; Soustelle, M.; Touret, O.; Nortier, P. Thermal stability of doped ceria: experiment and modelling. J. Chem. Soc. Faraday Trans. 1995, 91, 3941–3948. [Google Scholar] [CrossRef]
- Fornasiero, P.; Balducci, G.; di Monte, R.; Kašpar, J.; Sergo, V.; Gubitosa, G.; Ferrero, A.; Graziani, M. Modification of the Redox Behaviour of CeO2Induced by Structural Doping with ZrO2. J. Catal. 1996, 164, 173–183. [Google Scholar] [CrossRef]
- Fan, L.; Fujimoto, K. Reaction Mechanism of Methanol Synthesis from Carbon Dioxide and Hydrogen on Ceria-Supported Palladium Catalysts with SMSI Effect. J. Catal. 1997, 172, 238–242. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, P. Production of hydrogen by steam reforming of methanol on CeO2 promoted Cu/Al2O3 catalysts. J. Mol. Catal. A Chem. 2003, 194, 99–105. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosinska, M.; Maniukiewicz, W.; Nowosielska, M.; Czylkowska, A.; Szynkowska, M.I. Oxy-steam reforming of methanol on copper catalysts. React. Kinet. Mech. Catal. 2019, 127, 857–874. [Google Scholar] [CrossRef]
- Iwasa, N.; Yoshikawa, M.; Nomura, W.; Arai, M. Transformation of methanol in the presence of steam and oxygen over ZnO-supported transition metal catalysts under stream reforming conditions. Appl. Catal. A Gen. 2005, 292, 215–222. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO: Effect of the formation of PdZn alloys upon the reaction. Appl. Catal. A Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Iwasa, N.; Takezawa, N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Ichihashi, Y.; Kuraoka, K.; Matsumura, Y. Catalytic methanol decomposition over palladium deposited on thermally stable mesoporous titanium oxide. J. Mol. Catal. A Chem. 2003, 198, 303–308. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Ichihashi, Y.; Kuraoka, K.; Shen, W.-J.; Matsumura, Y. Catalytic Methanol Decomposition Over Palladium Deposited on Mesoporous Cerium Oxide. Catal. Lett. 2003, 88, 83–87. [Google Scholar] [CrossRef]
- Lenarda, M.; Moretti, E.; Storaro, L.; Patrono, P.; Pinzari, F.; Rodríguez-Castellón, E.; Jiménez-López, A.; Busca, G.; Finocchio, E.; Montanari, T.; et al. Finely dispersed Pd-Zn catalyst supported on an organized mesoporous alumina for hydrogen production by methanol steam reforming. Appl. Catal. A Gen. 2006, 312, 220–228. [Google Scholar] [CrossRef]
- Usami, Y.; Kagawa, K.; Kawazoe, M.; Yasuyuki, M.; Sakurai, H.; Haruta, M. Catalytic methanol decomposition at low temperatures over palladium supported on metal oxides. Appl. Catal. A Gen. 1998, 171, 123–130. [Google Scholar] [CrossRef]
- Udani, P.P.C.; Gunawardana, P.V.D.S.; Lee, H.C.; Kim, D.H. Steam reforming and oxidative steam reforming of methanol over CuO–CeO2 catalysts. Int. J. Hydrog. Energy 2009, 34, 7648–7655. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Seelam, P.K.; Huuhtanen, M.; Sápi, A.; Szabó, M.; Kordás, K.; Turpeinen, E.; Tóth, G.; Keiski, R.L. CNT-based catalysts for H2 production by ethanol reforming. Int. J. Hydrog. Energy 2010, 35, 12588–12595. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Keller, N.; Ehret, G.; Charbonniere, L.c.J.; Ziessel, R.; Ledoux, M.J. Carbon nanofiber supported palladium catalyst for liquid-phase reactions: An active and selective catalyst for hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. J. Mol. Catal. A Chem. 2001, 170, 155–163. [Google Scholar] [CrossRef]
