Nano targeted Therapies Made of Lipids and Polymers have Promising Strategy for the Treatment of Lung Cancer
Abstract
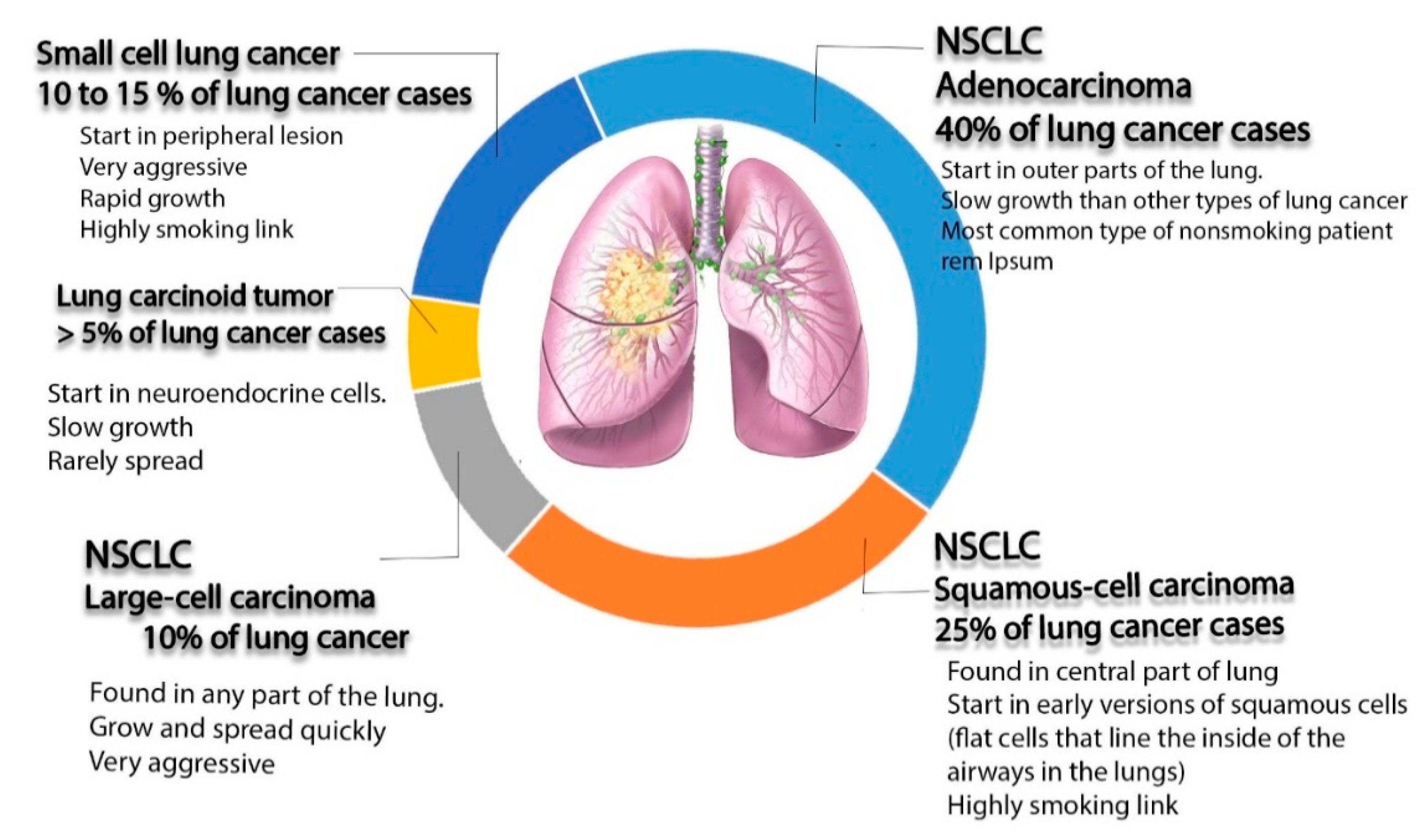
1. Introduction
2. Nanotechnology-Mediated Pulmonary Drug Delivery
2.1. Liposomes
| Drug | Composition | Preparation Technique | Target | Ref. |
|---|---|---|---|---|
| Paclitaxel (PTX) | A PTX liposome coated with hyaluronic acid (HA) that acts as an active drug targeting the mitochondria to overcome multidrug resistance. | Thin-film hydration | Adenocarcinomic human alveolar basal epithelial cells and taxol cells (Mitochondria) | [62] |
| PTX | The specific ligand peptide (T7) and the cationic cell-penetrating peptide TAT were connected with phospholipids via a polyethylene glycol (PEG) spacer to prepare dual-ligand liposomes | Thin-film hydration methods | Adenocarcinomic human alveolar basal epithelial cells (transferrin receptor) | [63] |
| Afatinib (AFT) | pH-sensitive liposomes for tyrosine kinase inhibitor AFT | Thin-film. Hydration followed by extrusion | Non-small cell lung cancer (NSCLC) | [64] |
| Docetaxel (DTX) | A15 cell surface modified DTX liposome | Thin-film hydration | Lung cancer stem cells (CD133) | [65] |
| DTX | Hyaluronic acid (HA)-coated PLGA nano-particulate DTX | Modified nano-precipitation method | Non-small cell lung cancer (NSCLC) (CD44) | [66] |
| DTX | DTX-loaded folic-acid-conjugated liposomes | Thin-film hydration and ultrasonic dispersion technology | Non-small cell lung cancer (NSCLC) (Folate receptor) | [67] |
| Erlotinib | Multifunctional liposomal complexes, where anti- epidermal growth factor receptor (EGFR) Apt-conjugated chitosan (Apt-Cs) was anchored into liposomes to co-administrate erlotinib and perfluorooctylbromide (PFOB) for the reversal of hypoxia-induced drug resistance | Thin-film hydration | Non-small cell lung cancer (NSCLC) (EGFR) | [68] |
| Doxorubicin (DOX) | DOX-loaded thermo-sensitive hybrid liposome formulation composed of dipalmitoyl phosphatidyl cholineas the phospholipid and Poloxamer 188 | Thin-film hydration flow by the remote-loading method | Lung cancer (PLA2) | [69] |
| Pixantrone (PIX) | Poly (sialic acid)–octa decylamine conjugate was synthesized and used to decorate the surface of pixantrone loaded liposomes | Remote loading method | Non-small cell lung cancer (NSCLC) | [70] |
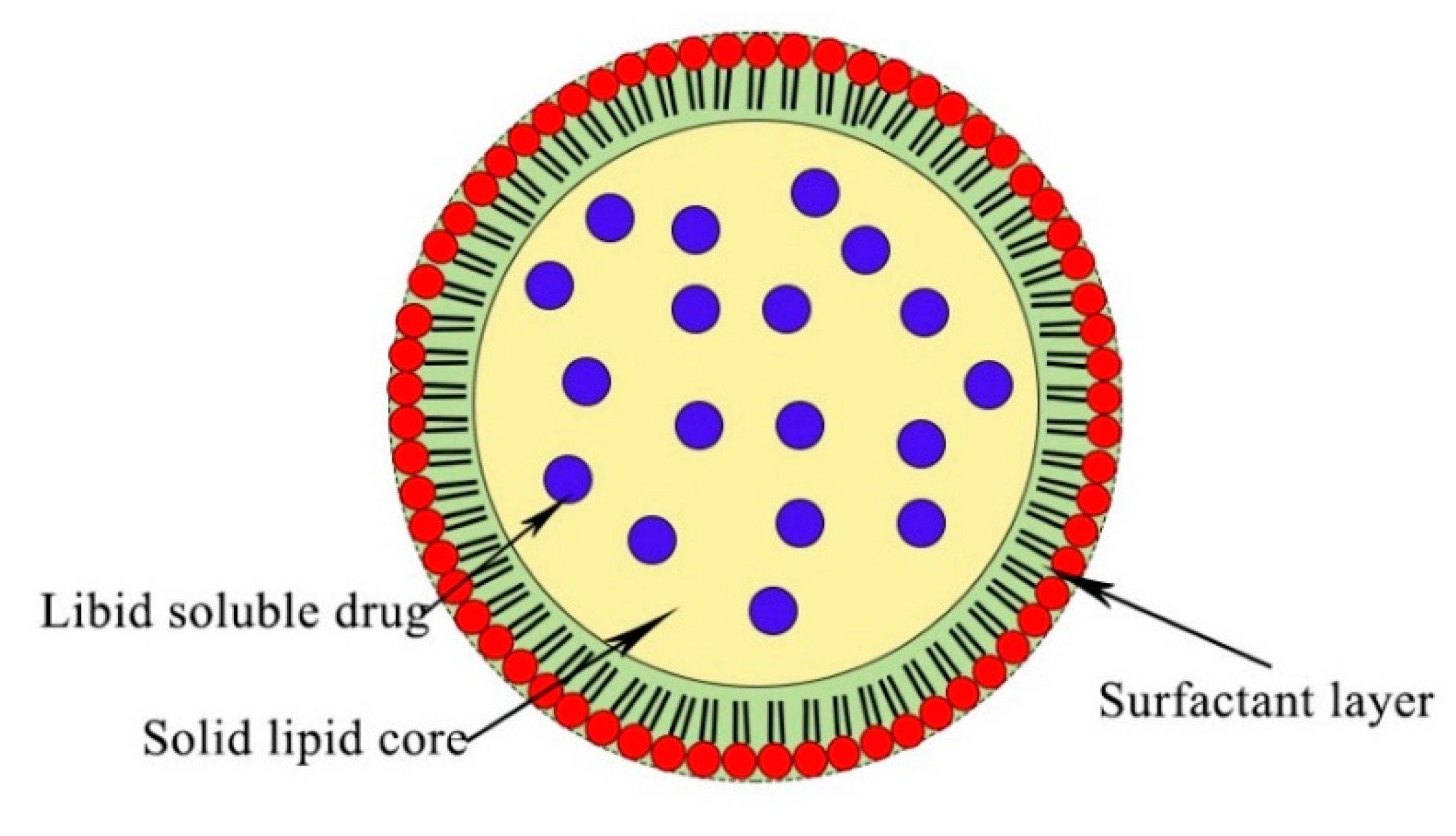
2.2. Solid Lipid Nanoparticle (SLNs)
| Drug | Composition | Preparation Techniques | Target | Ref. |
|---|---|---|---|---|
| Paclitaxel (PTX) and Erlotinib (ERL) | PTX- and ERL-co-loaded SLCN consisting of cores that accommodate both PTX and ERL and block copolymer coronae with PEGylated exterior. | Nanoprecipitation and sonication | Non-small cell lung carcinoma NSCLC (patients with activating-EGFR mutations) | [80] |
| Bberberine (BER) and Rapamycin (RAP) | Lactoferrin and HA were utilized to develop layer-by-layer lipid nanoparticles (NPs) for the dual delivery of BER and RAP to lung cancer. | Hot homogenization method | Adenocarcinomic human alveolar basal epithelial cells (HA targeted CD44 receptor) | [81] |
| Artemether (ART) | Oral anticancer drug ART-SLNs stabilized using MPEG2000- 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]-N-(Cyanine 5 (,DSPE) exhibited an anti-lipolytic effect | Pressure homogenization technique | Lung cancer | [82] |
| PTX | Inhaled drug delivery system. Paclitaxel-loaded SLN folate-grafted copolymer of PEG and N-((2-hydroxy-3-trimethylammonium)propyl) chitosan chloride coated liposomes. Surface modification was conducted to prolong the retention of PTX within the lungs | Carbodiimide-mediated coupling chemistry | Lung cancer (Folate receptors) | [83] |
