Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Powder Characterisation
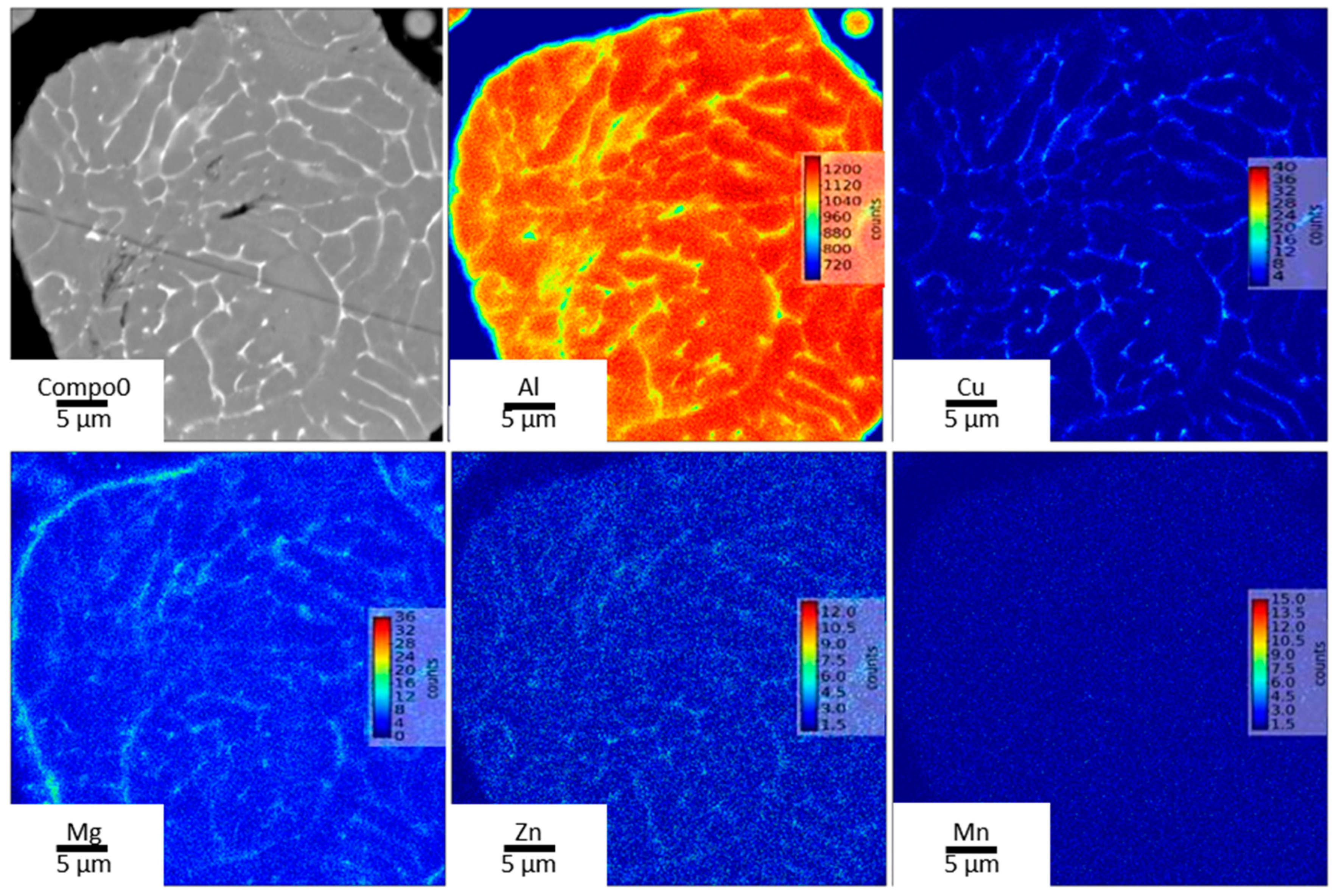
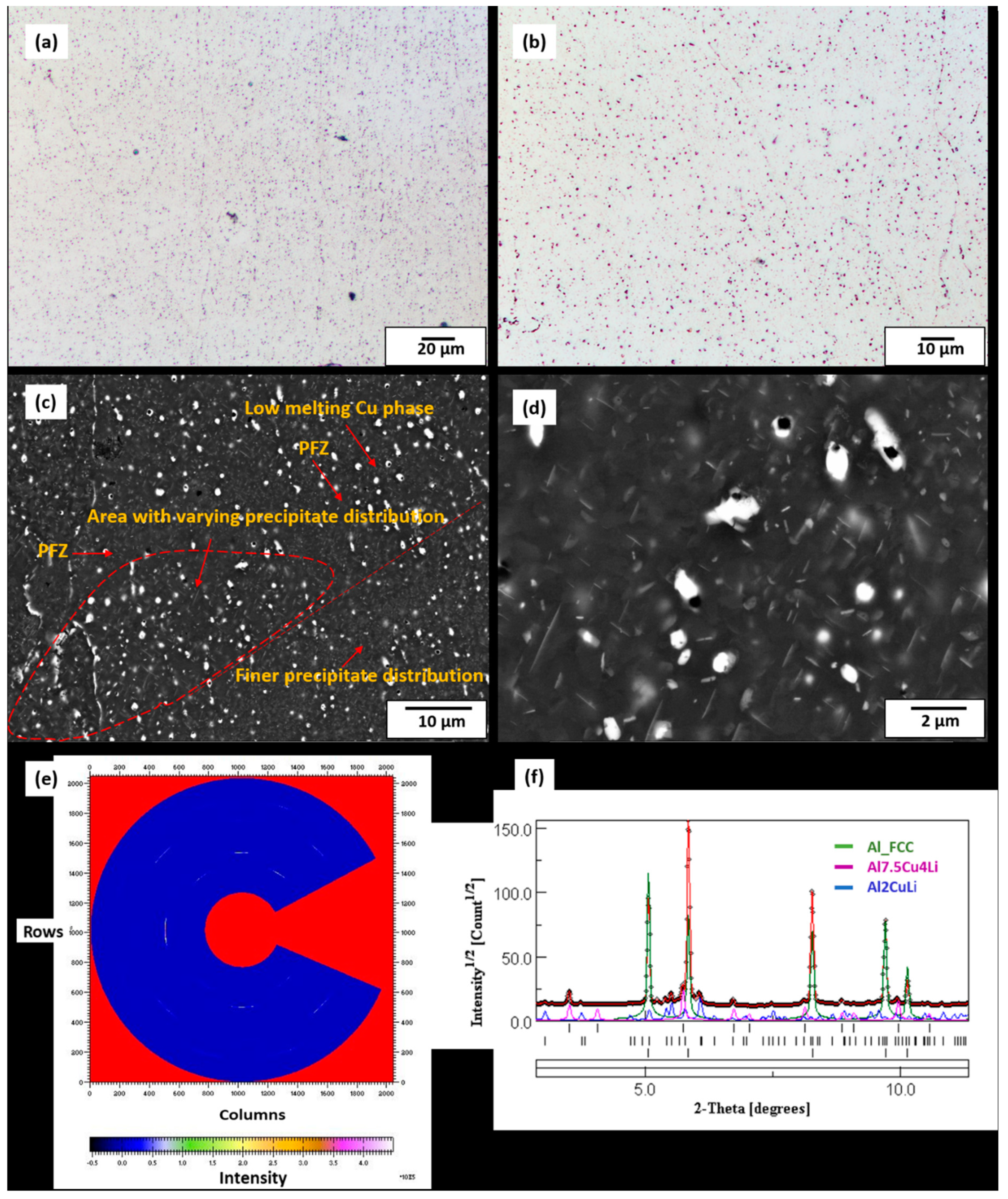
3.2. Microstructure of As-Built Sample
3.3. Phase Field Simulation
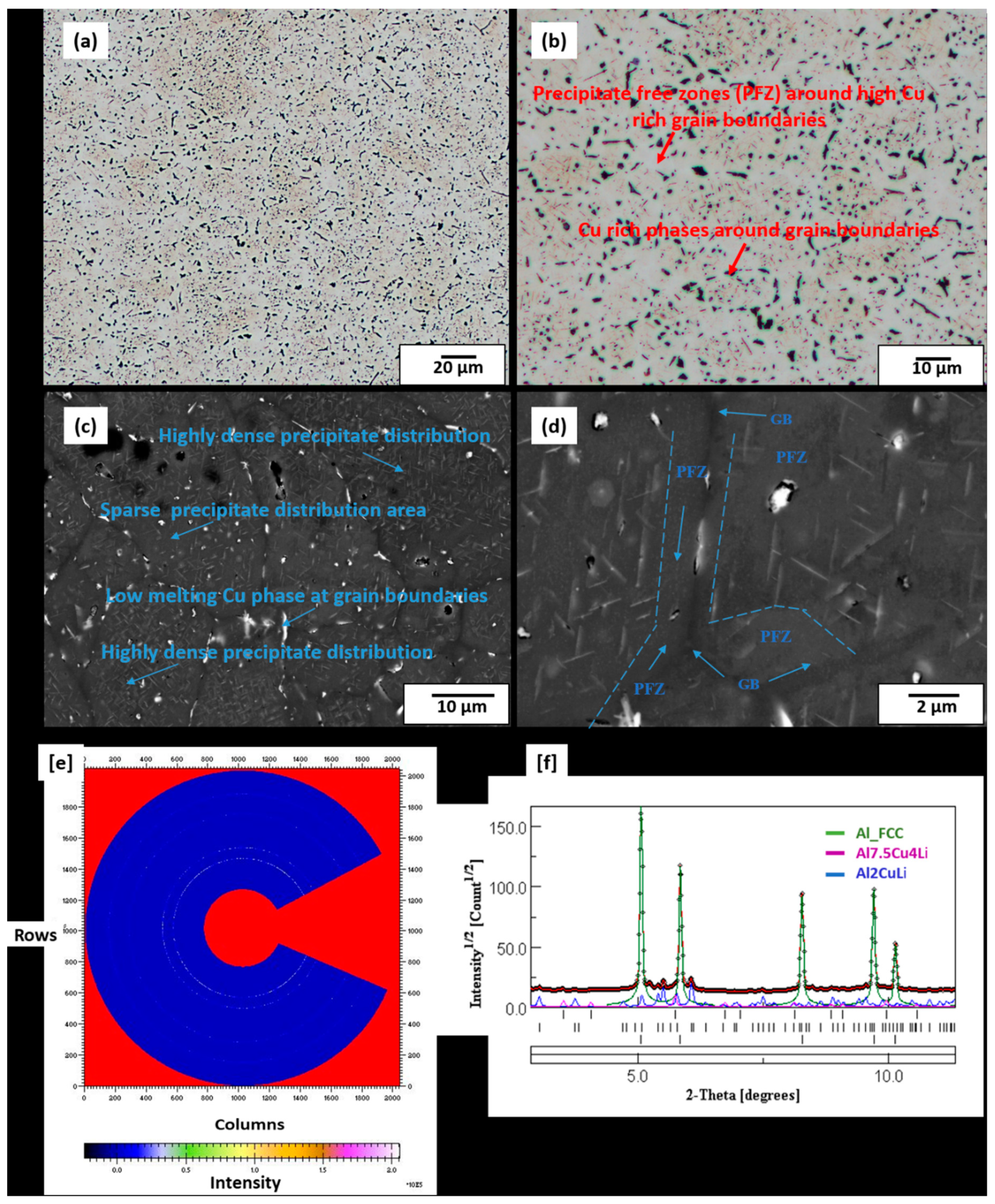
3.4. Microstructure of Preheat-Treated LPBF-Built Sample
3.5. EBSD Characterisation
3.6. Microhardness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Łukaszek-Sołek, A.; Motyka, M.; Baran-Sadleja, A. Numerical and Experimental Analysis of Isothermal Forging Process of Ribbed Plates Made of 2099 Aluminium Alloy for Aircraft Structures. Adv. Manuf. Sci. Technol. 2016, 40, 61–72. [Google Scholar] [CrossRef]
- Sauermann, R.; Arnold, A.; Friedrich, B.; Hallstedt, B.; Schneider, J.M. Development of Al-Li-Based Alloys for Thixoformed Automotive Parts. In Proceedings of the European Metallurgical Conference, EMC 2005, Dresden, Germany, 8–21 September 2005. [Google Scholar]
- Belov, N.A.; Eskin, D.; Aksenov, A.A. Alloys with Lithium. In Multicomponent Phase Diagrams; Elsevier BV: Amsterdam, The Netherlands, 2005; pp. 257–286. [Google Scholar]
- Kumar, K.; Brown, S.; Pickens, J.R. Microstructural evolution during aging of an AlCuLiAgMgZr alloy. Acta Mater. 1996, 44, 1899–1915. [Google Scholar] [CrossRef]
- Romios, M.; Tiraschi, R.; Parrish, C.; Babel, H.W.; Ogren, J.R.; Es-Said, O.S. Design of Multistep Aging Treatments of 2099 (C458) Al-Li Alloy. J. Mater. Eng. Perform. 2005, 14, 641–646. [Google Scholar] [CrossRef]
- Ovri, H. Micromechanisms Governing Plastic Instability in Al—Li Based Alloys. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Blankenship, C.; Hornbogen, E.; Starke, E. Predicting slip behavior in alloys containing shearable and strong particles. Mater. Sci. Eng. A 1993, 169, 33–41. [Google Scholar] [CrossRef]
- Dorin, T.; Deschamps, A.; De Geuser, F.; Sigli, C. Quantification and modelling of the microstructure/strength relationship by tailoring the morphological parameters of the T1 phase in an Al–Cu–Li alloy. Acta Mater. 2014, 75, 134–146. [Google Scholar] [CrossRef]
