Evaluation of the Flexural Strength, Water Sorption, and Solubility of a Glass Ionomer Dental Cement Modified Using Phytomedicine
Abstract
1. Introduction
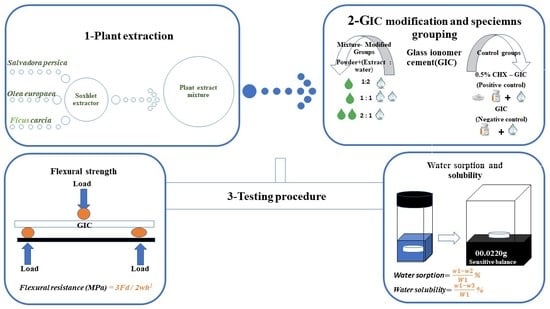
2. Materials and Methods
2.1. Preparation of the Plant Extract Mixture
2.2. Preparation of GIC, Extract and CHX Combinations and Specimen Grouping
Specimen Grouping
2.3. Water Sorption and Solubility
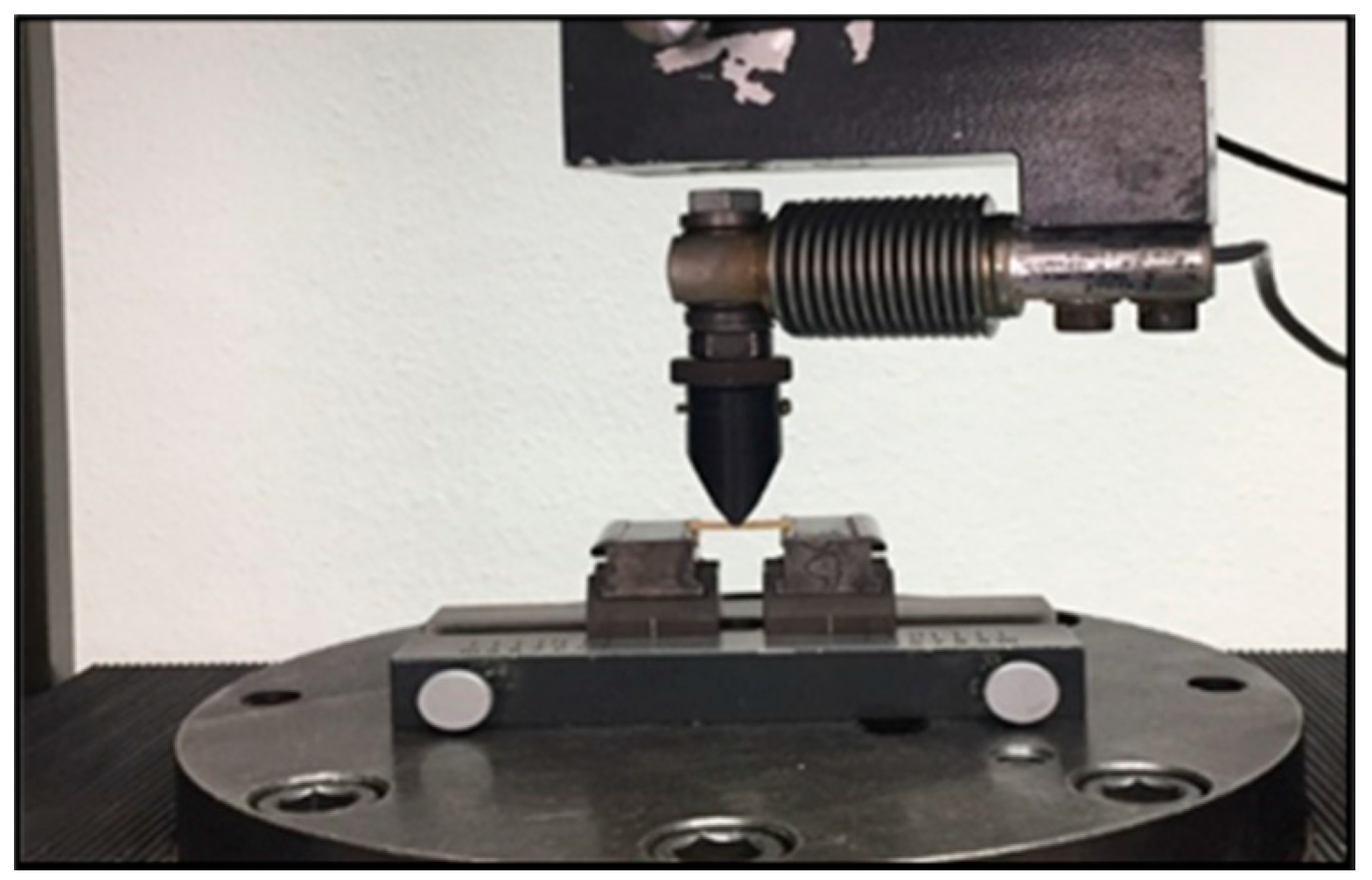
2.4. Flexural Strength
2.5. Statistical Analysis
3. Results
3.1. Water Sorption and Solubility
3.1.1. Water Sorption
3.1.2. Water Solubility
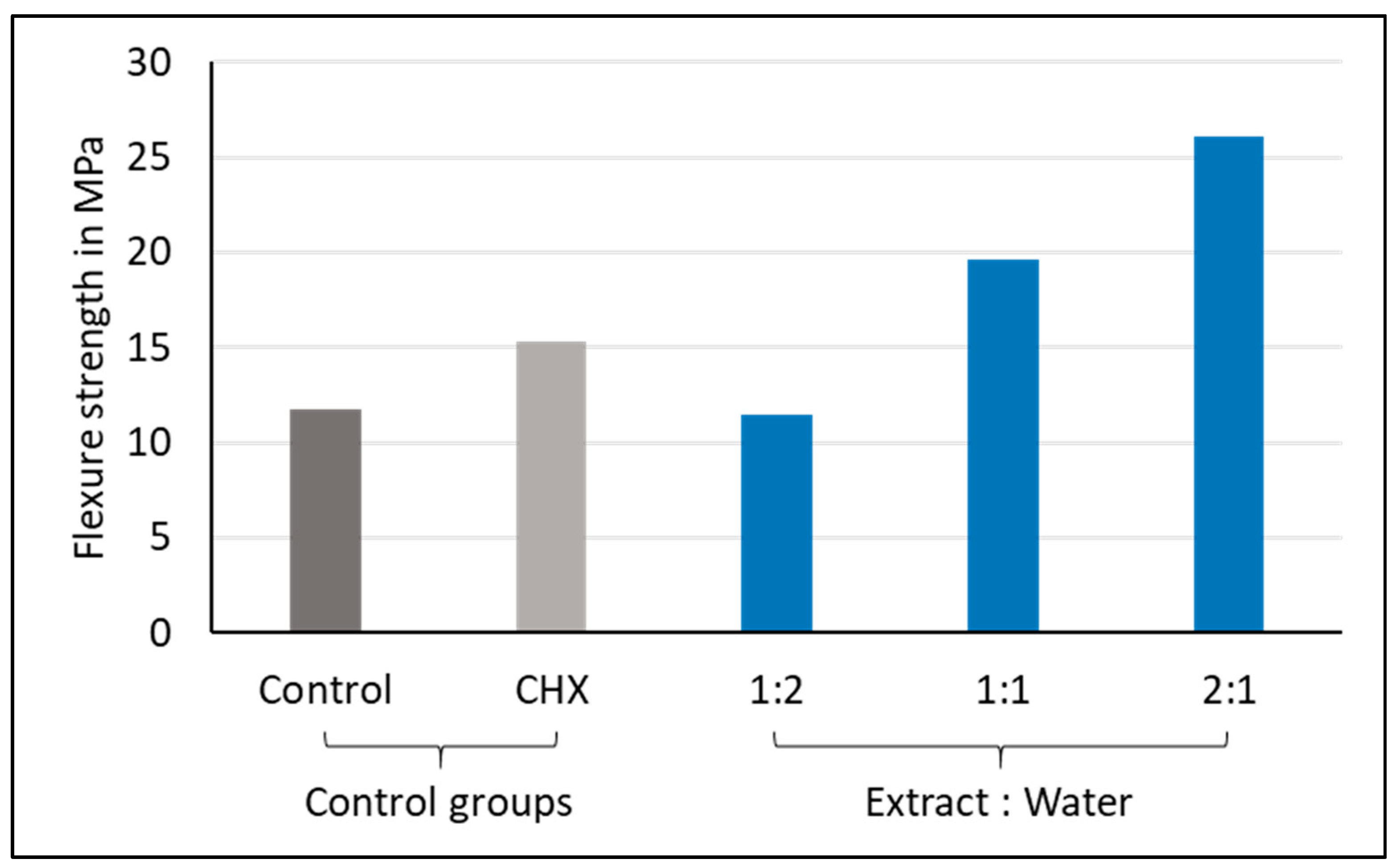
3.2. Flexural Strength
4. Discussion
4.1. Water Sorption and Solubility
4.2. Flexural Strength
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davidson, C.L.; Mjor, I.A. Advances in Glass Ionomner Cements; Quintessence Publishing Co., Inc.: Berlin, Germany; Chicago, IL, USA, 1999. [Google Scholar]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.W.; Nicholson, J.W.; Wilson, A.D. Proposed nomenclature for glass-ionomer dental cements and related materials. Quintessence Int. 1994, 25, 587–589. [Google Scholar]
- Davidson, C.L. Advances in glass-ionomer cements. J. Appl. Oral Sci. 2006, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Ciaramella, S.; Martorelli, M.; Lanzotti, A.; Gloria, A.; Watts, D.C. CAD-FE modeling and analysis of class II restorations incorporating resin-composite, glass ionomer and glass ceramic materials. Dent. Mater. 2017, 33, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef]
- Bonifácio, C.C.; Kleverlaan, C.; Raggio, D.P.; Werner, A.; De Carvalho, R.; Van Amerongen, W. Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust. Dent. J. 2009, 54, 233–237. [Google Scholar] [CrossRef]
- Toledano, M.B.; Osorio, R.; Osorio, E.; Fuentes, V.; Prati, C.; García-Godoy, F. Sorption and solubility of resin-based restorative dental materials. J. Dent. 2003, 31, 43–50. [Google Scholar] [CrossRef]