| Gemcitabine (GmcH) | GmcH loaded mannosylated SLNs for improving drug uptake into the lung cancer cells | Emulsification and solvent | Lung cancer (Mannose receptor) | [84] |
| Erlotinib (ETB) | ETB-loaded SLN-based formulation of inhaled dry powder | Hot homogenization method followed by sonication | Non-small cell lung carcinoma (NSCLC) | [85] |
| PTX | PTX- and DNA-loaded NLC was prepared, and surface modification was done by transferring receptor -containing ligands. | Micro-emulsion technique. | Lung adenocarcinoma cell (Transferring receptor) | [86] |
| PTX and DOX | Combined delivery of PTX and DOX was prepared as a synergistic anti-tumor drug | Melted ultrasonic dispersion method | Non-small cell lung carcinoma (NSCLC) | [87] |
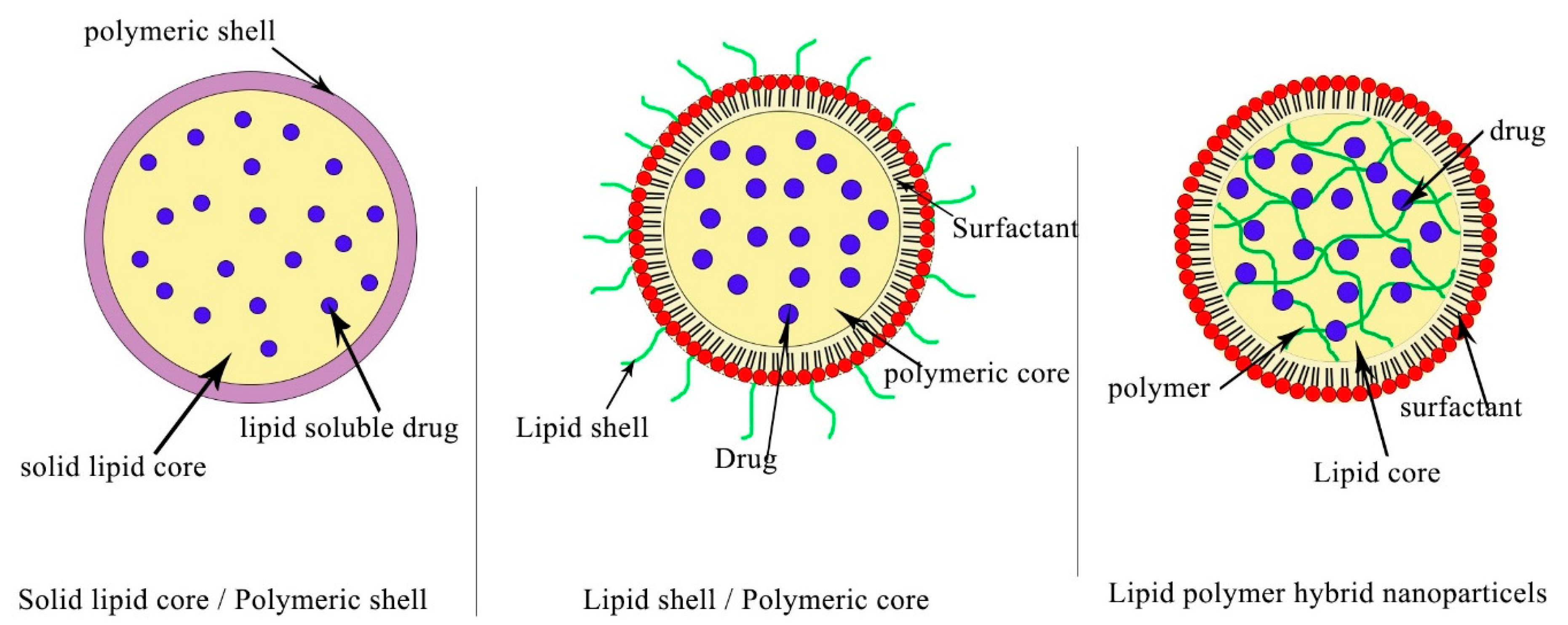
2.3. Polymeric Nanoparticles (PNPs)
| Drug | Composition | Preparation Technique | Target | Ref. |
|---|---|---|---|---|
| NU7441—radiosensitizer and Gemcitabine | A folate receptor targeting multifunctional, dual, drugs-loaded nanoparticles containing a poly(N-isopropylacrylamide)-carboxymethyl chitosan shell and a PLGA core to enhance localized chemo-radiotherapy | Standard emulsion method | Human dermal fibroblasts (HDFs) and Alveolar Type 1 epithelial cells | [96] |
| Erlotinib | Erlotinib-loaded core-shell-type lipid–polymer hybrid nanoparticles composed of polycaprolactone as the core and hydrogenated soy phosphatidylcholine/1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxyPEG2000) as the shell | Single-step sonication method | Non-small cell lung cancer (NSCLC). | [97] |
| Cisplatin (CDDP) and Metformin | Co-encapsulation of CDDP and metformin into single self-assembled core-membrane NPs. CDDP was first conjugated to PGA to form PGA-CDDP, which was electrostatically complexed with the cationic polymer metformin (polymet) and then coated with PEGylatedcationic liposomes to form the final core–membrane structure. | Non-small cell lung cancer (NSCLC). | [98] | |
| Gemcitabine (Gem) | Silk fibroin nanoparticles (SFNPs) used for the systemic delivery of gemcitabine (Gem) For targeting the tumorigenic lung tissue, SP5-52 peptide was conjugated to Gem-loaded SFNPs. | Electrospraying and desolvation method | Animal lung cancer | [99] |
| paclitaxel (PTX) | PTX-loaded polymeric nanoparticles (PTX-NPs) combined with circadian chronomodulated chemotherapy The polymer nanoparticles were prepared from two amphiphilic three-block copolymers: poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) | Thin film dispersion technique | Non-small cell lung cancer (NSCLC). | [100] |
3. Clinical Studies of Pulmonary Nanoparticles Can Be Finally Driven into the Industry Market
| Study Title | Conditions | Status | Assigned Number |
|---|---|---|---|
| Study of Irinotecan Liposome Injection (ONIVYDE®) in Patients With Small Cell Lung Cancer | Small Cell Lung Cancer | Recruiting | NCT03088813 |
| Irinotecan Hydrochloride Liposome Injection (LY01610) For Small Cell Lung Cancer | Small Cell Lung Cancer | Recruiting | NCT04381910 |
| Paclitaxel Liposome for Squamous Non-Small-cell Lung Cancer Study (LIPUSU) | Squamous Non-small-cell Lung Cancer | Active, not recruiting | NCT04381910 |
| Phase I Study of IV DOTAP: Cholesterol-Fus1 in Non-Small-Cell Lung Cancer | Lung Cancer | Completed | NCT00059605 |
| BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | Lung Cancer | Active, not recruiting | NCT00828009 |
| Efficacy and Safety Study of OSI-211 (Liposomal Lurtotecan) to Treat Recurrent Small Cell Lung Cancer | SCLC and Carcinoma, Small Cell | Completed | NCT00046787 |
| Study of Tecemotide (L-BLP25) in Participants With Stage III Unresectable Non-Small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy | Non-small Cell Lung Cancer | Completed | NCT00960115 |
