Evolutive Models for the Geometry and Heat Conductivity of an Intumescent EVA-ATH Composite during Its Thermal Degradation
Abstract
1. Introduction
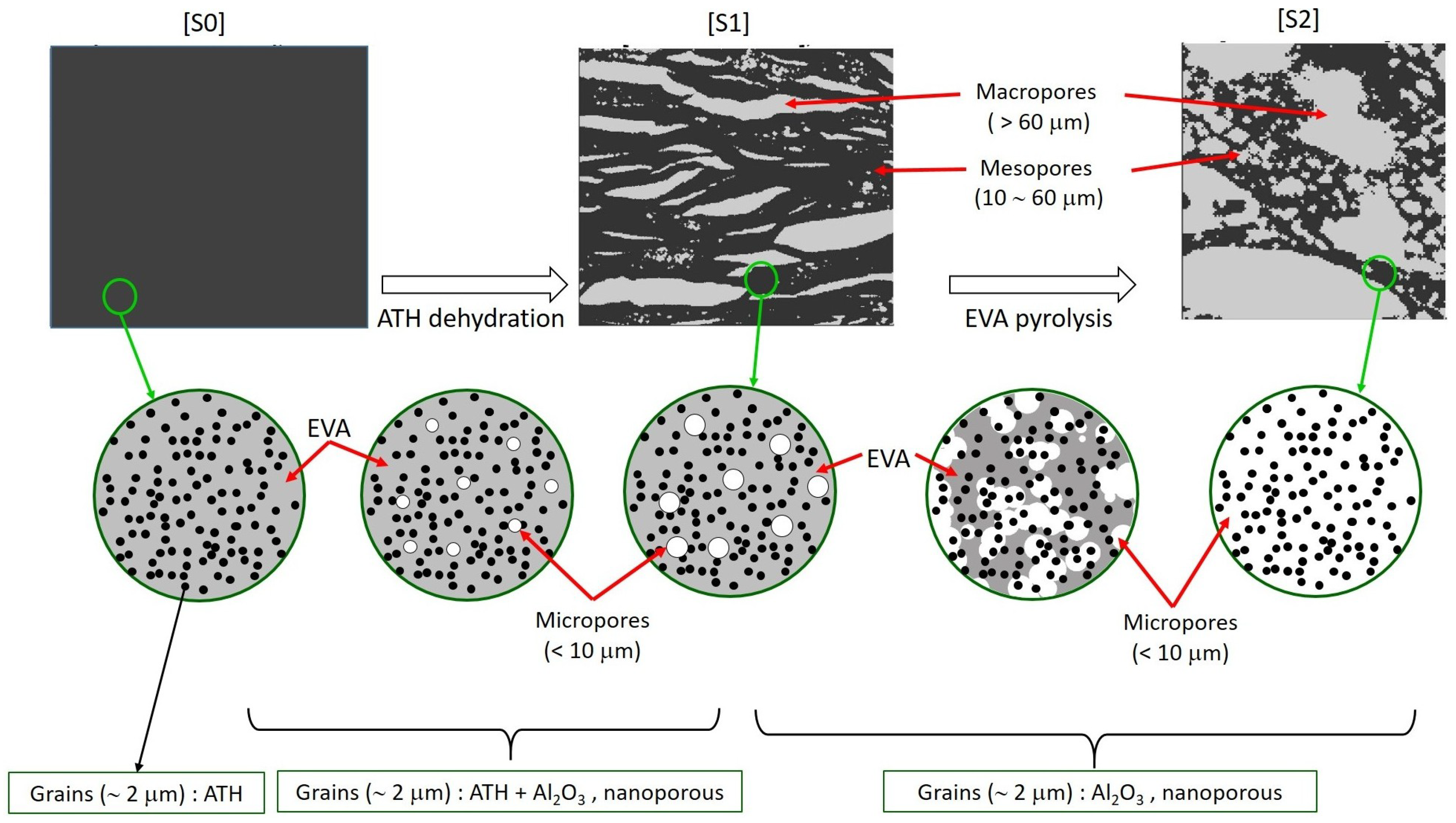
2. Geometric Characterization
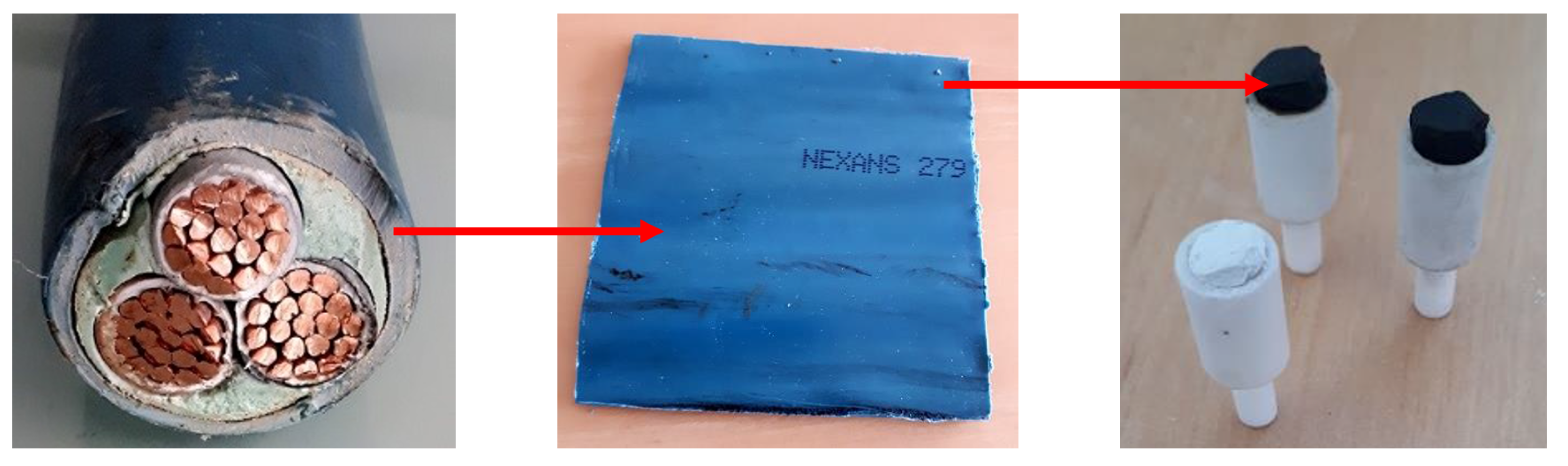
2.1. Characteristics of the Investigated Material