- Hajmiragha, H.; Nokar, S.; Alikhasi, M.; Nikzad, S.; Dorriz, H. Solubility of three luting cements in dynamic artificial saliva. J. Dent. 2008, 5, 95–98. [Google Scholar]
- McCabe, F.; Walls, A.W.G. (Eds.) Properties used to characterize materials. In Applied Dental Materials, 9th ed.; Blackwell: Oxford, UK, 2009. [Google Scholar]
- Mitra, S.B.; Kedrowski, B.L. Long-term mechanical properties of glass ionomers. Dent. Mater. 1994, 10, 78–82. [Google Scholar] [CrossRef]
- Glasspoole, E.A.; Erickson, R.; Davidson, C. A fluoride-releasing composite for dental applications. Dent. Mater. 2001, 17, 127–133. [Google Scholar] [CrossRef]
- Powers, J.; Sakaguchi, L. Craig’s Restorative Dental Materials; Elsevier Mosby: London, UK, 2006. [Google Scholar]
- Palmer, G.; Jones, F.; Billington, R.; Pearson, G. Chlorhexidine release from an experimental glass ionomer cement. Biomaterials 2004, 25, 5423–5431. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Simionato, M.R.L.; Imparato, J.C.P.; Oda, M. Antibacterial activity of glass-ionomer cement containing antibiotics on caries lesion microorganisms. Am. J. Dent. 2005, 18, 261–266. [Google Scholar] [PubMed]
- Santos, B.P.D.L.; Kanis, L.A.; Pereira, J.R. Herbal medicines in dentistry: History, obtainment methods, and properties of Copaifera multijuga hayne and Baccharis dracunculifolia dc. J. Res. Dent. 2016, 3, 859. [Google Scholar] [CrossRef][Green Version]
- Shafi, T.; Ashok, A.; Suresh, K. A study on the antibacterial effects of selected Western ghats against dental caries bacteria. Int. J. Phytomed. 2011, 3, 416–421. [Google Scholar]
- Okafor, A.C.; Igwesi, S.N.; David, E.E.; Okolo, V.K.; Agu, K. Presence of Bacteria with Pathogenic Potential Among Already-Used Toothbrushes from University Students. Am. J. Life Sci. Res. 2016, 4, 78–82. [Google Scholar] [CrossRef][Green Version]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Rugini, E.; Baldoni, L.; Muleo, R.; Sebastiani, L. The Olive Tree Genome; Springer: Cham, Switzerland, 2016; pp. 37–54. ISBN 978-972-579-041-0. [Google Scholar]
