Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructure Characterization
2.3. Mechanical Properties
2.4. Electrochemical Corrosion Measurements
2.5. Static Immersion Test
2.6. Cell Experiments
2.7. Hemolysis Ratio Test
2.8. Platelet Adhesion Test
2.9. Magnetic Susceptibility Measurement
2.10. Statistical Analysis
3. Results
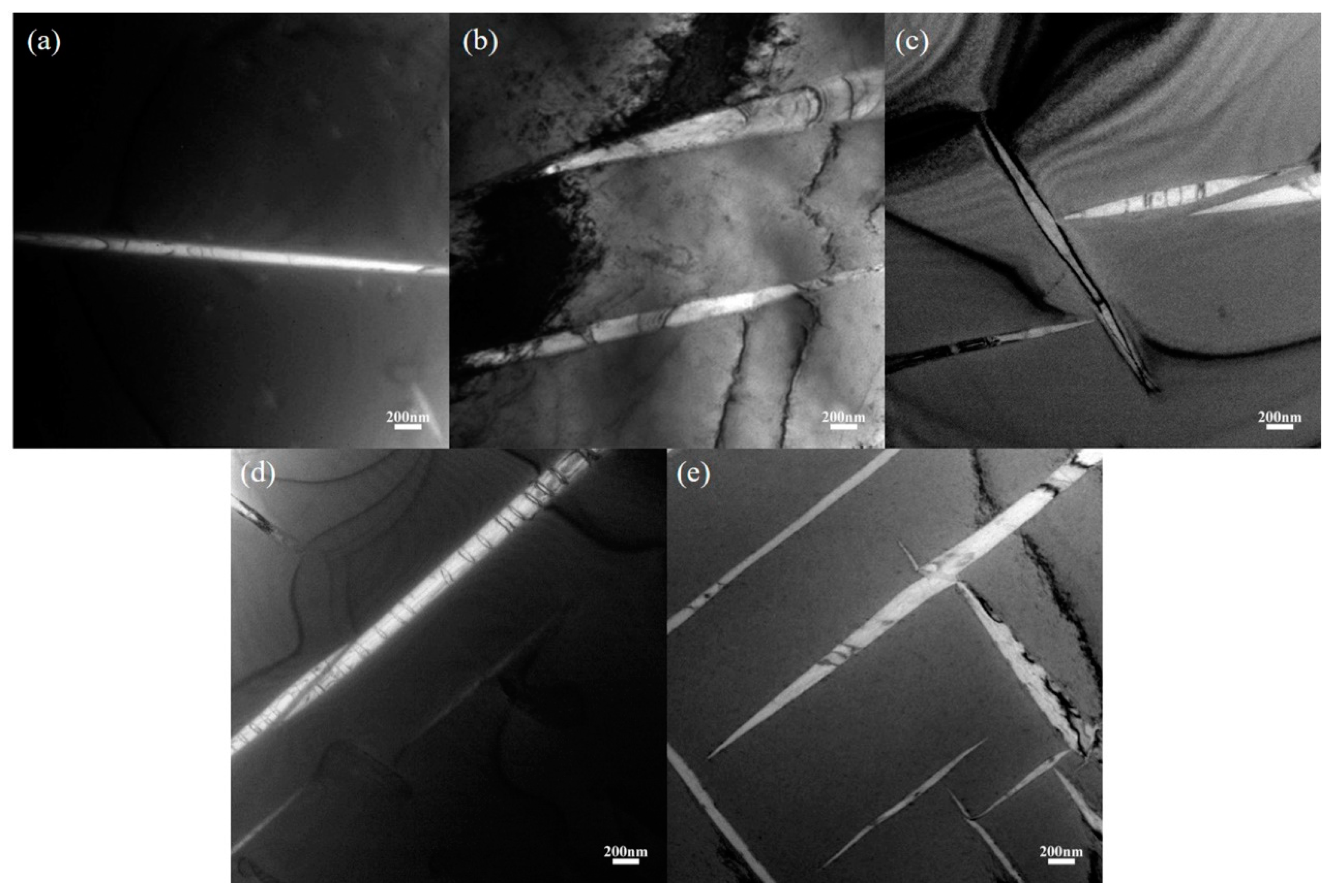
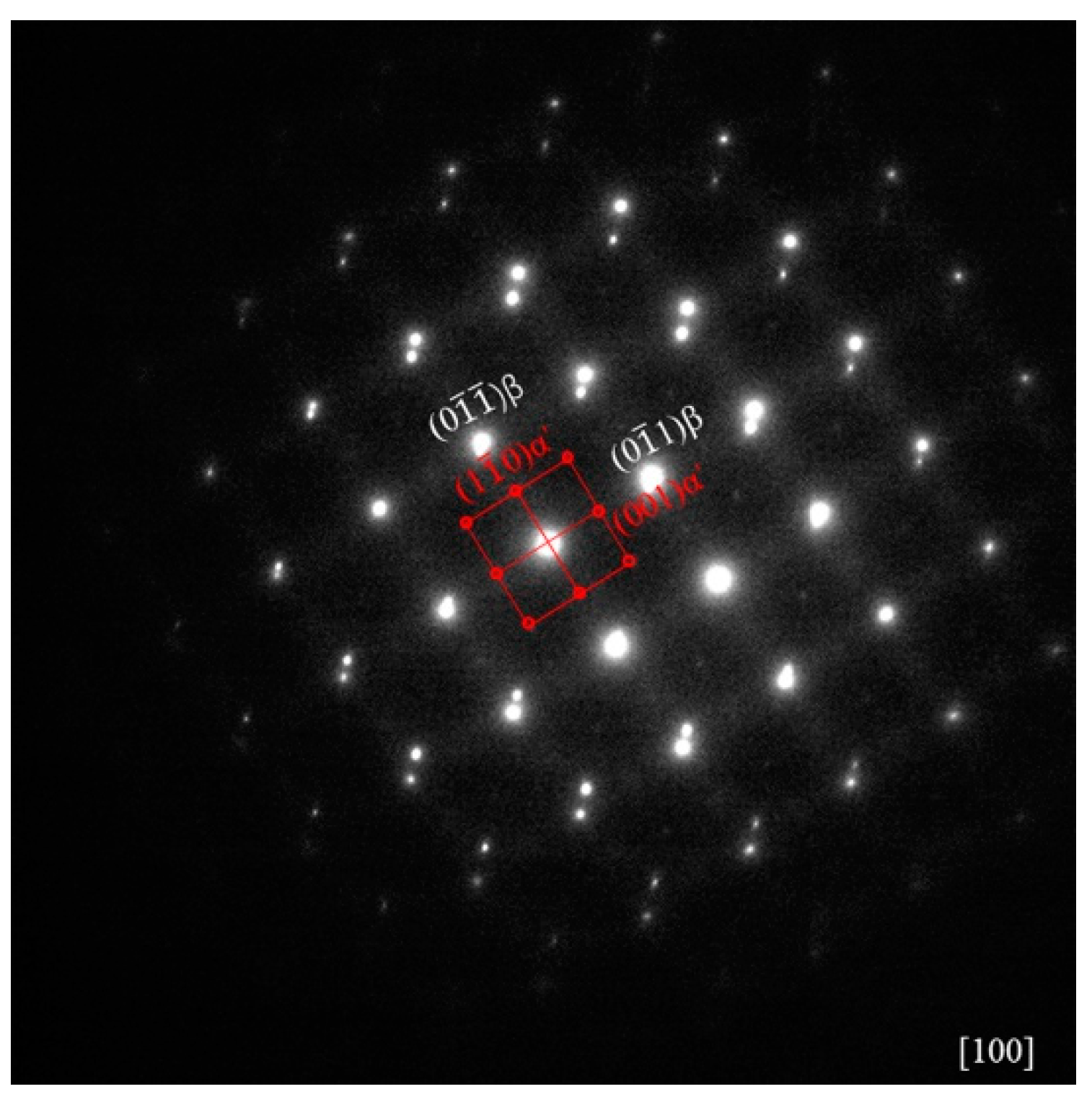
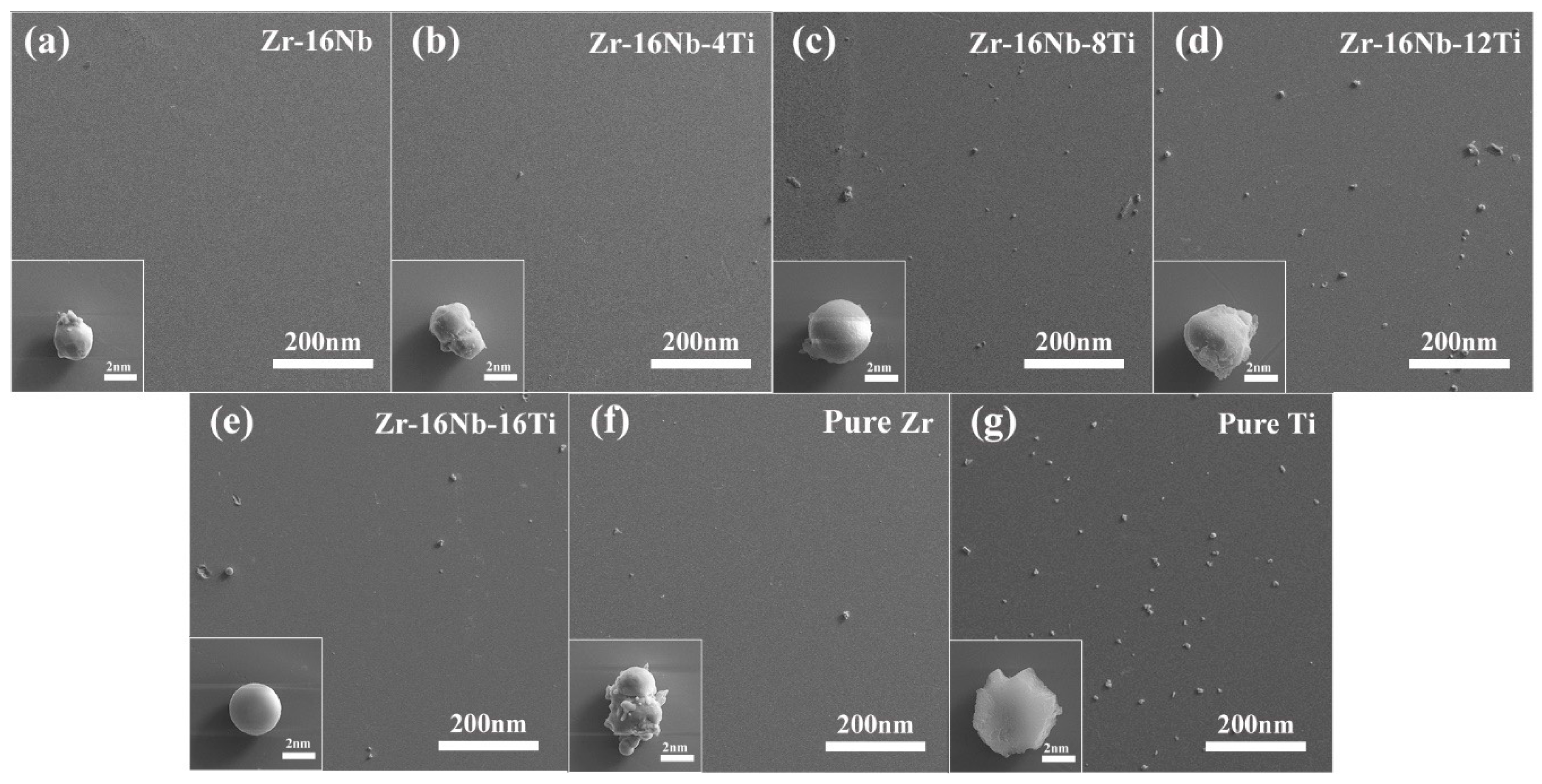