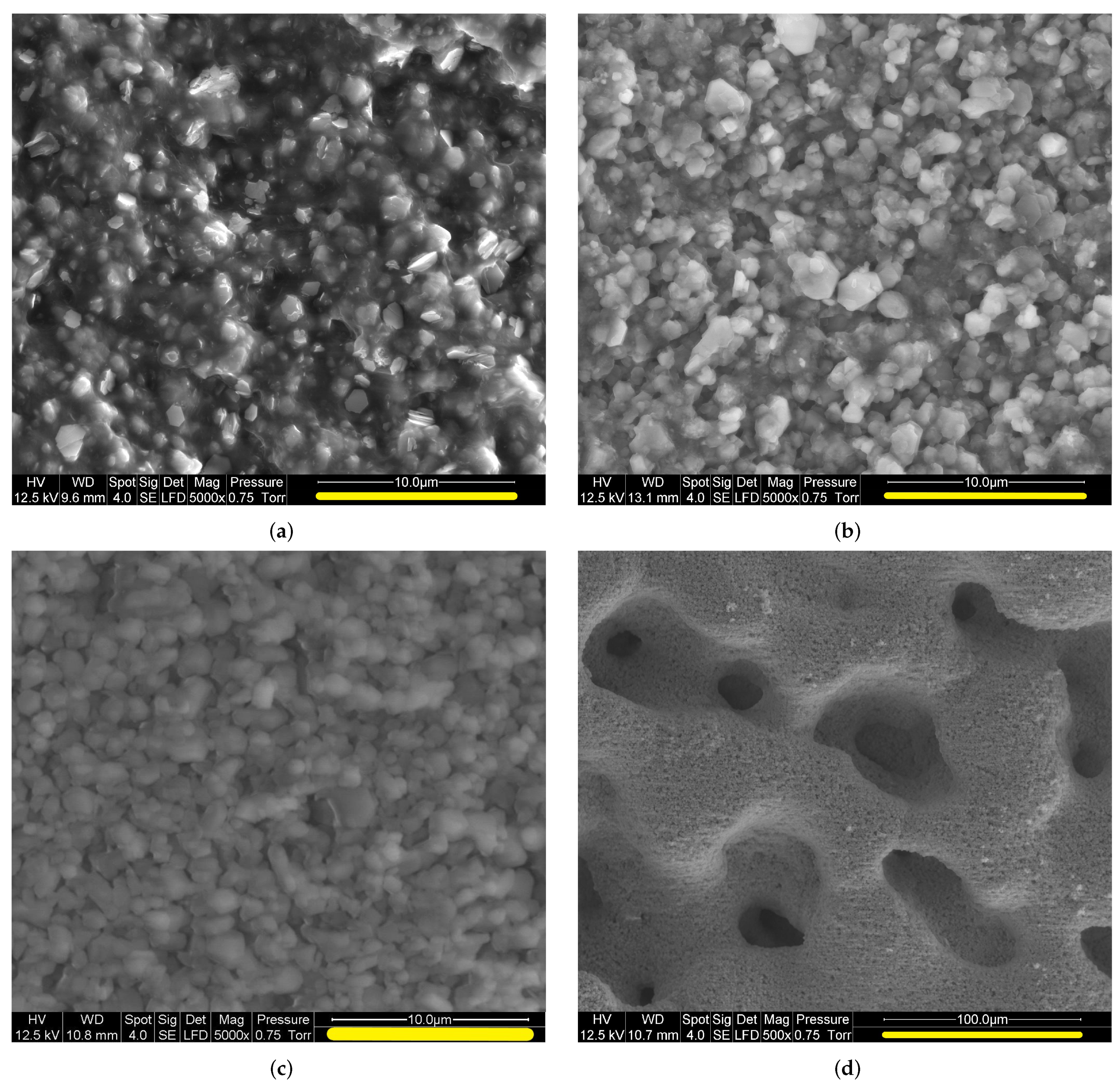
2.2. SEM Observation
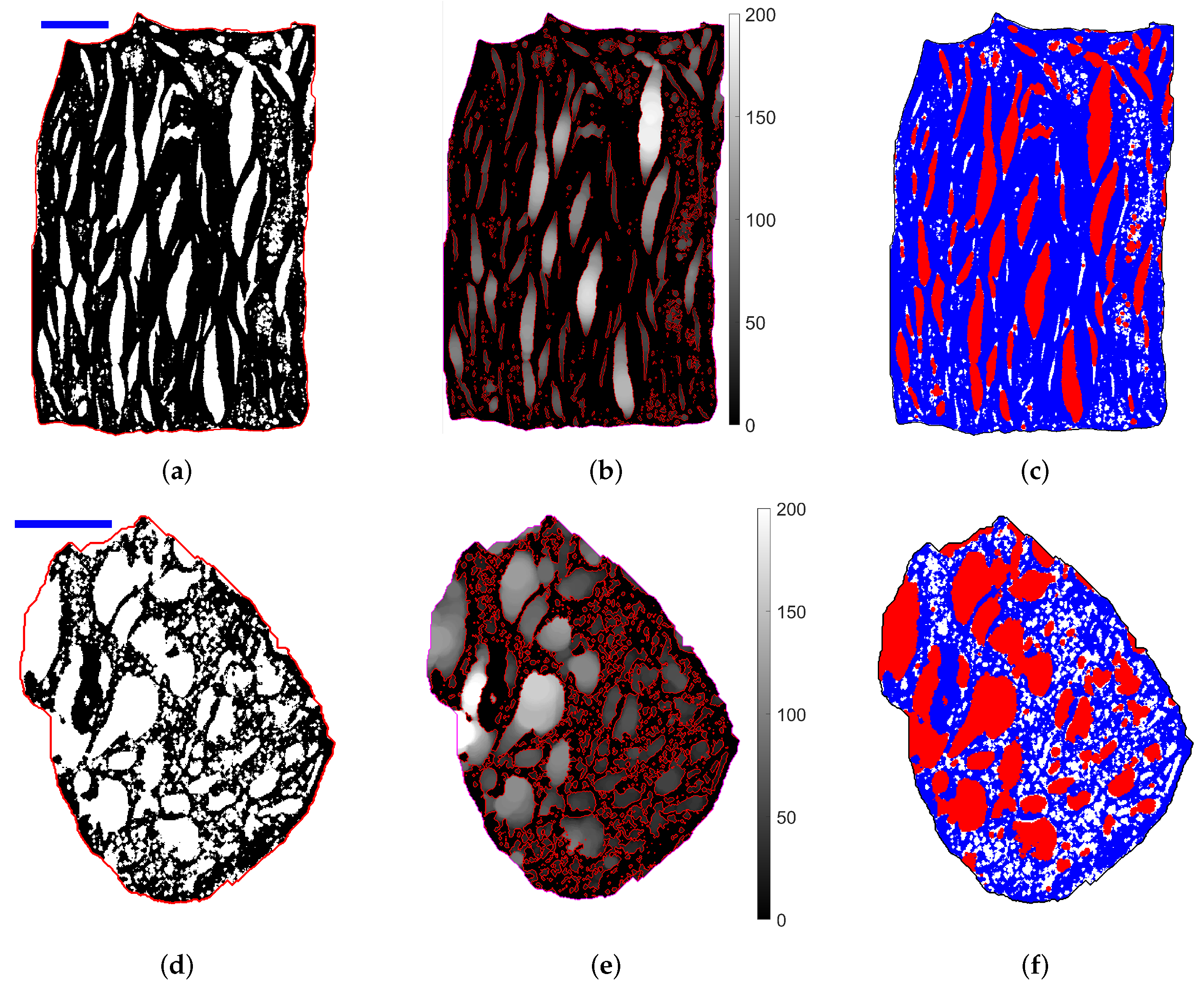
2.3. Tomography Observation
2.3.1. Pore Size Spectrum and Scale Separation
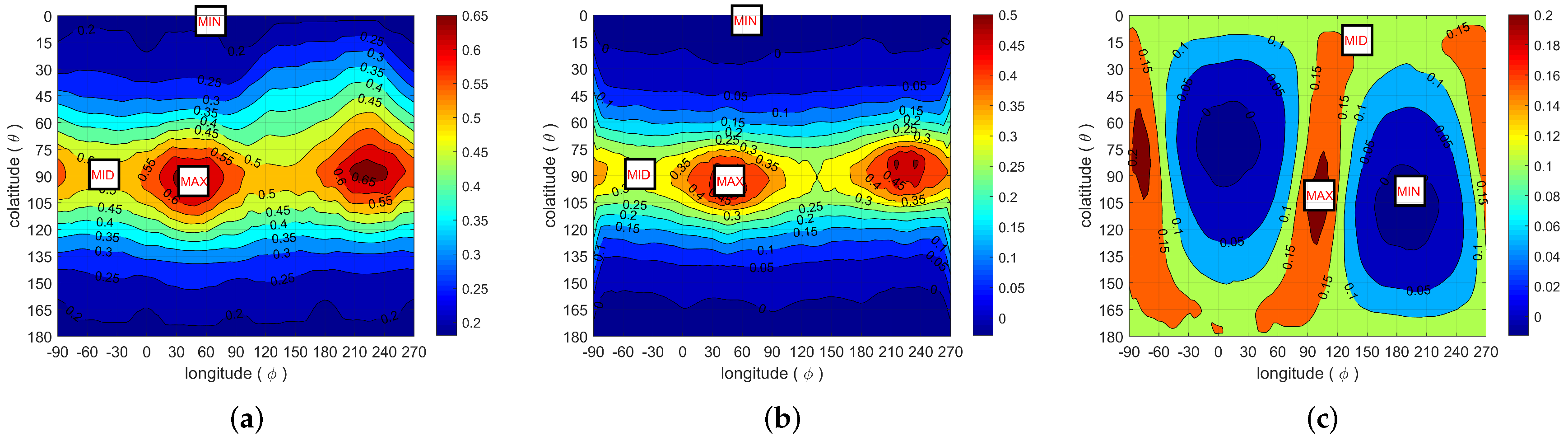
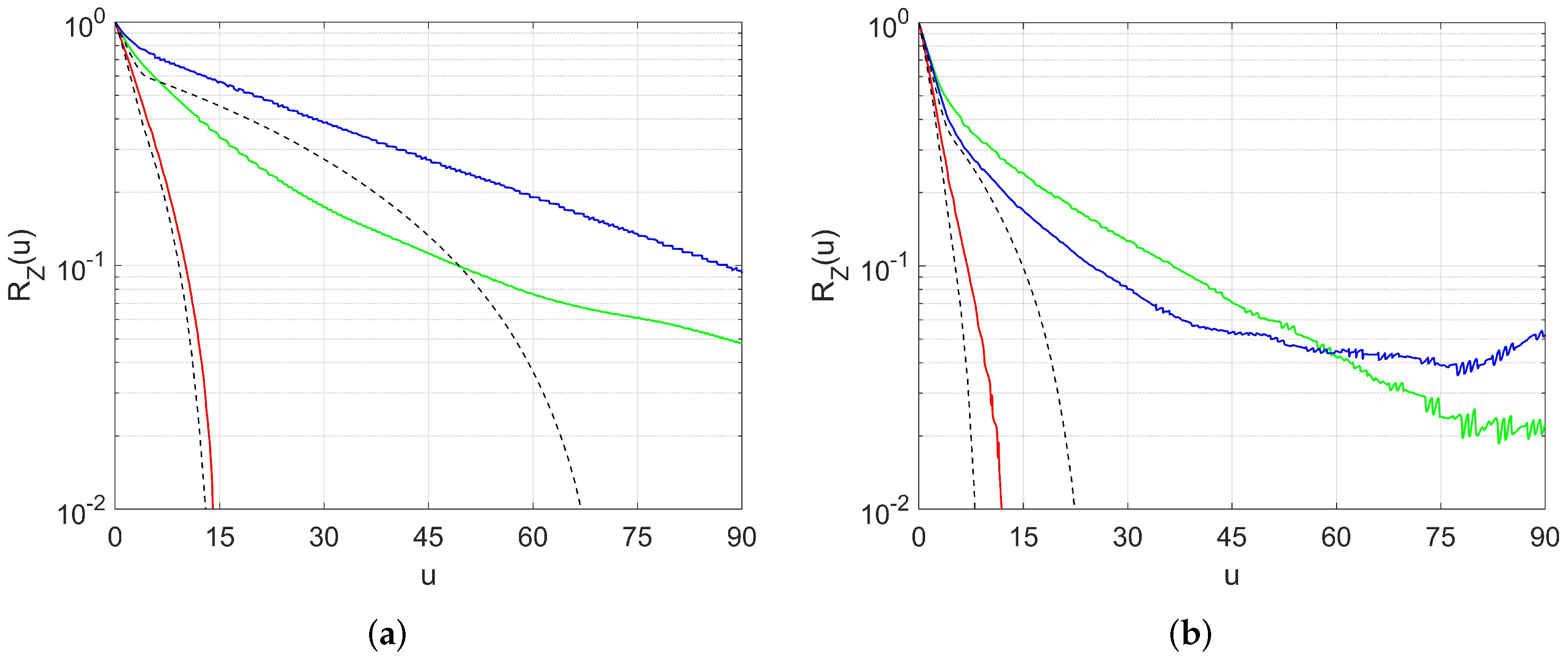
2.3.2. Spatial Correlation and Anisotropy Determination
2.3.3. Pore Segmentation and Equivalent Ellipsoids
- The mesopores are fairly isotropic and they can be represented by spheres;
- The macropores are anisotropic, and they can be represented by oblate ellipsoids, with their minor semi-axis oriented along the direction corresponding to the most rapidly decreasing correlations.