- Vogel, P.; Machado, I.K.; Garavaglia, J.; Zani, V.T.; De Souza, D.; Bosco, S.M.D. Polyphenols benefits of olive leaf (Olea europaea L) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar]
- Almas, K. The antimicrobial effects of seven different types of Asian chewing sticks. Trop. Dent. J. 2001, 24, 17–20. [Google Scholar]
- Niazi, F.; Naseem, M.; Khurshid, Z.; Zafar, M.S.; Almas, K. Role of Salvadora persica chewing stick (miswak): A natural toothbrush for holistic oral health. Eur. J. Dent. 2016, 10, 301–308. [Google Scholar] [CrossRef]
- Mawa, S.; Husain, K.; Jantan, I. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evid.-Based Complement. Altern. Med. 2013, 2013, 974256. [Google Scholar] [CrossRef] [PubMed]
- Tchombe, N.; Louajri, A. Therapeutic effects of Ficus carica leaves: A brief review. ARPN J. Sci. Technol. 2015, 5, 37–41. [Google Scholar]
- Türkün, L.S.; Türkün, M.; Ertugrul, F.; Ates, M.; Brugger, S. Long-Term Antibacterial Effects and Physical Properties of a Chlorhexidine-Containing Glass Ionomer Cement. J. Esthet. Restor. Dent. 2008, 20, 29–44. [Google Scholar] [CrossRef] [PubMed]
- El-Tatari, A.; De Soet, J.J.; De Gee, A.J.; Shelib, M.A.; Van Amerongen, W.E. Influence of salvadora persica (miswak) extract on physical and antimicrobial properties of glass ionomer cement. Eur. Arch. Paediatr. Dent. 2011, 12, 22–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonulol, N.; Ozer, S.; Tunc, E.S. Water Sorption, Solubility, and Color Stability of Giomer Restoratives. J. Esthet. Restor. Dent. 2014, 27, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Garoushi, S.K.; He, J.; Vallittu, P.K.; Lassila, L.V.J. Effect of discontinuous glass fibers on mechanical properties of glass ionomer cement. Acta Biomater. Odontol. Scand. 2018, 4, 72–80. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 9917-2:2017–Dentistry—Water Based Cements. Part 2: Resin-Modified Cements; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech. 2017, 7, 172. [Google Scholar] [CrossRef]
