Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Hydrotalcite
2.3. Material Characterization
2.4. Thermal Stability Testing of PVC–LDHs Composites
3. Results and Discussion
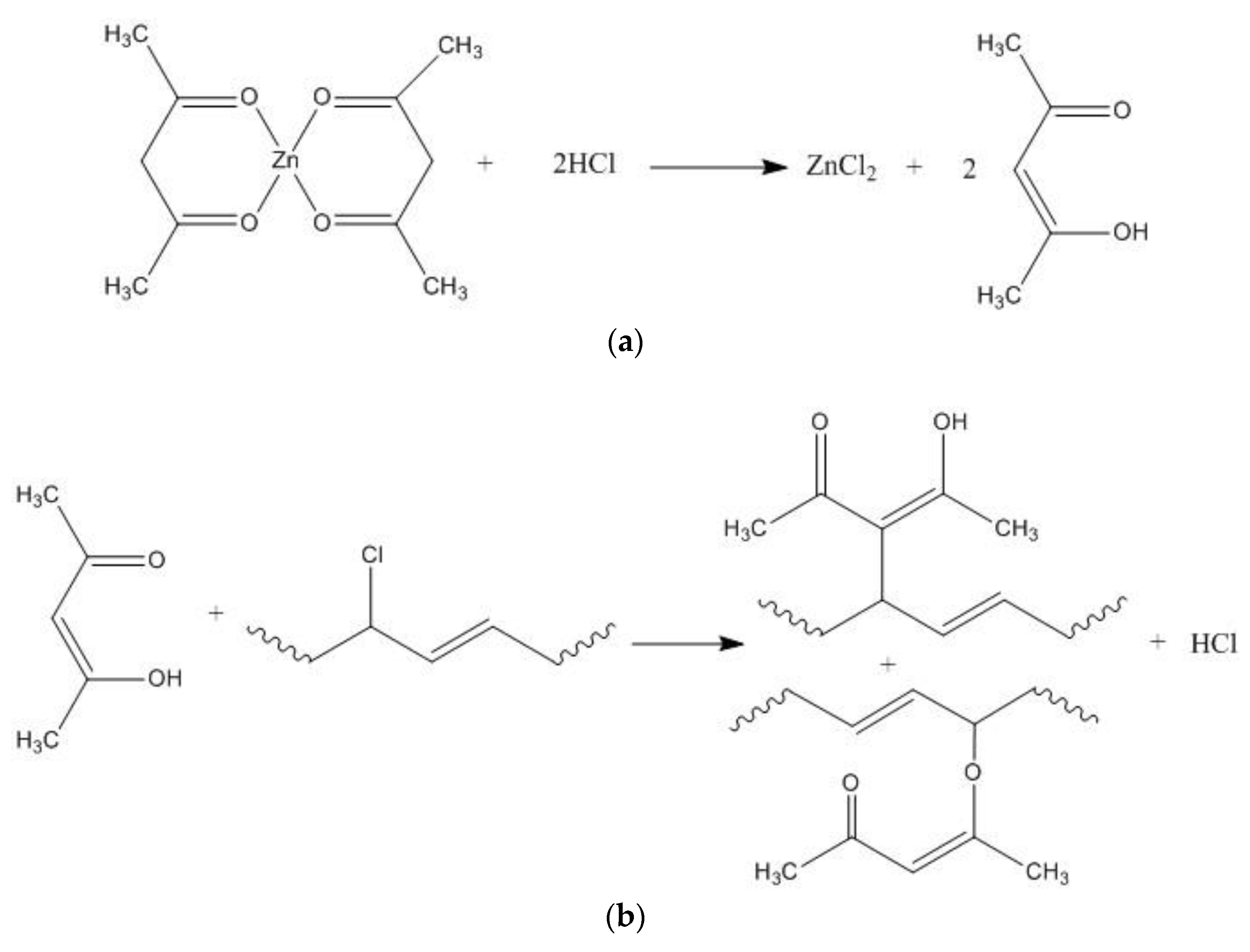
3.1. Characterization of Mg2Al-CO3 LDHs
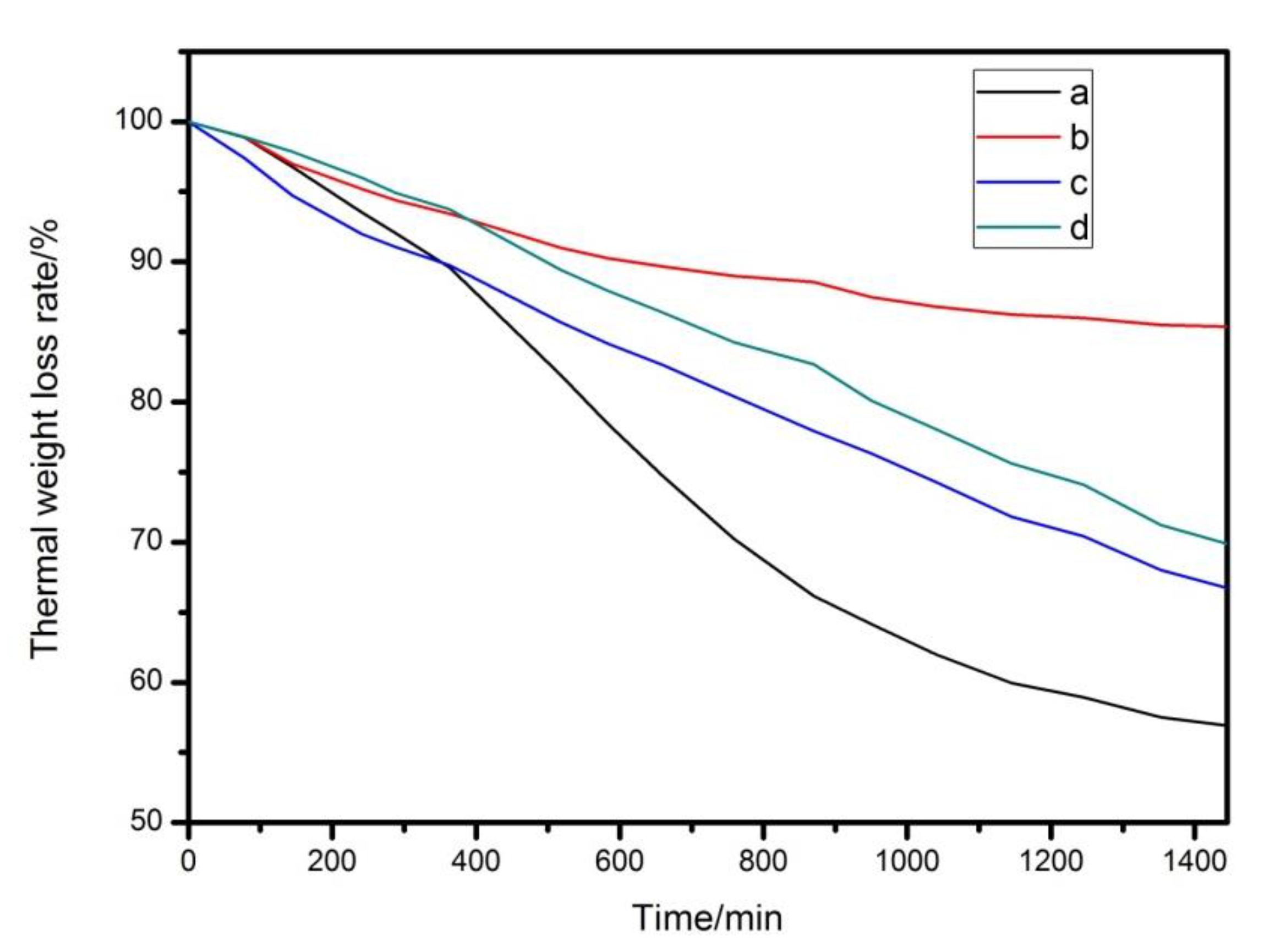
3.2. Thermal Stability of Mg2Al-CO3 LDHs on PVC
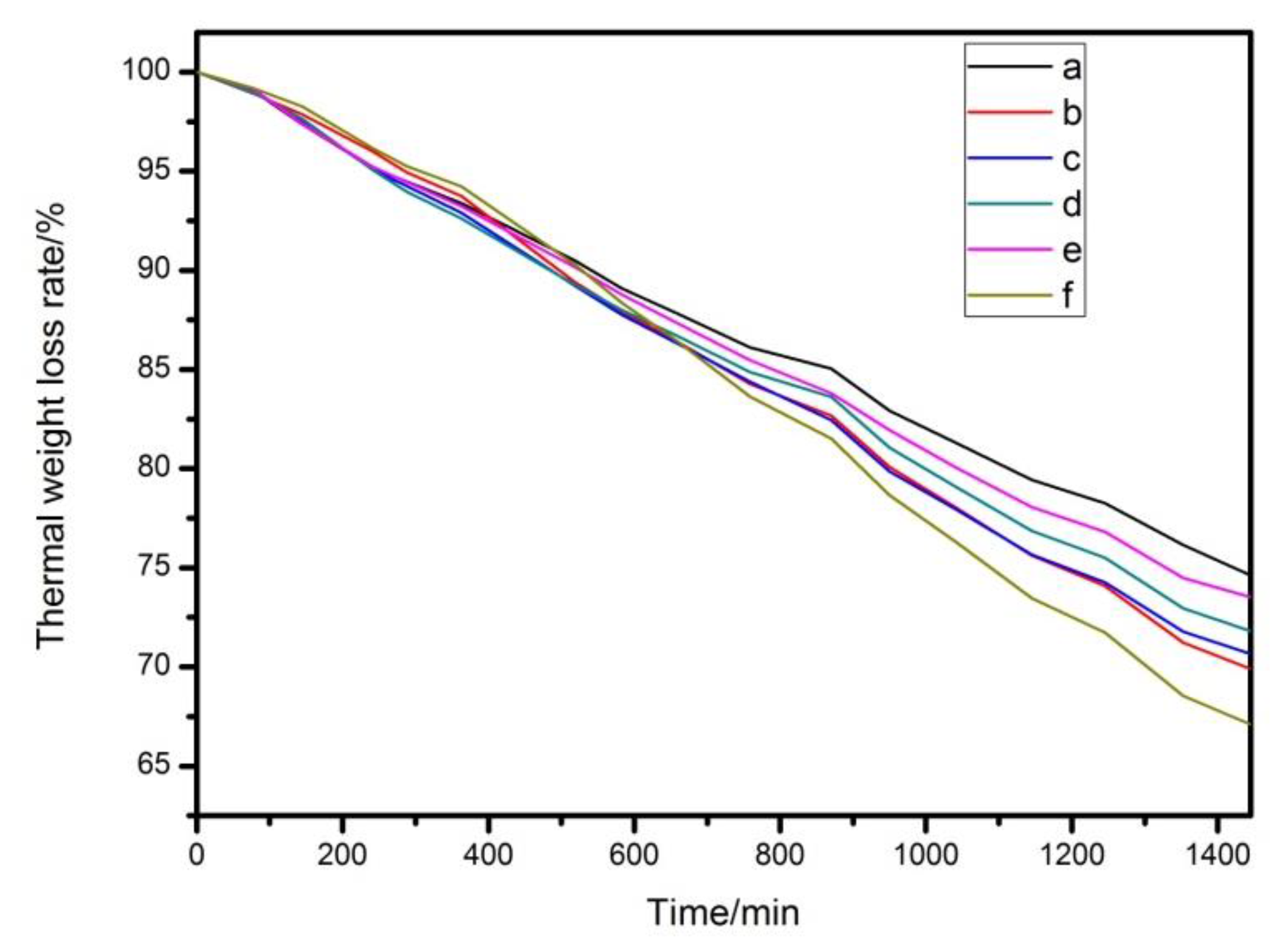
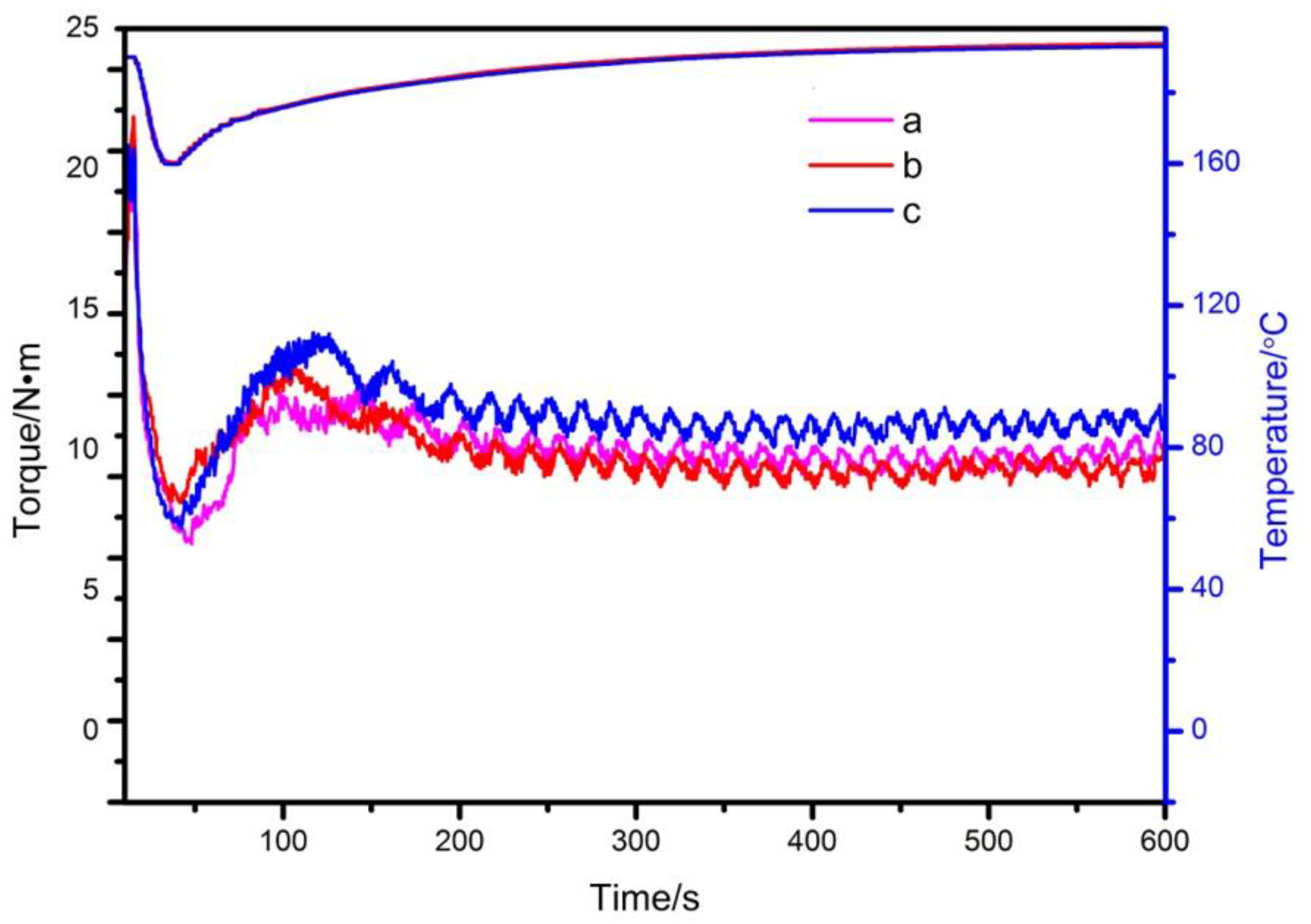
3.3. Processing Performance Test of Compound Heat Stabilizer Added to PVC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.; Yang, Z.H. The effect of sodium stearate-modified hydrocalumite on the thermal stability of poly(vinyl chloride). J. Appl. Polym. Sci. 2018, 135, 45758. [Google Scholar] [CrossRef]
- Korkusuz, Ç.; Demir, A.P.T. Evaluation of the thermal stabilization behavior of hydrotalcite against organic stabilizers for plasticized PVC films. Polym. Bull. 2019, 77, 4805–4831. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Sabaa, M.W.; Oraby, E.H.; Yassin, A.A. Organic thermal stabilizers for rigid poly(vinyl chloride) VII. Effect of mixing 2-benzimidazolyl-omega-phenylpropenylidineacetonitrile with some commercial stabilizers. Polym. Degrad. Stab. 2003, 79, 495–501. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Tang, W.; Qu, Y.; Wang, X. Synthesis and application of uracil derivatives as novel thermal stabilizers for rigid poly(vinyl chloride). Polym. Degrad. Stab. 2013, 98, 659–665. [Google Scholar] [CrossRef]
