Functional Materials Based on Active Carbon and Titanium Dioxide in Fog Seal
Abstract
:1. Introduction
2. Indoor Experiments
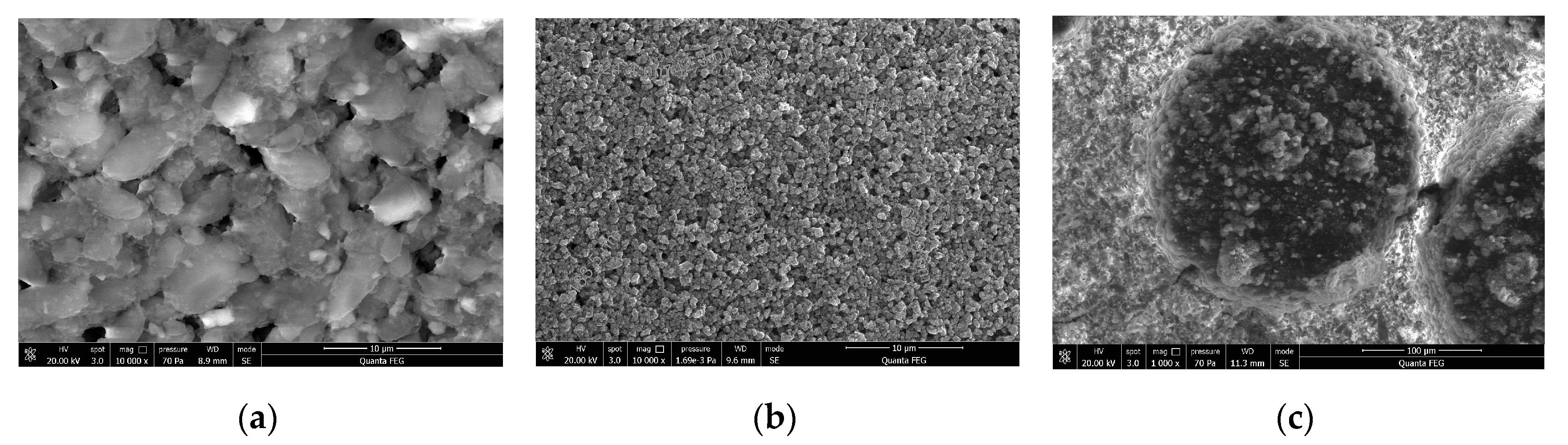
2.1. Material Characterization
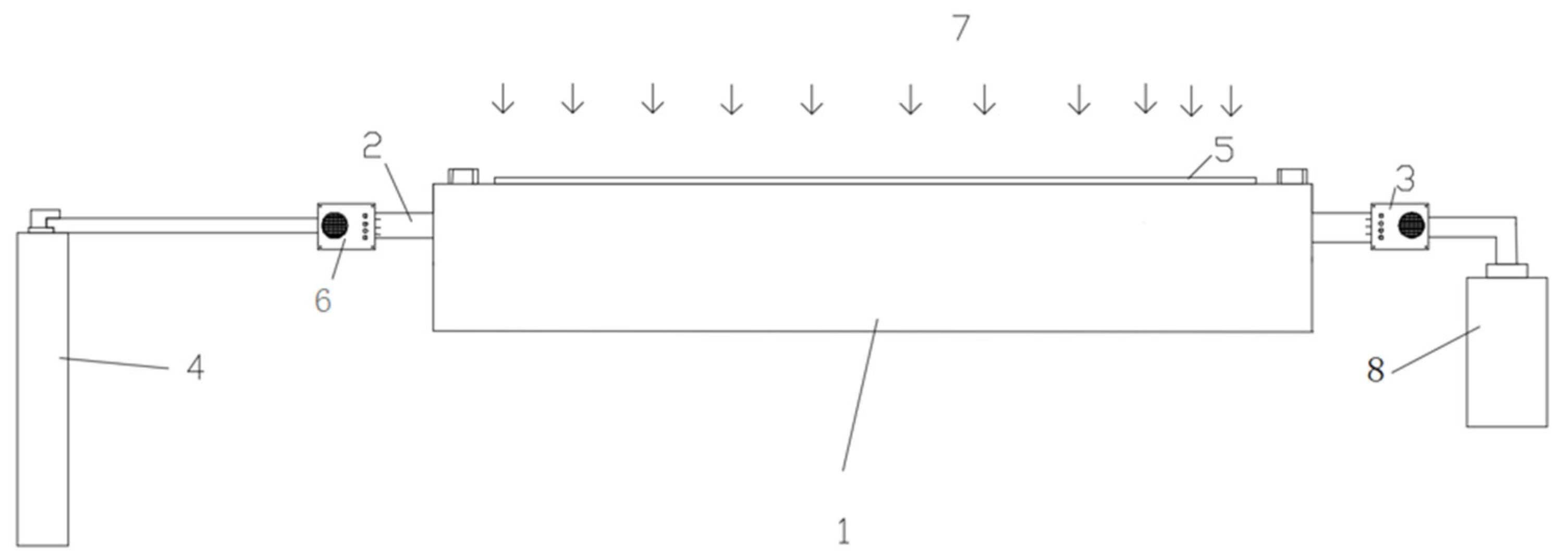
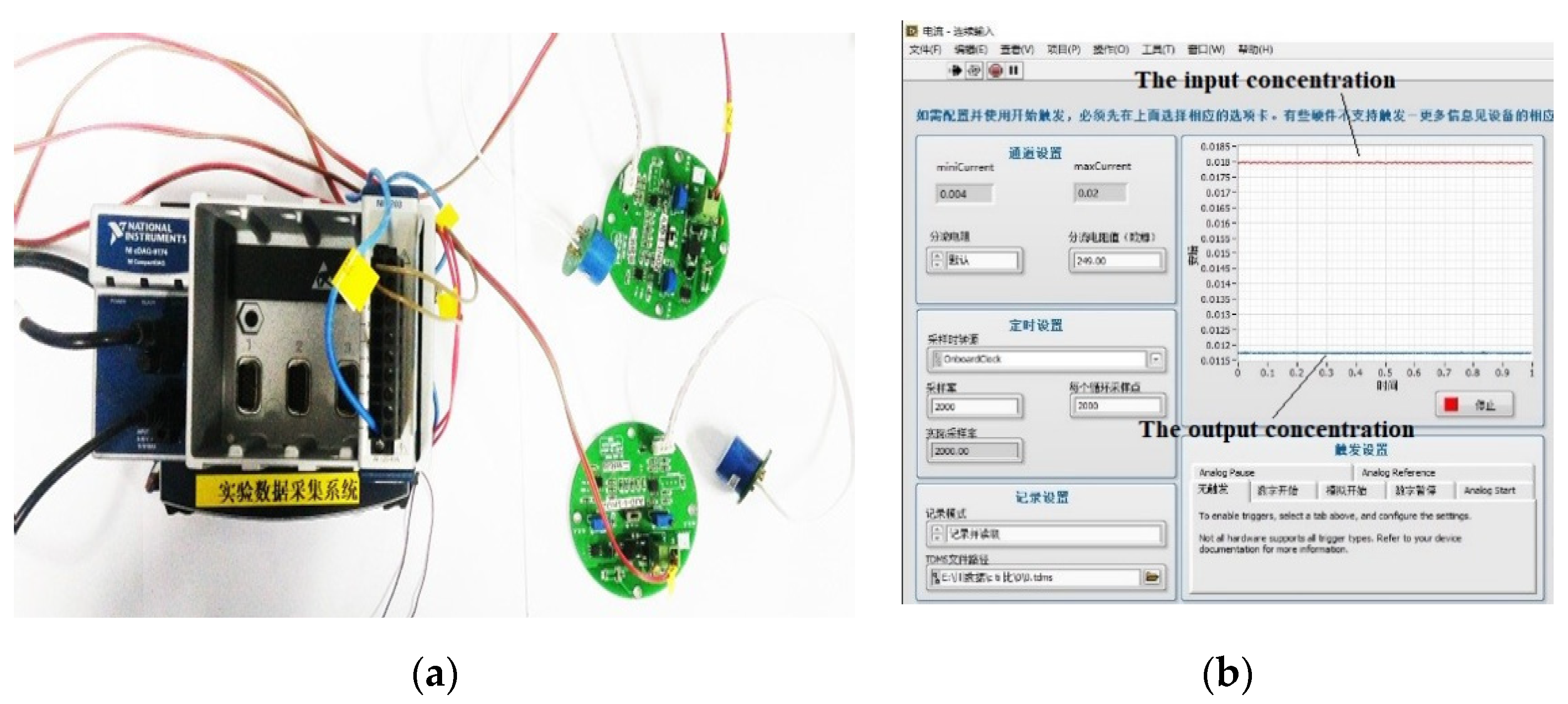
2.2. Testing System
2.3. Testing Principle
2.4. Photodegradation Process
2.4.1. Experimental Background
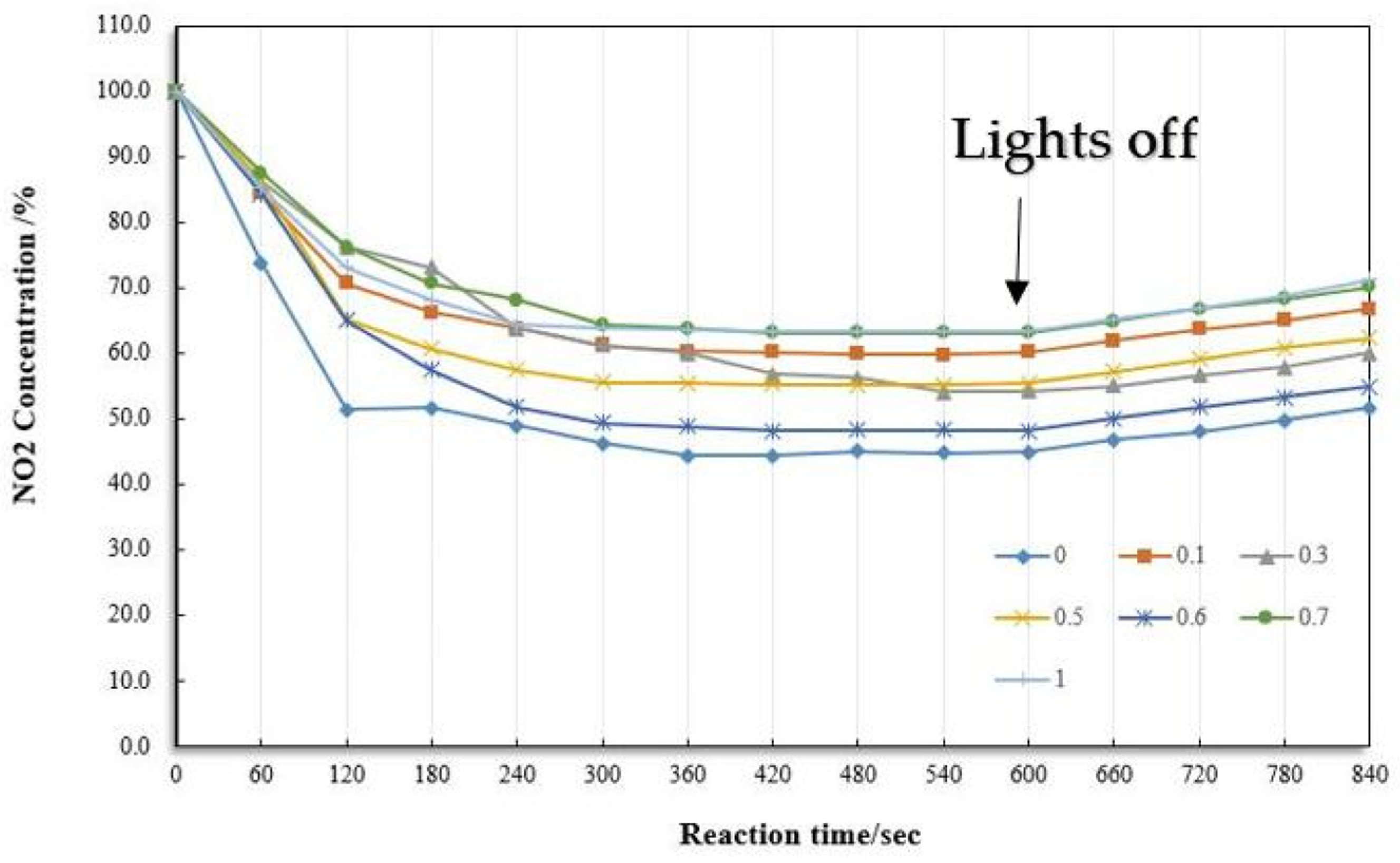
2.4.2. Selection of Photocatalytic Composite
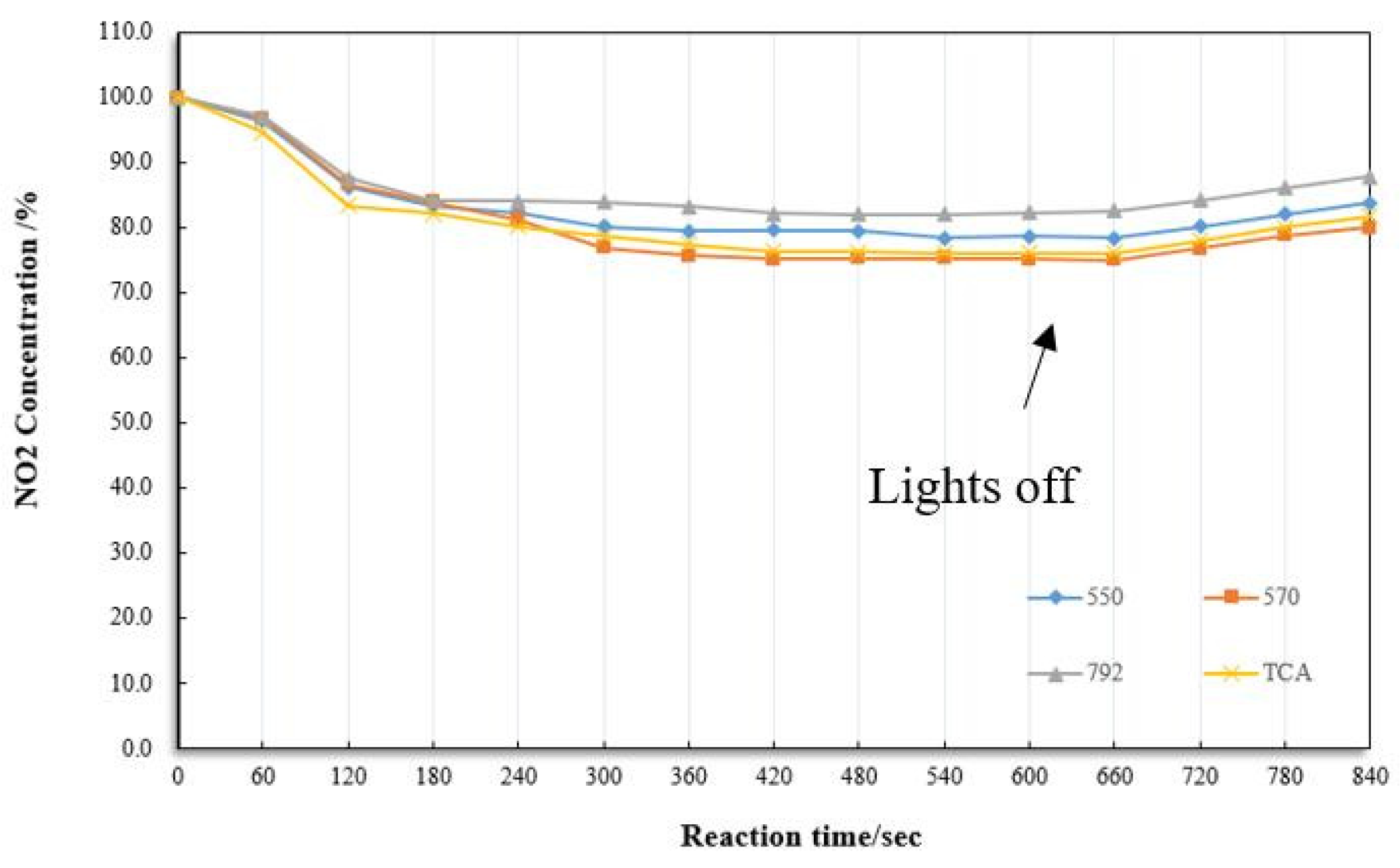
2.4.3. Modifier for Composite
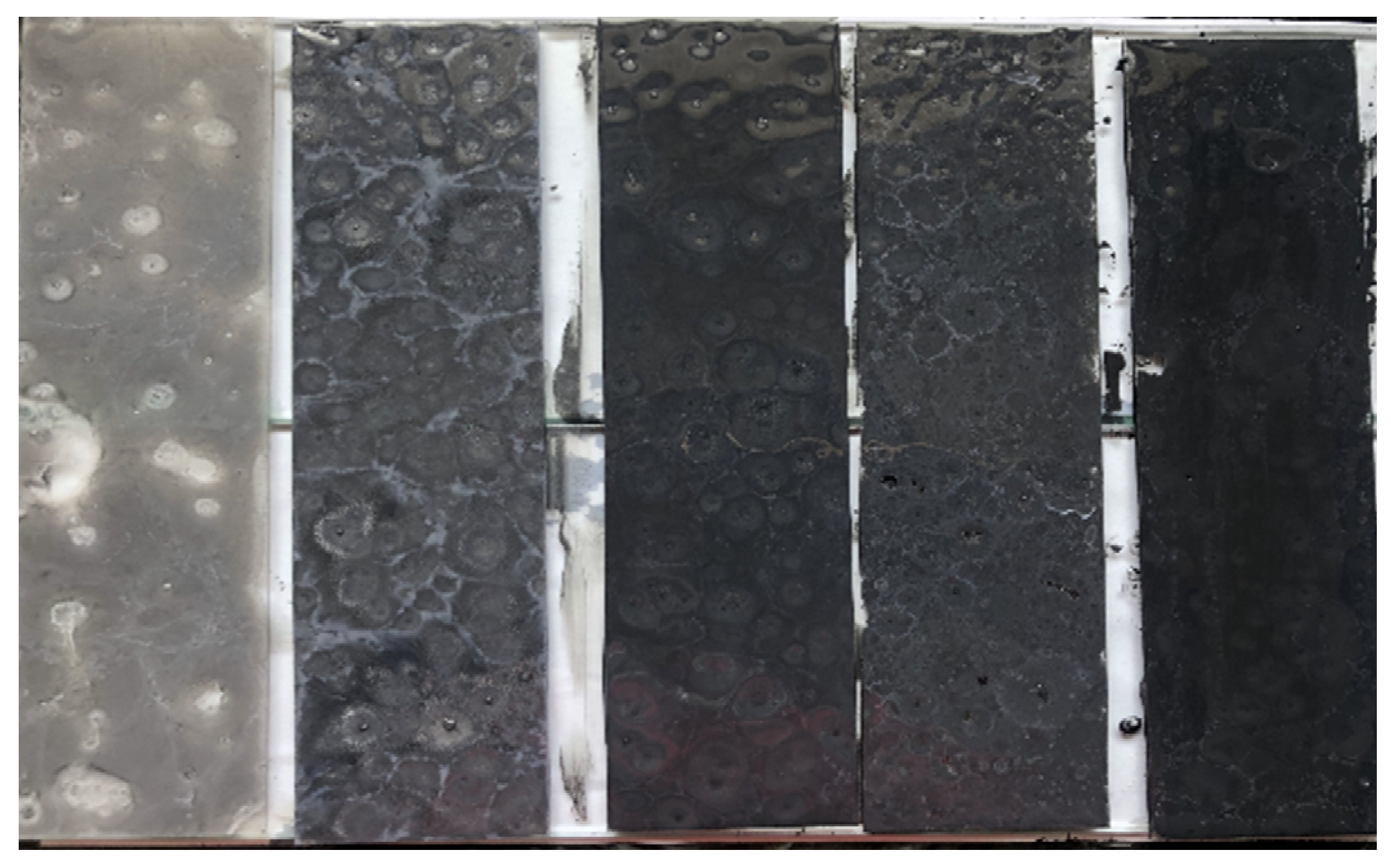
2.4.4. Composite Dosage for Fog Seal
3. Pavement Performance
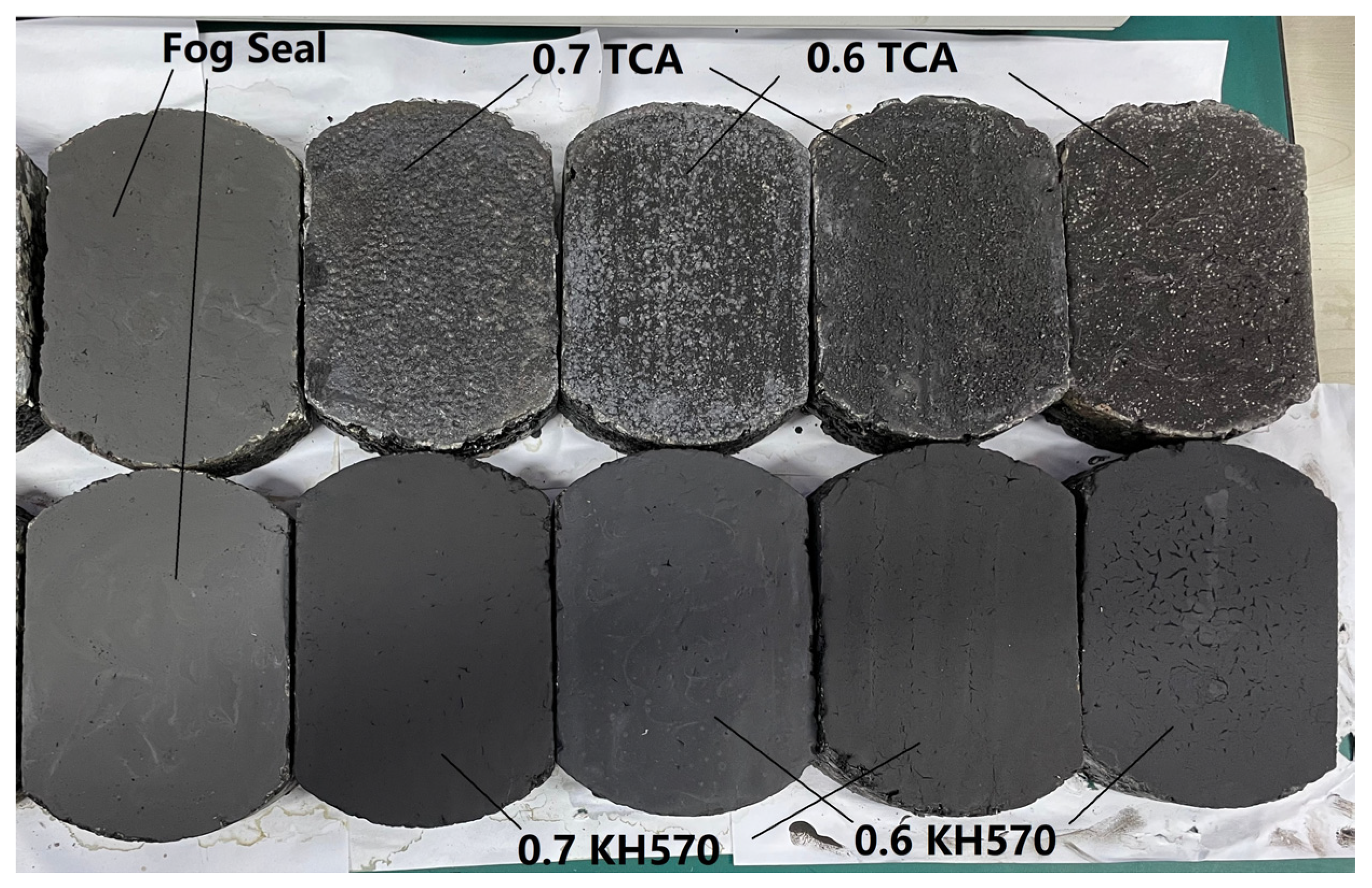
3.1. Skid Resistance Performance
3.2. Nitrogen Oxide Detection
4. Results
4.1. Removal of NO2
4.2. BPN Value
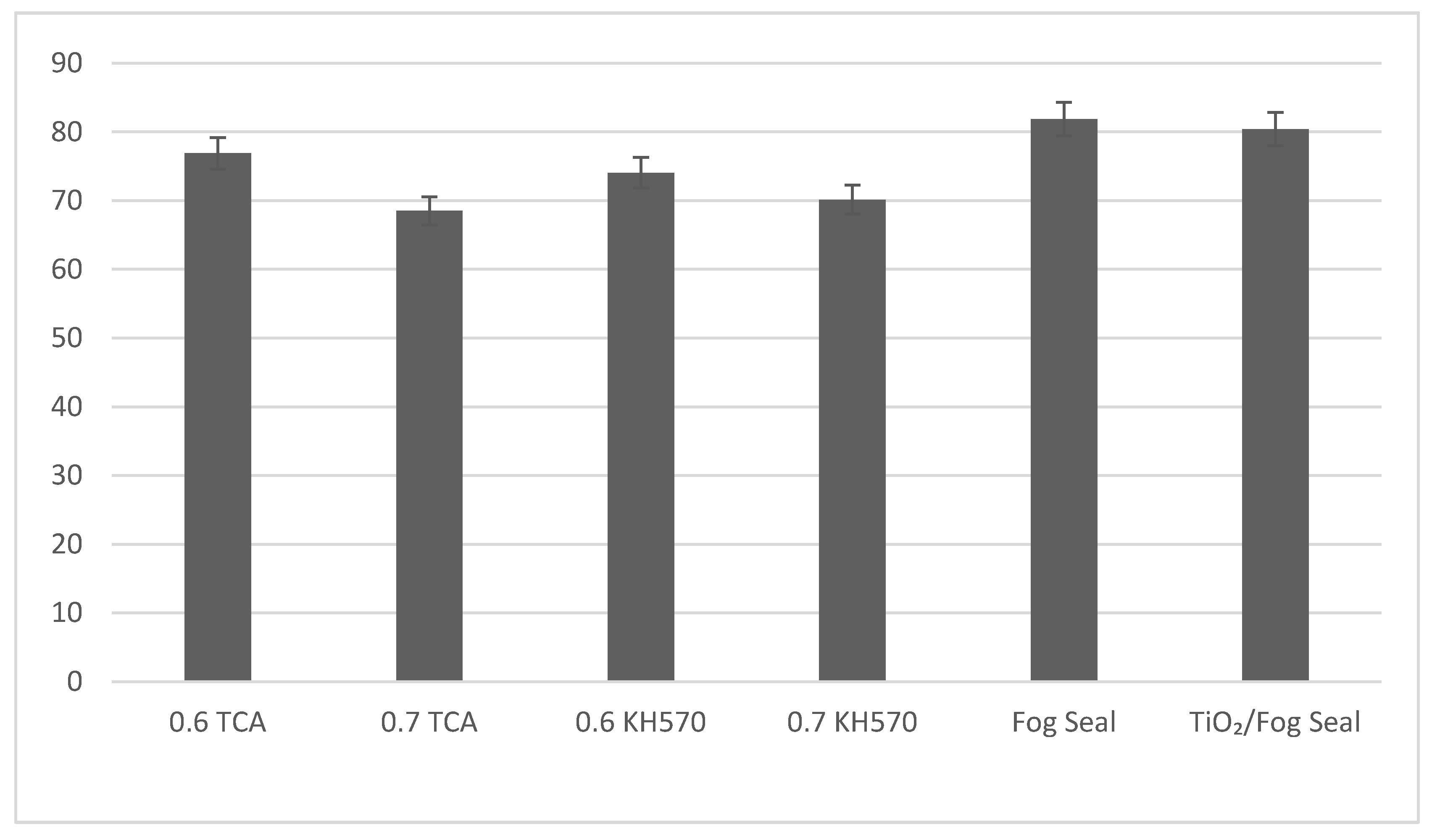
4.3. Outdoor Degradation Performance
5. Discussion
6. Conclusions
7. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Hassan, M.; Mohammad, L.N.; Asadi, S.; Dylla, H.; Cooper, S. Sustainable photocatalytic asphalt pavements for mitigation of Nitrogen Oxide and Sulfur Dioxide vehicle emissions. J. Mater. Civ. Eng. 2013, 25, 365–371. [Google Scholar] [CrossRef]
- Nadiri, A.; Hassan, M.M.; Asadi, S.; Information, R. Supervised intelligence committee machine to evaluate field performance of photocatalytic asphalt pavement for ambient air purification. Transp. Res. Rec. J. Transp. Res. Board 2019, 2528, 96–105. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes. Rendus. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Wengang, Z. Research on Catalytic Decomposition of Automobile Exhaust Asphalt Pavement Material by TiO2; Chang’an University: Xi’an, China, 2014. [Google Scholar]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 43, 229–251. [Google Scholar] [CrossRef]
- Chen, M.; Baglee, D.; Chu, J.W.; Du, D.; Guo, X. Photocatalytic oxidation of NOx under visible light on asphalt-pavement surface. J. Mater. Civ. Eng. 2017, 29, 04017133. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Hassan, M.; Asadi, S.; Kevern, J.; Rupnow, T. Nitrogen Oxide reduction and Nitrate measurements on TiO2 photocatalytic pervious concrete pavement. In Construction Research Congress 2012: Construction Challenges in a Flat World; American Society of Civil Engineers: Reston, VA, UAS, 2012; pp. 1920–1930. [Google Scholar] [CrossRef]
- Wang, D.; Leng, Z.; Yu, H.; Hüben, M.; Kollmann, J.; Oeser, M. Durability of epoxy-bonded TiO2-modified aggregate as a photocatalytic coating layer for asphalt pavement under vehicle tire polishing. Wear 2017, 382, 1–7. [Google Scholar] [CrossRef]
- Wang, D.; Leng, Z.; Hüben, M.; Oeser, M.; Steinauer, B. Photocatalytic pavements with epoxy-bonded TiO2-containing spreading material. Constr. Build. Mater. 2016, 107, 44–51. [Google Scholar] [CrossRef]
- Hu, C.; Ma, J.; Jiang, H.; Zhao, J.; Chen, Z.; Singh, D.; Valentin, J.; Liu, Z. Study on exhaust degradation material for asphalt pavement. Geo-China 2016, 20162016, 38–44. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A 2019, 382, 111941. [Google Scholar] [CrossRef]