| Liposomal Lurtotecan Plus Cisplatin in Treating Patients With Advanced or Metastatic Solid Tumors | Head and Neck Cancer, Lung Cancer, Ovarian Cancer | Completed | NCT00006036 |
| Study of Autologous CIK Cell Immunotherapy Combination With PD-1 Inhibitor and Chemotherapy in Advanced NSCLC | Non-small Cell Lung Cancer | Recruiting | NCT03987867 |
| A Study of FF-10850 Topotecan Liposome Injection in Advanced Solid Tumors | Advanced Solid Tumors | Recruiting | NCT04047251 |
| TUSC2-Nanoparticles and Erlotinib in Stage IV Lung Cancer | Lung Cancer | Active, not recruiting | NCT01455389 |
| Doxil Topotecan, Doublet Cancer Study | Small Cell Lung Cancer, Pancreatic Cancer, Head and Neck Cancer | Completed | NCT00252889 |
| TUSC2-nanoparticles (GPX-001) and Osimertinib in Patients With Stage IV Lung Cancer Who Progressed on Osimertinib Alone | Carcinoma, Non-Small-Cell Lung | Not yet recruiting | NCT04486833 |
| Intrathecal Pemetrexed for Recurrent Leptomeningeal Metastases From Non-Small Cell Lung Cancer | Leptomeningeal Metastases | Completed | NCT03101579 |
| Inhaled Doxorubicin in Treating Patients With Primary Lung Cancer or Lung Metastases | Lung Cancer, Metastatic Cancer | Completed | NCT00004930 |
| VX-710, Doxorubicin, and Vincristine for the Treatment of Patients With Recurrent Small Cell Lung Cancer | Lung Cancer | Terminated | NCT00003847 |
| Topotecan Hydrochloride and Doxorubicin Hydrochloride in Treating Patients With Relapsed or Refractory Small Cell Lung Cancer | Recurrent Small Cell Lung Carcinoma | Completed | NCT00856037 |
| Inhaled Doxorubicin in Treating Patients With Advanced Solid Tumors Affecting the Lungs | Lung Cancer, Malignant Mesothelioma, Metastatic Cancer | Completed | NCT00020124 |
| Effects of STM 434 Alone or in Combination With Liposomal Doxorubicin in Patients With Ovarian Cancer or Other Advanced Solid Tumors | Ovarian Cancer, Fallopian Tube Cancer, Endometrial Cancer, Solid Tumors | Completed | NCT02262455 |
| An Open-label, Phase I/IIa, Dose Escalating Study of 2B3-101 in Patients With Solid Tumors and Brain Metastases or Recurrent Malignant Glioma. | Brain Metastases, Lung Cancer Breast Cancer | Completed | NCT01386580 |
| Radiation Therapy Plus Combination Chemotherapy In Treating Patients With Limited Stage Small Cell Lung Cancer | Lung Cancer | Completed | NCT00003364 |
| A Phase II Study of Doxorubicin, Cyclophosphamide and Vindesine With Valproic Acid in Patients With Refractory or Relapsing Small Cell Lung Cancer After Platinum Derivatives and Etoposide | Small Cell Lung Carcinoma | Completed | NCT00759824 |
| Combination Chemotherapy Followed by Radiation Therapy in Patients With Small Cell Lung Cancer | Lung Cancer | Completed | NCT00002822 |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cokkinides, V.; Albano, J.; Samuels, A.; Ward, M.; Thum, J. American Cancer Society: Cancer Facts and Figures; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.; Goggins, V.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Pichler, M. Non-Smoking-Associated Lung Cancer: A distinct Entity in Terms of Tumor Biology, Patient Characteristics and Impact of Hereditary Cancer Predisposition. Cancers 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I. The morphological and molecular diagnosis of lung cancer. Dtsch. Arztebl. Int. 2011, 108, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.J. Lung cancer in never smokers—A review. Eur. J. Cancer 2012, 48, 1299–1311. [Google Scholar] [CrossRef]
- Noguchi, M.; Morikawa, A.; Kawasaki, M.; Matsuno, Y.; Yamada, T.; Hirohashi, S.; Kondo, H.; Shimosato, Y. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995, 75, 2844–2852. [Google Scholar] [CrossRef]
