1. Introduction
There are two main types of no-inclusions in Si-killed stainless steel are CaO-SiO
2-Al
2O
3-based and MnO-SiO
2-Al
2O
3-based systems [
1,
2,
3,
4,
5]. They have different properties and different effects on the surface quality of stainless steel strips. Researchers [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10] found that an effective way to improve the surface quality of stainless steel strip by controlling the nonmetallic inclusion as plasticity MnO-SiO
2-Al
2O
3-based inclusion instead of CaO-SiO
2-Al
2O
3-based ones. This could be realized by using different basicity refining slags and [Al]
s contents during the smelting process. The melting points of CaO-SiO
2-Al
2O
3 and MnO-SiO
2-Al
2O
3 inclusions, in particular the latter, are relatively low and the liquid phase is likely to present at the soaking process, which is before the rolling process. In addition, the high Cr content (18 wt%) containing in 304 stainless steel is more likely to react with the liquid inclusions or solid–liquid mixes in the steel during the soaking process. This reaction will change the morphology, size, composition, and even crystal structure of the inclusions. Correspondingly, the mechanical properties of inclusions and their effects on the final products will be perhaps changed as well. Therefore, it is important to investigate the evolution behaviors of two main types of noninclusion during the soaking process.
Many researchers have noted that the nonmetallic inclusion would change in shape, size, and/or composition during heat treatment. Takahashi et al. [
11] reported the inclusion in 18Cr-8Ni stainless steel would evolve from CaO-SiO
2-Al
2O
3 to MnO-Cr
2O
3 during heat treatment at 800 °C to 1200 °C. Takano et al. [
12] found that a large number of MnO-Cr
2O
3 inclusions precipitated during heat treatment and they could somehow pin austenite grain boundary in a 17Cr-9Ni stainless steel. Shibata et al. [
13,
14] discovered that inclusion transferred from MnO-SiO
2 to MnO-Cr
2O
3 during heat treatment and they also discussed the effects of Si, Mn, Ni and Cr contents on the evolutions of inclusions. Taniguchi et al. [
15] investigated the inclusion variation in martensitic stainless steel and proposed three steps for nonmetallic inclusion phase transformation during heat treatment. Ren et al. [
16] also reported that the MnO-SiO
2 type inclusion would transform to MnO-Cr
2O
3 spinel-type inclusion during heat treatment, and they proposed that the modification was as Cr reduced the SiO
2 in SiO
2-MnO-based inclusion.
Our previous work [
17] has investigated the plasticized MnO-Al
2O
3-SiO
2-based inclusion evolution during heat treatment and found that the chemical reaction between Cr and SiO
2 as well as Ostwald ripening plays a significant role in the inclusion behavior during heat treatment. Those results indicate that behaviors of nonmetallic inclusion during the soaking process are still not fully understood. For example, the differences between two main types of inclusions, i.e., CaO-SiO
2-Al
2O
3-based and MnO-SiO
2-Al
2O
3-based during the industrial soaking process (soaking temperature and time are 1250 °C and 120 min, respectively) and their evolution mechanisms are rarely reported. Furthermore, the properties of the two types of inclusions after the soaking process are not clarified.
In the present study, two 304 steel slabs, containing mainly CaO-SiO2-Al2O3-based and MnO-SiO2-Al2O3-based inclusions, respectively, were manufactured and isothermal heat treatment for the different time at 1250 °C to reveal the nonmetallic inclusion transformation process during the industrial soaking process. Furthermore, a thermodynamic analysis was performed and Ostwald ripening was introduced to explain the inclusion evolution mechanism.