- Araña, J. TiO2 activation by using activated carbon as a support Part II. Photoreactivity and FTIR study. Appl. Catal. B Environ. 2003, 44, 153–160. [Google Scholar] [CrossRef]
- Araña, J. TiO2 activation by using activated carbon as a support Part I. Surface characterisation and decantability study. Appl. Catal. B Environ. 2003, 44, 161–172. [Google Scholar] [CrossRef]
- Zohoori, S.; Karimi, L. Effect of nano SrTiO3 supporting nano TiO2 on self-cleaning of cotton fabric. Fibers Polym. 2013, 14, 996–1000. [Google Scholar] [CrossRef]
- Reis, H.; Papadopoulos, M.; Calaminici, P.; Jug, K.; Köster, A. Calculation of macroscopic linear and nonlinear optical susceptibilities for the naphthalene, anthracene and meta-nitroaniline crystals. Chem. Phys. 2000, 261, 359–371. [Google Scholar] [CrossRef]
- Chao, Y.; Guochao, G.; Xiping, L.; Chujie, Y.; Lude, L.; Xin, W. Study on surface modification of nano-titanium dioxide by silane coupling agent. J. Inorg. Mater. 2007, 2, 61–67. [Google Scholar]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Effect of surfactant in a modified sol on the physicochemical properties and photocatalytic activity of crystalline TiO2 nanoparticles. Top. Catal. 2007, 44, 513–521. [Google Scholar] [CrossRef]
- Jiang, W.; Yuan, D.; Shan, J.; Ye, W.; Lu, H.; Sha, A. Experimental study of the performance of porous ultra-thin asphalt overlay. Int. J. Pavement Eng. 2020, 1–13. [Google Scholar] [CrossRef]
- Gong, F.; Guo, S.; Chen, S.; You, Z.; Liu, Y.; Dai, Q. Strength and durability of dry-processed stone matrix asphalt containing cement pre-coated scrap tire rubber particles. Constr. Build. Mater. 2019, 214, 475–483. [Google Scholar] [CrossRef]
- Gong, F.; Liu, Y.; Zhou, X.; You, Z. Lab assessment and discrete element modeling of asphalt mixture during compaction with elongated and flat coarse aggregates. Constr. Build. Mater. 2018, 182, 573–579. [Google Scholar] [CrossRef]