3.1. Initial Microstructure
3.2. Mechanical Properties
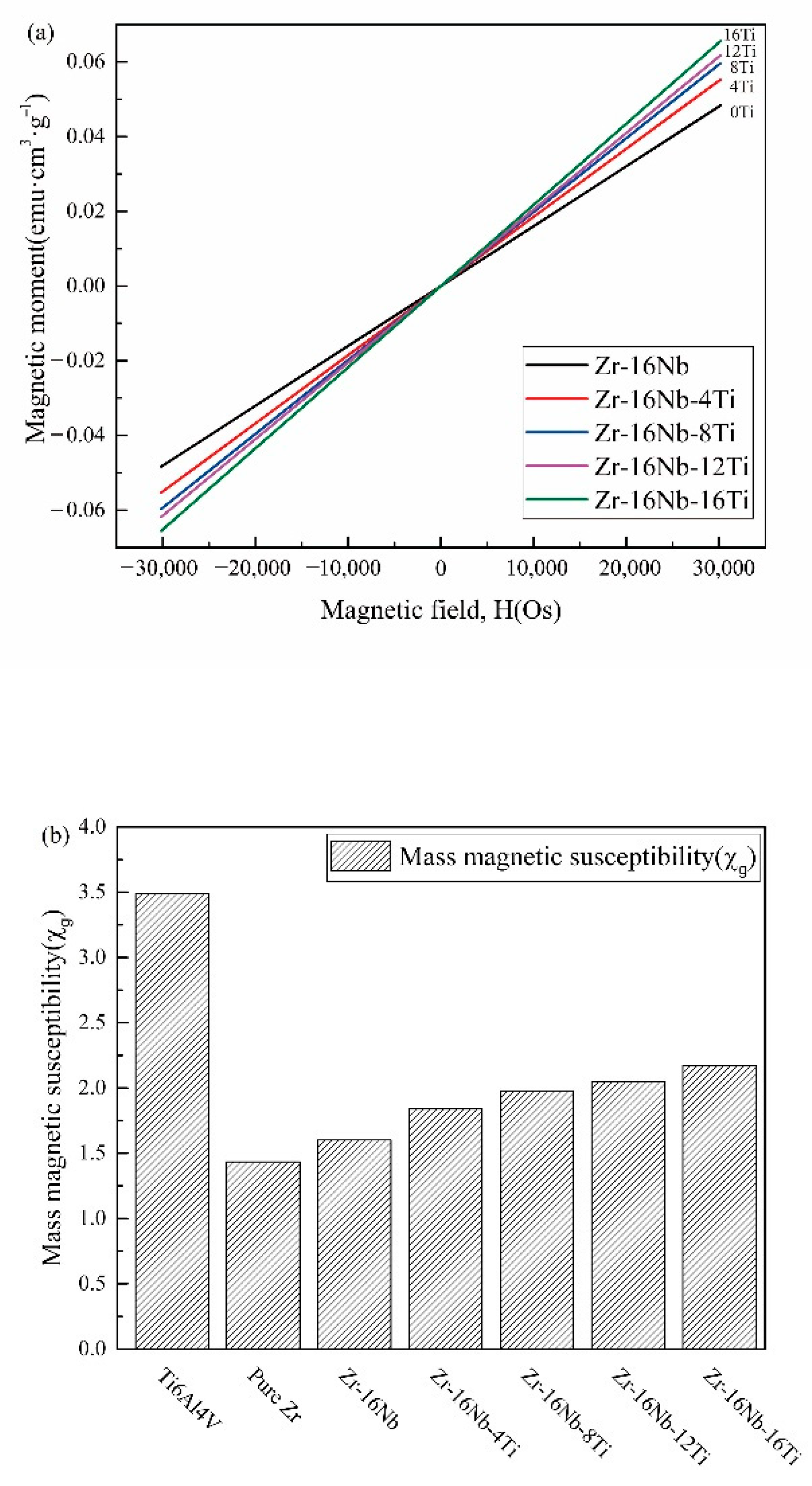
3.3. Magnetic Susceptibilities
3.4. Ion Release
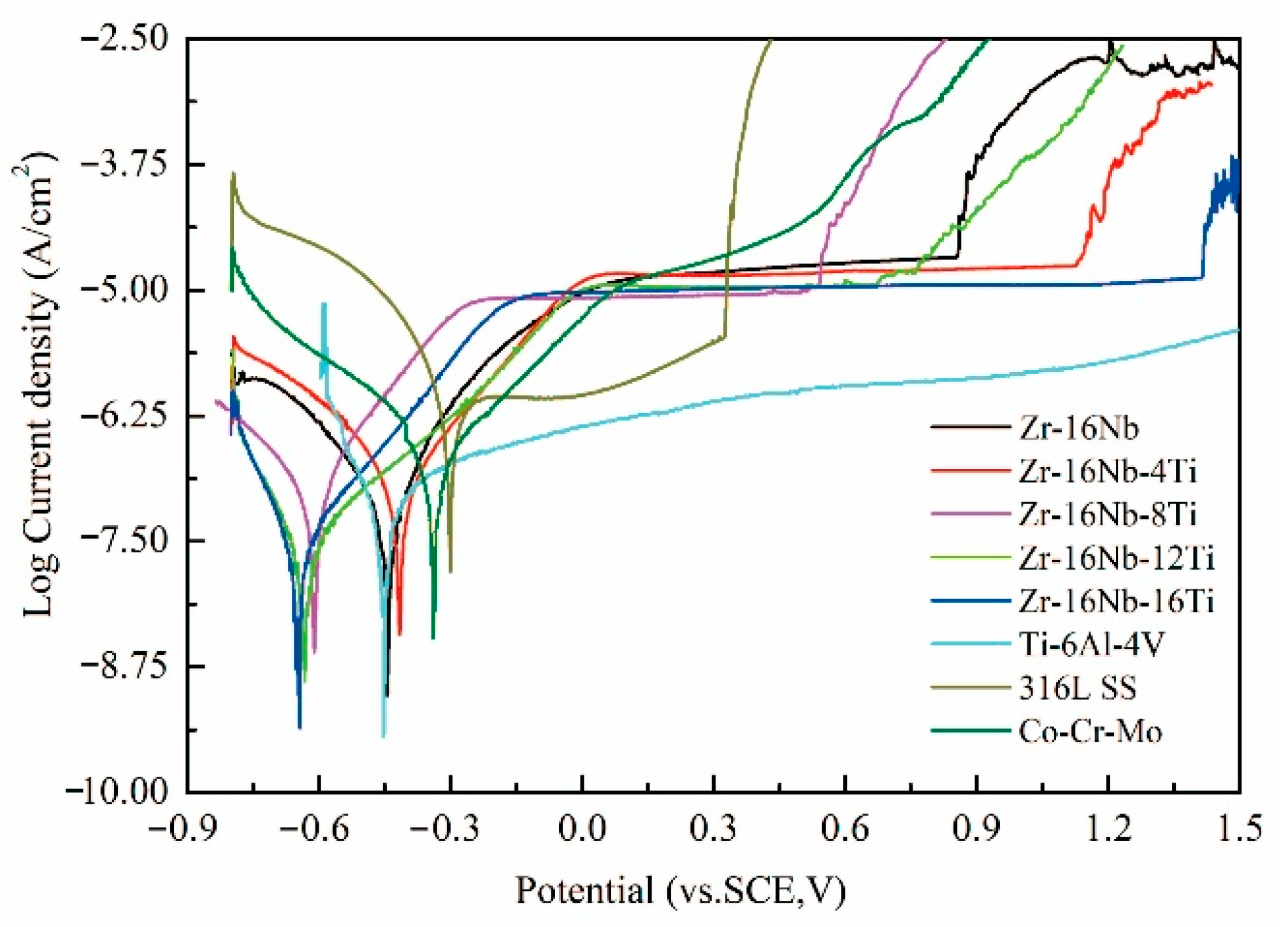
3.5. Electrochemical Corrosion Properties of the Zr-16Nb-xTi Alloys
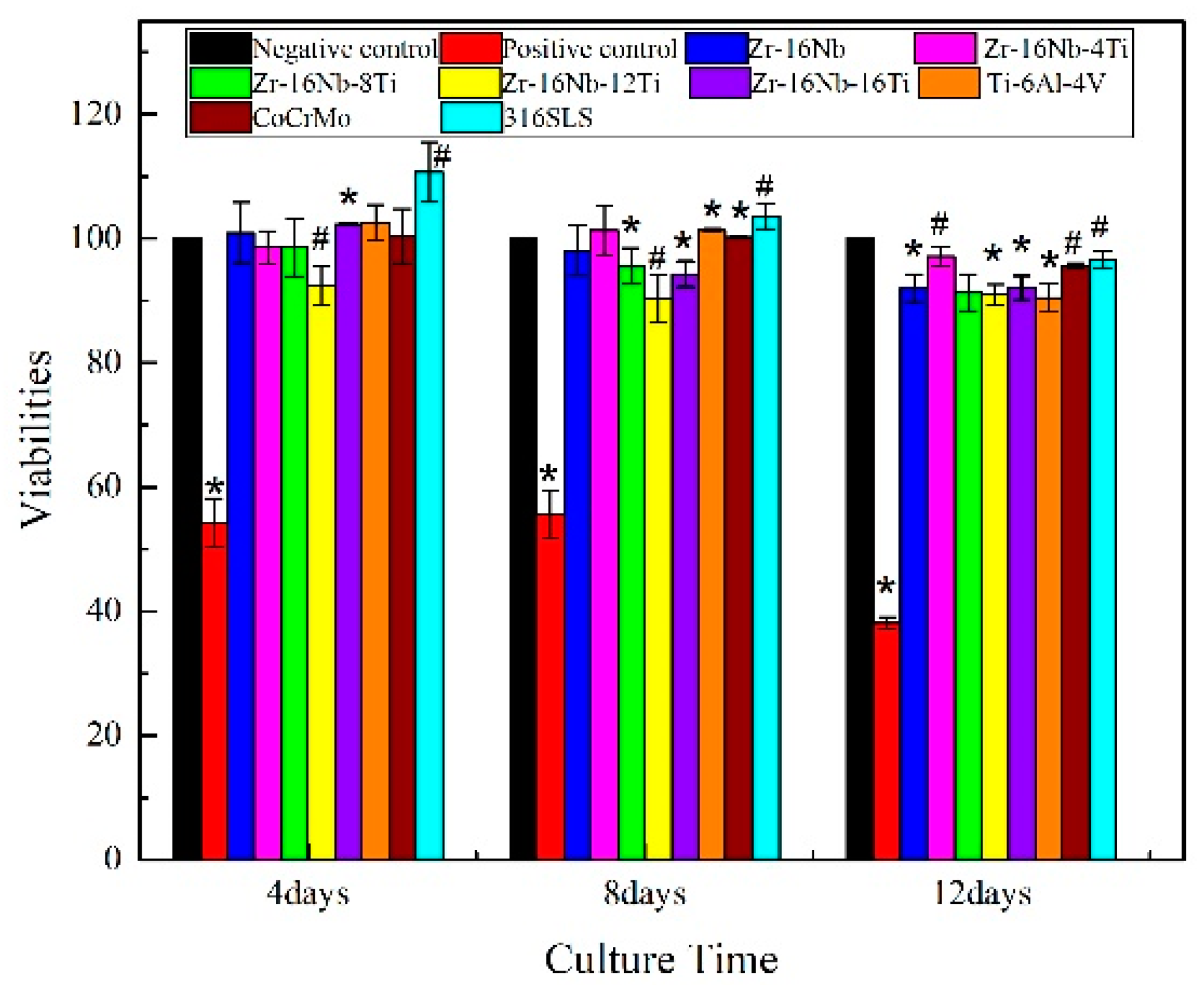
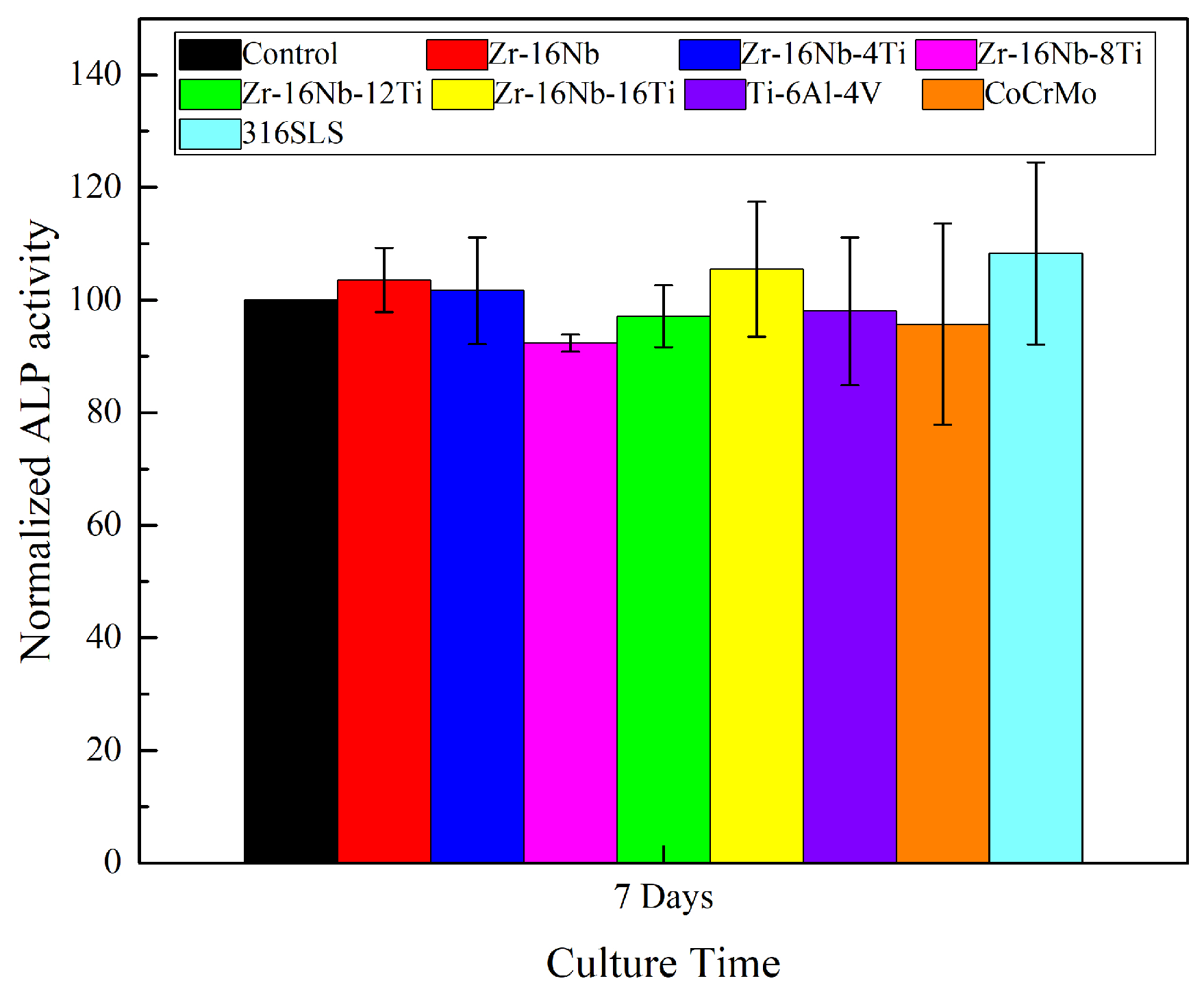
3.6. Assessment of In Vitro Cytocompatibility
3.7. Blood Compatibility Test
4. Discussion