- Decreus, B.; Deschamps, A.; De Geuser, F.; Donnadieu, P.; Sigli, C.; Weyland, M. The influence of Cu/Li ratio on precipitation in Al–Cu–Li–x alloys. Acta Mater. 2013, 61, 2207–2218. [Google Scholar] [CrossRef]
- Gable, B.M.; Csontos, A.; Starke, E.J. The Role of Mechanical Stretch on Processing-Microstructure-Property Relationships of AF/C 458. Mater. Sci. Forum 2000, 331, 1341–1346. [Google Scholar] [CrossRef]
- Decreus, B.; Deschamps, A.; De Geuser, F.; Sigli, C. Influence of Natural Ageing and Deformation on Precipitation in an Al-Cu-Li Alloy. Adv. Eng. Mater. 2013, 15, 1082–1085. [Google Scholar] [CrossRef]
- Balducci, E.; Ceschini, L.; Messieri, S.; Wenner, S.; Holmestad, R. Thermal stability of the lightweight 2099 Al-Cu-Li alloy: Tensile tests and microstructural investigations after overaging. Mater. Des. 2017, 119, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Donnadieu, P.; Shao, Y.; De Geuser, F.; Botton, G.A.; Lazar, S.; Cheynet, M.; De Boissieu, M.; Deschamps, A. Atomic structure of T1 precipitates in Al–Li–Cu alloys revisited with HAADF-STEM imaging and small-angle X-ray scattering. Acta Mater. 2011, 59, 462–472. [Google Scholar] [CrossRef]
- Araullo-Peters, V.; Gault, B.; De Geuser, F.; Deschamps, A.; Cairney, J.M. Microstructural evolution during ageing of Al–Cu–Li–x alloys. Acta Mater. 2014, 66, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Khushaim, M.; Boll, T.; Seibert, J.; Haider, F.; Al-Kassab, T. Characterization of Precipitation in Al-Li Alloy AA2195 by means of Atom Probe Tomography and Transmission Electron Microscopy. Adv. Condens. Matter Phys. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Grant, N.J. Rapidly solidified P/M X2020 aluminum alloys. Met. Mater. Trans. A 1987, 18, 2037–2046. [Google Scholar] [CrossRef]
- Ludwiczak, E.; Rioja, R. TB precipitates in an Al-Cu-Li alloy. Scr. Met. Mater. 1991, 25, 1415–1419. [Google Scholar] [CrossRef]