- Gong, F.; Zhou, X.; You, Z.; Liu, Y.; Chen, S. Using discrete element models to track movement of coarse aggregates during compaction of asphalt mixture. Constr. Build. Mater. 2018, 189, 338–351. [Google Scholar] [CrossRef]
- Hassan, M.M.; Dylla, H.; Mohammad, M.L.N.; Rupnow, T. Evaluation of the durability of titanium dioxide photocatalyst coating for concrete pavement. Constr. Build. Mater. 2010, 24, 1456–1461. [Google Scholar] [CrossRef]
- Dylla, H.; Hassan, M.M.; Osborn, D. Field evaluation of ability of photocatalytic concrete pavements to remove nitrogen oxides. Transp. Res. Rec. J. Transp. Res. Board 2012, 2290, 154–160. [Google Scholar] [CrossRef]
- Hassan, M.M.; Dylla, H.; Asadi, S.; Mohammad, L.N.; Cooper, S. Laboratory evaluation of environmental performance of photocatalytic titanium dioxide warm-mix asphalt pavements. J. Mater. Civ. Eng. 2012, 24, 599–605. [Google Scholar] [CrossRef]
- Krýsa, J.; Baudys, M.; Vislocka, X.; Neumann-Spallart, M. Composite photocatalysts based on TiO2—Carbon for air pollutant removal: Aspects of adsorption. Catal. Today 2020, 340, 34–39. [Google Scholar] [CrossRef]







