Phase Transition Kinetics in Austempered Ductile Iron (ADI) with Regard to Mo Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Composition
2.2. Heat Treatment
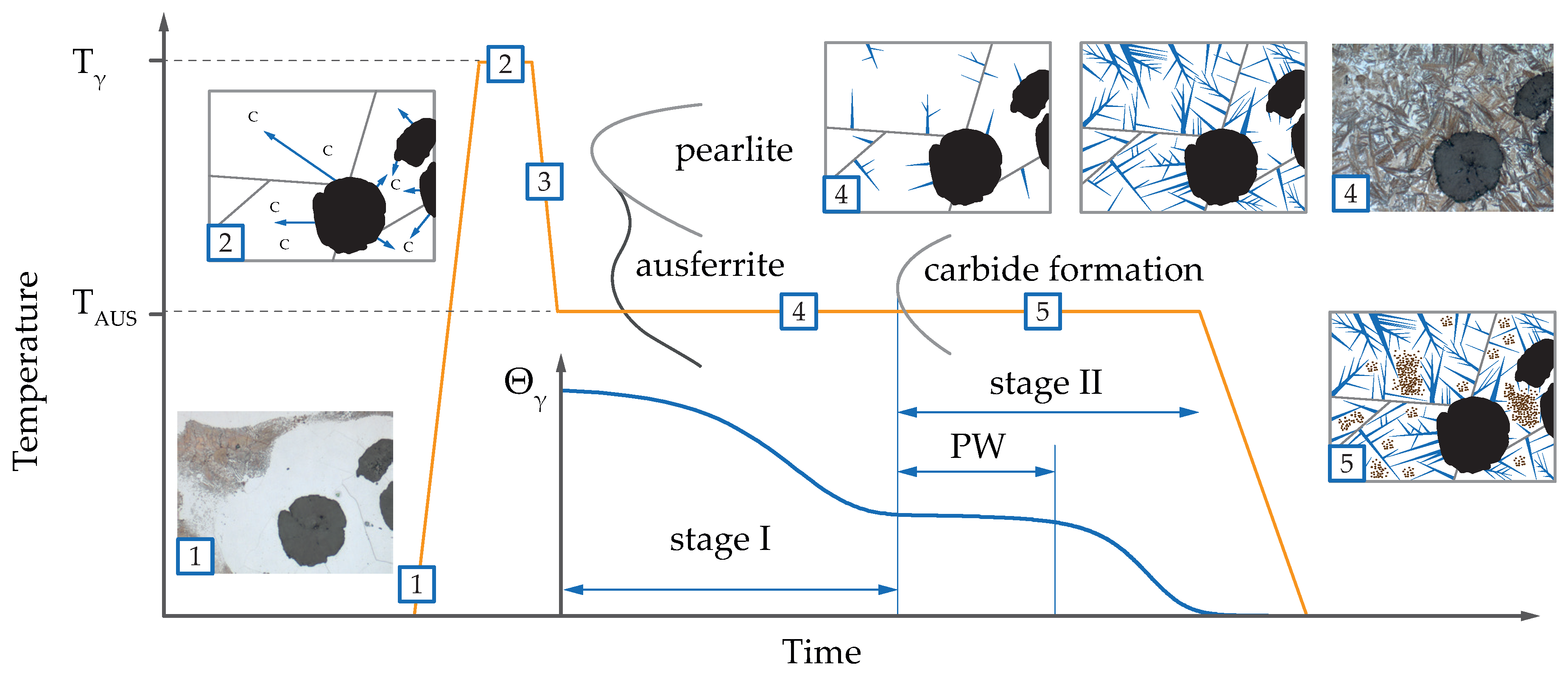
2.3. Metallography and Interrupted Heat Treatment
2.4. Neutron Diffraction
2.5. In-Situ Dilatometry
2.6. Atom Probe Tomography
3. Results and Discussion
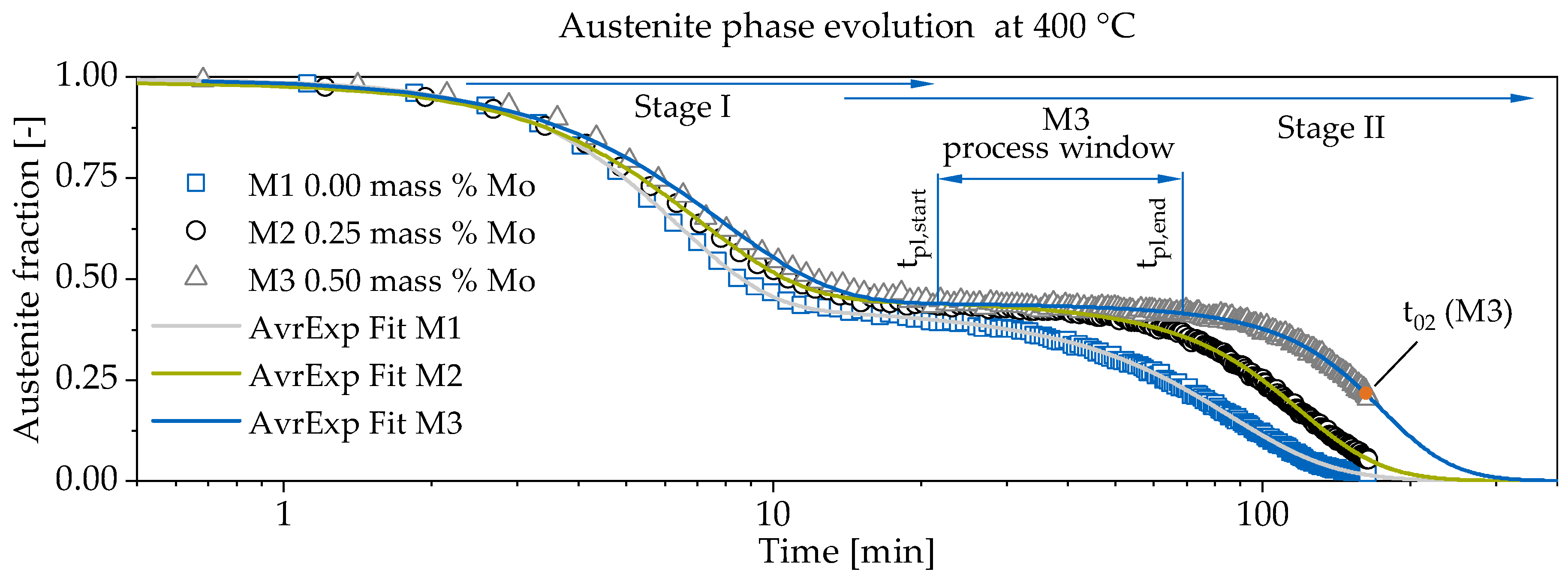
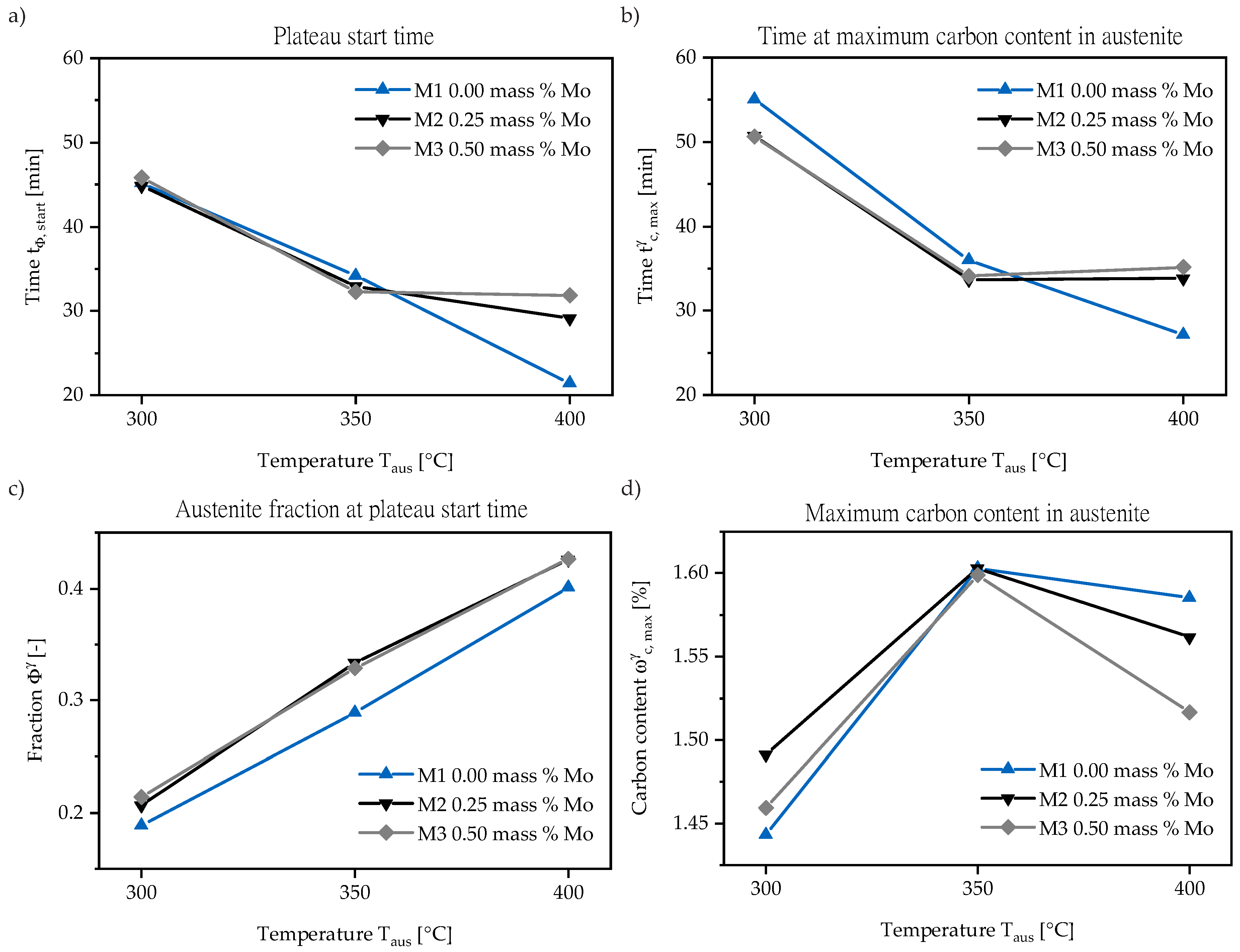
3.1. In-Situ Neutron Diffraction
3.2. Metallography
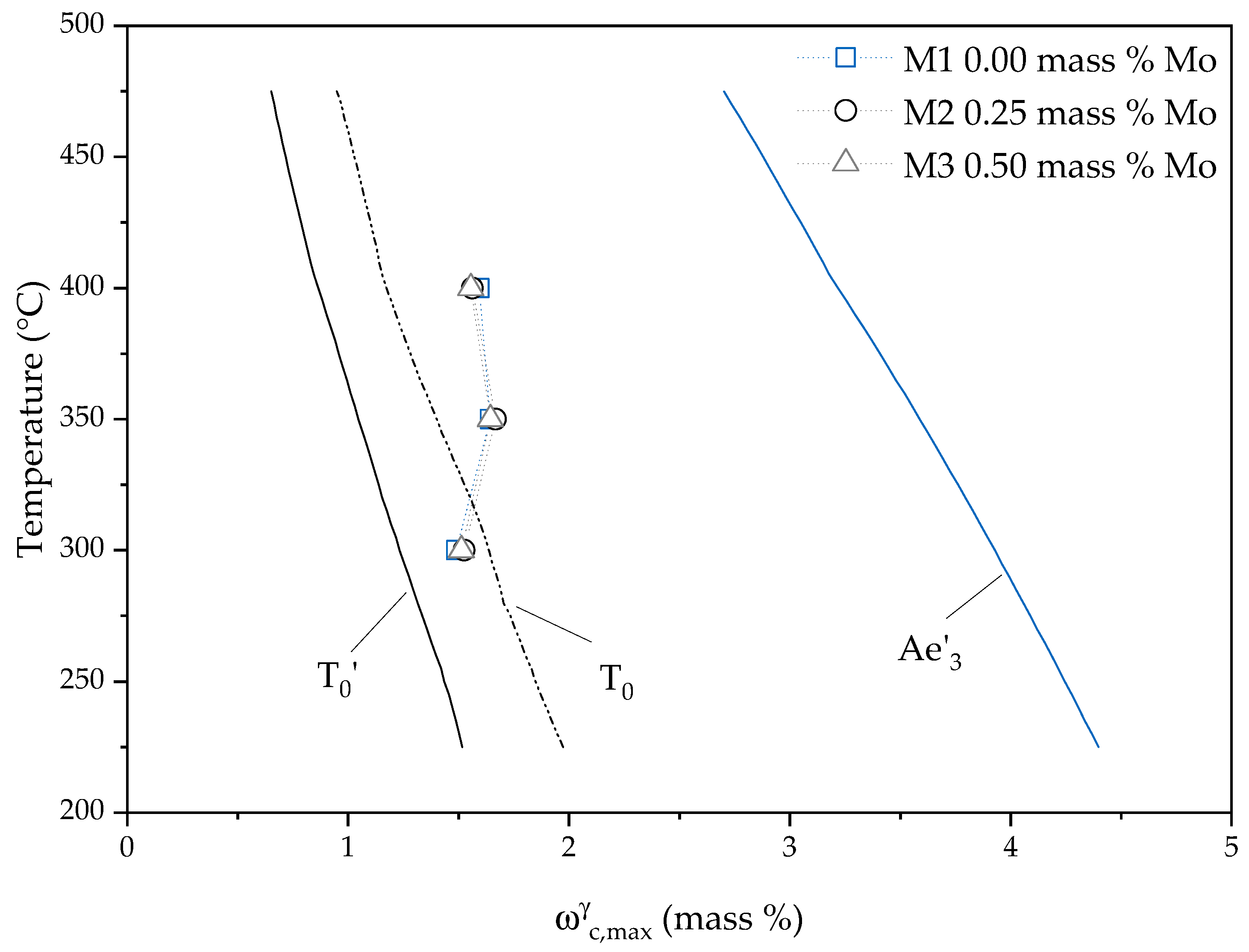
3.3. Comparison of Neutron Diffraction to Dilatometry
3.4. Atom Probe Tomography (APT)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boccardo, A.D.; Dardati, P.M.; Godoy, L.A.; Celentano, D.J. Sensitivity of Austempering Heat Treatment of Ductile Irons to Changes in Process Parameters. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 1522–1536. [Google Scholar] [CrossRef]
- Gazda, A.; Warmuzek, M. Analysis of thermal stability of ausferrite obtained by means of cyclic heat treatment of Cu–Ni (Mn, Mo) ADI. Thermochim. Acta 2018, 663, 58–66. [Google Scholar] [CrossRef]
- Eric Cekic, O.; Rajnovic, D.; Sidjanin, L.; Janjatovic, P.; Balos, S. Dual Phase Austempered Ductile Iron—The Material Revolution and Its Engineering Applications. In Computational and Experimental Approaches in Materials Science and Engineering; Lecture Notes in Networks and Systems; Mitrovic, N., Mitrovic, M., Mitrovic, G., Eds.; Springer: Cham, Switzerland, 2020; Volume 90, pp. 22–38. [Google Scholar] [CrossRef]
- Górny, M.; Angella, G.; Tyrała, E.; Kawalec, M.; Paź, S.; Kmita, A. Role of Austenitization Temperature on Structure Homogeneity and Transformation Kinetics in Austempered Ductile Iron. Met. Mater. Int. 2019, 4, 415. [Google Scholar] [CrossRef]
