Oxidized Low-Density Lipoprotein Promotes In Vitro Calcification
Abstract
1. Introduction
2. Materials and Methods
2.1. Effect of Biomaterials on Calcium Phosphate Sedimentation
2.1.1. Experimental Procedure
2.1.2. Statistical Analysis
2.2. Solid-Phase Analysis
2.2.1. Transmission Electron Microscopy Observation
2.2.2. Scanning Electron Microscopy Observation
2.2.3. Analysis of the Calcium Phosphate Molar Ratio with Electron Probe Micro-Analyzer (EPMA)
2.3. Dentin Mineralization Experiments
2.3.1. Dentin Sample Preparation
2.3.2. Mineralization of Root Surface
3. Results
3.1. Effect of Biomaterials on the Calcium Phosphate Crystallization
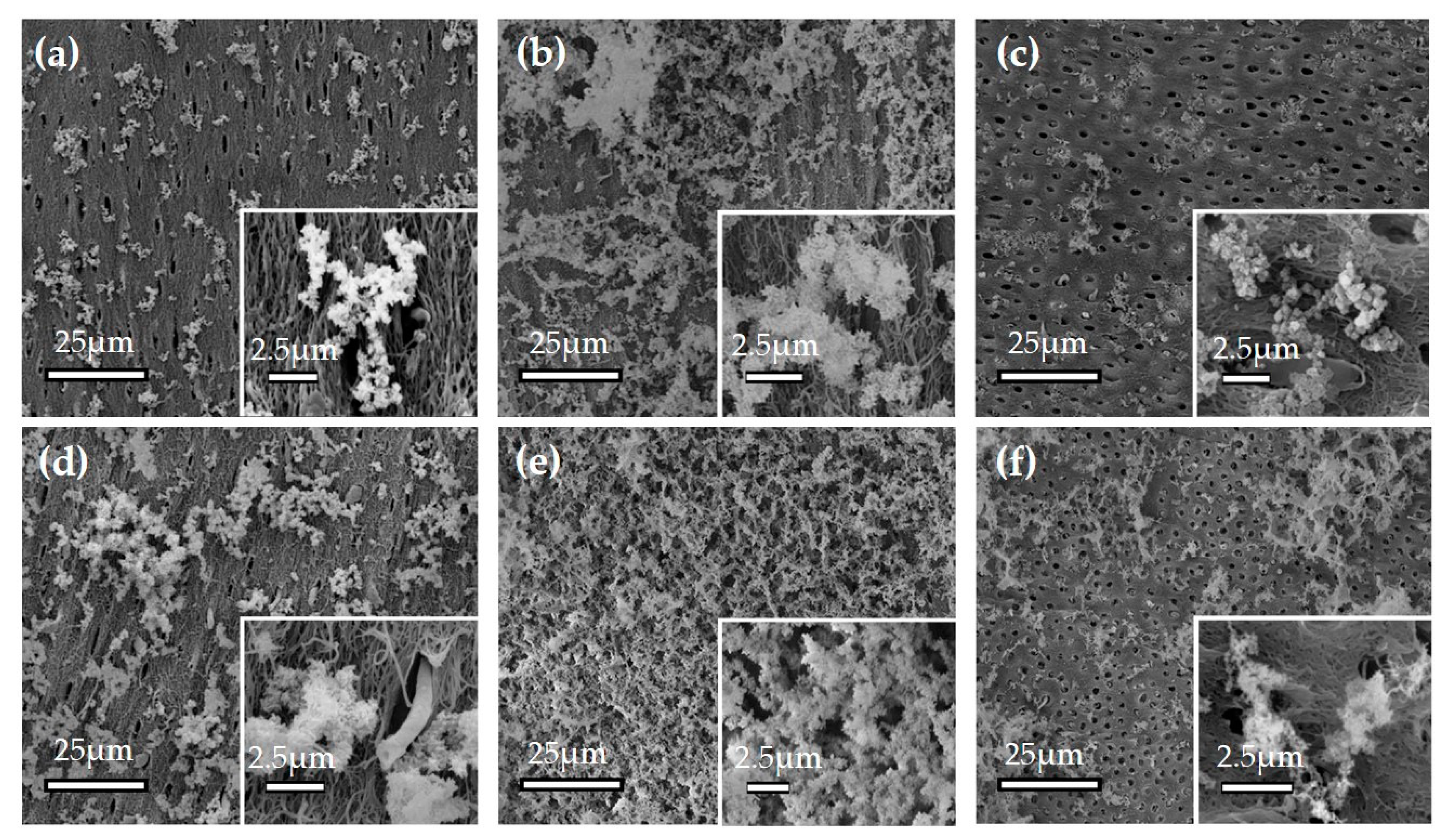
3.1.1. Effect of ox-LDL on the Mineralization on the Dentin Surface
3.1.2. Ca/P Analysis of Precipitates with EPMA
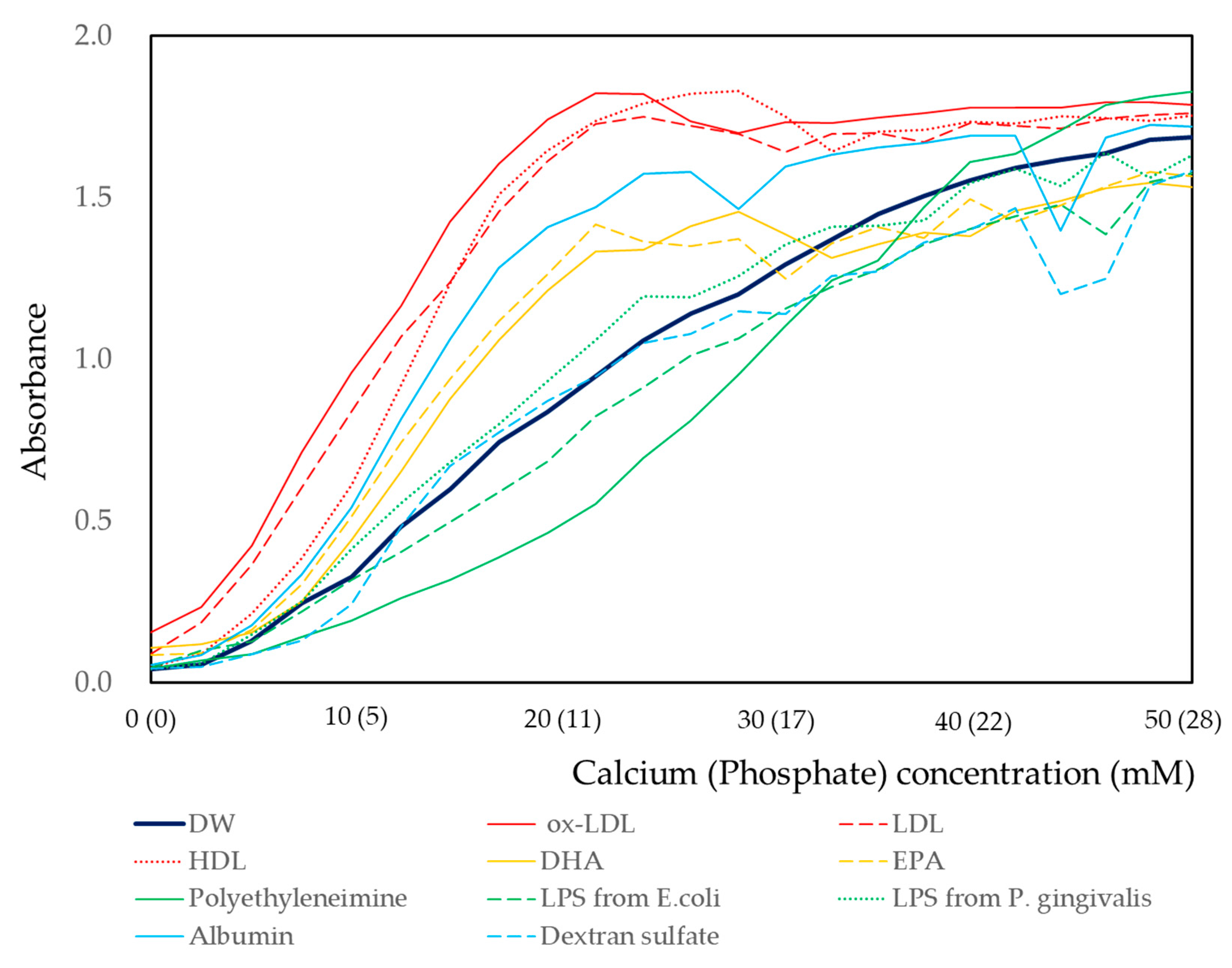
3.1.3. Effects of Calcium and Phosphate Concentrations
3.1.4. Effect of Concentration of Biomaterials on Calcium and Phosphate Reaction
3.2. Solid-Phase Analysis of Precipitates by Electron Microscopy
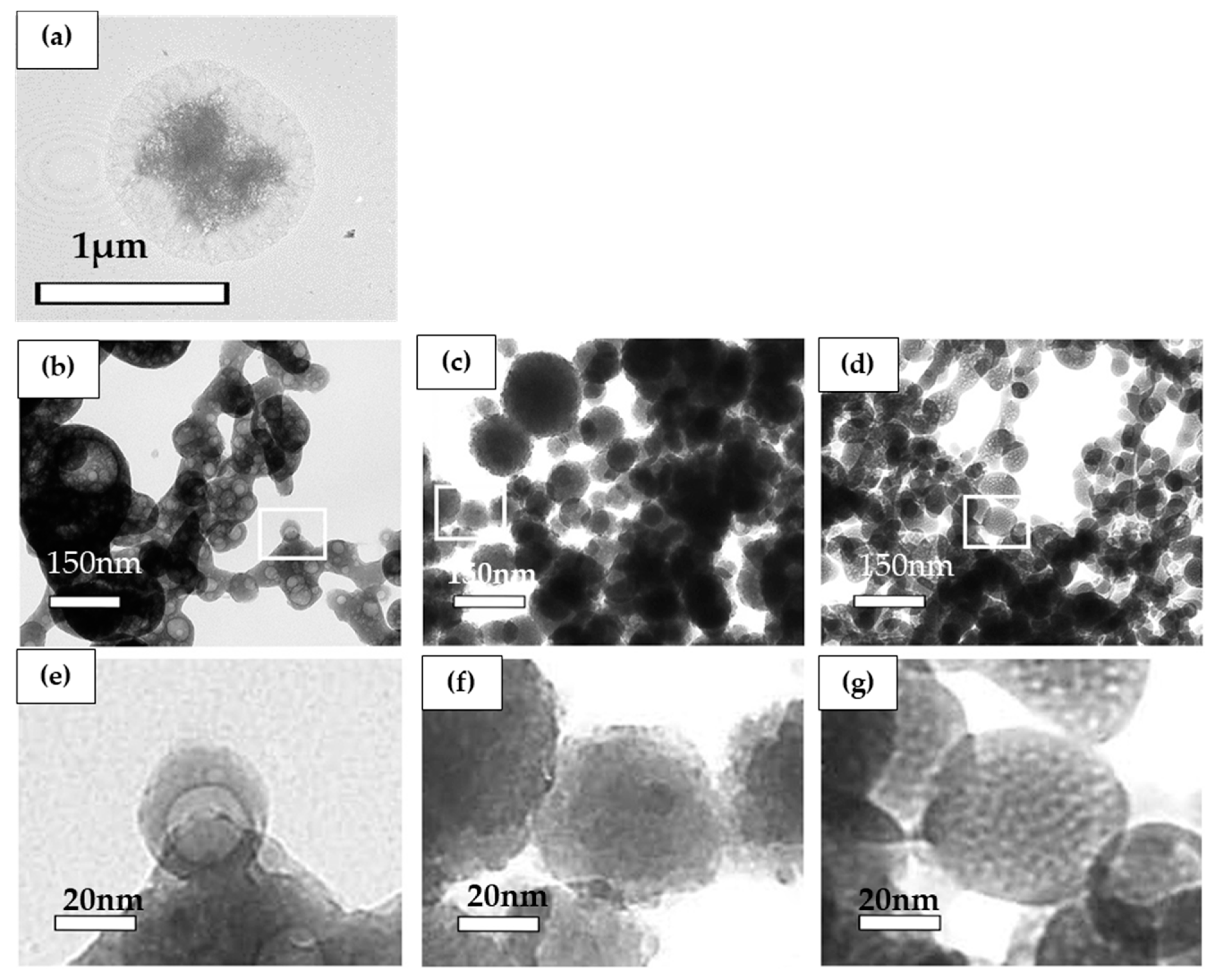
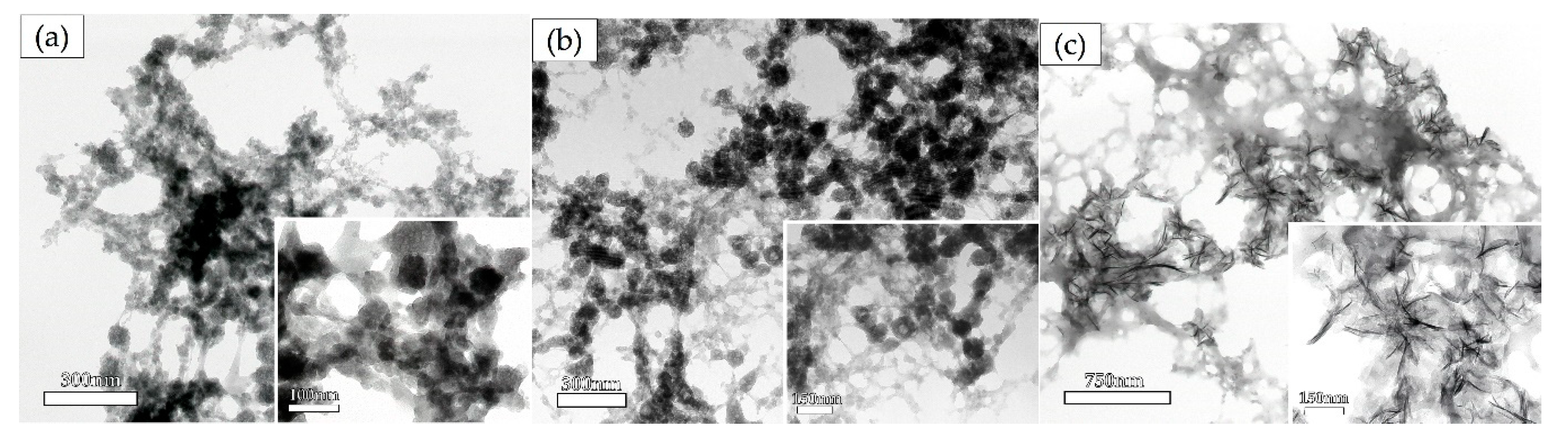
3.2.1. Transmission Electron Microscopy Analysis
Effect of Biomaterials on the Morphology of Precipitates as Determined by TEM Observation
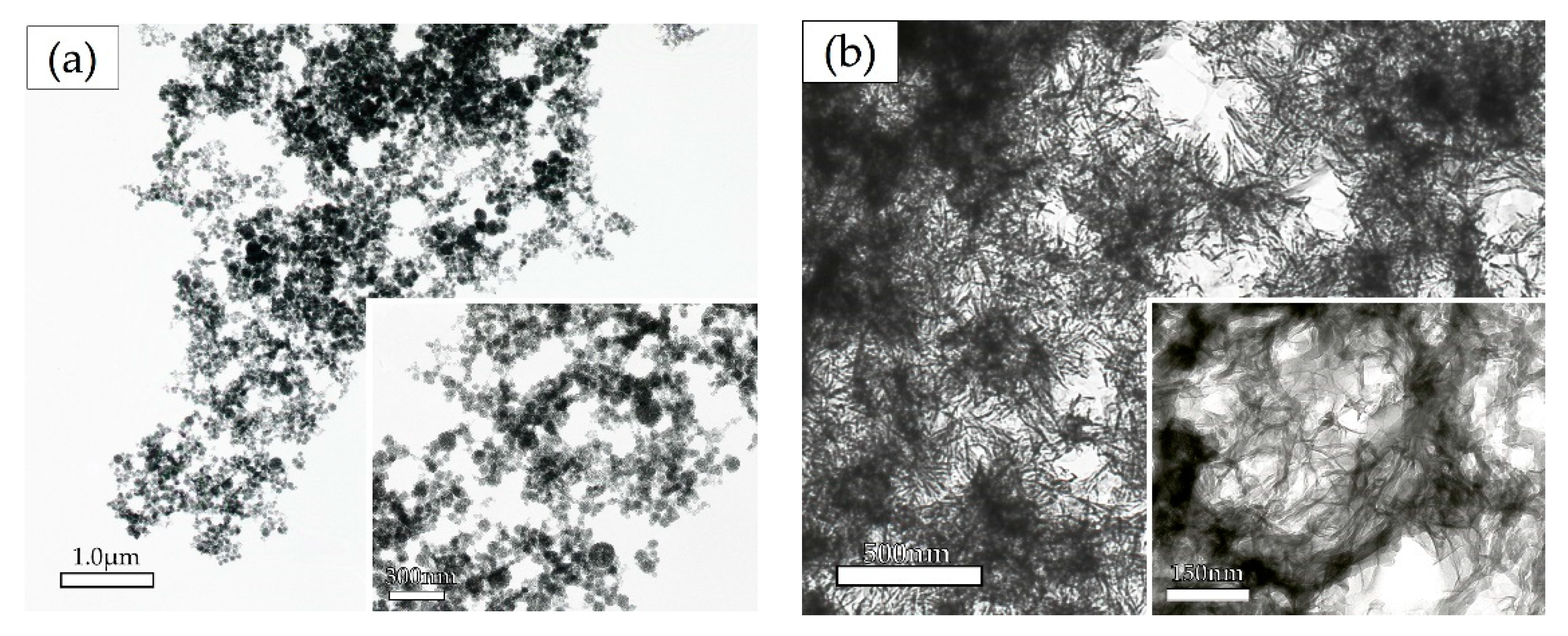
The Effect of Calcium and Phosphate Concentrations on the Ox-LDL-Containing Precipitates as Determined by Electron Microscopy
Time Course Analysis of the Crystal Shape in the Solution Containing 0.025% Ox-LDL