3. Results
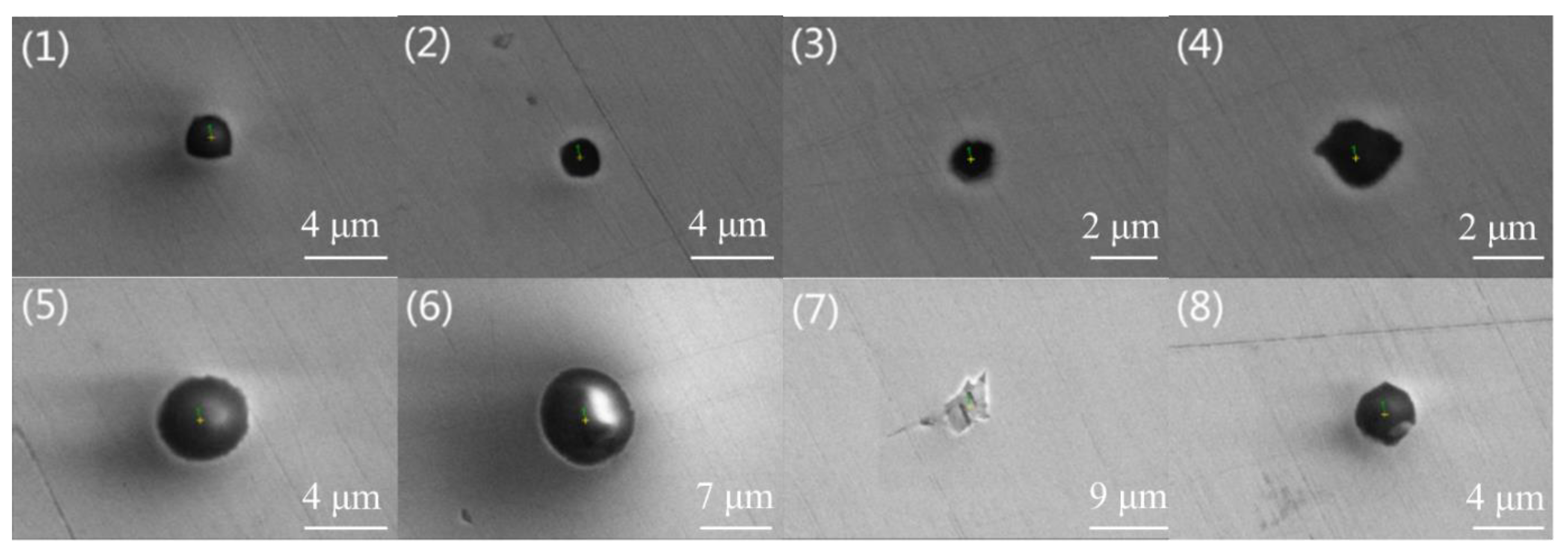
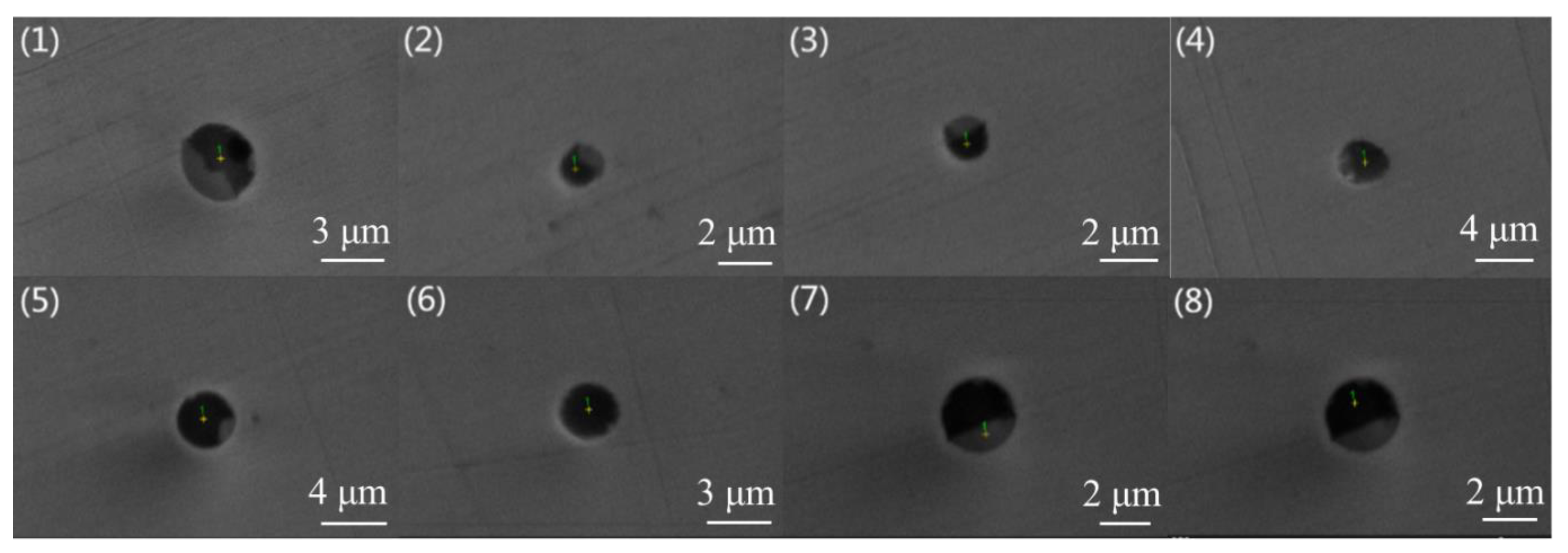
Figure 1 and
Figure 2 are typical inclusion morphologies in steel A and Steel B, respectively. In addition, their melting points calculated by the submodule
Equilib of FactSage 7.2 according to their compositions, are also listed in the
Table 3 and
Table 4, respectively. The morphologies of inclusions in the two steel samples are mainly spherical, and their sizes are mainly lower than 5 μm. However, the inclusion components and their corresponding melting points are significantly different. As shown in the
Table 3, the inclusions in steel A are mainly composed of CaO, SiO
2 and Al
2O
3, as well as small amounts of MnO, MgO and/or TiO
2. Calculated by FactSage 7.2, the melting points of those inclusions are between 1391 °C to 2050 °C, and the average melting point is 1561 °C, which is much higher than the open-rolling temperature region (i.e., 1150–1250 °C).