4.1. Microstructure and Mechanical Properties of the Zr-16Nb-xTi Alloys
4.2. Magnetic Susceptibilities of the Zr-16Nb-xTi Alloys
4.3. In Vitro Cytocompatibility Assessment of the Zr-16Nb-xTi Alloys
4.4. Corrosion Properties of the Zr-16Nb-xTi Alloys
4.5. Blood Compatibility Test
5. Conclusions
- The martensite α’ phase was formed in the Zr-16Nb-xTi alloys during the quenching process. The content of the α’ phase in Zr-16Nb was observed to be less than the other samples via TEM; however, the determination of their accurate constitution needs further research. The average Young’s modulus of Zr-16Nb-xTi (52 GPa) was lower than most of the Ti-based alloys. The Zr-16Nb-xTi alloys exhibited much lower magnetic susceptibility as compared to Ti-6Al-4V because of the presence of the martensite α’ phase caused by Ti addition and the lower magnetic susceptibility of Zr. It is suggested that the α’ + β phase is suitable for achieving a low modulus and magnetic susceptibility.
- Immersion in Hank’s simulated body fluid for 30 days exhibited a very low level of the ion release from Zr-16Nb-xTi. The observed results aligned well with the findings from the electrochemical corrosion behavior test. The addition of Ti enhanced the corrosion resistance of Zr-16Nb-xTi, with Zr-16Nb-16Ti exhibiting a small Icorr value and the widest passivation region, demonstrating the best corrosion resistance among the test samples.
- The MG63 cells retained high viability (91% to 97%) after cultivation in the extraction media of Zr-16Nb-xTi for 12 days. The hemolysis rate for the Zr-16Nb-xTi value was observed to be less than 0.3%. The SEM analysis revealed that the platelets maintained spherical shape and did not undergo aggregation or activation, displaying superior hemocompatibility.