- Suryawanshi, J.; Kaban, I.; Scudino, S.; Eckert, J.; Prakash, O.; Ramamurty, U. Simultaneous enhancements of strength and toughness in an Al-12Si alloy synthesized using selective laser melting. Acta Mater. 2016, 115, 285–294. [Google Scholar] [CrossRef]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Tillmann, W.; Tröster, T.; Hoyer, K.-P.; Schaper, M. Duplex stainless steel fabricated by selective laser melting - Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Raffeis, I.; Adjei-Kyeremeh, F.; Vroomen, U.; Suwanpinij, P.; Ewald, S.; Bührig-Polazcek, A. Investigation of the Lithium-Containing Aluminum Copper Alloy (AA2099) for the Laser Powder Bed Fusion Process [L-PBF]: Effects of Process Parameters on Cracks, Porosity, and Microhardness. JOM 2019, 71, 1543–1553. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Nie, X.; Hu, Z.; Zhu, H.; Zeng, X. A high strength Al–Li alloy produced by laser powder bed fusion: Densification, microstructure, and mechanical properties. Addit. Manuf. 2020, 35, 101346. [Google Scholar] [CrossRef]
- Mullis, A.; Farrell, L.; Cochrane, R.F.; Adkins, N.J. Estimation of Cooling Rates During Close-Coupled Gas Atomization Using Secondary Dendrite Arm Spacing Measurement. Met. Mater. Trans. A 2013, 44, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, M.; StJohn, D.; Easton, M.; Yuan, L.; Dargusch, M. Revealing the Mechanisms of Grain Nucleation and Formation During Additive Manufacturing. JOM 2020, 72, 1065–1073. [Google Scholar] [CrossRef]
- Todaro, C.J.; Easton, M.A.; Qiu, D.; Zhang, D.; Bermingham, M.J.; Lui, E.W.; Brandt, M.; StJohn, D.H.; Qian, M. Grain structure control during metal 3D printing by high-intensity ultrasound. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Debroy, T.; Wei, H.; Zuback, J.; Mukherjee, T.; Elmer, J.; Milewski, J.; Beese, A.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Bai, Q. Defect Formation Mechanisms in Selective Laser Melting: A Review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.K.; Ahmad, B.; Guo, H.; Machry, T.; Eatock, D.; Meyer, J.; Fitzpatrick, M.E.; Zhang, X. An experimental study of residual stress and direction-dependence of fatigue crack growth behaviour in as-built and stress-relieved selective-laser-melted Ti6Al4V. Mater. Sci. Eng. A 2019, 755, 246–257. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Deng, S.; Liao, H.; Coddet, C. Microstructure and tensile properties of iron parts fabricated by selective laser melting. Opt. Laser Technol. 2014, 56, 451–460. [Google Scholar] [CrossRef]