- Moulton, J.E. Tumors in Domestic Animals; University of California Press: Berkeley, CA, USA, 1978; pp. 203–205. [Google Scholar]
- Kenfield, S.A.; Wei, E.K.; Stampfer, M.J.; Rosner, B.A.; Colditz, G.A. Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control. 2008, 17, 198–204. [Google Scholar] [CrossRef]
- Brambilla, E.; Pugatch, B.; Geisinger, K.; Gal, A.; Sheppard, M.; Guinee, D. Large cell carcinoma, World Health Organization Classification of Tumours. Pathol. Genet. Tumours Lung Pleura Thymus Heart 2004, 10, 45–50. [Google Scholar]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Curran, W.J., Jr. Therapy of limited stage small cell lung cancer. Cancer Treat. Res. 2001, 105, 229–252. [Google Scholar]
- Mulshine, J.L.; Treston, A.M.; Brown, P.H.; Birrer, M.J.; Shaw, G.L. Initiators and promoters of lung cancer. Chest 1993, 103, 4S–11S. [Google Scholar] [CrossRef] [PubMed]
- Zöchbauer-Müller, S.; Pirker, R.; Huber, H. Treatment of small cell lung cancer patients. Ann. Oncol. 1999, 10, S83–S91. [Google Scholar] [CrossRef]
- Tamura, T. New state of the art in small-cell lung cancer. Oncology (Williston Park NY) 2001, 15, 8–10. [Google Scholar]
- Lad, T.; Piantadosi, S.; Thomas, P.; Payne, D.; Ruckdeschel, J.; Giaccone, G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994, 106, 320S–323S. [Google Scholar] [CrossRef]
- Lassen, U.; Hansen, H.H. Surgery in limited stage small cell lung cancer. Cancer Treat. Rev. 1999, 25, 67–72. [Google Scholar] [CrossRef]
- Fund, W.C.R. Research, Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Arlington, VA, USA, 2007. [Google Scholar]
- Hansen, H. Textbook of Lung Cancer; Informa Health Care: London, UK, 2008. [Google Scholar]
- Cho, L.C.; Dowell, J.E.; Garwood, D.; Spangler, A.; Choy, H. Prophylactic Cranial Irradiation with Combined Modality Therapy for Patients with Locally Advanced Non-Small Cell Lung Cancer. Semin. Oncol. 2005, 32, 293–298. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta-Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The Microenvironment of Lung Cancer and Therapeutic Implications. In Lung Cancer and Personalized Medicine: Novel Therapies and Clinical Management. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; pp. 75–110. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M. Mucoadhesive Hydrogel Nanoparticles as Smart Biomedical Drug Delivery System. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Hanafy, N.A.N. The growth of hepatocellular carcinoma can be inhibited by encapsulation of TGF ß1 antagonists. SL Pharm. Toxicol. 2018, 1, 112. [Google Scholar]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Understanding TGF β1 signalling pathway is well strategy to use its encapsulated antagonist as nano therapeutic molecules. Transl. Sci. 2018, 5, 1–2. [Google Scholar]
- Satalkar, P.; Elger, B.S.; Shaw, D.M. Defining nano, nanotechnology and nanomedicine: Why should it matter? Sci. Eng. Ethics 2016, 22, 1255–1276. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2015, 3, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Sivarajakumar, R.; Mallukaraj, D.; Kadavakollu, M.; Neelakandan, N.; Chandran, S.; Bhojaraj, S.; Reddy Karri, V.V.S. Nanoparticles for the Treatment of Lung Cancers. J. Young Pharm. 2018, 10, 276–281. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef]
- Greish, K. Enhanced Permeability and Retention (EPR) Effect for Anticancer Nanomedicine Drug Targeting, Cancer Nanotechnology. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar]
- Chari, R.V. Targeted delivery of chemotherapeutics: Tumor-activated prodrug therapy. Adv. Drug Deliv. Rev. 1998, 31, 89–104. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Vranic, B.Z.; Samy, W.M.; Elgindy, N.A. Swellable floating tablet based on spray-dried casein nanoparticles: Near-infrared spectral characterization and floating matrix evaluation. Int. J. Pharm. 2015, 491, 113–122. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [PubMed]