- DIN Deutsches Institut für Normung e. V. Gießereiwesen—Ausferritisches Gusseisen mit Kugelgraphit; Deutsche Fassung EN 1564:2011; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2012. [Google Scholar]
- Laino, S.; Sikora, J.A.; Dommarco, R.C. Development of wear resistant carbidic austempered ductile iron (CADI). Wear 2008, 265, 1–7. [Google Scholar] [CrossRef]
- Masaggia, S. The development of ADI and IDI in Italy. Fatigue 2010, 2, 1459–1476. [Google Scholar] [CrossRef]
- Daber, S.; Ravishankar, K.S.; Prasad Rao, P. Influence of austenitising temperature on the formation of strain induced martensite in austempered ductile iron. J. Mater. Sci. 2008, 43, 4929–4937. [Google Scholar] [CrossRef]
- Daber, S.; Prasad Rao, P. Formation of strain-induced martensite in austempered ductile iron. J. Mater. Sci. 2008, 43, 357–367. [Google Scholar] [CrossRef]
- Saal, P.; Meier, L.; Li, X.; Hofmann, M.; Hoelzel, M.; Wagner, J.N.; Volk, W. In Situ Study of the Influence of Nickel on the Phase Transformation Kinetics in Austempered Ductile Iron. Metall. Mater. Trans. A 2016, 47, 661–671. [Google Scholar] [CrossRef]
- Górny, M.; Tyrała, E.; Sikora, G. Transformation Kinetics and Mechanical Properties of Copper-Alloyed and Copper-Nickel Alloyed ADI. Mater. Sci. Forum 2018, 925, 181–187. [Google Scholar] [CrossRef]
- Benam, A.S. Effect of alloying elements on austempered ductile iron (ADI) properties and its process: Review. China Foundry 2015, 12, 54–70. [Google Scholar]
- Yazdani, S.; Elliott, R. Influence of molybdenum on austempering behaviour of ductile iron Part 4—Austempering behaviour of ductile iron containing 0·45%Mo. Mater. Sci. Technol. 1999, 15, 896–902. [Google Scholar] [CrossRef]