| Project | Units | Indicators |
|---|---|---|
| Specific surface area | m2/g | ≥280 |
| Particle size | nm | 30 |
| Purity | % | ≥99.9 |
| Bulk density | g/cm3 | 0.2–0.3 |
| Project | Units | Indicators |
|---|---|---|
| Specific surface area | m2/g | 900–1100 |
| Purity | % | ≥99.9 |
| Filling density | g/cm3 | 0.45–0.55 |
| Project | Units | Indicators |
|---|---|---|
| Proportion of 25 °C | - | ≥1.18 |
| Dry time | h | ≤8 |
| Volatile | - | Maximum loss of 10% weight |
| Model | Chemical Name | Molecular Formula | Commercial Name |
|---|---|---|---|
| KH550 | 3-Aminopropyltriethoxysilane | C9H23NO3Si | Union Carbide Corporation: A-1100 |
| KH570 | 3-Glycidoxypropyltrimethoxysilane | C10H20O5Si | Union Carbide Corporation: A-174 |
| KH792 | [3-(2-Aminoethyl)aminopropyl]trimethoxysilane | C8H22N2O3Si | Union Carbide Corporation: A-1120 |
| TCA | Isopropyl tri(dioctylpyrophosphate) titanate | C16H37O7P2 | Kenrich Corporation: KR-38S |