Compared with the inclusions in steel A, CaO contents of the inclusions in steel B are much less while the MnO contents are much more. Furthermore, the Al2O3 contents in the inclusions are much less as well. The melting points of the inclusions in steel B are between 1143 °C to 1479 °C, and their average is 1238 °C that is close to the open-rolling temperature region, indicating the inclusions are well plasticized since they are likely to soften at the rolling temperature. In a word, the inclusion in steel A is CaO-SiO2-Al2O3-based, and have a relative high melting point due to being smelted by the high basicity refining slag. The inclusion in steel B is plasticized MnO-SiO2-Al2O3-based, and have a low melting point due to the low basicity slag refining.
Figure 3a,b show the average inclusion composition evolution during the heat treatment process of steel A and steel B, respectively. For the steel A as shown in
Figure 3a, the average Cr
2O
3 and MnO contents gradually increase with increasing heat treatment time. In contrast, the CaO and SiO
2 contents decrease significantly. The contents of Al
2O
3 and MgO are somewhat fluctuant, and do not show obvious variation during heat treatment. As shown in
Figure 3b, the change of each component is similar to that of
Figure 3a. However, the average Cr
2O
3 content of inclusion in steel B increases much more than that in steel A, while the SiO
2 content decreases significantly along with the heat treatment time. In a word, during heat treatment, Cr
2O
3 and MnO of the inclusions in the two samples both increase while the SiO
2 and CaO contents decrease. However, there are also some differences between the inclusion composition evolution in the two steel: Cr
2O
3 in steel B increases much more than that in steel A; the SiO
2 content in steel B decreases more obviously than in steel A.
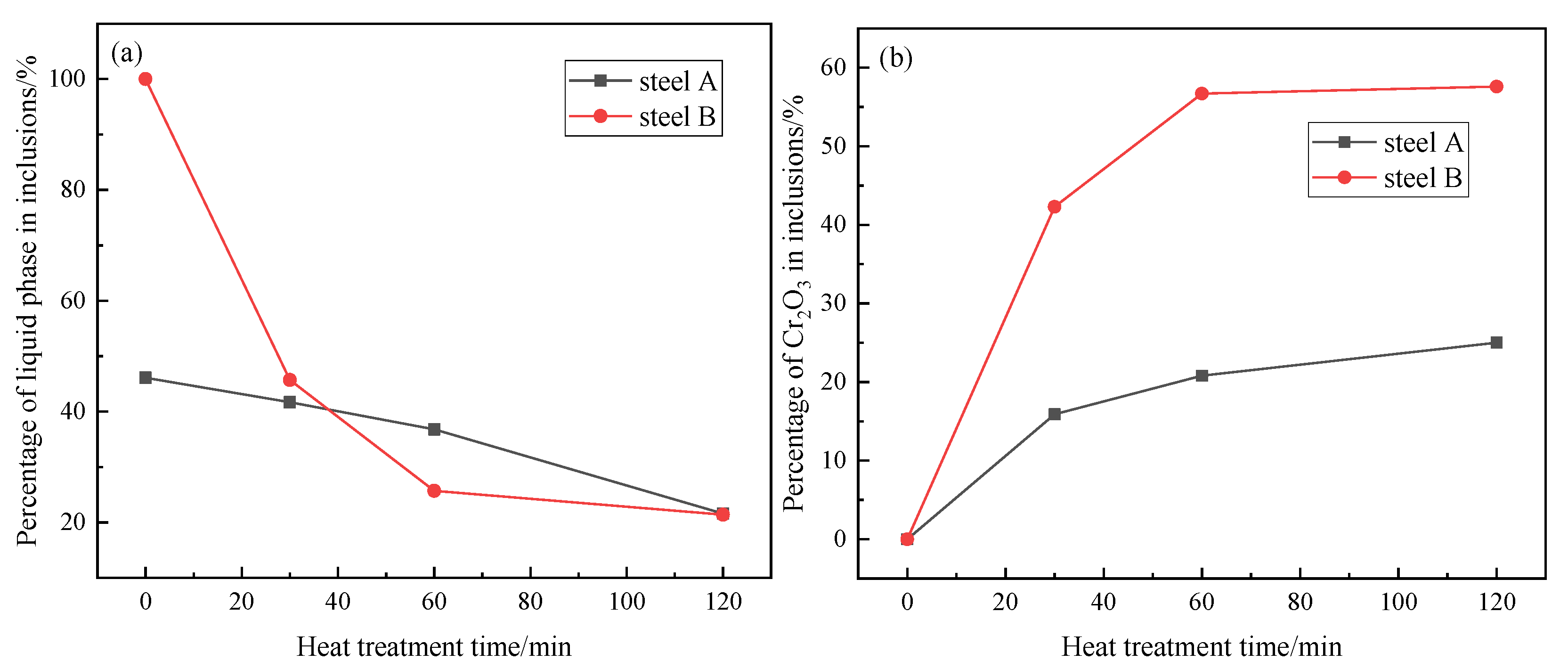
Figure 4 shows the variations of the liquid phase and Cr