Identification of the Precipitates by Selected Area Electron Diffraction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giachelli, C.M. Ectopic Calcification. Am. J. Pathol. 1999, 154, 671–675. [Google Scholar] [CrossRef]
- Okamoto, M.; Murai, J.; Yoshikawa, H.; Tsumaki, N. Bone Morphogenetic Proteins in Bone Stimulate Osteoclasts and Osteoblasts During Bone Development. J. Bone Miner. Res. 2006, 21, 1022–1033. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Kinoshita, S.; Izuhara, L.; Karakida, T.; Fukae, M.; Oida, S. DPP and DSP are Necessary for Maintaining TGF-β1 Activity in Dentin. J. Dent. Res. 2014, 93, 671–677. [Google Scholar] [CrossRef]
- Griffin, B.A. Lipoprotein atherogenicity: An overview of current mechanisms. Proc. Nutr. Soc. 1999, 58, 163–169. [Google Scholar] [CrossRef]
- Tabas, I. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death Differ. 2004, 11, S12–S16. [Google Scholar] [CrossRef]
- Kumon, H.; Matsumoto, A.; Uehara, S.; Abarzua, F.; Araki, M.; Tsutsui, K.; Tomochika, K.-I. Detection and isolation of nanobacteria-like particles from urinary stones: Long-withheld data. Int. J. Urol. 2011, 18, 458–465. [Google Scholar] [CrossRef]
- Kumon, H.; Matsuura, E.; Nagaoka, N.; Yamamoto, T.; Uehara, S.; Araki, M.; Matsunami, Y.; Kobayashi, K.; Matsumoto, A. Ectopic calcification: importance of common nanoparticle scaffolds containing oxidized acidic lipids. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 441–450. [Google Scholar] [CrossRef]
- Brecher, G.; Bookstein, N.; Redfearn, W.; Nečas, E.; Pallavicini, M.G.; Cronkite, E.P. Self-renewal of the long-term repopulating stem cell. Proc. Natl. Acad. Sci. USA 1993, 90, 6028–6031. [Google Scholar] [CrossRef]