- Shu, W.Y.; Liu, Y.N.; Chen, Q.Y. Synthesis of antimony tris(mercaptoethyl carboxylates) as thermal stabilizer for polyvinyl chloride. Trans. Nonferrous Metal Soc. China 2002, 12, 997–1000. [Google Scholar]
- Gupta, S.; Agarwal, D.; Banerjee, S. Thermal stabilization of poly(vinyl chloride) by hydrotalcites, zeolites, and conventional stabilizers. J. Vinyl Addit. Technol. 2009, 15, 164–170. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Tang, W.; Qu, Y.; Wang, X. Investigation of basic zinc cyanurate as a novel thermal stabilizer for poly(vinyl chloride) and its synergistic effect with calcium stearate. Polym. Degrad. Stab. 2014, 99, 211–218. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Wu, H.; Guo, S. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride). Polym. Degrad. Stab. 2006, 91, 2101–2109. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, Z. Improved performance of flower-like ZnAl LDH growing on carbon nanotubes used in zinc–nickel secondary battery. Electrochim. Acta 2018, 277, 67–76. [Google Scholar] [CrossRef]
- Wang, G.; Yang, M.; Li, Z.; Lin, K.; Jin, Q.; Xing, C.; Hu, Z.; Wang, D. Synthesis and characterization of Zn-doped MgAl-layered double hydroxide nanoparticles as PVC heat stabilizer. J. Nanopart. Res. 2013, 15, 1882. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.; Lakzian, A. Adsorption–desorption characteristics of nitrate, phosphate and sulfate on Mg–Al layered double hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Takahashi, N.; Hata, H.; Kuroda, K. Anion Exchangeable Layered Silicates Modified with Ionic Liquids on the Interlayer Surface. Chem. Mater. 2010, 22, 3340–3348. [Google Scholar] [CrossRef]
- Meloni, D.; Monaci, R.; Solinas, V.; Auroux, A.; Dumitriu, E. Characterisation of the active sites in mixed oxides derived from LDH precursors by physico-chemical and catalytic techniques. Appl. Catal. A Gen. 2008, 350, 86–95. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.-H.; Wang, R.; Zhang, Z.; Feng, Z.; Xie, X. Zn–Al layered double oxides as high-performance anode materials for zinc-based secondary battery. J. Mater. Chem. A 2015, 3, 7429–7436. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Liu, L.; Meng, J.; Cui, F. Zn-Al layered double hydroxide growing on the substrate of graphene and polypyrrole composite as anode material for Zn-Ni secondary battery. Mater. Lett. 2019, 255, 126558. [Google Scholar] [CrossRef]
- Meng, J.; Yang, Z.; Liu, L.; Cui, F.; Jiang, Y. The in-situ growth of zinc-aluminum hydrotalcite on hollow carbon spheres and its application as anode material with long cycle life for zinc-nickel secondary battery. J. Alloys Compd. 