- Yesilyurt, C.; Er, K.; Tasdemir, T.; Buruk, K.; Celik, D. Antibacterial Activity and Physical Properties of Glass-ionomer Cements Containing Antibiotics. Oper. Dent. 2009, 34, 18–23. [Google Scholar] [CrossRef]
- Tüzüner, T.; Dimkov, A.; Nicholson, J.W. The effect of antimicrobial additives on the properties of dental glass-ionomer cements: A review. Acta Biomater Odontol Scand. 2019, 5, 9–21. [Google Scholar] [CrossRef]
- Botelho, M. Compressive strength of glass ionomer cements with dental antibacterial agents. SADJ J. S. Afr. Dent. Assoc. 2004, 59, 51–53. [Google Scholar]
- Marti, L.M.; Da Mata, M.; Ferraz-Santos, B.; Azevedo, E.R.; Giro, E.M.A.; Zuanon, A.C.C. Addition of Chlorhexidine Gluconate to a Glass Ionomer Cement: A Study on Mechanical, Physical and Antibacterial Properties. Braz. Dent. J. 2014, 25, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Becci, A.C.D.O.; Marti, L.M.; Zuanon, A.C.C.; Brighenti, F.L.; Spolidório, D.M.P.; Giro, E.M.A. Influence of the addition of chlorhexidine diacetate on bond strength of a high-viscosity glass ionomer cement to sound and artificial caries-affected dentin. Rev. Odontol. UNESP 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Somani, R.; Jaidka, S.; Singh, D.J.; Shafat, S. Comparative evaluation of compressive strength, diametral tensile strength and shear bond strength of GIC type IX, chlorhexidine-incorporated GIC and triclosan-incorporated GIC: An in vitro study. J. Int. Soc. Prev. Community Dent. 2016, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Takahashi, Y.; Hisama, K.; Shimizu, H. Water sorption and dimensional stability of three glass fiber-reinforced composites. Int. J. Prosthodont. 2004, 17, 195–199. [Google Scholar] [PubMed]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Zankuli, M.; Devlin, H.; Silikas, N. Water sorption and solubility of core build-up materials. Dent. Mater. 2014, 30, e324–e329. [Google Scholar] [CrossRef]
- Kucukyilmaz, E.; Savas, S.; Colgecen, O.; Yasa, B. Color stability, roughness, and water sorption/solubility of glass ionomer–Based restorative materials. Niger. J. Clin. Pract. 2019, 22, 824–832. [Google Scholar] [CrossRef]