- Pentikainen, M.O.; Oorni, K.; Ala-Korpela, M.; Kovanen, P.T. Modified LDL—trigger of atherosclerosis and inflammation in the arterial intima. J. Intern. Med. 2000, 247, 359–370. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Kato, R.; Inoue, S.; Suzuki, K.; Itabe, H.; Yamamoto, M. Detection of oxidized low-density lipoproteins in gingival crevicular fluid from dental patients. J. Periodontal Res. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Türer, Ç.C.; Ballı, U.; Güven, B. Fetuin-A, serum amyloid A and tumor necrosis factor alpha levels in periodontal health and disease. Oral Dis. 2017, 23, 379–386. [Google Scholar] [CrossRef]
- Que, X.; Kuang-Hung, H.; Yeang, C.; Egonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nat. Cell Biol. 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Ribeiro, P.V.D.M.; Andrade, P.A.; Hermsdorff, H.; Dos Santos, C.A.; Cotta, R.M.M.; Estanislau, J.D.A.S.G.; Campos, A.A.D.O.; Rosa, C.D.O.B. Dietary non-nutrients in the prevention of non-communicable diseases: Potentially related mechanisms. Nutrition 2019, 66, 22–28. [Google Scholar] [CrossRef]
- Baghbani-Oskouei, A.; Tohidi, M.; Asgari, S.; Ramezankhani, A.; Azizi, F.; Hadaegh, F. Serum Lipids During 20 Years in the Tehran Lipid and Glucose Study: Prevalence, Trends and Impact on Non-Communicable Diseases. Int. J. Endocrinol. Metab. 2018, 16, e84750. [Google Scholar] [CrossRef]
- Shimoda, S.; Aoba, T.; Moreno, E.; Miake, Y. Effect of Solution Composition on Morphological and Structural Features of Carbonated Calcium Apatites. J. Dent. Res. 1990, 69, 1731–1740. [Google Scholar] [CrossRef]