- Yazdani, S.; Elliott, R. Influence of molybdenum on austempering behaviour of ductile iron Part 3—Austempering kinetics, mechanical properties, and hardenability of ductile iron containing 0·25%Mo. Mater. Sci. Technol. 1999, 15, 885–895. [Google Scholar] [CrossRef]
- Yazdani, S.; Elliott, R. Influence of molybdenum on austempering behaviour of ductile iron Part 2—Influence of austenitising temperature on austempering kinetics, mechanical properties, and hardenability of ductile iron containing 0·13%Mo. Mater. Sci. Technol. 1999, 15, 541–546. [Google Scholar] [CrossRef]
- Yazdani, S.; Elliott, R. Influence of molybdenum on austempering behaviour of ductile iron Part 1—Austempering kinetics and mechanical properties of ductile iron containing 0·13%Mo. Mater. Sci. Technol. 1999, 15, 531–540. [Google Scholar] [CrossRef]
- Dekker, L.; Tonn, B. Occurrence and behaviour of Mo containing precipitates in nodular cast iron at high temperatures. Int. J. Cast Met. Res. 2016, 29, 85–91. [Google Scholar] [CrossRef]
- Gazda, A.; Warmuzek, M.; Bitka, A. Optimization of mechanical properties of complex, two-stage heat treatment of Cu–Ni (Mn, Mo) austempered ductile iron. J. Therm. Anal. Calorim. 2018, 132, 813–822. [Google Scholar] [CrossRef]
- Dorazil, E. High Strength Austempered Ductile Cast Iron; Ellis Horwood Ltd.: Hemel Hempstead, UK, 1991. [Google Scholar]
- Domeij, B. On the Solidification of Compacted and Spheroidal Graphite Irons; JTH Research Report; Jönköping University, School of Engineering: Jönköping, Sweden, 2017. [Google Scholar]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Cornide, J.; Santofimia, M.J. Temperature dependence of carbon supersaturation of ferrite in bainitic steels. Scr. Mater. 2012, 67, 846–849. [Google Scholar] [CrossRef]
- DIN Deutsches Institut für Normung e. V. Gießereiwesen—Gusseisen mit Kugelgraphit; Deutsche und Englische Fassung prEN 1563:2016; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2016. [Google Scholar]