- Hou, T.; Yuan, L.; Ye, T.; Gong, L.; Tu, J.; Yamamoto, M.; Torimoto, Y.; Li, Q. Hydrogen production by low-temperature reforming of organic compounds in bio-oil over a CNT-promoting Ni catalyst. Int. J. Hydrog. Energy 2009, 34, 9095–9107. [Google Scholar] [CrossRef]
- Solhy, A.; Machado, B.F.; Beausoleil, J.; Kihn, Y.; Gonçalves, F.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Faria, J.L.; Serp, P. MWCNT activation and its influence on the catalytic performance of Pt/MWCNT catalysts for selective hydrogenation. Carbon 2008, 46, 1194–1207. [Google Scholar] [CrossRef]
- Tang, J.M.; Jensen, K.; Waje, M.; Li, W.; Larsen, P.; Pauley, K.; Chen, Z.; Ramesh, P.; Itkis, M.E.; Yan, Y.; et al. High Performance Hydrogen Fuel Cells with Ultralow Pt Loading Carbon Nanotube Thin Film Catalysts. J. Phys. Chem. C 2007, 111, 17901–17904. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Wang, C.-X.; Liu, Z.-W.; Lu, J. Selective hydrogenation of cinnamaldehyde over Pt-supported multi-walled carbon nanotubes: Insights into the tube-size effects. Appl. Catal. A Gen. 2008, 344, 114–123. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M.; Melián-Cabrera, I.; Navarro, R.M.; Fierro, J.L.G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Huang, X.; Ma, L.; Wainwright, M.S. The influence of Cr, Zn and Co additives on the performance of skeletal copper catalysts for methanol synthesis and related reactions. Appl. Catal. A Gen. 2004, 257, 235–243. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Uematsu, K.; Ayabe, M. Hydrogen production by oxidative methanol reforming on Pd/ZnO catalyst: effects of the addition of a third metal component. Appl. Catal. A Gen. 2004, 277, 265–270. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-Temperature Oxidation of CO over Gold Supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Ciesielski, R.; Rogowski, J.; Szynkowska, I.M.; Trifonov, A.Y.; Dubkov, S.V.; Gromov, D.G.; et al. The effect of gold on modern bimetallic Au–Cu/MWCNT catalysts for the oxy-steam reforming of methanol. Catal. Sci. Technol. 2016, 6, 4168–4183. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Nowosielska, M.; Kubicki, J.; Maniukiewicz, W.; Czylkowska, A.; Maniecki, T.P. Monometallic copper catalysts supported on multi-walled carbon nanotubes for the oxy-steam reforming of methanol. React. Kinet. Mech. Catal. 2016, 117, 675–691. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Dagle, R.; Hu, J.; Dohnalkova, A.C.; Wang, Y. Steam reforming of methanol over highly active Pd/ZnO catalyst. Catal. Today 2002, 77, 79–88. [Google Scholar] [CrossRef]
- Cubeiro, M.L.; Fierro, J.L.G. Partial oxidation of methanol over supported palladium catalysts. Appl. Catal. A Gen. 1998, 168, 307–322. [Google Scholar] [CrossRef]