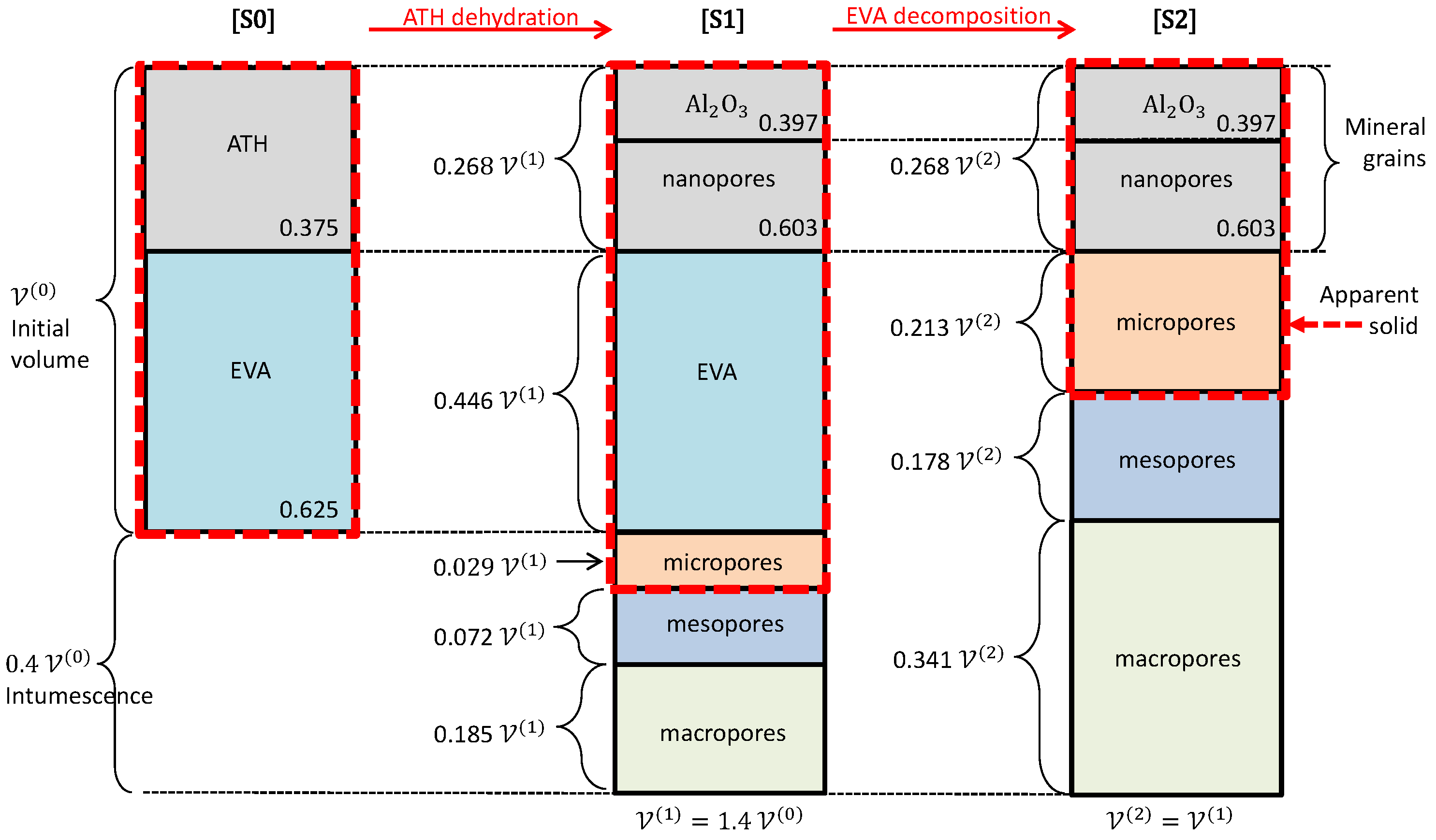
2.4. Apparent Solid, Nano and Micro-Porosity
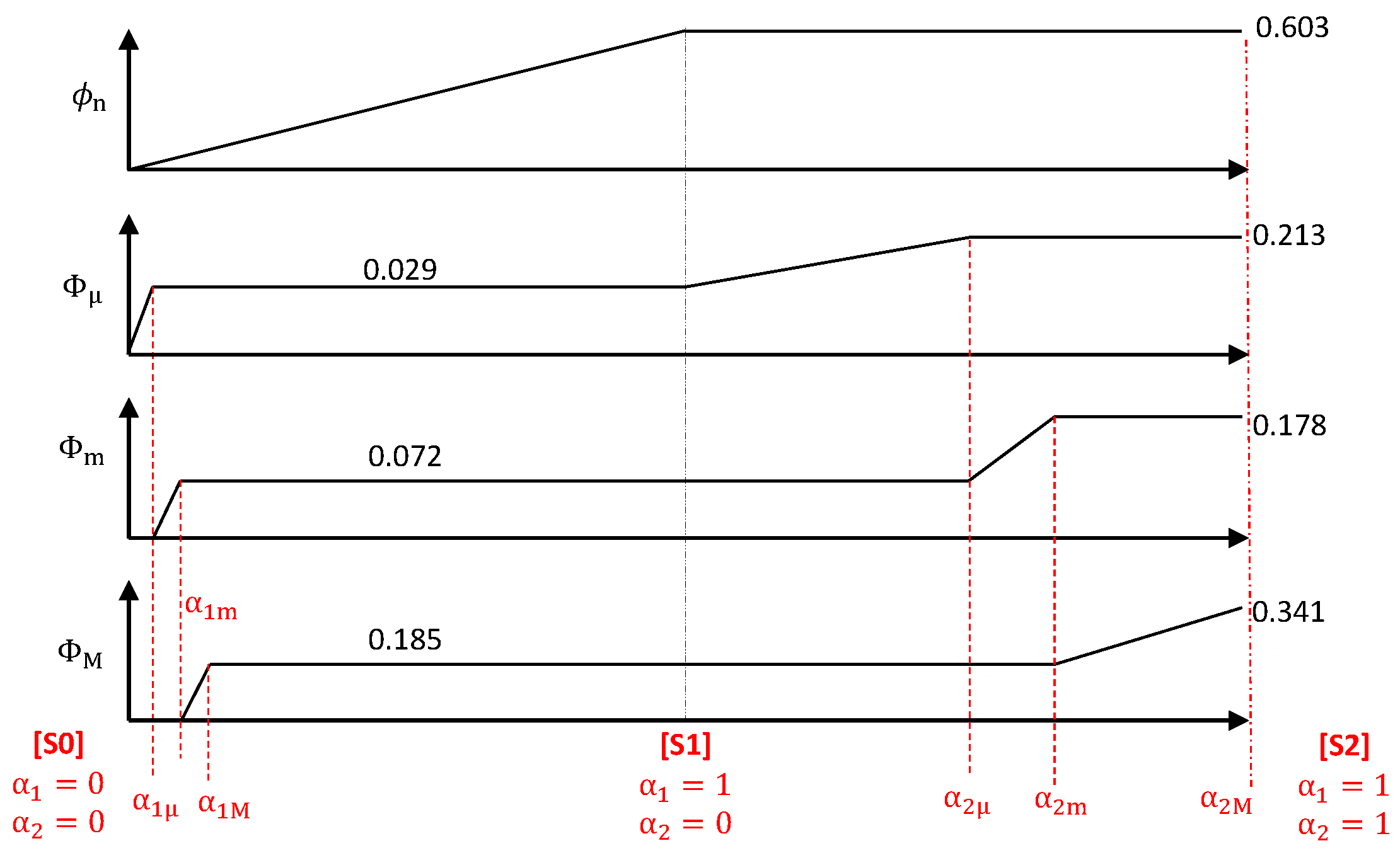
3. Evolutive Geometrical Model
3.1. Pem/Psm—Morphological Model for [S1] and [S2]
3.2. A Scenario for the Morphological Evolution
4. Thermal Conductivity Modeling
4.1. DNS Based on the Tomographies of [S1] and [S2]
4.2. DNS in Pem/Psm Samples
4.3. Differential Effective-Medium Model (DEM)
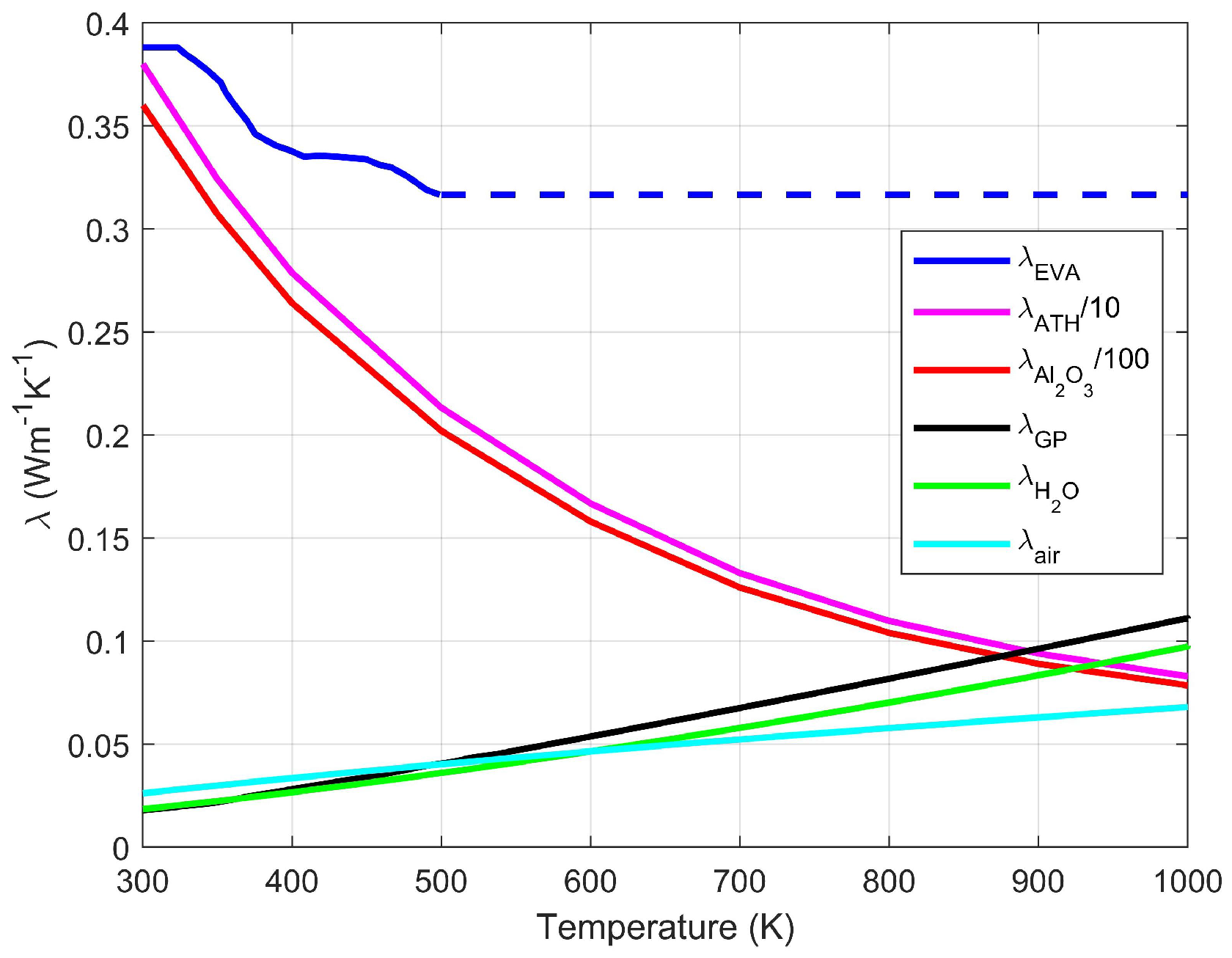
4.4. Conductivity of the Apparent Solid
4.5. Evolutive Conductivity Model
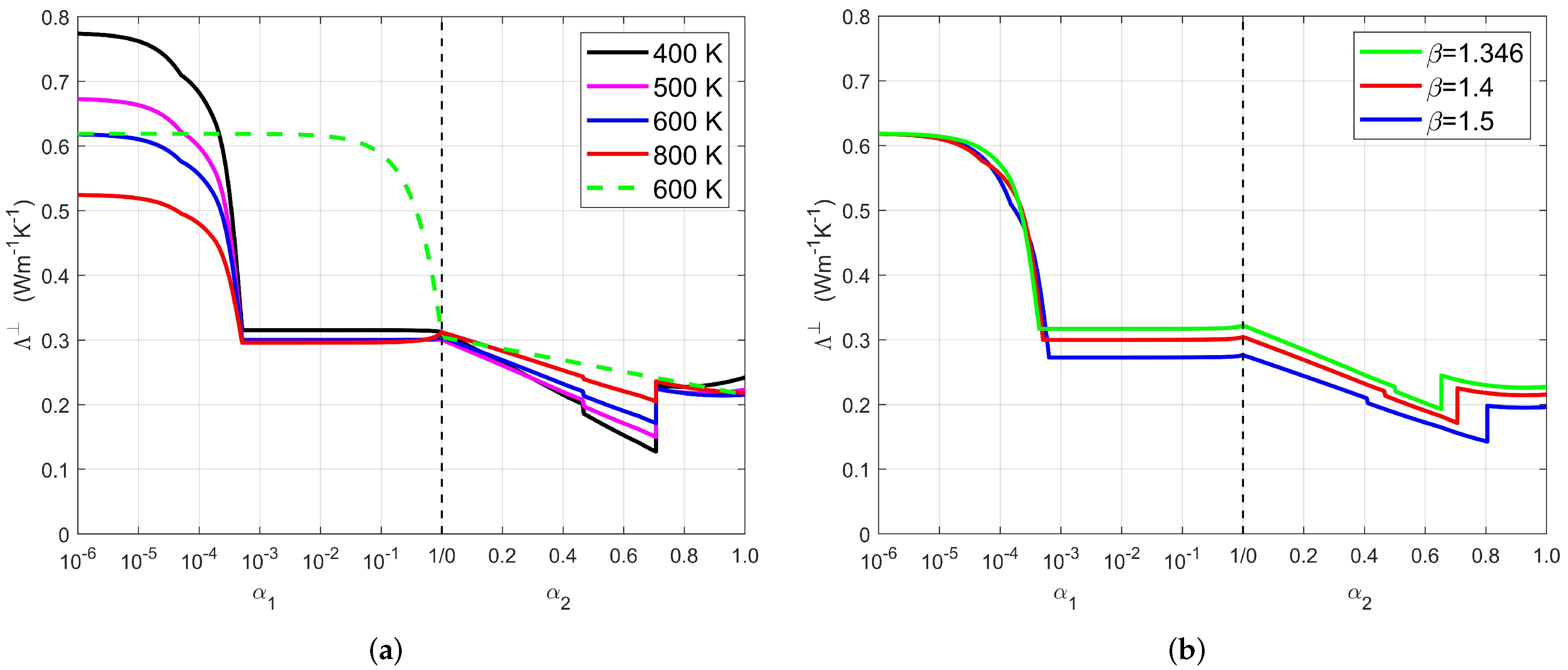
4.6. Application of the Model
5. Discussion
5.1. Sensitivity to Some Possible Sources of Error
5.2. Confrontation with Experiments
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATH | Alumina Trihydrate |
| EVA | Ethylvinyl Acetate |
| SEM | Scanning Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| DNS | Direct Numerical Simulation |
| PEM | Penetrable Ellipsoid Model |
| PSM | Penetrable Sphere Model |
| DEM | Differential Effective-Medium model |
| SSC | Symmetric Self-Consistent Scheme |
Appendix A. Sizes and Shapes of the Individual Inclusions
Appendix A.1. Measurements
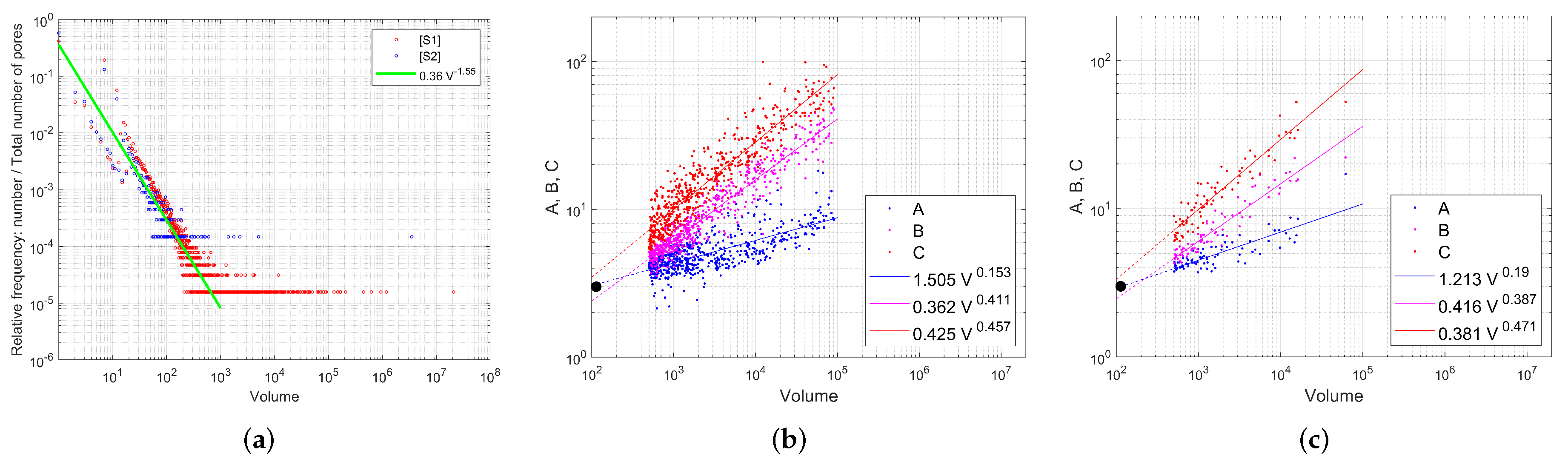
Appendix A.2. Modeling
Appendix B. Tensorial Distance
References
- Zavaleta, P.; Audouin, L. Fire spreading from a real open-doors electrical cabinet to overhead multiple cable trays into a confined and mechanically-ventilated facility. In Proceedings of the International Conference on Fire Research and Engineering (Interflam), London, UK, 4–6 July 2016; Committee, I., Ed.; Interscience Communications Ltd.: London, UK, 2016; Volume 14, pp. 1563–1574. [Google Scholar]