2O
3 content in the inclusion of steel A and steel B with the heat treatment time at 1250 °C calculated by FactSage 7.2 based on the average composition of inclusion. With the Cr
2O
3 content increase during heat treatment, MnO-Cr
2O
3 spinel phase in the inclusion increase correspondingly. The MnO-Cr
2O
3 spinel phase in the inclusion of steel B increases much rapidly than that in steel A. In addition, the percentage of the liquid phase in steel A and steel B decreases with increasing heat treatment time, which is due to the change of inclusion composition. Associated with the aforementioned phenomena (
Figure 3 and
Figure 4), it is therefore indicated that MnO-Cr
2O
3 spinel continuously precipitates during heat treatment.
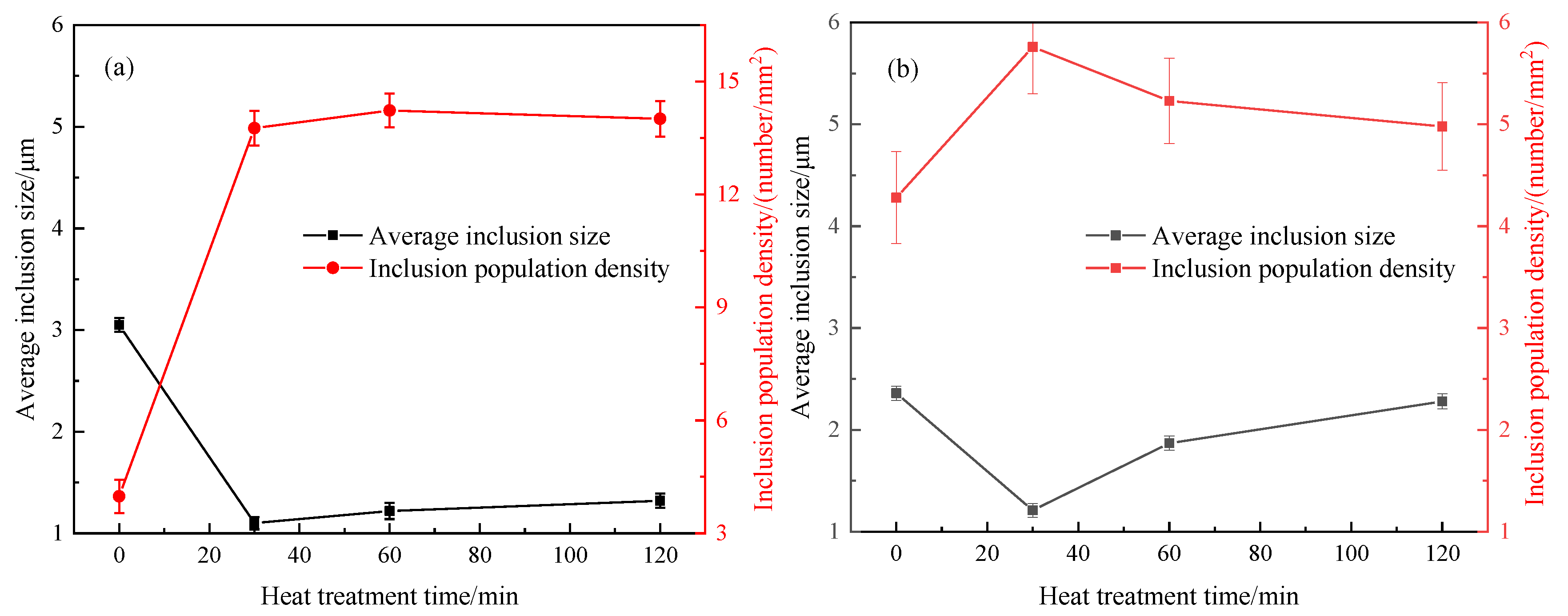
Figure 5a,b show the changes of inclusion population density and average inclusion size during heat treatment in steel A and steel B, respectively. Interestingly, the above indexes in steel A and steel B show a similar tendency. After heat treatment for 30 min, the inclusion population density increases as many small size Cr
2O
3-MnO-based inclusions precipitate during the heat treatment process, and the average inclusion size decreases correspondingly. With increasing heat treatment time, the inclusion population density decreases, and their average size gradually increases. This is because the inclusion growth follows the Ostwald ripening during heat treatment: the small inclusions dissolve and the larger inclusions grow. Ren et al. [
16] also observed a similar inclusion number evolution and size distribution, which is consistent with the findings in
Figure 5. Those phenomena in the
Figure 5 are more popular for inclusion transformation during heat treatment. Compared with steel A, steel B has had an even more volatile ride after 30 min due to the higher transformation speed caused by lower melting points.