- Agu, R.U.; Ugwoke, M.I.; Armand, M.; Kinget, R.; Verbeke, N. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir. Res. 2001, 2, 198. [Google Scholar] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Mucoadhesive curcumin crosslinked carboxy methyl cellulose might increase inhibitory efficiency for liver cancer treatment. Mater. Sci. Eng. C 2020, 116, 111119. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano-and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015, 1, 2. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int. J. Nanomed. 2012, 7, 1551. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Merkel, O.M.; Kissel, T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J. Control. Release 2010, 161, 214–224. [Google Scholar] [CrossRef]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.M.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012, 7, 4391. [Google Scholar]
- Sarkar, S.; Osama, K.; Mohammad Sajid Jamal, Q.; Amjad Kamal, M.; Sayeed, U.; Khan, K.A.; Siddiqui, H.; Akhtar, S. Advances and implications in nanotechnology for lung cancer management. Curr. Drug Metab. 2017, 18, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [PubMed]
- Solaro, R.; Chiellini, F.; Battisti, A. Targeted delivery of protein drugs by nanocarriers. Materials 2010, 3, 1928–1980. [Google Scholar] [CrossRef]
- Gaber, M.; Medhat, W.; Hany, M.; Saher, N.; Fang, J.Y. Elzoghby, Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: Challenges and outcomes. J. Control. Release 2017, 254, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent developments in protein and peptide parenteral delivery approaches. Ther. Deliv. 2014, 5, 337–365. [Google Scholar] [CrossRef]
- Pisal, D.S.; Kosloski, M.P.; Balu-Iyer, S.V. Delivery of therapeutic proteins. J. Pharm. Sci. 2010, 99, 2557–2575. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine (London) 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Bardania, H.; Tarvirdipour, S.; Dorkoosh, F. Liposome-targeted delivery for highly potent drugs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1478–1489. [Google Scholar] [CrossRef]
- Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, A.; Destyorini, F.; Irmawati, Y.; Yang, M.C.; Liu, C.M.; Chaldun, E.R.; Yung, M.C.; Liu, T.Y. characterization of doxorubicin loaded PEGylated magnetic liposomes for cancer therapy. J. Polym. Res. 2019, 26, 282. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Kleinerman, E.S.; Waldrep, C.; Jia, S.F.; Worth, L.L.; Gilbert, B.E.; Knight, V. 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin. Cancer Res. 2000, 6, 2876–2880. [Google Scholar] [PubMed]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef]
- Miatmoko, A.; Kawano, K.; Yoda, H.; Yonemochi, E.; Hattori, Y. Tumor delivery of liposomal doxorubicin prepared with poly-L-glutamic acid as a drug-trapping agent. J. Liposome Res. 2017, 27, 99–107. [Google Scholar] [CrossRef]
- Ieranò, C.; Portella, L.; Lusa, S.; Salzano, G.; D’Alterio, C.; Napolitano, M.; Buoncervello, M.; Macchia, D.; Spada, M.; Barbieri, A. CXCR4-antagonist Peptide R-liposomes for combined therapy against lung metastasis. Nanoscale 2016, 8, 7562–7571. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Qin, Y.; Li, D.; Liu, Y.; Wang, H.; Gan, L. Overcoming drug-resistant lung cancer by paclitaxel-loaded hyaluronic acid-coated liposomes targeted to mitochondria. Drug Dev. Ind. Pharm. 2018, 44, 2071–2082. [Google Scholar] [CrossRef]