- DIN Deutsches Institut für Normung e. V. Mikrostruktur von Gusseisen—Teil 1: Graphitklassifizierung Durch Visuelle Auswertung (ISO 945-1:2008 + Cor. 1:2010): Deutsche Fassung EN ISO 945-1:2008 + AC:2010; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2010. [Google Scholar]
- Meier, L.; Hofmann, M.; Saal, P.; Volk, W.; Hoffmann, H. In-situ measurement of phase transformation kinetics in austempered ductile iron. Mater. Charact. 2013, 85, 124–133. [Google Scholar] [CrossRef]
- Hofmann, M.; Schneider, R.; Seidl, G.A.; Rebelo-Kornmeier, J.; Wimpory, R.C.; Garbe, U.; Brokmeier, H.G. The new materials science diffractometer STRESS-SPEC at FRM-II. Phys. B Condens. Matter 2006, 385, 1035–1037. [Google Scholar] [CrossRef]
- Zeitelhack, K.; Schanzer, C.; Kastenmüller, A.; Röhrmoser, A.; Daniel, C.; Franke, J.; Gutsmiedl, E.; Kudryashov, V.; Maier, D.; Päthe, D.; et al. Measurement of neutron flux and beam divergence at the cold neutron guide system of the new Munich research reactor FRM-II. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2006, 560, 444–453. [Google Scholar] [CrossRef]
- Hofmann, M.; Gan, W.; Rebelo-Kornmeier, J. STRESS-SPEC: Materials science diffractometer. J. Large-Scale Res. Facil. JLSRF 2015, 1. [Google Scholar] [CrossRef]
- Rebelo Kornmeier, J.; Gibmeier, J.; Hofmann, M. Minimization of spurious strains by using a Si bent-perfect-crystal monochromator: Neutron surface strain scanning of a shot-peened sample. Meas. Sci. Technol. 2011, 22, 065705. [Google Scholar] [CrossRef]
- Rebelo-Kornmeier, J.; Hofmann, M.; Gan, W.M.; Randau, C.; Braun, K.; Zeitelhack, K.; Defendi, I.; Krueger, J.; Faulhaber, E.; Brokmeier, H.G. New Developments of the Materials Science Diffractometer STRESS-SPEC. Mater. Sci. Forum 2017, 905, 151–156. [Google Scholar] [CrossRef]