2019, 809, 151842. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Efficient flame-retardant and smoke-suppression properties of MgAlCO3-LDHs on the intumescent fire retardant coating for steel structures. Prog. Org. Coat. 2019, 135, 291–298. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Fireproof performance of the intumescent fire retardant coatings with layered double hydroxides additives. Constr. Build. Mater. 2020, 256, 119445. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Xie, X.; Huang, J.; Wen, X. Layered Double Oxides Nano-flakes Derived from Layered Double Hydroxides: Preparation, Properties and Application in Zinc/Nickel Secondary Batteries. Electrochim. Acta 2015, 185, 190–197. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Z. Intercalated hydrotalcite-like materials and their application as thermal stabilizers in poly(vinyl chloride). J. Appl. Polym. Sci. 2017, 134, 44896. [Google Scholar] [CrossRef]
- Yi, S.; Yang, Z.; Wang, S.-W.; Liu, D.-R.; Wang, S.-Q.; Liu, Q.-Y.; Chi, W.-W. Effects of MgAlCe-CO3 layered double hydroxides on the thermal stability of PVC resin. J. Appl. Polym. Sci. 2011, 119, 2620–2626. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Li, D.-Q.; Evans, D.G.; Duan, X. Modulating effect of Mg–Al–CO3 layered double hydroxides on the thermal stability of PVC resin. Polym. Degrad. Stab. 2005, 88, 286–293. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Pi, H.; Guo, S. Preparation of intercalated Mg-Al layered double hydroxides and its application in PVC thermal stability. J. Appl. Polym. Sci. 2011, 124, 5180–5186. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, J.-P.; Zhao, J.; Hu, G.-H.; Dang, Z.-M. A hybrid Mg–Al layered double hydroxide/graphene nanostructure obtained via hydrothermal synthesis. Chem. Phys. Lett. 2014, 605–606, 77–80. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, D.D.; Banerjee, S. Role of Hydrotalcites Cations in Thermal Stabilization of Poly (Vinyl Chloride). Int. J. Polym. Mater. 2012, 61, 124–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, A.D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Feng, G.-H.; Chen, W.; Song, Y.-F.; Dong, X.; Li, G.-H.; Zhang, H.-J.; Wei, W. Artificial bioconversion of carbon dioxide. Chin. J. Catal. 2019, 40, 1421–1437. [Google Scholar] [CrossRef]
- Bao, T.N.; Tegus, O.; Hasichaolu; Ning, J.; Narengerile. Preparation of Black Phosphorus by the Mechanical Ball Milling Method and its Characterization. Solid State Phenom. 2018, 271, 18–22. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Greenwell, H.C.; Krzhizhanovskaya, M.G.; Apperley, D.C.; Pekov, I.V.; Yakovenchuk, V.N. Thermal Evolution of Natural Layered Double Hydroxides: Insight from Quintinite, Hydrotalcite, Stichtite, and Iowaite as Reference Samples for CO3- and Cl-Members of the HydrotalciteSupergroup. Minerals 2020, 10, 961. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Li, X.; He, X.; Zhang, Q. Precursor Preparation to Promote the Adsorption of Mg-Al Layered Double Hydroxide. J. Am. Ceram. Soc. 2016, 99, 2882–2885. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ai, Z.; Wu, L.; Zhang, Q. Mechanochemical synthesis of CdS/MgAl LDH-precursor as improved visible-light driven photocatalyst for organic dye. Appl. Clay Sci. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Eliseev, A.; Lukashin, A.V.; Vertegel, A.A.; Tarasov, V.P.; Tret’Yakov, Y.D. A Study of Crystallization of Mg–Al Double Hydroxides. Dokl. Chem. 2002, 387, 339–343. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Zhu, Y.; Zhang, F. Transformation Mechanism of Magnesium and Aluminum Precursor Solution into Crystallites of Layered Double Hydroxide. Chem. Mater. 2011, 24, 81–87. [Google Scholar] [CrossRef]
- Belhanechebensemra, N.; Van, H.T.; Guyot, A.; Gay, M.; Carette, L. Thermal dehydrochlorination and stabilization of poly(vinylchloride) in solution: Part IV—Synergistic effects of β-diketone compounds and metal soap stabilizers. Polym. Degrad. Stab. 1989, 24, 89–111. [Google Scholar] [CrossRef]