- Kanagarajah, P.; Brenne, F.; Niendorf, T.; Maier, H. Inconel 939 processed by selective laser melting: Effect of microstructure and temperature on the mechanical properties under static and cyclic loading. Mater. Sci. Eng. A 2013, 588, 188–195. [Google Scholar] [CrossRef]
- Gumbmann, E.; De Geuser, F.; Lefebvre, W.; Sigli, C.; Deschamps, A. The Influence of Mg and Ag on the Precipitation Kinetics and the Formation of the T1 Phase in Al-Cu-Li Alloys. Mater. Sci. Forum 2014, 794, 945–950. [Google Scholar] [CrossRef]
- Gumbmann, E.; Lefebvre, W.; De Geuser, F.; Sigli, C.; Deschamps, A. The effect of minor solute additions on the precipitation path of an Al Cu Li alloy. Acta Mater. 2016, 115, 104–114. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Role of Ag and Mg on precipitation of T1 phase in an Al-Cu-Li-Mg-Ag alloy. Scr. Mater. 2001, 44, 701–706. [Google Scholar] [CrossRef]
- Mertens, R.; Vrancken, B.; Holmstock, N.; Kinds, Y.; Kruth, J.-P.; Van Humbeeck, J. Influence of Powder Bed Preheating on Microstructure and Mechanical Properties of H13 Tool Steel SLM Parts. Phys. Procedia 2016, 83, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Ronneberg, T.; Davies, C.; Hooper, P. Revealing relationships between porosity, microstructure and mechanical properties of laser powder bed fusion 316L stainless steel through heat treatment. Mater. Des. 2020, 189, 108481. [Google Scholar] [CrossRef]
- Lee, Y. Microstructures in Over-aged Al–Cu–Li–Mg–Ag Alloys. J. Mater. Sci. Lett. 1998, 17, 1161–1164. [Google Scholar] [CrossRef]
- Totten, G.E.; Tiryakioglu, M.; Kessler, O. Encyclopedia of Aluminum and Its Alloys, Volume 2; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Padgett, B.N.; Buchheit, R.; Frankel, G.; Morral, J. Investigation into the Stress Corrosion Cracking Properties of Aa2099, an Al-Li-Cu Alloy. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2008. [Google Scholar]