- Merry, J.C.; Gibson, I.R.; Best, S.M.; Bonfield, W. Synthesis and characterization of carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 779–783. [Google Scholar] [CrossRef]
- Cottignoli, V.; Relucenti, M.; Agrosì, G.; Cavarretta, E.; Familiari, G.; Salvador, L.; Maras, A. Biological Niches within Human Calcified Aortic Valves: Towards Understanding of the Pathological Biomineralization Process. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Nancollas, G.; Mohan, M. The growth of hydroxyapatite crystals. Arch. Oral Biol. 1970, 15, 731–745. [Google Scholar] [CrossRef]
- Aoba, T.; Moreno, E. Preparation of Hydroxyapatite Crystals and Their Behavior as Seeds for Crystal Growth. J. Dent. Res. 1984, 63, 874–880. [Google Scholar] [CrossRef]
- Harries, J.; Hukins, D.; Holt, C.; Hasnain, S. Conversion of amorphous calcium phosphate into hydroxyapatite investigated by EXAFS spectroscopy. J. Cryst. Growth 1987, 84, 563–570. [Google Scholar] [CrossRef]
- Holt, C.; Van Kemenade, M.; Harries, J.; Nelson, L.; Bailey, R.; Hukins, D.; Hasnain, S.; De Bruyn, P. Preparation of amorphous calcium-magnesium phosphates at pH 7 and characterization by x-ray absorption and fourier transform infrared spectroscopy. J. Cryst. Growth 1988, 92, 239–252. [Google Scholar] [CrossRef]
- Holt, C.; van Kemenade, M.J.J.M.; Nelson, L.S.; Sawyer, L.; Harries, J.E.; Bailey, R.T.; Hukins, D.W.L. Composition and Structure of Micellar Calcium Phosphate. J. Dairy Res. 1989, 56, 411–416. [Google Scholar] [CrossRef]