- Shnawa, H.A. Characterization of processing, rheological and dynamic mechanical thermal properties of PVC stabilized with polyphenol-based thermal stabilizer. J. Therm. Anal. Calorim. 2020, 139, 125–135. [Google Scholar] [CrossRef]
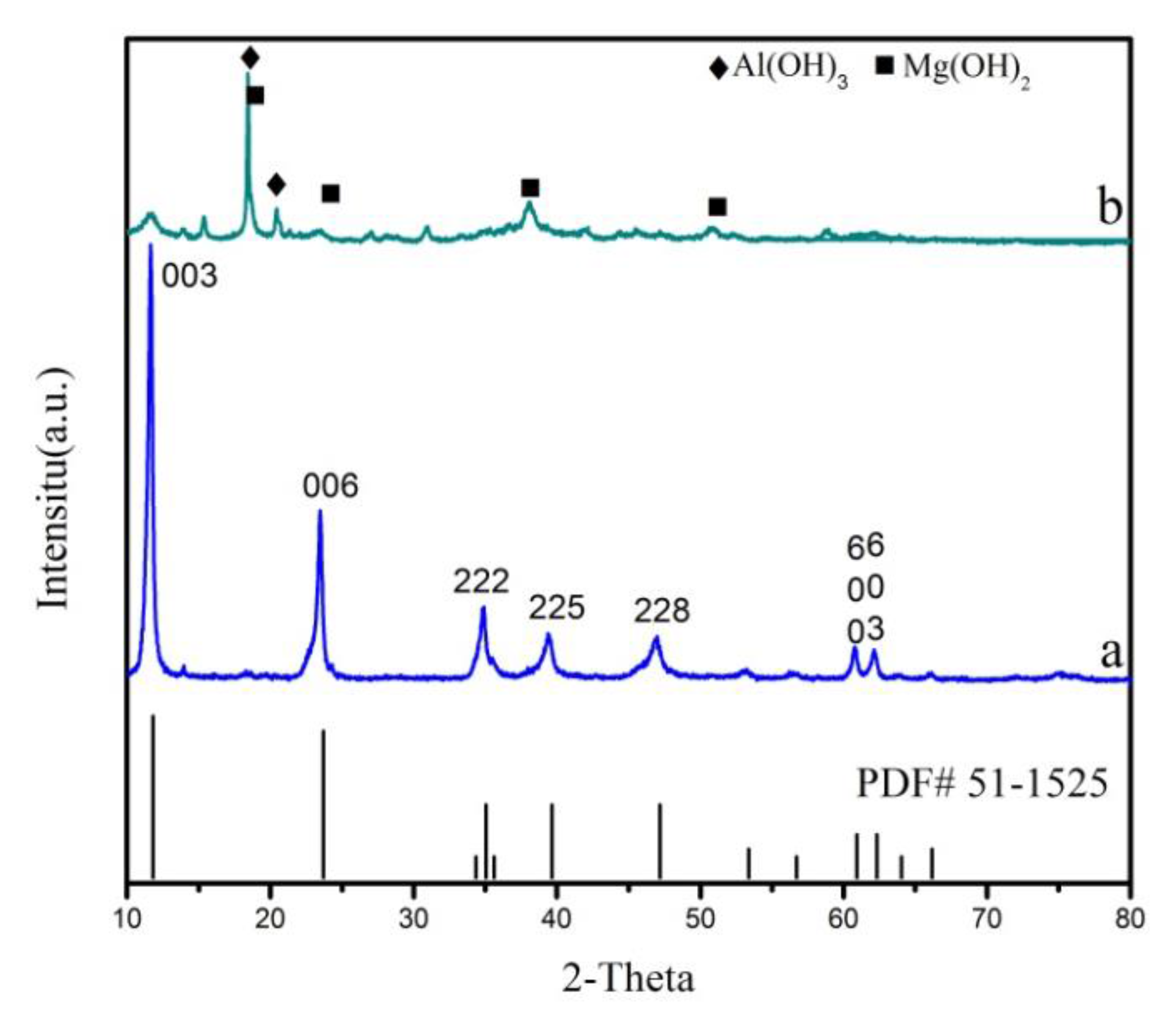


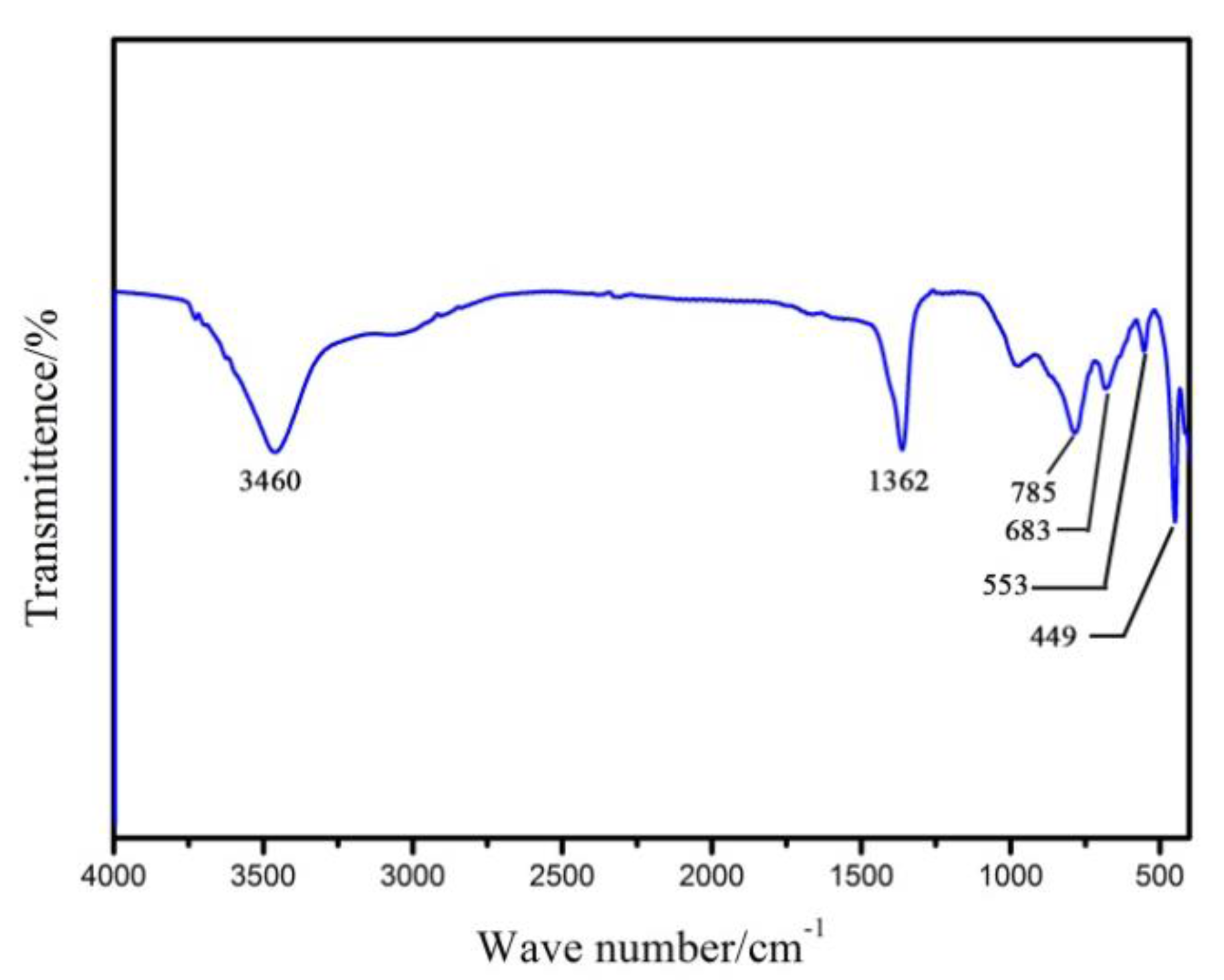
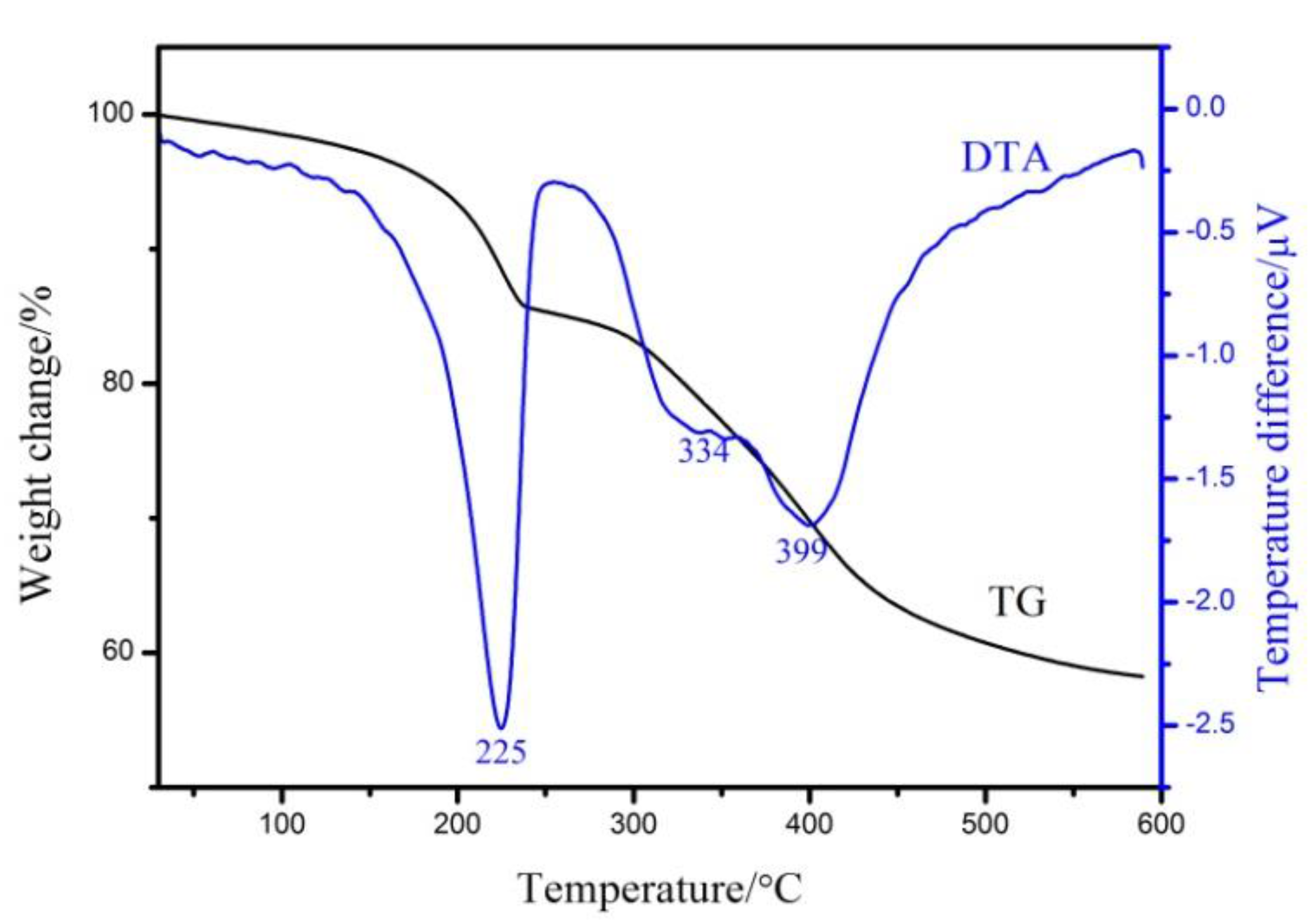
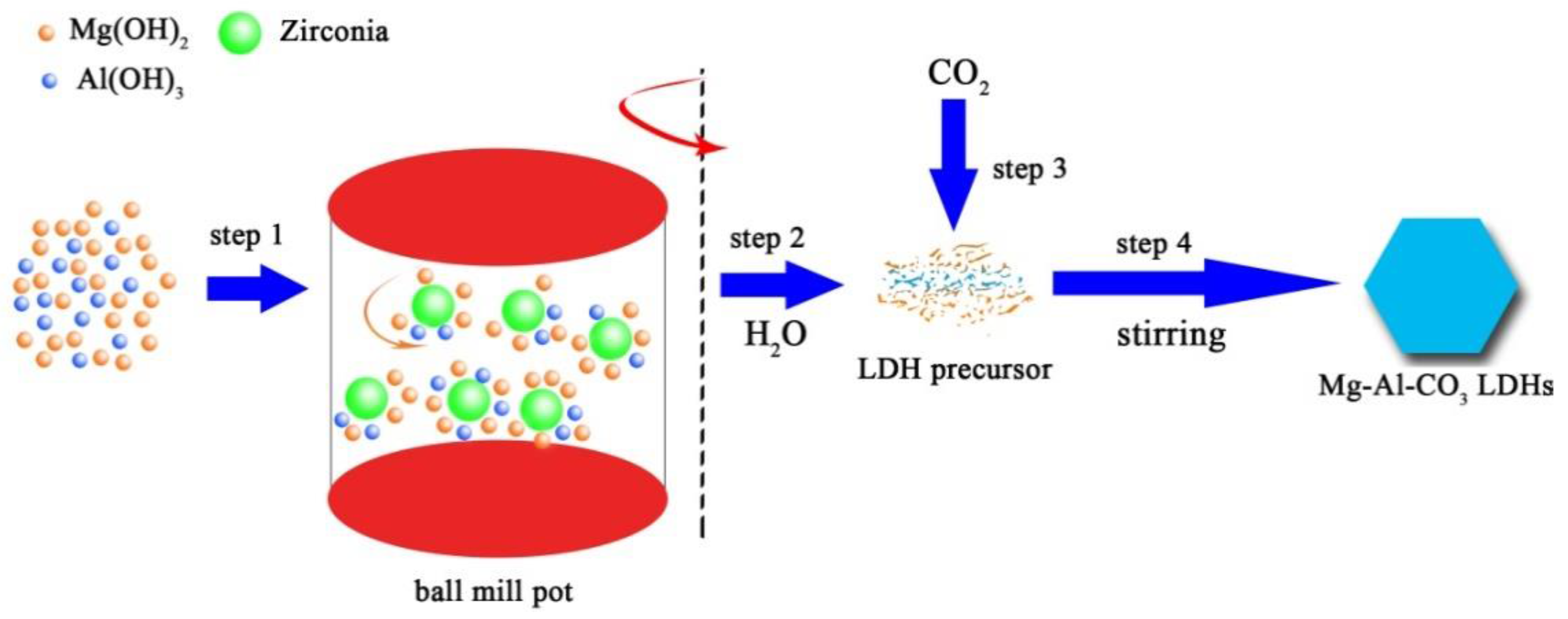


| Sample | d003/Å | d006/Å | d222/Å | d225/Å | d228/Å | d600/Å | d603/Å |
|---|---|---|---|---|---|---|---|
| Standard card | 7.570 | 3.778 | 2.570 | 2.281 | 1.932 | 1.524 | 1.493 |
| a | 7.586 | 3.789 | 2.576 | 2.286 | 1.938 | 1.524 | 1.493 |
| Label | PVC/g | LDHs/phr | ZnSt2/phr | Thermal Stability Time of PVC Samples (min) |
|---|---|---|---|---|
| a | 100 | 0 | 0 | 8 |
| b | 100 | 3.0 | 0 | 30 |
| c | 100 | 0 | 0.6 | 12 |
| d | 100 | 2.4 | 0.6 | 36 |
| Label | LDHs Category | PVC/g | LDHs/phr | ZnSt2/phr | Zinc Acetylacetonate/phr | Thermal Stability Time of PVC Samples (min) |
|---|---|---|---|---|---|---|
| a | commercial | 100 | 2.4 | 0.6 | 0 | 39 |
| b | experimental | 100 | 2.4 | 0.6 | 0 | 36 |
| c | experimental | 100 | 2.4 | 0.5 | 0.1 | 38 |
| d | experimental | 100 | 2.4 | 0.4 | 0.2 | 45 |
| e | experimental | 100 | 2.4 | 0.3 | 0.3 | 46 |
| f | experimental | 100 | 2.4 | 0.2 | 0.4 | 41 |
| Label | PVC/g | DOP/g | CaCO3/g | Stearic Acid/g | LDHs Category | LDHs/phr | ZnSt2/phr | zinc Acetylacetonate/phr |