- Kucukyilmaz, E.; Savas, S. Evaluation of shear bond strength, penetration ability, microleakage and remineralisation capacity of glass ionomer-based fissure sealants. Eur. J. Paediatr. Dent. 2016, 17, 17–23. [Google Scholar]
- Nicholson, J.W. Chemistry of glass-ionomer cements: A review. Biomaterials 1998, 19, 485–494. [Google Scholar] [CrossRef]
- Nomoto, R.; Uchida, K.; Momoi, Y.; McCabe, J.F. Erosion of water-based cements evaluated by volumetric and gravimetric methods. Dent. Mater. 2003, 19, 240–244. [Google Scholar] [CrossRef]
- Toledano, M.B.; Osorio, R.; Osorio, E.; Aguilera, F.S.; Romeo, A.; De La Higuera, B.; García-Godoy, F. Sorption and solubility testing of orthodontic bonding cements in different solutions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Keyf, F.; Tuna, S.H.; Şen, M.; Safrany, A. Water sorption and solubility of different luting and restorative dental cements. Turk. J. Med. Sci. 2007, 37, 47–55. [Google Scholar]
- Sinthawornkul, S.; Thunyakitpisal, P.; Thunyakitpisal, N.; Jiemsirilers, S. Comparison of Shear Bond Strength, Water Sorption and Solubility of 3 Glass Ionomer Cements for Direct Bonding of Orthodontic Brackets in vitro. J. Dent. Assoc. Thail. 2017, 67, 225–235. [Google Scholar] [CrossRef]
- Lohbauer, U. Dental Glass Ionomer Cements as Permanent Filling Materials? —Properties, Limitations and Future Trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef]
- Azillah, M.; Anstice, H.; Pearson, G. Long-term flexural strength of three direct aesthetic restorative materials. J. Dent. 1998, 26, 177–182. [Google Scholar] [CrossRef]
- Bresciani, E.; Barata, T.D.J.E.; Fagundes, T.C.; Adachi, A.; Terrin, M.M.; Navarro, M.F.D.L. Compressive and diametral tensile strength of glass ionomer cements. J. Appl. Oral Sci. 2004, 12, 344–348. [Google Scholar] [CrossRef]
- Kutuk, Z.B.; Vural, U.K.; Cakir, F.Y.; Miletic, I.; Gurgan, S. Mechanical properties and water sorption of two experimental glass ionomer cements with hydroxyapatite or calcium fluorapatite formulation. Dent. Mater. J. 2019, 38, 471–479. [Google Scholar] [CrossRef]