- Termine, J.D.; Eanes, E.D. Comparative chemistry of amorphous and apatitic calcium phosphate preparations. Calcif. Tissue Int. 1972, 10, 171–197. [Google Scholar] [CrossRef]
- Eanes, E.D.; Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tissue Int. 1977, 23, 259–269. [Google Scholar] [CrossRef]
- Eanes, E.D.; Termine, J.D.; Nylen, M.U. An electron microscopic study of the formation of amorphous calcium phosphate and its transformation to crystalline apatite. Calcif. Tissue Int. 1973, 12, 143–158. [Google Scholar] [CrossRef]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Accounts Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Eanes, E.D. The interaction of supersaturated calcium phosphate solutions with apatitic substrates. Calcif. Tissue Int. 1976, 20, 75–89. [Google Scholar] [CrossRef]
- Eanes, E. The Influence of Fluoride on the Seeded Growth of Apatite from Stable Supersaturated Solutions at pH 7.4. J. Dent. Res. 1980, 59, 144–150. [Google Scholar] [CrossRef]
- Landis, W.J.; Glimcher, M.J. Electron optical and analytical observations of rat growth plate cartilage prepared by ultracryomicrotomy: The failure to detect a mineral phase in matrix vesicles and the identification of heterodispersed particles as the initial solid phase of calcium phosphate deposited in the extracellular matrix. J. Ultrastruct. Res. 1982, 78, 227–268. [Google Scholar] [CrossRef]
- Doi, Y.; Eanes, E.; Shimokawa, H.; Termine, J. Inhibition of Seeded Growth of Enamel Apatite Crystals by Amelogenin and Enamelin Proteins in vitro. J. Dent. Res. 1984, 63, 98–105. [Google Scholar] [CrossRef]