- Stoliarov, S.I.; Crowley, S.; Lyon, R.E.; Linteris, G.T. Prediction of the burning rates of non-charring polymers. Combust. Flame 2009, 156, 1068–1083. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Crowley, S.; Walters, R.N.; Lyon, R.E. Prediction of the burning rates of charring polymers. Combust. Flame 2010, 157, 2024–2034. [Google Scholar] [CrossRef]
- Matala, A.; Hostikka, S. Pyrolysis Modelling of PVC Cable Materials. In Proceedings of the Fire Safety Science—Tenth International Symposium, College Park, MD, USA, 19–24 June 2011; pp. 917–930. [Google Scholar]
- Boyer, G. Fully coupled CFD simulation of the pyrolysis of non-charring polymers: A predictive approach. Fire Saf. J. 2017, 91, 208–217. [Google Scholar] [CrossRef]
- Swann, J.D.; Ding, Y.; Stoliarov, S.I. Characterization of pyrolysis and combustion of rigid poly(vinyl chloride) using two-dimensional modeling. Int. J. Heat Mass Transf. 2019, 132, 347–361. [Google Scholar] [CrossRef]
- Kaviany, M. Principles of Heat Transfer in Porous Media, 2nd ed.; Mechanical Engineering Series; Springer: New York, NY, USA, 1995. [Google Scholar]
- Torquato, S. Random Heterogeneous Materials—Microstructure and Macroscopic Properties, 1st ed.; Interdisciplinary Applied Mathematics; Springer: New York, NY, USA, 2002; Volume 16. [Google Scholar]
- Thovert, J.-F.; Wary, F.; Adler, P.M. Thermal conductivity of random media and regular fractals. J. Appl. Phys. 1990, 68, 3872–3883. [Google Scholar] [CrossRef]
- Yuan, J. Intumescent Coating Performance on Steel Structures under Realistic Fire Conditions. Ph.D. Thesis, University of Manchester, Manchester, UK, 2009. [Google Scholar]
- Wang, L.L.; Wang, Y.C.; Yuan, J.F.; Li, G.Q. Thermal conductivity of intumescent coating char after accelerated aging. Fire Mater. 2013, 37, 440–456. [Google Scholar] [CrossRef]