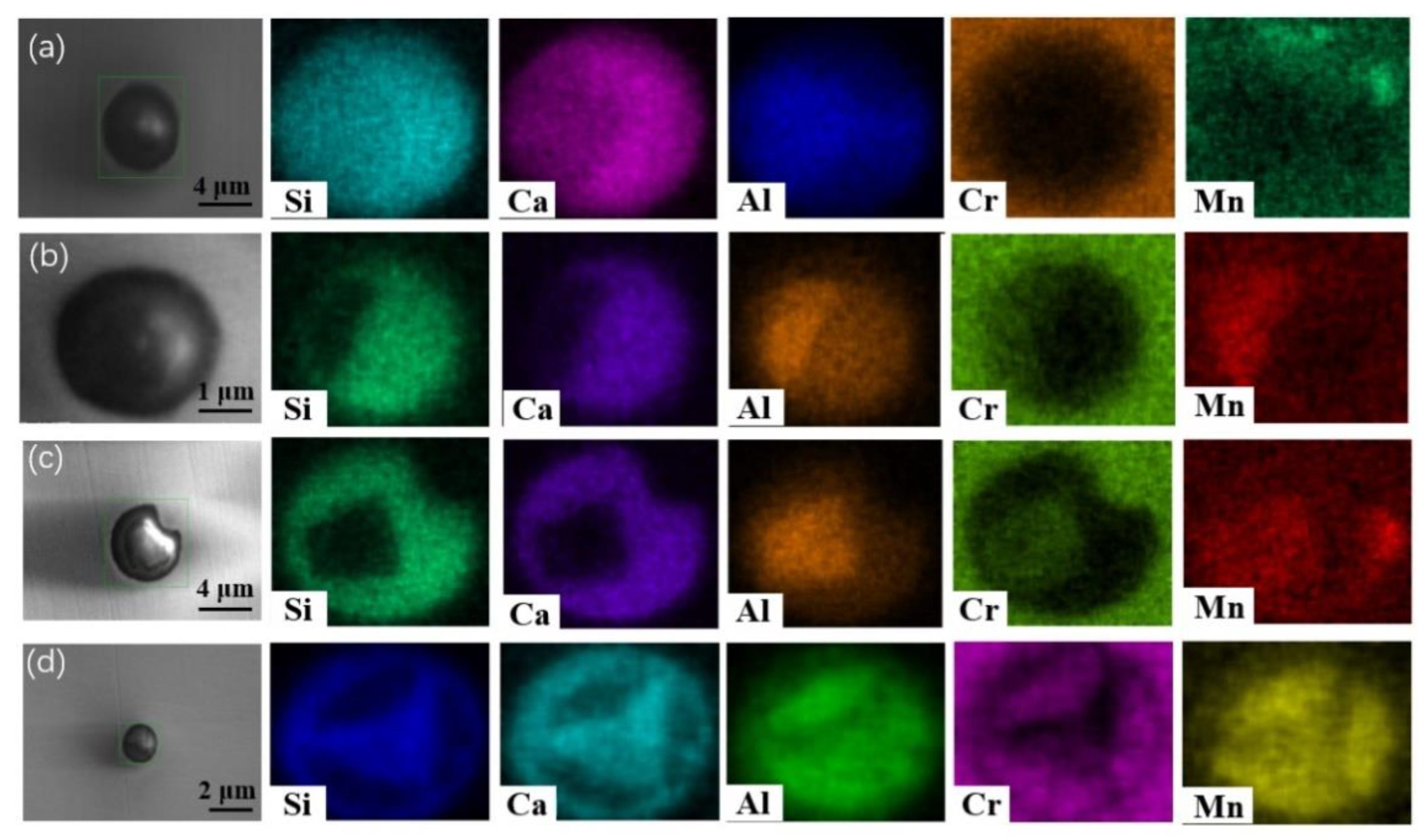
Figure 6 and
Figure 7 show SEM-mapping images of some typical inclusions in the steel samples at different stages of the heat treatment process. At the beginning of the heat treatment as shown in
Figure 6a and
Figure 7a, no Cr oxide-concentrated regions were found within all inclusions. The differences are that: (1) there is a triangular Al-concentrated region in the left part of inclusion of
Figure 6a while Al distributes homogeneously within the inclusion in
Figure 7a; and (2) very little Mn was detected in the inclusion in
Figure 6a, while much more Mn content was detected uniformly distributed in the inclusion in
Figure 7a. As shown in
Figure 6b and
Figure 7b, some quadrate Cr
2O
3-MnO-Al
2O
3-concentrated regions were observed in the outer layer within the inclusion, and the other parts are CaO-SiO
2 became enriched in the composition after heat treatment for 30 min. However, the inclusions in
Figure 6c and
Figure 7c are different: a faceted Cr
2O
3-MnO-Al
2O
3-concentrated core with a light color in the center of inclusion listed in
Figure 6c, while most of inclusions were Cr
2O
3-MnO-concentrated region in the outer layer and only a small CaO-SiO
2-concentrated region in the top left corner of inclusion in
Figure 7c. Interestingly, some Cr
2O
3-MnO-Al
2O
3-concentrated regions were inserted in the inclusion in steel A after heat treatment for 120 min as shown in
Figure 6d. In the contrast, a Cr
2O
3-MnO-Al
2O
3-concentrated core wrapped by a ring-like CaO-SiO
2-concentrated region was observed in the inclusion of steel B after heat treatment for 120 min as shown in
Figure 7d. In short, there are some interesting similar behaviors of the inclusions evolution during heat treatment, although the compositions at the beginning are quite different. The Cr
2O
3 contents in the inclusions are increasing with increasing heat treatment time, but the Cr element does not always diffusion from the outer layer to the inner part; Cr
2O
3-MnO or Cr
2O
3-MnO-Al
2O
3-enriched regions and the CaO-SiO