- Gillotte, K.L.; Hӧrkko, S.; Witztum, J.L.; Steinberg, D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. J. Lipid Res. 2000, 41, 824–833. [Google Scholar]
- Fujita, Y.; Kakino, A.; Nishimichi, N.; Yamaguchi, S.; Sato, Y.; Machida, S.; Cominacini, L.; Delneste, Y.; Matsuda, H.; Sawamura, T. Oxidized LDL Receptor LOX-1 Binds to C-Reactive Protein and Mediates Its Vascular Effects. Clin. Chem. 2009, 55, 285–294. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chemie. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Chow, L.C. Development of Self-Setting Calcium Phosphate Cements. J. Ceram. Soc. Jpn. 1991, 99, 954–964. [Google Scholar] [CrossRef]
- Fukae, M. Enamel Formation-Biochemical Aspect-. J. Oral Biosci. 2009, 51, 46–60. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Lipoprotein Metabolism in the Macrophage: Implications for Cholesterol Deposition in Atherosclerosis. Annu. Rev. Biochem. 1983, 52, 223–261. [Google Scholar] [CrossRef]
- Kita, T.; Nagano, Y.; Yokode, M.; Ishii, K.; Kume, N.; Ooshima, A.; Yoshida, H.; Kawai, C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA 1987, 84, 5928–5931. [Google Scholar] [CrossRef]
- Steinberg, D. LDL-Cell Interaction In Vivo and In Vitro. Ann. N. Y. Acad. Sci. 1980, 348, 256–264. [Google Scholar] [CrossRef]

| - | Coefficient (95% CI) | p-Value | - | |
| Calcium and Phosphate | 0.138 (0.133–0.143) | <0.001 | ||
| Biomaterials | ||||
| DW | Reference | |||
| Biomaterials | Crude | Interaction with Calcium and Phosphate | ||
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | |
| Ox-LDL | 0.600 (0.519–0.680) | <0.001 | −0.015 (−0.020–−0.009) | <0.001 |
| LDL | 0.497 (0.417–0.578) | <0.001 | −0.015 (−0.020–−0.010) | <0.001 |