- Lin, Y.; Zheng, Z.; Li, S.; Kong, X.; Han, Y. Microstructures and properties of 2099 Al-Li alloy. Mater. Charact. 2013, 84, 88–99. [Google Scholar] [CrossRef]
- Carrick, D.M.; Hogg, S.C.; Wilcox, G.D. Influence of Li Additions on the Microstructure and Corrosion Response of 2XXX Series Aluminium Alloys. Mater. Sci. Forum 2014, 794, 193–198. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Place, T.A. Microstructure, tensile properties and fracture behaviour of an Al-Cu-Li-Mg-Zr alloy 8090. J. Mater. Sci. 1989, 24, 1543–1551. [Google Scholar] [CrossRef]
- Lütjering, G.; Albrecht, J.; Sauer, C.; Krull, T. The influence of soft, precipitate-free zones at grain boundaries in Ti and Al alloys on their fatigue and fracture behavior. Mater. Sci. Eng. A 2007, 201–209. [Google Scholar] [CrossRef]
- Srivatsan, T.S.; Lavernia, E.J. The presence and consequences of precipitatefree zones in an aluminium-copper-lithium alloy. J. Mater. Sci. 1991, 26, 940–950. [Google Scholar] [CrossRef]
- Rioja, R.J.; Liu, J. The Evolution of Al-Li Base Products for Aerospace and Space Applications. Met. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Nagahari, T.; Kakehi, K. The Effect of Post-Processes on the Microstructure and Creep Properties of Alloy718 Built Up by Selective Laser Melting. Mater. 2018, 11, 996. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, Z.; Xiao, Y.; Horstemeyer, M.F.; Cui, X.; Chen, L.-Q. Insight into the mechanisms of columnar to equiaxed grain transition during metallic additive manufacturing. Addit. Manuf. 2019, 26, 22–29. [Google Scholar] [CrossRef]
- Sun, S.; Brandt, M.; Easton, M. Powder bed fusion processes. In Laser Additive Manufacturing; Elsevier BV: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Li, H.; Li, H.-Y.; Zeng, Z.; Zheng, Z.; Zheng, F. Effect of ageing time on strength and microstructures of an Al–Cu–Li–Zn–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2008, 498, 314–320. [Google Scholar] [CrossRef]