2-concentrated region seem incompatible and complementary among the inclusions in most inclusions in the two steels. The difference is that the Mn element concentration of inclusion in steel A is much less than that of inclusions in steel B, which agrees with the inclusion composition in
Figure 3.
4. Discussion
Thermodynamic calculated results (
Figure 4) and the experimental results show a large number of Cr
2O
3-MnO tetragonal spinel contained some amount of Al
2O
3 would precipitate during heat treatment process. They result in the change of inclusion composition as shown in
Figure 3 and inclusion size and density, as shown in
Figure 5.
According to the density functional theory calculations [
19,
20], the solute atoms can enter the oxide defect and produce a mixed oxide structure, which indicates that Cr atoms may have a chemical reaction with oxygen in inclusions. Some researchers [
14,
16] developed the idea that the formation of MnCr
2O
4 spinel during the heat treatment process is due to the reaction between Cr in the solid steel matrix and 2MnO·SiO
2-type inclusion, as shown in Equation (1).
In fact, the Cr in steel matrix has the possibilities to react with MnO, SiO2, Al2O3 and even CaO, thus the thermodynamic analysis among these chemical reactions should be performed to understand the inclusion evolution mechanism.
The reaction between [Cr] and (MnO) and its standard Gibbs energy
are expressed as Equations (2) and (3) [
21]. Equation (4) is the Gibbs free energy
of the actual reaction.
where
R is gas constant, 8.314 J/(mol·K);
T represents temperature (i.e., 1523 K);
and
are the element activity in steel matrix relative to 1% standard state;
and indicate the MnO and Cr
2O
3 activities in the inclusion. The Al
2O
3-SiO
2-MnO inclusions are in liquid phase, or most of them are in liquid at 1523 K; thus the activity of the MnO and Cr
2O
3 are calculated by applying of the thermodynamic software FactSage 7.2 [
18].
Similarly, the Gibbs free energies of the other reactions can be obtained according to their corresponding standard Gibbs free energy as shown in
Table 5.