- Wang, R.H.; Cao, H.M.; Tian, Z.J.; Jin, B.; Wang, Q.; Ma, H.; Wu, J. Efficacy of dual-functional liposomes containing paclitaxel for treatment of lung cancer. Oncol. Rep. 2015, 33, 783–791. [Google Scholar] [CrossRef]
- Almurshedi, A.S.; Radwan, M.; Omar, S.; Alaiya, A.A.; Badran, M.M.; Elsaghire, H.; Saleem, L.Y.; Hutcheon, G.A. A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: Impact on lung cancer therapy. J. Mol. Liq. 2018, 259, 154–166. [Google Scholar] [CrossRef]
- Ma, J.; Zhuang, H.; Zhuang, Z.; Lu, Y.; Xia, R.; Gan, L.; Wu, Y. Development of docetaxel liposome surface modified with CD133 aptamers for lung cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1864–1871. [Google Scholar] [CrossRef]
- Wu, J.; Deng, C.; Meng, F.; Zhang, J.; Sun, H.; Zhong, Z. Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J. Control. Release 2017, 259, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharm. 2019, 55, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in nanotechnology-based delivery systems for EGFR tyrosine kinases inhibitors in cancer therapy. Asian J. Pharm. Sci. 2020, 15, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Kubota, M.; Ozeki, T. Effective Remote Loading of Doxorubicin into DPPC/Poloxamer 188 Hybrid Liposome to Retain Thermosensitive Property and the Assessment of Carrier-Based Acute Cytotoxicity for Pulmonary Administration. J. Pharm. Sci. 2015, 104, 3824–3832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, S.; Hu, L.; Peng, B.; Liu, Y.; Luo, X.; Song, Y.; Liu, X.; Deng, Y. Polysialic acid-modifying liposomes for efficient delivery of epirubicin, in-vitro characterization and in-vivo evaluation. Int. J. Pharm. 2016, 515, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery–liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595. [Google Scholar]
- Pignon, J.P.; Arriagada, R.; Ihde, D.C.; Johnson, D.H.; Perry, M.C.; Souhami, R.L.; Brodin, O.; Joss, R.A.; Kies, M.S.; Lebeau, B. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N. Engl. J. Med. 1992, 327, 1618–1624. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Subedi, R.K.; Kang, H.K.; Choi, H.K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur. J. Pharm. Sci. 2009, 37, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Videira, M.; Almeida, A.J.; Fabra, A. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Levet, V.; Rosière, R.; Merlos, R.; Fusaro, L.; Berger, G.; Amighi, K.; Wauthoz, N. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 2016, 515, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Poudel, B.K.; Regmi, S.; Pathak, S.; Ruttala, H.B.; Gautam, M.; An, G.J.; Jeong, J.H.; Choi, H.G.; Yong, C.S. Paclitaxel and Erlotinib-co-loaded Solid Lipid Core Nanocapsules: Assessment of Physicochemical Characteristics and Cytotoxicity in Non-small Cell Lung Cancer. Pharm. Res. 2018, 35, 96. [Google Scholar] [CrossRef]
- Kabary, D.M.; Helmy, M.W.; Elkhodairy, K.A.; Fang, J.Y.; Elzoghby, A.O. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf. B Biointerfaces 2018, 169, 183–194. [Google Scholar] [CrossRef]
- Khatri, H.; Chokshi, N.; Rawal, S.; Patel, M.M. Fabrication, characterization and optimization of artemether loaded PEGylated solid lipid nanoparticles for the treatment of lung cancer. Mater. Res. Express. 2019, 6, 045014. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New Folate-Grafted Chitosan Derivative to Improve Delivery of Paclitaxel-Loaded Solid Lipid Nanoparticles for Lung Tumor Therapy by Inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Soni, N.; Pandey, H.; Maheshwari, R.; Kesharwani, P.; Tekade, R.K. Augmented delivery of gemcitabine in lung cancer cells exploring mannose anchored solid lipid nanoparticles. J. Colloid Interface Sci. 2016, 481, 107–116. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef]