- Sajjad, A.; Bakar, W.Z.W.; Mohamad, D.; Kannan, T.P. Characterization and enhancement of physico-mechanical properties of glass ionomer cement by incorporating a novel nano zirconia silica hydroxyapatite composite synthesized via sol-gel. AIMS Mater. Sci. 2019, 6, 730–747. [Google Scholar] [CrossRef]
- Prentice, L.H.; Tyas, M.J. The effect of oxalic acid incorporation on the setting time and strength of a glass-ionomer cement. Acta Biomater. 2006, 2, 109–112. [Google Scholar] [CrossRef]
- Yap, A.U.; Mudambi, S.; Chew, C.L.; Neo, J.C. Mechanical properties of an improved visible light-cured resin-modified glass ionomer cement. Oper. Dent. 2001, 26, 295–301. [Google Scholar]
- Behr, M.; Rosentritt, M.; Loher, H.; Handel, G. Effect of variations from the recommended powder/liquid ratio on some properties of resin-modified cements. Acta Odontol. Scand. 2006, 64, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Ausiello, P.; Ciaramella, S.; Di Rienzo, A.; Lanzotti, A.; Ventre, M.; Watts, D.C. Adhesive class I restorations in sound molar teeth incorporating combined resin-composite and glass ionomer materials: CAD-FE modeling and analysis. Dent. Mater. 2019, 35, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Cefaly, D.F.G.; Franco, E.B.; Mondelli, R.F.L.; Francisconi, P.A.S.; Navarro, M.F.D.L. Diametral tensile strength and water sorption of glass-ionomer cements used in Atraumatic Restorative Treatment. J. Appl. Oral Sci. 2003, 11, 96–101. [Google Scholar] [CrossRef]
- Hwang, S.; Chung, S.H.; Lee, J.-T.; Kim, Y.-T.; Kim, Y.J.; Oh, S.; Yeo, I.-S.L. Influence of Acid, Ethanol, and Anthocyanin Pigment on the Optical and Mechanical Properties of a Nanohybrid Dental Composite Resin. Materials 2018, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Vallittu, P.K.; Lassila, L.; Viola, A.; Tessera, P.; Gandini, P.; Sfondrini, M.F. Effect of Long-Term Brushing on Deflection, Maximum Load, and Wear of Stainless Steel Wires and Conventional and Spot Bonded Fiber-Reinforced Composites. Int. J. Mol. Sci. 2019, 20, 6043. [Google Scholar] [CrossRef] [PubMed]