| Ratio | 0 | 0.1 | 0.3 | 0.5 | 0.6 | 0.7 | 1 |
|---|---|---|---|---|---|---|---|
| Active Carbon (g) | 0 | 0.1 | 0.3 | 0.5 | 0.6 | 0.7 | 1 |
| Titanium Dioxide (g) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Xiao, Q.; Gong, F.; Yang, Y.; Ren, X. Functional Materials Based on Active Carbon and Titanium Dioxide in Fog Seal. Materials 2020, 13, 5267. https://doi.org/10.3390/ma13225267
He C, Xiao Q, Gong F, Yang Y, Ren X. Functional Materials Based on Active Carbon and Titanium Dioxide in Fog Seal. Materials. 2020; 13(22):5267. https://doi.org/10.3390/ma13225267
Chicago/Turabian StyleHe, Chuan, Qingyi Xiao, Fangyuan Gong, Yun Yang, and Xipeng Ren. 2020. "Functional Materials Based on Active Carbon and Titanium Dioxide in Fog Seal" Materials 13, no. 22: 5267. https://doi.org/10.3390/ma13225267
APA StyleHe, C., Xiao, Q., Gong, F., Yang, Y., & Ren, X. (2020). Functional Materials Based on Active Carbon and Titanium Dioxide in Fog Seal. Materials, 13(22), 5267. https://doi.org/10.3390/ma13225267