Author Contributions
Funding
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Park, J.B. Biomaterials: Introduction. In The Biomedical Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2000; Volume 1, pp. IV1–IV8. [Google Scholar]
- Karthika, P.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar]
- Davies, J.R. Handbook of Materials for Medical Devices; ASM International: Columbus, OH, USA, 2003. [Google Scholar]
- Park, J.; Lakes, R.S. Biomaterials: An Introduction; Springer: New York, NY, USA, 2007. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Kennady, M.C.; Tucker, M.R.; Lester, G.E.; Buckley, M.J. Stress shielding effect of rigid internal fixation plates on mandibular bone grafts. A photon absorption densitometry and quantitative computerized tomographic evaluation. Int. J. Oral Maxillofac. Surg. 1989, 18, 307–310. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van, R.B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Nomura, N.; Tanaka, Y.; Kondo, R.; Doi, H.; Tsutsumi, Y.; Hanawa, T. Effects of Phase Constitution of Zr-Nb Alloys on Their Magnetic Susceptibilities. Mater. Trans. 2009, 50, 2466–2472. [Google Scholar] [CrossRef]
- Bui, F.M.; Bott, K.; Mintchev, M.P. A quantitative study of the pixel-shifting, blurring and nonlinear distortions in MRI images caused by the presence of metal implants. J. Med. Eng. Technol. 2000, 24, 20. [Google Scholar]
- Matsuura, H.; Inoue, T.; Ogasawara, K.; Sasaki, M.; Konno, H.; Kuzu, Y.; Nishimoto, H.; Ogawa, A. Quantitative analysis of magnetic resonance imaging susceptibility artifacts caused by neurosurgical biomaterials: Comparison of 0.5, 1.5, and 3.0 Tesla magnetic fields. Neurol. Med. Chir. 2005, 45, 395. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Muller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Gepreel, M.A.-H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Planell, J.A. Bone Repair Biomaterials; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Eisenbarth, E.; Breme, J.; Hildebrand, H. Einfluss des Vanadiumgehalts von Ti-Al-V-Legierungen. Biomaterialien 2001, 2, 203–210. [Google Scholar] [CrossRef]
- Rogers, S.D.; Howie, D.W.; Graves, S.E.; Pearcy, M.J.; Haynes, D.R. In vitro human monocyte response to wear particles of titanium alloy containing vanadium or niobium. J. Bone Jt. Surg. Br. 1997, 79, 311–315. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Blac, J. Systemic effects of biomaterials. Biomaterials 1984, 5, 11–18. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic toxicity related to metal hip prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef]
- Lide, D.R. Magnetic Susceptibility of the Elements and Inorganic Compounds. In CRC Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Milton Park, Abingdon, UK, 2006. [Google Scholar]
- Zhou, F.Y.; Qiu, K.J.; Li, H.F.; Huang, T.; Wang, B.L.; Li, L.; Zheng, Y.F. Screening on binary Zr-1X (X = Ti, Nb, Mo, Cu, Au, Pd, Ag, Ru, Hf and Bi) alloys with good in vitro cytocompatibility and magnetic resonance imaging compatibility. Acta Biomater. 2013, 9, 9578–9587. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase stability change with Zr content in β-type Ti–Nb alloys. Script. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Yang, R. Effect of Zr and Sn on Young’s modulus and superelasticity of Ti-Nb-based alloys. Mater. Sci. Eng. A 2006, 441, 112–118. [Google Scholar] [CrossRef]
- Kondo, R.; Nomura, N.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and mechanical properties of as-cast Zr-Nb alloys. Acta Biomater. 2011, 7, 4278–4284. [Google Scholar] [CrossRef]
- Nomura, N.; Oya, K.; Tanaka, Y.; Kondo, R.; Doi, H.; Tsutsumi, Y.; Hanawa, T. Microstructure and magnetic susceptibility of as-cast Zr-Mo alloys. Acta Biomater. 2010, 6, 1033–1038. [Google Scholar]
- Kondo, R.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Hanawa, T. Effects of phase constitution on magnetic susceptibility and mechanical properties of Zr-rich Zr–Mo alloys. Acta Biomater. 2011, 7, 4259–4266. [Google Scholar]
- American Society for Testing and Materials (Filadelfia, Pennsylvania). ASTM G31-72: Standard Practice for Laboratory Immersion Corrosion Testing of Metals; American Society for Testing and Materials: Filadelfia, PA, USA, 2004. [Google Scholar]
- ISO 10993-12:2007. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; American National Standards Institute: New York, NY, USA, 2012. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Milton Park, Abingdon, UK, 2006. [Google Scholar]
- ASTM-F756-00. Standard Practice for Assessment of Hemolytic Properties of Materials. In Annual Book of Astm Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2000.