- Shao, Z.; Shao, J.; Tan, B.; Guan, S.; Liu, Z.; Zhao, Z.; He, F.; Zhao, J. Targeted lung cancer therapy: Preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int. J. Nanomed. 2015, 10, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Allouche, J. Synthesis of Organic and Bioorganic Nanoparticles: An Overview of the Preparation Methods, Nanomaterials: A Danger or a Promise? Springer: London, UK, 2013; pp. 27–74. [Google Scholar]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, G.; Evangelatov, A.; Skrobanska, R. Epirubicin loaded to pre-polymerized poly(butyl cyanoacrylate) nanoparticles: Preparation and in vitro evaluation in human lung adenocarcinoma cells. Colloids Surf. B Biointerfaces 2013, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.; Malfettone, A.; Soukupova, J. Hybrid polymeric-protein nano-carriers (HPPNC) for targeted delivery of TGFβ inhibitors to hepatocellular carcinoma cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Hitzman, C.J.; Elmquist, W.F.; Wattenberg, L.W.; Wiedmann, T.S. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J. Pharm. Sci. 2006, 95, 1114–1126. [Google Scholar] [CrossRef]
- Ray, L. Polymeric Nanoparticle-Based Drug/Gene Delivery for Lung Cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Academic Press: Cambridge, MA, USA, 2019; pp. 77–93. [Google Scholar]
- Jiang, Z.M.; Dai, S.P.; Xu, Y.Q.; Li, T.; Xie, J.; Li, C.; Zhang, Z.H. Crizotinib-loaded polymeric nanoparticles in lung cancer chemotherapy. Med. Oncol. 2015, 32, 193. [Google Scholar] [CrossRef]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core-shell type lipid-polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, Y.; Miao, L.; Lin, C.M.; Huang, L. Co-delivery of polymeric metformin and cisplatin by self-assembled core-membrane nanoparticles to treat non-small cell lung cancer. J. Control. Release 2016, 244 Pt A, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Kiani, M.; Farokhi, M.; Kundu, S.C.; Reis, R.L.; Gholami, M.; Bardania, H.; Dinarvand, R.; Geramifar, P.; Beiki, D.; et al. Targeted Delivery System Based on Gemcitabine-Loaded Silk Fibroin Nanoparticles for Lung Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31600–31611. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fu, S.; Peng, Q.; Han, Y.; Xie, J.; Zan, N.; Chen, Y.; Fan, J. Paclitaxel-loaded polymeric nanoparticles combined with chonomodulated chemotherapy on lung cancer: In vitro and in vivo. Int. J. Pharm. 2017, 516, 313–322. [Google Scholar]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 13, 264. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Arshinova, O.Y.; Sanarova, E.V.; Lantsova, A.V.; Oborotova, N.A. Lyophilization of liposomal drug forms (Review). Pharm. Chem. J. 2012, 46, 228–233. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Sarika, S.G.; Bhatt, L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020, 37, 557–565. [Google Scholar]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Duong, V.A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Fabregat, I.; Leporatti, S.; Kemary, M.E. Encapsulating TGF-β1 Inhibitory Peptides P17 and P144 as a Promising Strategy to Facilitate Their Dissolution and to Improve Their Functionalization. Pharmaceutics 2020, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inhibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterial 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Fatma, S.E.; Maggy, S.; Ahmed, M.; Maged, E.; Magdy, M.; Ola, E.; Sara, B.; Nemany, A.H. Drug Delivery Systems from Bench to Clinical Trials. Glob. J. Nano 2018, 4, 555636. [Google Scholar]
- Data Retrieved from US National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/help/glossary/recruitment-status (accessed on 21 August 2011).