- Cubeiro, M.L.; Fierro, J.L.G. Selective Production of Hydrogen by Partial Oxidation of Methanol over ZnO-Supported Palladium Catalysts. J. Catal. 1998, 179, 150–162. [Google Scholar] [CrossRef]
- Xu, J.B.; Zhao, T.S.; Yang, W.W.; Shen, S.Y. Effect of surface composition of Pt-Au alloy cathode catalyst on the performance of direct methanol fuel cells. Int. J. Hydrog. Energy 2010, 35, 8699–8706. [Google Scholar] [CrossRef]
- Manzoli, M.; Chiorino, A.; Boccuzzi, F. Decomposition and combined reforming of methanol to hydrogen: A FTIR and QMS study on Cu and Au catalysts supported on ZnO and TiO2. Appl. Catal. B Environ. 2005, 57, 201–209. [Google Scholar] [CrossRef]
- Vizcaíno, A.; Carrero, A.; Calles, J. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrog. Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Khzouz, M.; Wood, J.; Pollet, B.; Bujalski, W. Characterization and activity test of commercial Ni/Al2O3, Cu/ZnO/Al2O3 and prepared Ni–Cu/Al2O3 catalysts for hydrogen production from methane and methanol fuels. Int. J. Hydrog. Energy 2013, 38, 1664–1675. [Google Scholar] [CrossRef]
- De Rogatis, L.; Montini, T.; Lorenzut, B.; Fornasiero, P. NixCuy/Al2O3 based catalysts for hydrogen production. Energy Environ. Sci. 2008, 1, 501–509. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Mondragón Galicia, G.; Mendoza Anaya, D.; Palacios, J.; Angeles-Chavez, C.; Arenas-Alatorre, J. Synthesis and characterization of bimetallic Cu–Ni/ZrO2 nanocatalysts: H2 production by oxidative steam reforming of methanol. Int. J. Hydrog. Energy 2008, 33, 4569–4576. [Google Scholar] [CrossRef]
- Eaimsumang, S.; Chollacoop, N.; Luengnaruemitchai, A.; Taylor, S.H. Ceria nanorod supported gold nanoparticles as structured catalysts for the oxidative steam reforming of methanol: Effect of CTAB concentration on physiochemical properties and catalyst performance. J. Catal. 2020, 392, 254–265. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Pan, L.; Wang, S.; Yuan, Z.; Wang, S. Measurement of concentration profiles over ZnO–Cr2O3/CeO2–ZrO2 monolithic catalyst in oxidative steam reforming of methanol. Fuel Process. Technol. 2007, 88, 65–71. [Google Scholar] [CrossRef]






| POM-SRM | TOM-SRM | ||
|---|---|---|---|
| SRM | CH3OH + H2O CO2 + 3H2 | 3 | |
| POM | CH3OH + O2 CO2 + 2H2 | 1 | - |
| TOM | CH3OH + O2 CO2 + 2H2O | - | |
| 4CH3OH + 3H2O + O2 + 2N2 4CO2 + 11H2 + 2N2 | |||
| Catalyst | Ea (cal mol−1) |
|---|---|
| Cu(5)Zn(50)Al(45) | 24 |
| Cu(15)Zn(48)Al(37) | 27 |
| Cu(18)Zn(33)Al(49) | 16 |
| Cu(45)Zn(31)Al(24) | 22 |
| Cu(75)Zn(25)Al(0) | - |
| References | Investigated Mechanism | Employed Catalyst | Operating Conditions | ||||
|---|---|---|---|---|---|---|---|
| Total Flow [cm3/min] | Temperature [K] | Pressure [atm] | H2O/CH3OH | O2/CH3OH | |||
| [56] | Selective oxidation of CH3OH to H2CO | Copper (110) | - | 295 | - | - | - |
| [59] | SRM | Copper containing catalyst | - | 360–573 | 1 | 1.5 | - |
| [60] | SRM | Cu/ZnO/Al2O3 | - | 433–473 | 1 | - | - |