- Randau, C.; Garbe, U.; Brokmeier, H.G. StressTextureCalculator: A software tool to extract texture, strain and microstructure information from area-detector measurements. J. Appl. Crystallogr. 2011, 44, 641–646. [Google Scholar] [CrossRef]
- Roberts, C.S. Effect of Carbon on the Volume Fractions and Lattice Parameters of Retained Austenite and Martensite. JOM 1953, 5, 203–204. [Google Scholar] [CrossRef]
- Voigt, R.C.; Loper, C.R. Austempered Ductile Iron—Process Control and Quality Assurance. J. Heat Treat. 1984, 3, 291–309. [Google Scholar] [CrossRef]
- Chang, L.C. An analysis of retained austenite in austempered ductile iron. Metall. Trans. A 2003, 34, 211–217. [Google Scholar] [CrossRef]
- Li, X.H.; Saal, P.; Gan, W.M.; Hoelzel, M.; Volk, W.; Petry, W.; Hofmann, M. Strain-Induced Martensitic Transformation Kinetic in Austempered Ductile Iron (ADI). Metall. Mater. Trans. A 2017, 85, 124. [Google Scholar] [CrossRef]
- Li, X.; Wagner, J.N.; Stark, A.; Koos, R.; Landesberger, M.; Hofmann, M.; Fan, G.; Gan, W.; Petry, W. Carbon Redistribution Process in Austempered Ductile Iron (ADI) During Heat Treatment—APT and Synchrotron Diffraction Study. Metals 2019, 9, 789. [Google Scholar] [CrossRef]
- Boll, T.; Zhu, Z.Y.; Al-Kassab, T.; Schwingenschlögl, U. Atom probe tomography simulations and density functional theory calculations of bonding energies in Cu3Au. Microsc. Microanal. 2012, 18, 964–970. [Google Scholar] [CrossRef]
- Hellman, O.C.; Vandenbroucke, J.A.; Rüsing, J.; Isheim, D.; Seidman, D.N. Analysis of Three-dimensional Atom-probe Data by the Proximity Histogram. Microsc. Microanal. 2000, 6, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hellman, O.C.; Du Rivage, J.B.; Seidman, D.N. Efficient sampling for three-dimensional atom probe microscopy data. Ultramicroscopy 2003, 95, 199–205. [Google Scholar] [CrossRef]