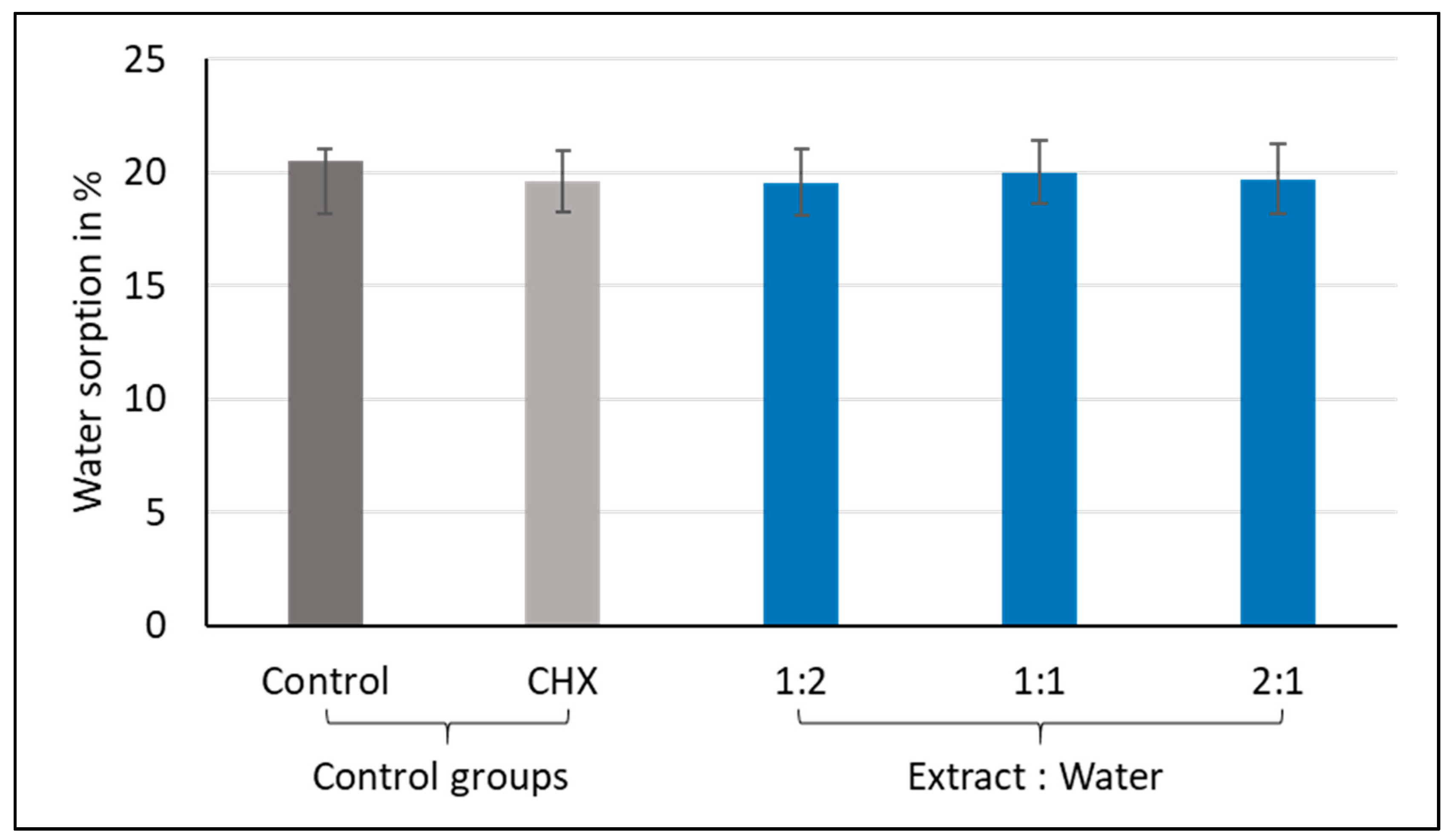

| Group | N | Mean % | St Dev | p-Value * |
|---|---|---|---|---|
| Control | 10 | 20.5 | 2.7 | 0.908 |
| CHX-GIC | 10 | 19.6 | 1.2 | |
| 1:2 | 10 | 19.5 | 2.6 | |
| 1:1 | 10 | 20.0 | 1.3 | |
| 2:1 | 10 | 19.7 | 4.3 |
| Group | N | Mean % | St Dev | p-Value * | Pairwise Comparison ** | ||
|---|---|---|---|---|---|---|---|
| Control | 10 | −4.9 | 1.5 | <0.001 | A | ||
| CHX-GIC | 10 | −3.5 | 1.0 | B | |||
| 1:2 | 10 | −5.1 | 0.6 | A | |||
| 1:1 | 10 | −2.4 | 0.9 | BC | |||
| 2:1 | 10 | −0.3 | 1.4 | D | |||
| Variable | N | Median (MPa) | IQR (MPa) | p-Value * | Pairwise Comparison ** | ||
|---|---|---|---|---|---|---|---|
| Control | 10 | 11.8 | 5.1 | <0.001 | A | ||
| CHX-GIC | 10 | 15.3 | 10.3 | A | B | ||
| 1:2 | 10 | 11.5 | 5.9 | A | B | ||
| 1:1 | 10 | 19.6 | 4.6 | B | C | ||
| 2:1 | 10 | 26.1 | 9.0 | C | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, L.; Bierbaum, G.; Kehl, K.; Bourauel, C. Evaluation of the Flexural Strength, Water Sorption, and Solubility of a Glass Ionomer Dental Cement Modified Using Phytomedicine. Materials 2020, 13, 5352. https://doi.org/10.3390/ma13235352
Singer L, Bierbaum G, Kehl K, Bourauel C. Evaluation of the Flexural Strength, Water Sorption, and Solubility of a Glass Ionomer Dental Cement Modified Using Phytomedicine. Materials. 2020; 13(23):5352. https://doi.org/10.3390/ma13235352
Chicago/Turabian StyleSinger, Lamia, Gabriele Bierbaum, Katja Kehl, and Christoph Bourauel. 2020. "Evaluation of the Flexural Strength, Water Sorption, and Solubility of a Glass Ionomer Dental Cement Modified Using Phytomedicine" Materials 13, no. 23: 5352. https://doi.org/10.3390/ma13235352
APA StyleSinger, L., Bierbaum, G., Kehl, K., & Bourauel, C. (2020). Evaluation of the Flexural Strength, Water Sorption, and Solubility of a Glass Ionomer Dental Cement Modified Using Phytomedicine. Materials, 13(23), 5352. https://doi.org/10.3390/ma13235352