- Staggs, J. Thermal conductivity estimates of intumescent chars by direct numerical simulation. Fire Saf. J. 2010, 45, 228–237. [Google Scholar] [CrossRef]
- Muller, M.; Bourbigot, S.; Duquesne, S.; Klein, R.; Giannini, G.; Lindsay, C.; Vlassenbroeck, J. Investigation of the synergy in intumescent polyurethane by 3D computed tomography. Polym. Degrad. Stab. 2013, 98, 1638–1647. [Google Scholar] [CrossRef]
- Okyay, G.; Naik, A.D.; Samyn, F.; Jimenez, M.; Bourbigot, S. Fractal conceptualization of intumescent fire barriers, toward simulations of virtual morphologies. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.C.; Bailey, C.G.; Taylor, A.P. Global modelling of fire protection performance of an intumescent coating under different furnace fire conditions. J. Fire Sci. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Cirpici, B.K.; Wang, Y.; Rogers, B. Assessment of the thermal conductivity of intumescent coatings in fire. Fire Saf. J. 2016, 81, 74–84. [Google Scholar] [CrossRef]
- Girardin, B.; Fontaine, G.; Duquesne, S.; Forsth, M.; Bourbigot, S. Characterization of Thermo-Physical Properties of EVA/ATH: Application to Gasification Experiments and Pyrolysis Modeling. Materials 2015, 161, 7837–7863. [Google Scholar] [CrossRef] [PubMed]
- Girardin, B. Numerical Modelling and Small Scale Testing of Fire Performances for Halogen-Free Cable. Ph.D. Thesis, Université Lille 1—Sciences et Technologies, Lille, France, 2016. [Google Scholar]
- Shi, J. Simulation de la Pyrolyse de Gaines de Câbles électriques Exposés au feu: Caractérisation et Modélisation de la Morphologie et de la Conductivité Thermique Selon L’état de Dégradation. Ph.D. Thesis, Université de Poitiers, Poitiers, France, 2019. Available online: http://www.theses.fr/2019POIT2311 (accessed on 5 November 2020).