| [61] | SRM | CuO/ZnO/ZrO2/Al2O3 | - | 473 and 573 | 1 | 1.3 | - |
| [62] | SRM | Cu/ZnO/Al2O3 | 230 | 448–623 | 1 | 1.3 | - |
| [77] | SRM | Cu/ZnO/Al2O3 | 50 | 433–533 | 1–35 | 0–1.2 | - |
| [45] | SRM | Cu/ZnO/Al2O3 | 50 | 433–533 | 1–35 | 0–1.2 | - |
| [63] | SRM | Cu/ZrO2/CeO2 | - | 523 | 1 | 1.0 | - |
| [66] | SRM | Cu/ZnO/Al2O3 | - | 443–533 | 1 | - | - |
| [67] | SRM | Cu/Zn/Zr/Al | 38.6 | 413–618 | 1 | 1.3 | - |
| [47] | SRM | copper–silica | - | 433 and 453 | 1 | - | - |
| [69] | SRM | copper–silica aerogel | - | 423–673 | 1 | 2.0 | - |
| [70] | OSRM | Cu/ZnO/Al2O3 | 120 | 473–633 | 1 | 1.1 | 0.3 |
| [71] | POM | Cu/ZnO/Al2O3 | - | 453 and 493 | - | - | 0.1–0.5 |
| [72] | OSRM | Cu/ZrO2/CeO2/Al2O3 | - | 473–573 | 1 | 1.5 | 0.1–0.2 |
| [73] | OSRM | Cu/CeO2/Al2O3 | - | 473–573 | 1 | 1.5 | 0–0.5 |
| [74] | OSRM | Cu/ZnO/Al2O3 Cu/ZnO/Al2O3/Cr2O3 | 100 | 538–548 | 1.28 | 1 | 0.9 |
| [48] | OSRM | Cu/ZnO/Al2O3 | - | 473–673 | - | 1.1 | 0.12 |
| Catalyst | Preparation Method | SBET (m2/g) | Metal Dispersion (%) | Reduction Temp. (°C) | Reduction Time (h) | W/F (gscm−3) | GHSV (h−1) | TOSR (°C) | Catalyst Weight (g) | CH3OH Conv. (%) | H2 | CO | CO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CuO/ZnO/Al2O3 [42] | CP | 94 | - | 350–450 | 2 | 1.43 | 0.47 | - | - | 230 | 0.3 | 100.0 | 71.0 y | 0.1 y | 28.9 y |
| Cu0.30Mn0.70 [39] | UNC | 8 | - | 320 | 2 | 1.26 | 0.10 | 0.257 | - | 240 | 0.3 | 100.0 | 97.0 s | 3.0 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 1.2) [79] | UNC | 10 | - | - | - | 1.50 | 0.10 | 0.257 | - | 300 | 0.3 | 56.0 | 90.3 s | 2.1 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 1.2) [79] | UNC | 10 | - | - | - | 1.50 | 0.10 | 0.257 | - | 340 | 0.3 | 5.7 | 75.0 s | 0.8 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 2.38) [79] | UNC | 5 | - | - | - | 1.50 | 0.10 | 0.257 | - | 300 | 0.3 | 45.0 | 84.3 s | 4.8 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 2.38) [79] | UNC | 5 | - | - | - | 1.50 | 0.10 | 0.257 | - | 240 | 0.3 | 12.0 | 69.0 s | 3.1 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 3.30) [79] | UNC | 20 | - | - | - | 1.50 | 0.10 | 0.257 | - | 300 | 0.3 | 95.0 | 96.6 s | 3.4 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 3.30) [79] | UNC | 20 | - | - | - | 1.50 | 0.10 | 0.257 | - | 240 | 0.3 | 32.4 | 92.6 s | 1.0 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 4.17) [79] | UNC | 43 | - | - | - | 1.50 | 0.10 | 0.257 | - | 300 | 0.3 | 100 | 95.9 s | 4.0 s | - |
| CuO–CeO2 (mol.rat. = 0.15, u/n rat. = 4.17) [79] | UNC | 43 | - | - | - | 1.50 | 0.10 | 0.257 | - | 240 | 0.3 | 36.5 | 93.7 s | 0.8 s | - |
| Cu(5)/CeO2·Al2O3 [91] | IP | 126 | 2.14 * | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 97.7 | 69.1 s | 0 s | 30.6 s |
| Cu(20)/CeO2·Al2O3 [91] | IP | 101 | 0.16 * | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 99.6 | 68.2 s | 0 s | 31.7 s |
| Cu(40)/CeO2·Al2O3 [91] | IP | 90 | 0.13 * | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 87.9 | 65.7 s | 0 s | 33.8 s |