- Darwish, N.; Elliott, R. Austempering of low manganese ductile irons: Part 2 Influence of austenitising temperature. Mater. Sci. Technol. 1993, 9, 586–602. [Google Scholar] [CrossRef]
- Lee, S.J.; Matlock, D.K.; Van Tyne, C.J. An Empirical Model for Carbon Diffusion in Austenite Incorporating Alloying Element Effects. ISIJ Int. 2011, 51, 1903–1911. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys, 3rd ed.; Pergamon: Oxford, UK; Boston, MA, USA, 2002. [Google Scholar]
- Bhadeshia, H.K.D.H. Bainite in Steels: Theory and Practice, 3rd ed.; Maney Publishing: Leeds, UK; The Institute of Materials Minerals and Mining: London, UK, 2015. [Google Scholar]
- Lee, J.L.; Bhadeshia, H. A methodology for the prediction of time-temperature-transformation diagrams. Mater. Sci. Eng. A 1993, 171, 223–230. [Google Scholar] [CrossRef]
- Hall, D.J.; Bhadeshia, H.K.; Stobbs, W.M. The incomplete bainite reaction: Possible reasons for the apparent differences in TEM and atom probe determination of austenite carbon content. J. Phys. 1982, 43, 449–454. [Google Scholar] [CrossRef]
- Onuki, Y.; Hirano, T.; Hoshikawa, A.; Sato, S.; Tomida, T. In Situ Observation of Bainite Transformation and Simultaneous Carbon Enrichment in Austenite in Low-Alloyed TRIP Steel Using Time-of-Flight Neutron Diffraction Techniques. Metall. Mater. Trans. A 2019, 50, 4977–4986. [Google Scholar] [CrossRef]
- Li, X.H.; Saal, P.; Gan, W.M.; Landesberger, M.; Hoelzel, M.; Hofmann, M. Strain Induced Martensitic Transformation in Austempered Ductile Iron (ADI). J. Phys. Conf. Ser. 2016, 746, 012055. [Google Scholar] [CrossRef]