Table 6 shows the average contents of the two typical inclusions and the corresponding activities calculated by FactSage 7.2.
The activity coefficients
and
are calculated by the Wagner formula in Equation (5), where
is the first-order activity interaction coefficient of elements
j to
i relative to the diluted solution. These values are from reference [
22]. It should be pointed out that the activity interaction coefficients applied in the present calculations are selected from those measured based on Fe-Cr-Ni stainless steel hot metal, or those are confirmed applicable for the 18 pct Cr-8 pct Ni stainless steel. In addition, the second interaction coefficients of Cr and Ni to the elements in steel are performed because of high Cr and Ni content in 18 pct Cr-8 pct Ni stainless steel [
1,
23,
24,
25].
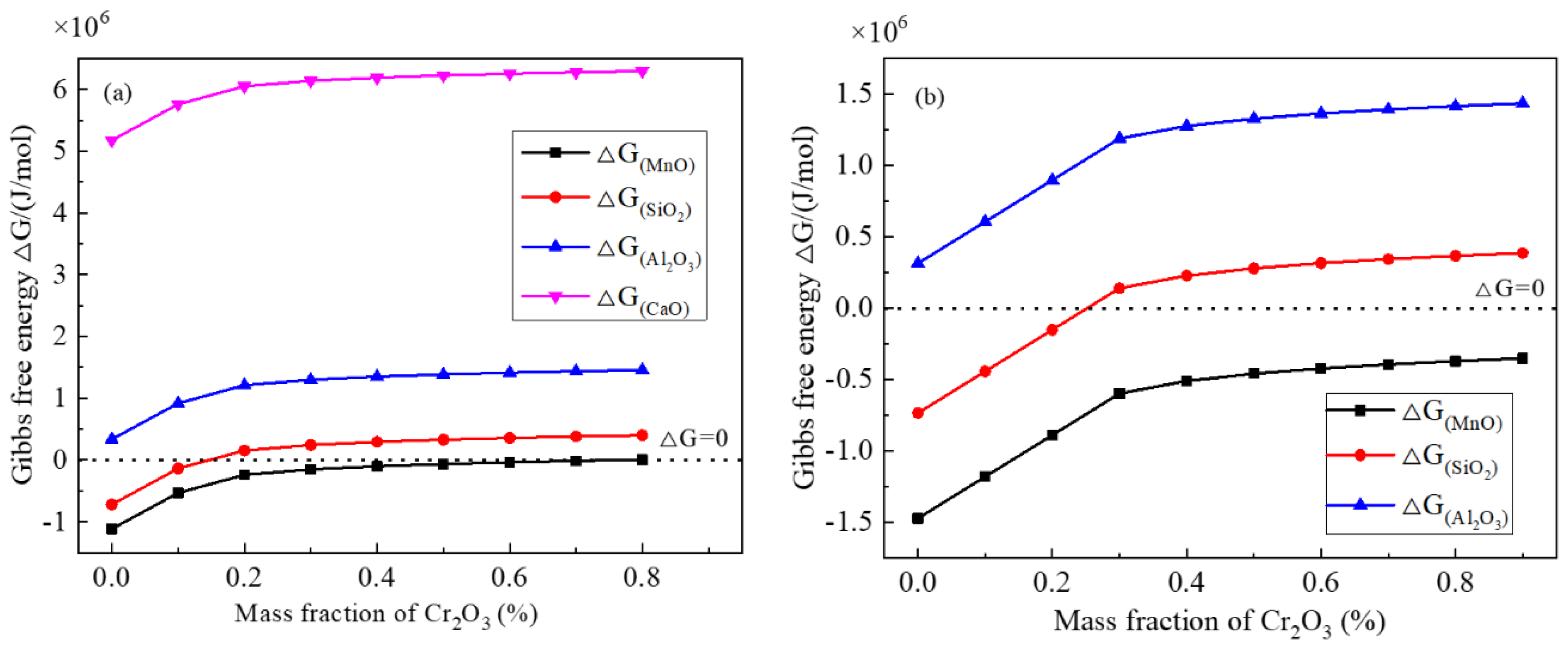
Figure 8a,b show the Gibbs free energy change of the foregoing typical chemical reactions with different Cr
2O
3 contents in inclusions at 1250 °C. In the two figures, with increasing Cr
2O
3 content, the Gibbs free energy increases, and finally are positive, indicating it is more and more difficult to take place for these chemical reactions. The Gibbs free energy for the reaction between Cr and MnO is lower than 0, even if a larger Cr
2O
3 content in an inclusion. Moreover, the Gibbs free energy between Cr and SiO
2 is larger than 0 in the case of the amount of Cr
2O
3 content larger than 20 mass%. For the reaction between Cr and Al
2O
3 or CaO, the change of free Gibbs energy is almost positive even if very low Cr
2O
3 content in an inclusion. Hence, Cr is most likely to reduce the MnO in the inclusion, followed by the SiO
2, but Al
2O
3 or CaO in the inclusion is not likely to be reduced by Cr during heat treatment process. Compared with steel A, inclusions in steel B are more likely to react with Cr, which accords with the changes of Cr
2O
3 in
Figure 3.