| HDL | 0.429 (0.349–0.501) | <0.001 | −0.011 (−0.016–−0.006) | <0.001 |
| DHA | 0.189 (0.109–0.207) | <0.001 | −0.011 (−0.016–−0.006) | <0.001 |
| EPA | 0.230 (0.150–0.311) | <0.001 | −0.008 (−0.013–−0.003) | 0.001 |
| Polyethyleneimine | −0.205 (−0.286–−0.124) | <0.001 | 0.003 (−0.002–0.008) | 0.266 |
| LPS from E. coli | −0.068 (−0.148–0.013) | 0 100 | −0.001 (−0.006–0.004) | 0.639 |
| LPS from P. gingivalis | 0.061 (0.020–0.142) | 0.137 | −0.002 (−0.007–0.003) | 0.448 |
| Albumin | 0.303 (0.223–0.384) | <0.001 | −0.011 (−0.016–−0.006) | <0.001 |
| Dextran sulfate | −0.010 (−0.076–0.055) | 0.756 | −0.002 (−0.007–0.003) | 0.354 |
| Intercept | −0.191 (−0.253–−0.129) | <0.001 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, M.; Nomura, Y.; Ishikawa, M.; Shimoda, S.; Hanada, N. Oxidized Low-Density Lipoprotein Promotes In Vitro Calcification. Materials 2020, 13, 5120. https://doi.org/10.3390/ma13225120
Yamashita M, Nomura Y, Ishikawa M, Shimoda S, Hanada N. Oxidized Low-Density Lipoprotein Promotes In Vitro Calcification. Materials. 2020; 13(22):5120. https://doi.org/10.3390/ma13225120
Chicago/Turabian StyleYamashita, Mamiko, Yoshiaki Nomura, Misao Ishikawa, Shinji Shimoda, and Nobuhiro Hanada. 2020. "Oxidized Low-Density Lipoprotein Promotes In Vitro Calcification" Materials 13, no. 22: 5120. https://doi.org/10.3390/ma13225120
APA StyleYamashita, M., Nomura, Y., Ishikawa, M., Shimoda, S., & Hanada, N. (2020). Oxidized Low-Density Lipoprotein Promotes In Vitro Calcification. Materials, 13(22), 5120. https://doi.org/10.3390/ma13225120