| Cu(60)/CeO2·Al2O3 [91] | IP | 30 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 9.6 | 28.5 s | 0 s | 71.5 s |
| Ni(5)/CeO2·Al2O3 [23] | IP | 132 | 1.06 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 2 | 30.5 s | - | 25 s |
| Ni(20)/CeO2·Al2O3 [23] | IP | 128 | 1.16 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 31 | 33 s | - | 55.6 s |
| Ni(40)/CeO2·Al2O3 [23] | IP | 78 | 0.56 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 98 | 68.9 s | 13.3 s | 14.3 s |
| Ni(60)/CeO2·Al2O3 [23] | IP | 133 | 0.69 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 77 | 58.2 s | 27.7 s | 14.1 s |
| Ni(40)/CeO2 [23] | IP | 34 | 0.63 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 85 | 68.6 s | 22.6 s | 0.5 s |
| Ni(40)/Al2O3 [23] | IP | 58 | 0.28 * | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 20.5 | 53.4 s | - | 25.2 s |
| Pd(2)–Ni(40)/CeO2·Al2O3 [23] | SIP | 42 | - | 300 | 1 | - | - | - | 26,700 | 250 | 0.2 | 99.9 | 71.5 s | 14.9 s | 9.1 s |
| Pd/ZnO [85] | IP | - | - | 400 | 2 | 1.50 | 0.10 | - | 110,000 | 250 | 0.3 | - | - | - | - |
| Pd/ZnO [85] | CP | - | - | 400 | 2 | 1.50 | 0.10 | - | 110,000 | 250 | 0.3 | - | - | - | - |
| Cu/ZnO [107] | CP | 49 | 9.6 * | 250–300 | 1 | 1.30 | 0.20 | - | - | 300 | 0.5 | 90.0 | 50.0 m | 0.07 m | 20.0 m |
| Cu/ZnO/Al2O3 [107] | CP | 92 | 11.3 * | 250–300 | 1 | 1.30 | 0.20 | - | - | 325 | 0.5 | 90.0 | - | 0.13 m | - |
| Cu/ZnO/ZrO2 [107] | CP | 82 | 13.2 * | 250–300 | 1 | 1.30 | 0.20 | - | - | 295 | 0.5 | 90.0 | - | 0.04 m | - |
| Cu/ZnO/ZrO2/Al2O3 [107] | CP | 116 | 23.2 * | 250–300 | 1 | 1.30 | 0.20 | - | - | 295 | 0.5 | 90.0 | - | 0.05 m | - |
| Cu(20)/ZrO2·Al2O3 (2:1) [18] | IP | 143 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 22 | 41 s | 0s | 59 s |
| Cu(20)/ZrO2·Al2O3 (1:1) [18] | IP | 138 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 46 | 70 s | 0 s | 29 s |
| Cu(20)/ZrO2·Al2O3 (1:2) [18] | IP | 167 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 58 | 68 s | 0 s | 31 s |
| Ni(20)/ZrO2·Al2O3 (1:2) [18] | IP | 116 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 94 | 70 s | 25 s | 5 s |
| Ni(20)/ZrO2·Al2O3 (1:2) [18] | IP | 116 | - | 500 | 1 | - | - | - | 26,700 | 300 | 0.2 | 61 | 65 s | 0 s | 22 s |
| Pd(1)–Cu(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | 171 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 60 | 66 s | 0 s | 33 s |
| Rh(0.5)–Cu(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | - | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 86 | 68 s | 14 s | 18s |
| Rh(1)–Cu(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | 164 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 74 | 71 s | 4 s | 25 s |
| Rh(2)–Cu(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | - | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 61 | 60 s | 18 s | 22 s |
| Pd(1)–Ni(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | 120 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 58 | 63 s | 19s | 18 s |
| Rh(1)–Ni(20)/ ZrO2·Al2O3 (1:2) [18] | SIP | 123 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 66 | 64 s | 18s | 18 s |