- Collings, E.W. Magnetic studies of phase equilibria in Ti-Al (30 to 57 At. Pct) alloys. Metall. Trans. A 1979, 10, 463–474. [Google Scholar] [CrossRef]
- Rempel, A.A.; Gusev, A.I.; Mulyukov, R.R.; Amirkhanov, N.M. Microstructure, microhardness and magnetic susceptibility of submicrocrystalline palladium. Nanostruct. Mater. 1996, 7, 667–674. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Semlitsch, M.F.; Weber, H.; Streicher, R.M.; Schon, R. Joint replacement components made of hot-forged and surface-treated Ti-6Al-7Nb alloy. Biomaterials 1992, 13, 781–788. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef]
- Tan, X.R.; Zhi, Q.; Yang, R.B.; Wang, F.Z.; Yang, J.R.; Liu, Z.X. Effects of milling on the corrosion behavior of Al2NbTi3V2Zr high-entropy alloy system in 10% nitric acid solution. Mater. Corros. 2017, 68, 1080–1089. [Google Scholar] [CrossRef]
- Goodman, S.L.; Lelah, M.D.; Lambrecht, L.K.; Cooper, S.L.; Albrecht, R.M. In vitro vs. ex vivo platelet deposition on polymer surfaces. Scanning Electron Microsc. 1984, 1 Pt 1, 279. [Google Scholar]
- Wang, B.L.; Li, L.; Zheng, Y.F. In vitro cytotoxicity and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and Ti-Nb-Hf biomedical shape memory alloys. Biomed. Mater. 2010, 5, 044102. [Google Scholar] [CrossRef]



| Element | Zr-16Nb | Zr-16Nb-4Ti | Zr-16Nb-8Ti | Zr-16Nb-12Ti | Zr-16Nb-16Ti |
|---|---|---|---|---|---|
| Zr | 83.453 | 79.587 | 75.770 | 71.444 | 67.012 |
| Nb | 16.547 | 16.370 | 16.323 | 16.398 | 16.563 |
| Ti | - | 4.043 | 7.907 | 12.158 | 16.425 |
| Samples | Nb (mg/L) | Ti (mg/L) | Zr (mg/L) |
|---|---|---|---|
| Zr-16Nb | 0.001 | - | 0.002 |
| Zr-16Nb-4Ti | 0.005 | 0.001 | 0.002 |
| Zr-16Nb-8Ti | - | - | 0.002 |
| Zr-16Nb-12Ti | 0.002 | - | - |
| Zr-16Nb-16Ti | - | 0.002 | - |
| Pure Zr | - | - | - |
| Pure Ti | - | 0.010 | - |
| Samples | OCP (V) | Ecorr (V) | Icorr (10−8/cm2) | Epit (V) | ΔE |
|---|---|---|---|---|---|
| Zr-16Nb | −0.417 (0.045) | −0.414 (0.045) | 14.76 (3.059) | 0.88 (0.028) | 1.294 |
| Zr-16Nb-4Ti | −0.492 (0.121) | −0.409 (0.084) | 5.075 (2.434) | 1.15 (0.0707) | 1.559 |
| Zr-16Nb-8Ti | −0.591 (0.087) | −0.617 (0.062) | 6.461 (3.068) | 0.535 (0.021) | 1.152 |
| Zr-16Nb-12Ti | −0.564 (0.028) | −0.620 (0.017) | 2.070 (0.220) | 0.722 (0.092) | 1.342 |
| Zr-16Nb-16Ti | −0.610 (0.032) | −0.621 (0.035) | 2.373 (1.426) | 1.367 (0.153) | 1.988 |
| Ti-6Al-4V | −0.247 (0.014) | −0.388 (0.093) | 4.501 (1.650) | - | - |
| 316L SS | −0.284 (0.024) | −0.306 (0.144) | 68.729 (11.634) | 0.230 (0.134) | 0.536 |
| Co-Cr-Mo | −0.257 (0.017) | −0.399 (0.087) | 16.610 (2.927) | 0.466 | 0.865 |
| Samples | Hemolysis Ratio | Hemolytic Standard |
|---|---|---|
| Zr-16Nb | 0.156% | Nonhemolytic |
| Zr-16Nb-4Ti | 0.207% | Nonhemolytic |
| Zr-16Nb-8Ti | 0.255% | Nonhemolytic |
| Zr-16Nb-12Ti | 0.219% | Nonhemolytic |
| Zr-16Nb-16Ti | 0.170% | Nonhemolytic |
| Pure Zr | 0.161% | Nonhemolytic |
| Pure Ti | 0.326% | Nonhemolytic |
| Samples | Phase Constitution | |
|---|---|---|
| Zr-3Nb (as-cast) [6] | α′ | ~1.13 |
| Zr-6Nb (as-cast) [6] | β, α′, ω | ~1.15 |
| Zr-9Nb (as-cast) [6] | β, ω | ~1.17 |
| Zr-12Nb(as-cast) [6] | β, ω | ~1.28 |
| Zr-14Nb (as-cast) [6] | β, ω | ~1.45 |
| Zr-14Nb (90% cold-rolled) [7] | β, ω | ~1.49 |
| Zr-16Nb (as-cast) [6] | β, ω | ~1.50 |
| Zr-16Nb (solution and quenching) [7] | β, ω | ~1.38 |
| Zr-16Nb (573 K aging) [7] | β, ω, α | ~1.19 |
| Zr-22Nb (as-cast) [6] | β | ~1.45 |
| Zr-1Nb (annealed) [8] | α | 1.28 |
| Zr-16Nb (in this work) | β, ω | 1.60 |
| Zr-16Nb-4Ti (in this work) | β, α′ | 1.83 |
| Zr-16Nb-8Ti (in this work) | β, α′ | 1.97 |
| Zr-16Nb-12Ti (in this work) | β, α′ | 2.04 |
| Zr-16Nb-16Ti (in this work) | β, α′ | 2.17 |
| Ti-6Al-4V (in this work) | - | 3.47 |
| Co-Cr-Mo [7] | - | ~7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, R.; Wang, D.; Yang, D.; Zhang, L.; Xu, X.; Liu, L.; Wu, D. Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Materials 2020, 13, 5130. https://doi.org/10.3390/ma13225130
Xue R, Wang D, Yang D, Zhang L, Xu X, Liu L, Wu D. Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Materials. 2020; 13(22):5130. https://doi.org/10.3390/ma13225130
Chicago/Turabian StyleXue, Renhao, Dong Wang, Dawei Yang, Ligang Zhang, Xiaoning Xu, Libin Liu, and Di Wu. 2020. "Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants" Materials 13, no. 22: 5130. https://doi.org/10.3390/ma13225130
APA StyleXue, R., Wang, D., Yang, D., Zhang, L., Xu, X., Liu, L., & Wu, D. (2020). Novel Biocompatible Zr-Based Alloy with Low Young’s Modulus and Magnetic Susceptibility for Biomedical Implants. Materials, 13(22), 5130. https://doi.org/10.3390/ma13225130