|---|---|---|---|---|---|---|---|---|
| a | 100 | 10 | 30 | 0.5 | commercial | 2.4 | 0.6 | 0 |
| b | 100 | 10 | 30 | 0.5 | experimental | 2.4 | 0.6 | 0 |
| c | 100 | 10 | 30 | 0.5 | experimental | 2.4 | 0.3 | 0.3 |
| Label | Maximum Torque/(Nm) | Balance Torque/(Nm) | Melting Time/s | Melting Temperature/°C | Balance Temperature/°C |
|---|---|---|---|---|---|
| A | 20.68 | 9.73 | 32 | 160.3 | 193.0 |
| B | 22.09 | 9.35 | 26 | 159.8 | 192.7 |
| C | 21.55 | 10.91 | 27 | 160.1 | 193.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Yang, Z.; Su, Q.; Chen, L.; Wu, J.; Meng, J. Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride). Materials 2020, 13, 5223. https://doi.org/10.3390/ma13225223
Jiang Y, Yang Z, Su Q, Chen L, Wu J, Meng J. Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride). Materials. 2020; 13(22):5223. https://doi.org/10.3390/ma13225223
Chicago/Turabian StyleJiang, Yinan, Zhanhong Yang, Qingsong Su, Linlin Chen, Jian Wu, and Jinlei Meng. 2020. "Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride)" Materials 13, no. 22: 5223. https://doi.org/10.3390/ma13225223
APA StyleJiang, Y., Yang, Z., Su, Q., Chen, L., Wu, J., & Meng, J. (2020). Preparation of Magnesium-Aluminum Hydrotalcite by Mechanochemical Method and Its Application as Heat Stabilizer in poly(vinyl chloride). Materials, 13(22), 5223. https://doi.org/10.3390/ma13225223