| Ni(20)/ZnO·Al2O3 (2:1) [22] | IP | 108 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 78 | 76 s | 0 s | 24 s |
| Ni(20)/ZnO·Al2O3 (1:1) [22] | IP | 123 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 99 | 76 s | 0 s | 24 s |
| Ni(20)/ZnO·Al2O3 (1:2) [22] | IP | 231 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 73 | 40 s | 10 s | 24 s |
| Ni(20)/ZnO·Al2O3 (1:4) [22] | IP | 246 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 83 | 65 s | 0 s | 21 s |
| Pd(0.5)–Ni(20)/ ZnO·Al2O3 (1:1) [22] | SIP | 106 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 99 | 73 s | 10 s | 17 s |
| Pd(2)–Ni(20)/ ZnO·Al2O3 (1:1) [22] | SIP | 104 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.2 | 99 | 72 s | 8 s | 20 s |
| Pd(6.5)/ZnO [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd(6.5)/ZnO–ZrO2 [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd(6.5)/ZnO–Fe3O4 [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd(6.5)/ZnO–MgO [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd/(6.5)ZnO–Cr2O3 [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd/(6.5)ZnO–Al2O3 [109] | CP | - | - | 400/500 | 2 | 1.50 | 0.10 | - | - | 250 | 0.3 | - | - | - | - |
| Pd(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 100 | 67 m | 6 m | 27 m |
| Pt(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 100 | 70 m | 2 m | 28 m |
| Co(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 53 | 43 m | 5 m | 30 m |
| Ni(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 96 | 54 m | 23 m | 13 m |
| Ir(10)/ZnO [92] | IP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 59 | 49 m | 3 m | 31 m |
| Ru(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 300 | 0.1 | 88 | 48 m | 25 m | 11 m |
| Pd(10)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 89 | 60 m | 3 m | 27 m |
| Pd(10)/SiO2 [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 22 | 2 m | 10 m | 21 m |
| Pd(1)/CeO2 [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 39 | 11 m | 14 m | 21 m |
| Pd(1)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 55 | 35 m | 14 m | 24 m |
| Pd(5)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 80 | 60 m | 10 m | 25 m |
| Cu(25)/ZnO [92] | CP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 99 | 20 c | - | - |
| Cu(25)/ZrO2 [92] | IP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 75 | 13 c | - | - |
| Cu(25)/SiO2 [92] | IP | - | - | 0–500 | 1 | - | - | - | - | 220 | 0.1 | 5 | 0.1 c | - | - |
| Cu/ZrO2 [121] | DP | 33 | - | 25–300 | 1 | - | - | - | 30,000 | 310 | 0.1 | 40 | 68 m | 2 m | 98 m |
| Cu/ZrO2 [121] | DP | 33 | - | 25–300 | 1 | - | - | - | 30,000 | 350 | 0.1 | 50 | 70 m | 10 m | 90 m |
| Ni/ZrO2 [121] | DP | 34 | - | 25–300 | 1 | - | - | - | 30,000 | 310 | 0.1 | 30 | 60 m | 19 m | 80 m |
| Ni/ZrO2 [121] | DP | 34 | - | 25–300 | 1 | - | - | - | 30,000 | 350 | 0.1 | 100 | 62 m | 80 m | 15 m |
| Cu–Ni/ZrO2 [121] | DP | 35 | - | 25–300 | 1 | - | - | - | 30,000 | 310 | 0.1 | 90 | 72 m | 87 m | 13 m |