- García de Andrés, C. Application of dilatometric analysis to the study of solid–solid phase transformations in steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Edmonds, D.V. The mechanism of bainite formation in steels. Acta Metall. 1980, 28, 1265–1273. [Google Scholar] [CrossRef]
- Rementeria, R.; Domínguez-Reyes, R.; Capdevila, C.; Garcia-Mateo, C.; Caballero, F.G. Positron Annihilation Spectroscopy Study of Carbon-Vacancy Interaction in Low-Temperature Bainite. Sci. Rep. 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Caballero, F.G.; Yen, H.W.; Miller, M.K.; Cornide, J.; Chang, H.T.; Garcia-Mateo, C.; Yang, J.R. Three phase crystallography and solute distribution analysis during residual austenite decomposition in tempered nanocrystalline bainitic steels. Mater. Charact. 2014, 88, 15–20. [Google Scholar] [CrossRef]
- Thuvander, M.; Weidow, J.; Angseryd, J.; Falk, L.K.L.; Liu, F.; Sonestedt, M.; Stiller, K.; Andrén, H.O. Quantitative atom probe analysis of carbides. Ultramicroscopy 2011, 111, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; de Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Isotopic compositions of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 293–306. [Google Scholar] [CrossRef]


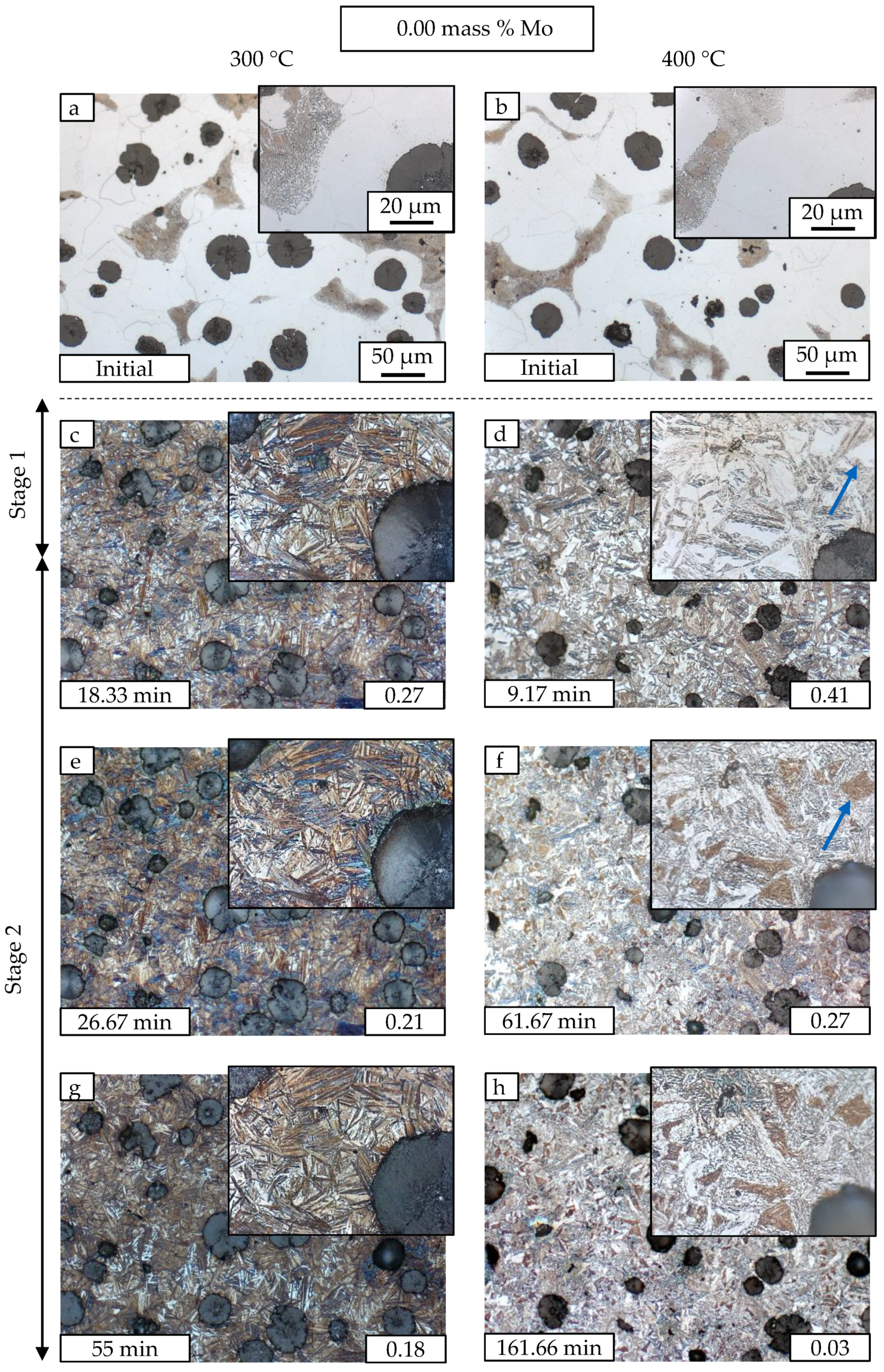
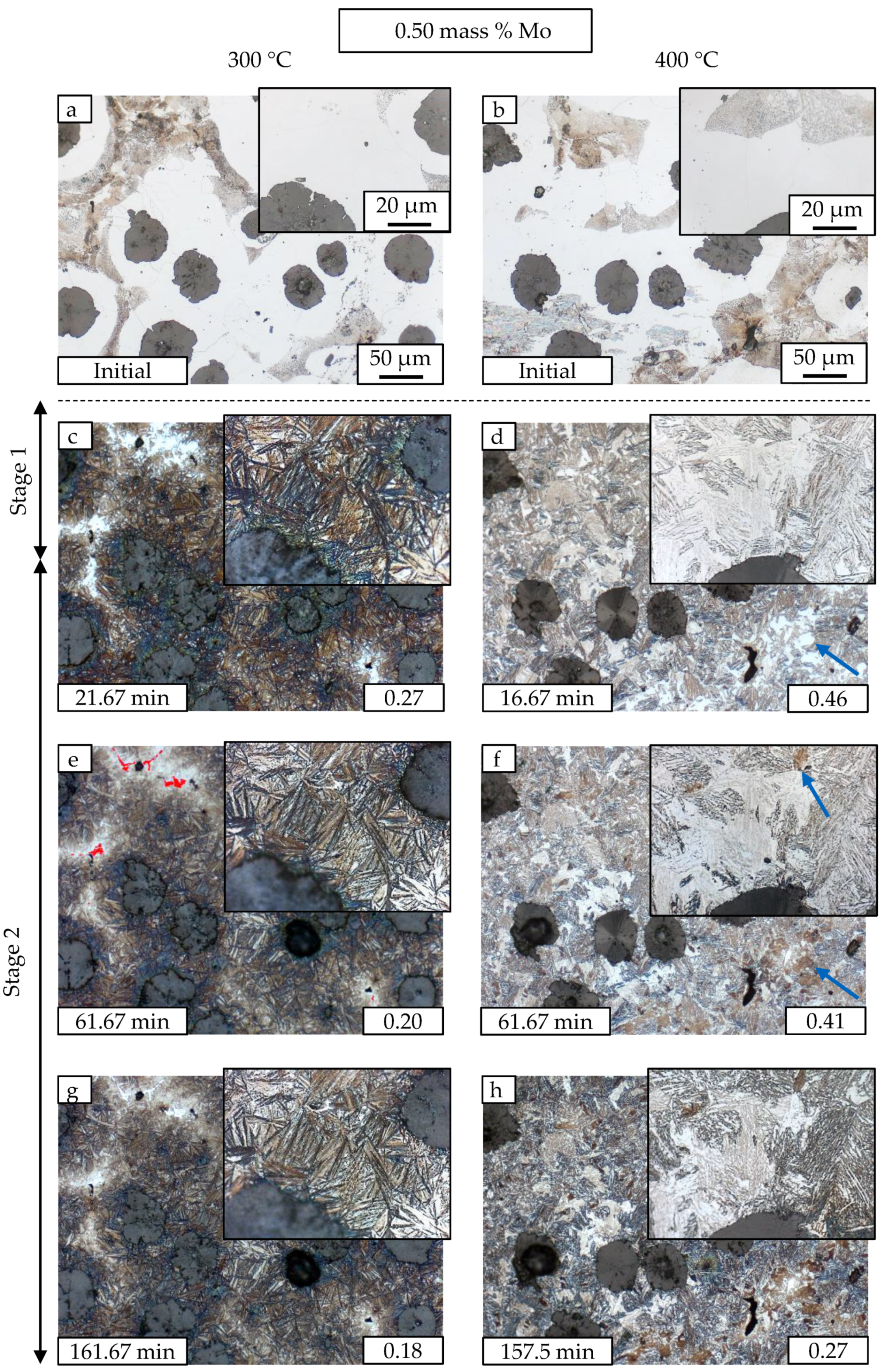

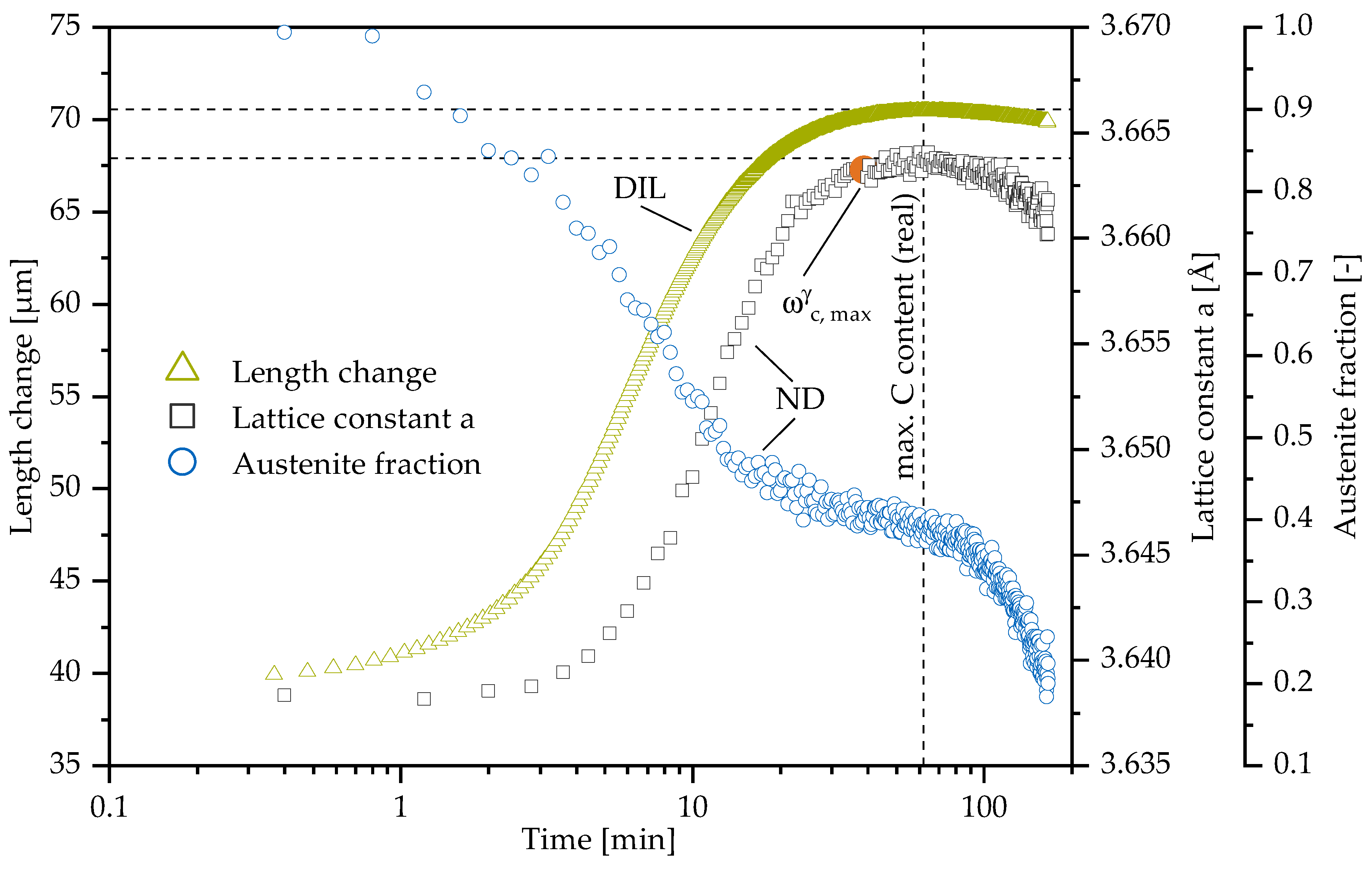

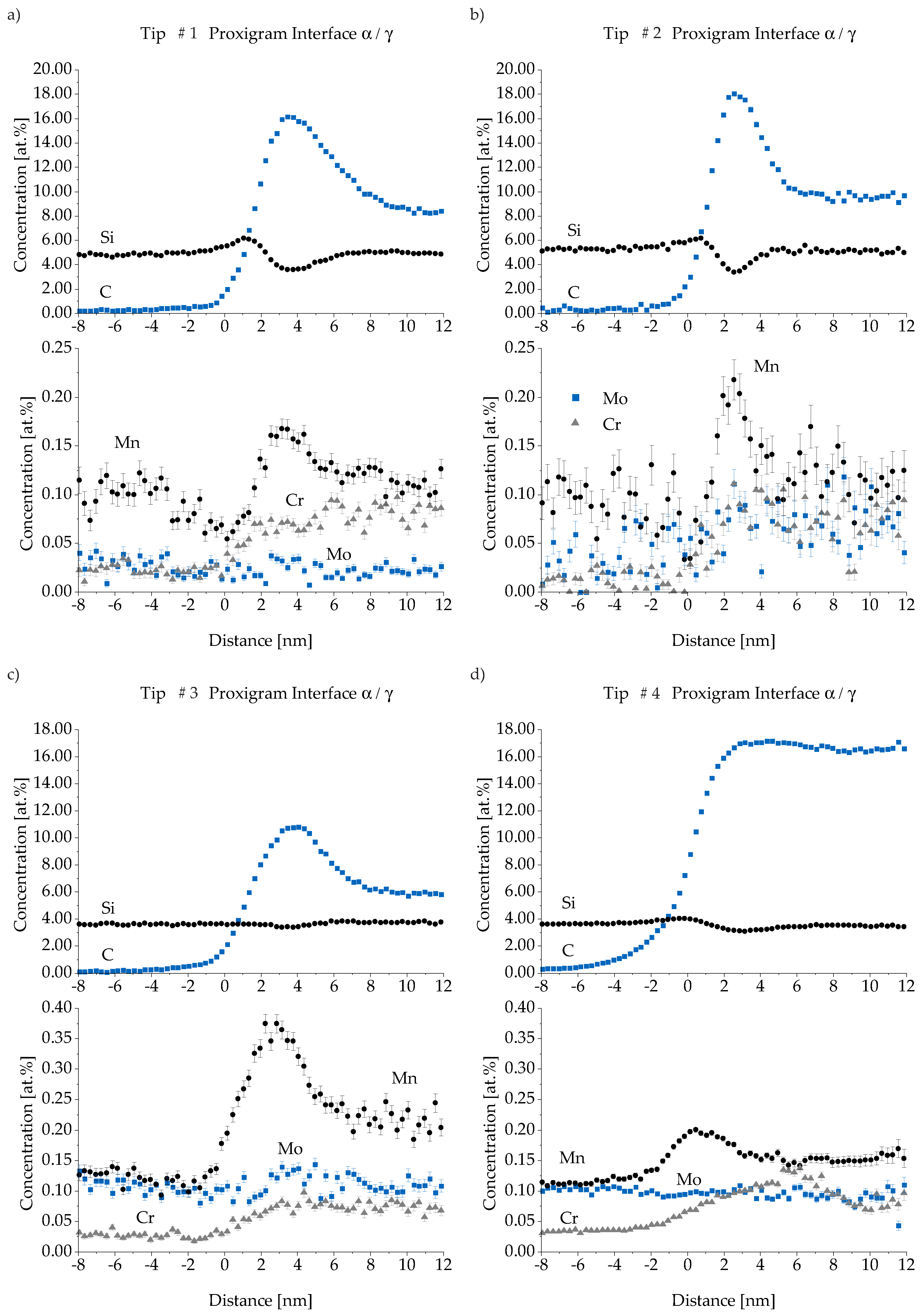
| Material | C | Si | Mn | Mo | Ni | Cu | Mg | S | P | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 3.73 | 2.37 | 0.18 | 0.00 | 0.02 | 0.02 | 0.05 | 0.01 | 0.04 | 0.01 | bal. |
| M2 | 3.68 | 2.35 | 0.18 | 0.25 | 0.02 | 0.03 | 0.04 | 0.02 | 0.04 | 0.01 | bal. |
| M3 | 3.63 | 2.38 | 0.18 | 0.49 | 0.02 | 0.03 | 0.04 | 0.01 | 0.04 | 0.01 | bal. |
| Material | Voigt [32] | Chang [33] |
|---|---|---|
| [mass %] | [mass %] | |
| M1 | 0.790 | 0.704 |
| M2 | 0.793 | 0.675 |
| M3 | 0.788 | 0.645 |