- Mishra, S.; Beura, V.K.; Singh, A.; Yadava, M.; Nayan, N. Deformation behaviour of Al–Cu–Li alloy containing T1 precipitates. Mater. Sci. Technol. 2018, 34, 2105–2113. [Google Scholar] [CrossRef]
- Prasad, N.E.; Ramachandran, T. Phase Diagrams and Phase Reactions in Al–Li Alloys. In Aluminum-lithium Alloys; Elsevier BV: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Yoshimura, R.; Konno, T.J.; Abe, E.; Hiraga, K. Transmission electron microscopy study of the evolution of precipitates in aged Al–Li–Cu alloys: The θ′ and T1 phases. Acta Mater. 2003, 51, 4251–4266. [Google Scholar] [CrossRef]







| Material | Nominal Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| AlCu2.7Li1.8Mg0.3 | Al | Cu | Li | Zn | Mg | Mn | Zr | Si |
| - | Bal | 2.63 | 1.56 | 0.67 | 0.28 | 0.17 | 0.09 | 0.02 |
| Parameter Table for Printed LPBF Samples | |||||
|---|---|---|---|---|---|
| LPBF Built Condition | Laser Power (W) | Scanning Speed (mm/s) | Hatch Distance (µm) | Layer Thickness (µm) | Relative Density (%) |
| As-built sample (no preheating) | 200 | 650 | 160 | 30 | 99.0 |
| Preheat-treated sample, 320 °C | 170 | 500 | 140 | 30 | 98.5 |
| 500 °C | 120 | 500 | 140 | 30 | 99.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffeis, I.; Adjei-Kyeremeh, F.; Vroomen, U.; Richter, S.; Bührig-Polaczek, A. Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy. Materials 2020, 13, 5188. https://doi.org/10.3390/ma13225188
Raffeis I, Adjei-Kyeremeh F, Vroomen U, Richter S, Bührig-Polaczek A. Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy. Materials. 2020; 13(22):5188. https://doi.org/10.3390/ma13225188
Chicago/Turabian StyleRaffeis, Iris, Frank Adjei-Kyeremeh, Uwe Vroomen, Silvia Richter, and Andreas Bührig-Polaczek. 2020. "Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy" Materials 13, no. 22: 5188. https://doi.org/10.3390/ma13225188
APA StyleRaffeis, I., Adjei-Kyeremeh, F., Vroomen, U., Richter, S., & Bührig-Polaczek, A. (2020). Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy. Materials, 13(22), 5188. https://doi.org/10.3390/ma13225188