It is true that some phenomena can be explained by chemical reactions. However, MnO content increases continuously or is almost not reduced obviously, while SiO
2 and even CaO contents are reduced, as shown in
Figure 3. Obviously, this is not in agreement with the thermodynamic results as shown in
Figure 8. In addition, if MnO-Cr
2O
3 inclusion is formed due to the chemical reaction Equation (1), the inclusion size would be almost constant but inclusion diameter is changed a lot in present experimental results (
Figure 5) as well as in other researchers’ results [
14,
16]. Therefore, it can be deduced that there is another driving force to cause inclusion transformation during heat treatment including chemical reactions between Cr and inclusions.
Ostwald ripening is a very common phenomenon for the secondary phase growth during heat treatment. It is characterized by that the smaller inclusions dissolve, and the larger inclusions grow by absorbing the smaller inclusions during heat treatment. The present observations, such as inclusion size and their population density evolution as shown in
Figure 5 and some SEM-mapping images in
Figure 6 and
Figure 7, accord with the features of Ostwald ripening very well. Therefore, the evolutions of some inclusions during heat treatment mainly follow the rule of Ostwald ripening rather than the chemical reactions.
According the classical Ostwald ripening [
26], the interface energy generates with the secondary phase precipitating during heat treatment, and total interface energy would generate when the inclusion with a smaller radius. The driving force of Ostwald ripening is the difference of interface energy between steel matrix and smaller size inclusion and between matrix and larger size inclusion.
In summary, according to the experimental observation and thermodynamic analysis, there are three steps for inclusion evolution during the heat treatment process as schematically shown in
Figure 9: (1) MnO-Cr
2O
3 spinel particles precipitate and normal grow; (2) Chemical reactions between Cr and CaO/MnO-SiO
2-Al
2O
3 inclusion; (3) CaO/MnO-SiO
2-Al
2O
3 inclusions and MnO-Cr
2O
3 inclusions grow by Ostwald ripening, namely by absorbing smaller MnO-Cr
2O
3 particles.
Three steps always take place simultaneously during the heat treatment process, in particular the later two steps are even likely to occur on the same one inclusion. Thus, it is difficult to distinguish quantitively, for which the step is the dominant mechanism for inclusion evolution during the heat treatment process, which is needed further investigated. But as shown in
Figure 3, average MnO contents increase or keep almost unchanged which is, obviously, not the effects of the chemical reactions, thus it could conclude that the Ostwald ripening play a very important role on the inclusion modification during heat treatment process.
5. Conclusions
Transformations of two common types of nonmetallic inclusion in 304 stainless steel, smelted by high basicity refining slag and low basicity slag, respectively, were investigated in laboratory-scale furnace at 1250 °C. The following conclusions were obtained.
Firstly, the slag system with high basicity of LF results in the formation of CaO-SiO2-Al2O3 inclusions with a high melting point. Moreover, MnO-SiO2-Al2O3 inclusions are mainly formed under slag with low basicity, which are almost in the liquid phase at the heat treatment temperature before rolling.
Secondly, inclusion population density increases at the first stage and then decreases, and their average size firstly decreases and then increases due to a large number of Cr2O3-MnO particles precipitating and their growth during heat treatment process.
Thirdly, there is almost no Cr2O3 before the heat treatment, but Cr2O3 precipitates gradually increase along with the heat treatment process. The increasing rate of Cr2O3 content in MnO-SiO2-Al2O3 inclusion is much higher due to its low melting point. The increasing of Cr2O3 content in the inclusion would increase their melting points and lower their plasticities.
Finally, both experimental results and thermodynamic analysis show that there are three steps for inclusion evolution during the heat treatment process: (1) many small size MnO-Cr2O3 spinel particles precipitate and grow; (2) Chemical reactions between Cr and CaO/MnO-SiO2-Al2O3 inclusion; (3) by Ostwald ripening, namely CaO/MnO-SiO2-Al2O3 inclusions and MnO-Cr2O3 inclusions grow by absorbing smaller MnO-Cr2O3 particles. Ostwald ripening plays an important role on inclusion evolution during the soaking process.