| Material | tpl,start | tpl,end | Duration | |
|---|---|---|---|---|
| [min] | [min] | [min] | [min] | |
| M1 | 17.09 | 27.36 | 10.27 | 27.15 |
| M2 | 21.57 | 42.89 | 21.32 | 33.85 |
| M3 | 27.22 | 72.51 | 45.29 | 35.15 |
| Material | Proportion p | Relax. Factor | Relax. Time | Max. Transf. Speed | Avrami Exp. n |
|---|---|---|---|---|---|
| [-] | [-] | [min] | [%/min] | [-] | |
| M1 | 0.5371 | 0.0137 | 6.38 | −8.45 | 2.315 |
| M2 | 0.5350 | 0.0191 | 7.03 | −6.64 | 2.029 |
| M3 | 0.5529 | 0.0208 | 7.81 | −5.86 | 1.884 |
| Material | Relax. Factor | Infliction Point | Max. Decay Speed | Complete Decay |
|---|---|---|---|---|
| [-] | [min] | [%/min] | [min] | |
| M1 | 0.0353 | 66.45 | −0.424 | 148.64 |
| M2 | 0.0340 | 104.32 | −0.395 | 188.79 |
| M3 | 0.0279 | 160.05 | −0.312 | 261.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landesberger, M.; Koos, R.; Hofmann, M.; Li, X.; Boll, T.; Petry, W.; Volk, W. Phase Transition Kinetics in Austempered Ductile Iron (ADI) with Regard to Mo Content. Materials 2020, 13, 5266. https://doi.org/10.3390/ma13225266
Landesberger M, Koos R, Hofmann M, Li X, Boll T, Petry W, Volk W. Phase Transition Kinetics in Austempered Ductile Iron (ADI) with Regard to Mo Content. Materials. 2020; 13(22):5266. https://doi.org/10.3390/ma13225266
Chicago/Turabian StyleLandesberger, Martin, Robert Koos, Michael Hofmann, Xiaohu Li, Torben Boll, Winfried Petry, and Wolfram Volk. 2020. "Phase Transition Kinetics in Austempered Ductile Iron (ADI) with Regard to Mo Content" Materials 13, no. 22: 5266. https://doi.org/10.3390/ma13225266
APA StyleLandesberger, M., Koos, R., Hofmann, M., Li, X., Boll, T., Petry, W., & Volk, W. (2020). Phase Transition Kinetics in Austempered Ductile Iron (ADI) with Regard to Mo Content. Materials, 13(22), 5266. https://doi.org/10.3390/ma13225266