- Laoutid, F.; Lorgouilloux, M.; Bonnaud, L.; Lesueur, D.; Dubois, P. Fire retardant behaviour of halogen-free calcium-based hydrated minerals. Polym. Degrad. Stab. 2017, 136, 89–97. [Google Scholar] [CrossRef]
- Ferry, L. Caractérisation de Résidus de Combustion de Câble électriques; Technical Report; IMT Mines Alès: Alès, France, 2018. [Google Scholar]
- Thovert, J.-F.; Yousefian, F.; Spanne, P.; Jacquin, C.G.; Adler, P.M. Grain reconstruction of porous media: Application to a low-porosity Fontainebleau sandstone. Phys. Rev. E 2001, 63, 61307–61323. [Google Scholar] [CrossRef]
- Duquesne, S.; Fontaine, G.; Cérin-Delaval, O.; Gardelle, B.; Tricot, G.; Bourbigot, S. Study of the thermal degradation of an aluminium phosphinate—Aluminium trihydrate combination. Thermochim. Acta 2013, 551, 175–183. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, P.; Wang, H.; Zhang, S.; Wang, S. Property of ethylene vinyl acetate copolymer in melting processing. J. Appl. Polym. Sci. 2006, 101, 2022–2026. [Google Scholar] [CrossRef]
- Moyano, M.A.; París, R.; Martín-Martínez, J.M. Viscoelastic and adhesion properties of hot-melts made with blends of ethylene-co-n-butyl acrylate (EBA) and ethylene-co-vinyl acetate (EVA) copolymers. Int. J. Adhes. Adhes. 2019, 88, 34–42. [Google Scholar] [CrossRef]
- Shi, J.; Boyer, G.; Mourzenko, V.; Thovert, J.-F. On the Influence of Boundary Conditions when Determining Transport Coefficients from Finite Samples of Porous Media: Assessment for Tomographic Images of Real Materials. Transp. Porous Media 2020, 132, 561–590. [Google Scholar] [CrossRef]
- Henriette, A.; Jacquin, C.G.; Adler, P.M. The effective permeability of heterogeneous porous media. Phys. Chem. Hydrodyn. 1989, 11, 63–80. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism, 1st ed.; Clarendon Press: Oxford, UK, 1873. [Google Scholar]
- Landauer, R. Electrical conductivity in inhomogeneous media. AIP Conf. Proc. 1978, 40, 2–43. [Google Scholar]
- Powell, R.W.; Ho, C.Y.; Liley, P.E. Thermal Conductivity of Selected Materials; Dept. of Commerce, National Bureau of Standards: Washington, DC, USA, 1966. [Google Scholar]
- McBride, B.J.; Gordon, S.; Reno, M.A. Coefficients for Calculating Thermodynamic and Transport Properties of Individual Species; NASA Technical Memorandum 4513; NASA: Cleveland, OH, USA, 1993. [Google Scholar]
- Horai, K. Thermal conductivity of rock-forming minerals. J. Geophys. Res. (1896–1977) 1971, 76, 1278–1308. [Google Scholar] [CrossRef]
- Roufosse, M.C.; Klemens, P.G. Lattice thermal conductivity of minerals at high temperatures. J. Geophys. Res. (1896–1977) 1974, 79, 703–705. [Google Scholar] [CrossRef]
- Malinouskaya, I.; Mourzenko, V.V.; Thovert, J.-F.; Adler, P.M. Random packings of spiky particles: Geometry and transport properties. Phys. Rev. E 2009, 80, 011304. [Google Scholar] [CrossRef] [PubMed]
- Lautenberger, C. Gpyro3D: A Three Dimensional Generalized Pyrolysis Model. Fire Saf. Sci. 2014, 11, 193–207. [Google Scholar] [CrossRef]
- Shi, J.; Boyer, G.; Thovert, J.-F. Simulation of the pyrolysis of charring polymers: Influence of the porous media properties. J. Phys. 2018, 1107, 032008. [Google Scholar] [CrossRef]