- Lu, C.; Stewart, D.J.; Lee, J.J.; Ji, L.; Ramesh, R.; Jayachandran, G.; Nunez, M.I.; Wistuba, I.I.; Erasmus, J.J.; Hicks, M.E.; et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 2012, 7, e34833. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Borner, M.; Chollet, P.; Vermorken, J.B.; Bloch, J.; Degardin, M.; Rolland, F.; Dittrich, C.; Baron, B.; Lacombe, D.; et al. EORTC-New Drug Development Group/New Drug Development Program. Phase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC New Drug Development Group study. Eur. J. Cancer 2004, 40, 2748–2752. [Google Scholar]
- Mitchell, P.; Thatcher, N.; Socinski, M.A.; Wasilewska-Tesluk, E.; Horwood, K.; Szczesna, A.; Martín, C.; Ragulin, Y.; Zukin, M.; Helwig, C.; et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: Updated overall survival and biomarker analyses. Ann. Oncol. 2015, 26, 1134–1142. [Google Scholar] [CrossRef]
- MacKenzie, M.J.; Hirte, H.W.; Siu, L.L.; Gelmon, K.; Ptaszynski, M.; Fisher, B.; Eisenhauer, E. A phase I study of OSI-211 and cisplatin as intravenous infusions given on days 1, 2 and 3 every 3 weeks in patients with solid cancers. Ann. Oncol. 2004, 15, 665–670. [Google Scholar] [CrossRef]
- Data Retrieved from US National Institutes of Health Website. Available online: http://clinicaltrials.gov/ (accessed on 21 August 2013).
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Reduction diameter of CaCO3 crystals by using poly acrylic acid might improve cellular uptake of encapsulated curcumin in breast cancer. J. Nanomed. Res. 2018, 7, 235–239. [Google Scholar]
- Hanafy, N.A.N. Thesis Development and Production of Multifunctional Bio-nano-engineered Drug Delivery Systems Loaded by TGF Inhibitors for Delivering into Hepatocellular Carcinoma Cells; Salento University: Salento, Italy, 2017. [Google Scholar]
- Hanafy, N.A.; Ferraro, M.M.; Gaballo, A.; Dini, L.; Tasco, V.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Rinaldi, R.; Leporatti, S. Fabrication and characterization of ALK1fc-loaded fluoro-magnetic nanoparticles rods for inhibiting TGF β1 in HCC. RSC Adv. 2016, 6, 48834–48842. [Google Scholar] [CrossRef]
- El-banna, F.S.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, N.A. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef]
- Hanafy, N.A.; De Giorgi, M.L.; Nobile, C.; Rinaldi, R.; Leporatti, S. Control of Colloidal CaCO3 suspension by using biodegradable polymers during fabrication” Beni-Suef University. J. Basic Appl. Sci. 2015, 4, 60–70. [Google Scholar]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 19, 1937–1952. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essa, M.L.; El-Kemary, M.A.; Ebrahem Saied, E.M.; Leporatti, S.; Nemany Hanafy, N.A. Nano targeted Therapies Made of Lipids and Polymers have Promising Strategy for the Treatment of Lung Cancer. Materials 2020, 13, 5397. https://doi.org/10.3390/ma13235397
Essa ML, El-Kemary MA, Ebrahem Saied EM, Leporatti S, Nemany Hanafy NA. Nano targeted Therapies Made of Lipids and Polymers have Promising Strategy for the Treatment of Lung Cancer. Materials. 2020; 13(23):5397. https://doi.org/10.3390/ma13235397
Chicago/Turabian StyleEssa, Marwa Labib, Maged Abdeltawab El-Kemary, Eman Mohammed Ebrahem Saied, Stefano Leporatti, and Nemany Abdelhamid Nemany Hanafy. 2020. "Nano targeted Therapies Made of Lipids and Polymers have Promising Strategy for the Treatment of Lung Cancer" Materials 13, no. 23: 5397. https://doi.org/10.3390/ma13235397
APA StyleEssa, M. L., El-Kemary, M. A., Ebrahem Saied, E. M., Leporatti, S., & Nemany Hanafy, N. A. (2020). Nano targeted Therapies Made of Lipids and Polymers have Promising Strategy for the Treatment of Lung Cancer. Materials, 13(23), 5397. https://doi.org/10.3390/ma13235397