| Cu–Ni/ZrO2 [121] | DP | 35 | - | 25–300 | 1 | - | - | - | 30,000 | 350 | 0.1 | 99 | 63 m | 80 m | 20 m |
| Cu(10)–Ni(30)/ ZrO2·Al2O3 [4] | CIP | 120 | - | 300 | 1 | - | - | - | 26,700 | 160 | 0.2 | 22 | 3.0 y | 0 s | 100 s |
| Cu(10)–Ni(30)/ ZrO2·Al2O3 [4] | CIP | 120 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 85 | 2.2 y | 48 s | 52 s |
| Cu(20)–Ni(20)/ ZrO2·Al2O3 [4] | CIP | 142 | - | 300 | 1 | - | - | - | 26,700 | 160 | 0.2 | 35 | 3.0 y | 0 s | 100 s |
| Cu(20)–Ni(20)/ ZrO2·Al2O3 [4] | CIP | 142 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 86 | 2.0 y | 48 s | 52 s |
| Cu(30)–Ni(10)/ ZrO2·Al2O3 [4] | CIP | 119 | - | 300 | 1 | - | - | - | 26,700 | 160 | 0.2 | 79 | 3.0 y | 0 s | 100 s |
| Cu(30)–Ni(10)/ ZrO2·Al2O3 [4] | CIP | 119 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 91 | 2.3 y | 39 s | 61 s |
| Cu(30)–Ni(10)/ CeO2·Al2O3 [4] | CIP | 120 | - | 300 | 1 | - | - | - | 26,700 | 160 | 0.2 | 26 | 3.0 y | 0 s | 100 s |
| Cu(30)–Ni(10)/ CeO2·Al2O3 [4] | CIP | 120 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 96 | 2.1 y | 30 s | 70 s |
| Cu(30)–Ni(10)/ ZnO·Al2O3 [4] | CIP | 150 | - | 300 | 1 | - | - | - | 26,700 | 160 | 0.2 | 19 | 3.0 y | 0 s | 100 s |
| Cu(30)–Ni(10)/ ZnO·Al2O3 [4] | CIP | 150 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.2 | 87 | 2.3 y | 23 s | 77 s |
| Cu(20)/MWCNTs [112] | IP | 290 | 0.35 * | 300 | 1 | - | - | - | 26,700 | 200 | 0.1 | 11 | 33 s | 0 s | 62 s |
| Cu(20)/MWCNTs [112] | IP | 290 | 0.35 * | 300 | 1 | - | - | - | 26,700 | 300 | 0.1 | 75 | 63 s | 8.5 s | 28.5 s |
| Ni(20)/MWCNTs [17] | IP | 271 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.1 | 7.5 | 78.5 s | 0 s | 21.5 s |
| Ni(20)/MWCNTs[17] | IP | 271 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.1 | 99.7 | 67.6 s | 16.5 s | 15.9 s |
| Au(1)–Cu(20)/MWCNTs [111] | DP | 272 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.1 | 14 | 29.8 s | 0 s | 70.2 s |
| Au(1)–Cu(20)/MWCNTs [111] | DP | 272 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.1 | 83 | 73 s | 8.6 s | 18.4 s |
| Au(1)–Ni(20)/MWCNTs [17] | DP | 311 | - | 300 | 1 | - | - | - | 26,700 | 200 | 0.1 | 8 | 63.2 s | 0 s | 36.8 s |
| Au(1)–Ni(20)/MWCNTs [17] | DP | 311 | - | 300 | 1 | - | - | - | 26,700 | 300 | 0.1 | 99.8 | 70.4 s | 0 s | 29.6 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosińska, M.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process. Materials 2020, 13, 5601. https://doi.org/10.3390/ma13245601
Mosińska M, Szynkowska-Jóźwik MI, Mierczyński P. Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process. Materials. 2020; 13(24):5601. https://doi.org/10.3390/ma13245601
Chicago/Turabian StyleMosińska, Magdalena, Małgorzata I. Szynkowska-Jóźwik, and Paweł Mierczyński. 2020. "Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process" Materials 13, no. 24: 5601. https://doi.org/10.3390/ma13245601
APA StyleMosińska, M., Szynkowska-Jóźwik, M. I., & Mierczyński, P. (2020). Catalysts for Hydrogen Generation via Oxy–Steam Reforming of Methanol Process. Materials, 13(24), 5601. https://doi.org/10.3390/ma13245601