- Snegirev, A.; Talalov, V.; Stepanov, V.; Harris, J. A new model to predict pyrolysis, ignition and burning of flammable materials in fire tests. Fire Saf. J. 2013, 59, 132–150. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Batiot, B.; Mourzenko, V.; Rogaume, T.; Thovert, J.-F. Thermal degradation of solid porous materials exposed to fire. In Proceedings of the 21ème Congrès Français de Mécanique, Bordeaux, France, 26–30 August 2013; Available online: http://hdl.handle.net/2042/52426 (accessed on 5 November 2020).
- Thovert, J.-F.; Mourzenko, V.V. On the influence of boundary conditions when determining transport coefficients from digital images of heterogeneous media. Adv. Water Resour. 2020, 141, 103612. [Google Scholar] [CrossRef]
- Thovert, J.-F.; Salles, J.; Adler, P.M. Computerized characterization of the geometry of real porous media: Their discretization, analysis and interpretation. J. Microsc. 1993, 170, 65–79. [Google Scholar] [CrossRef]


| Apparent Porosity | Meso-Porosity | Macro-Porosity | |||||
|---|---|---|---|---|---|---|---|
| = | |||||||
| [S1] | 0.257 | 0.072 | 30 m | 0.185 | 5.5 | 70 m | 385 m |
| [S2] | 0.519 | 0.178 | 30 m | 0.341 | 2.9 | 47 m | 136 m |
| ATH Dehydration | EVA Decomposition | |
|---|---|---|
| Microporosity | = 5 × 10 | = 0.413 |
| Mesoporosity | = 1.8 ×10 | = 0.652 |
| Macroporosity | = 5.1 ×10 | = 1 |
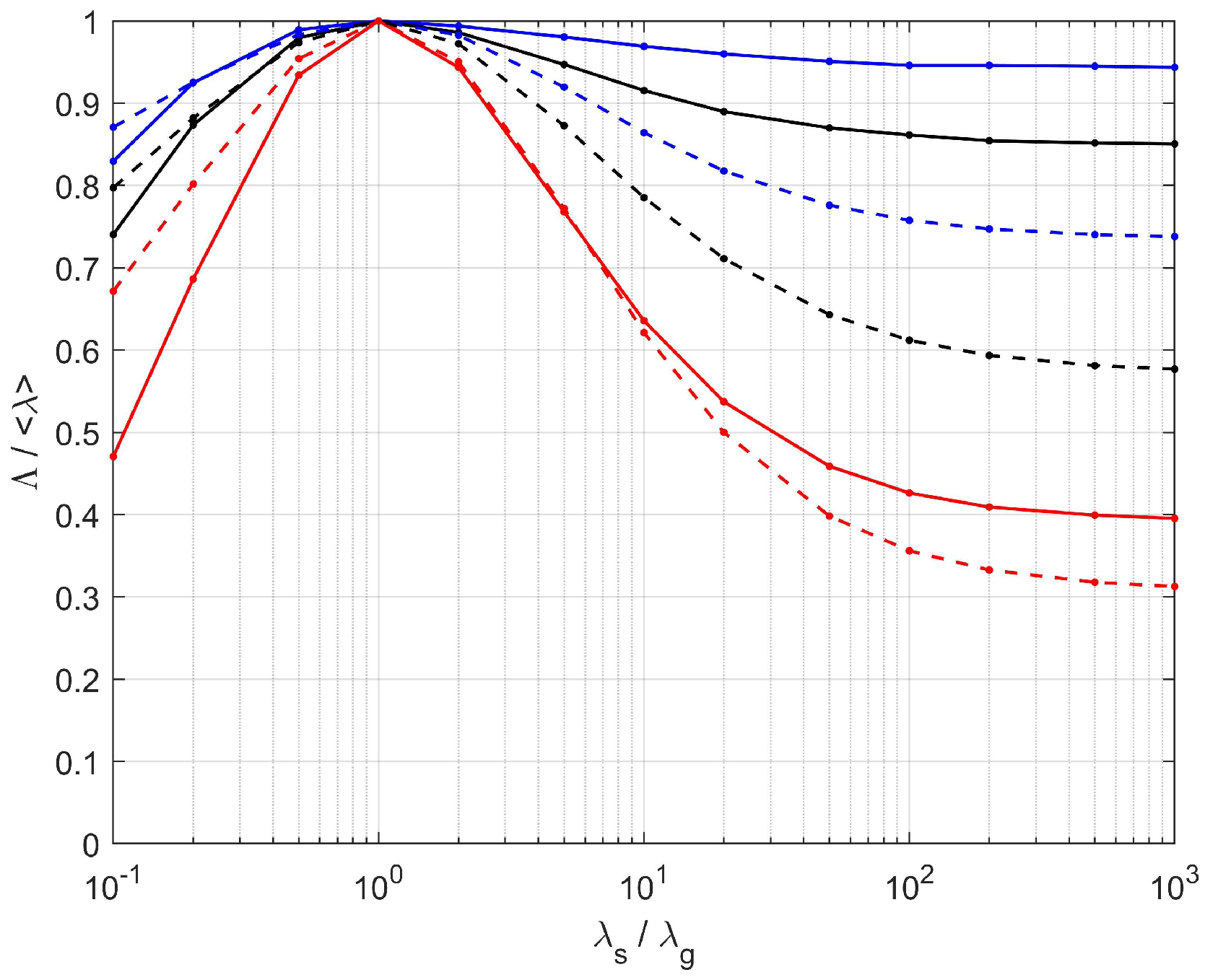
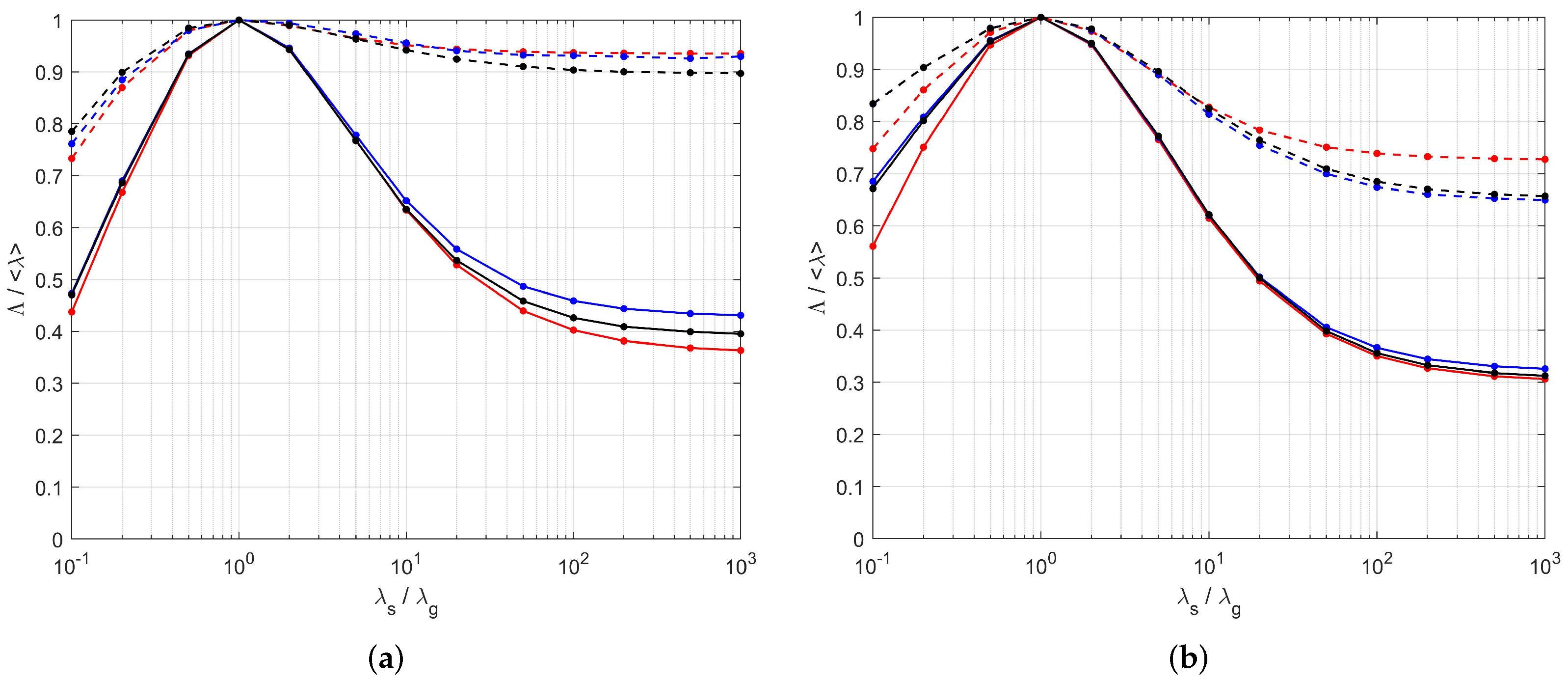
| PEM/PSM vs. Tomographies | Analytic Model vs. Tomographies | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 20 | 10 | 10 | 0.1 | 20 | 10 | 10 | |
| [S1], | 0.005 | 0.040 | 0.074 | 0.087 | −0.073 | −0.016 | −0.057 | −0.083 |
| [S1], | −0.030 | 0.017 | 0.031 | 0.037 | −0.066 | 0.021 | 0.037 | 0.043 |
| [S1], | 0.035 | 0.027 | 0.043 | 0.048 | 0.079 | 0.024 | 0.045 | 0.052 |
| [S2], | 0.020 | 0.004 | 0.028 | 0.041 | −0.179 | −0.011 | −0.016 | −0.020 |
| [S2], | −0.001 | −0.013 | −0.015 | −0.012 | −0.103 | 0.025 | 0.079 | 0.107 |
| [S2], | 0.017 | 0.014 | 0.018 | 0.024 | 0.151 | 0.028 | 0.092 | 0.124 |
| T (C) | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 500 | 600 |
|---|---|---|---|---|---|---|---|---|---|---|
| (sensor) | 0 | 0 | 0.0011 | 0.117 | 0.931 | 1 | 1 | 1 | 1 | 1 |
| (boundaries) | 0 | 0 | 0.0011 | 0.130 | 0.996 | 1 | 1 | 1 | 1 | 1 |
| (sensor) | 0 | 0 | 0 | 0 | 0 | 0.0004 | 0.036 | 0.759 | 1 | 1 |
| (boundaries) | 0 | 0 | 0 | 0 | 0 | 0.0004 | 0.037 | 0.832 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Boyer, G.; Mourzenko, V.; Thovert, J.-F. Evolutive Models for the Geometry and Heat Conductivity of an Intumescent EVA-ATH Composite during Its Thermal Degradation. Materials 2020, 13, 5258. https://doi.org/10.3390/ma13225258
Shi J, Boyer G, Mourzenko V, Thovert J-F. Evolutive Models for the Geometry and Heat Conductivity of an Intumescent EVA-ATH Composite during Its Thermal Degradation. Materials. 2020; 13(22):5258. https://doi.org/10.3390/ma13225258
Chicago/Turabian StyleShi, Jianwei, Germain Boyer, Valeri Mourzenko, and Jean-François Thovert. 2020. "Evolutive Models for the Geometry and Heat Conductivity of an Intumescent EVA-ATH Composite during Its Thermal Degradation" Materials 13, no. 22: 5258. https://doi.org/10.3390/ma13225258
APA StyleShi, J., Boyer, G., Mourzenko, V., & Thovert, J.-F. (2020). Evolutive Models for the Geometry and Heat Conductivity of an Intumescent EVA-ATH Composite during Its Thermal Degradation. Materials, 13(22), 5258. https://doi.org/10.3390/ma13225258





