Efficiency of DNA Isolation Methods Based on Silica Columns and Magnetic Separation Tested for the Detection of Mycobacterium avium Subsp. Paratuberculosis in Milk and Faeces
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MAP Culture for Artificial Contamination Experiments
2.2. Preparation of Milk and Faecal Samples Used for Artificial Contamination
2.3. DNA Isolation and qPCR Detection of MAP
3. Results
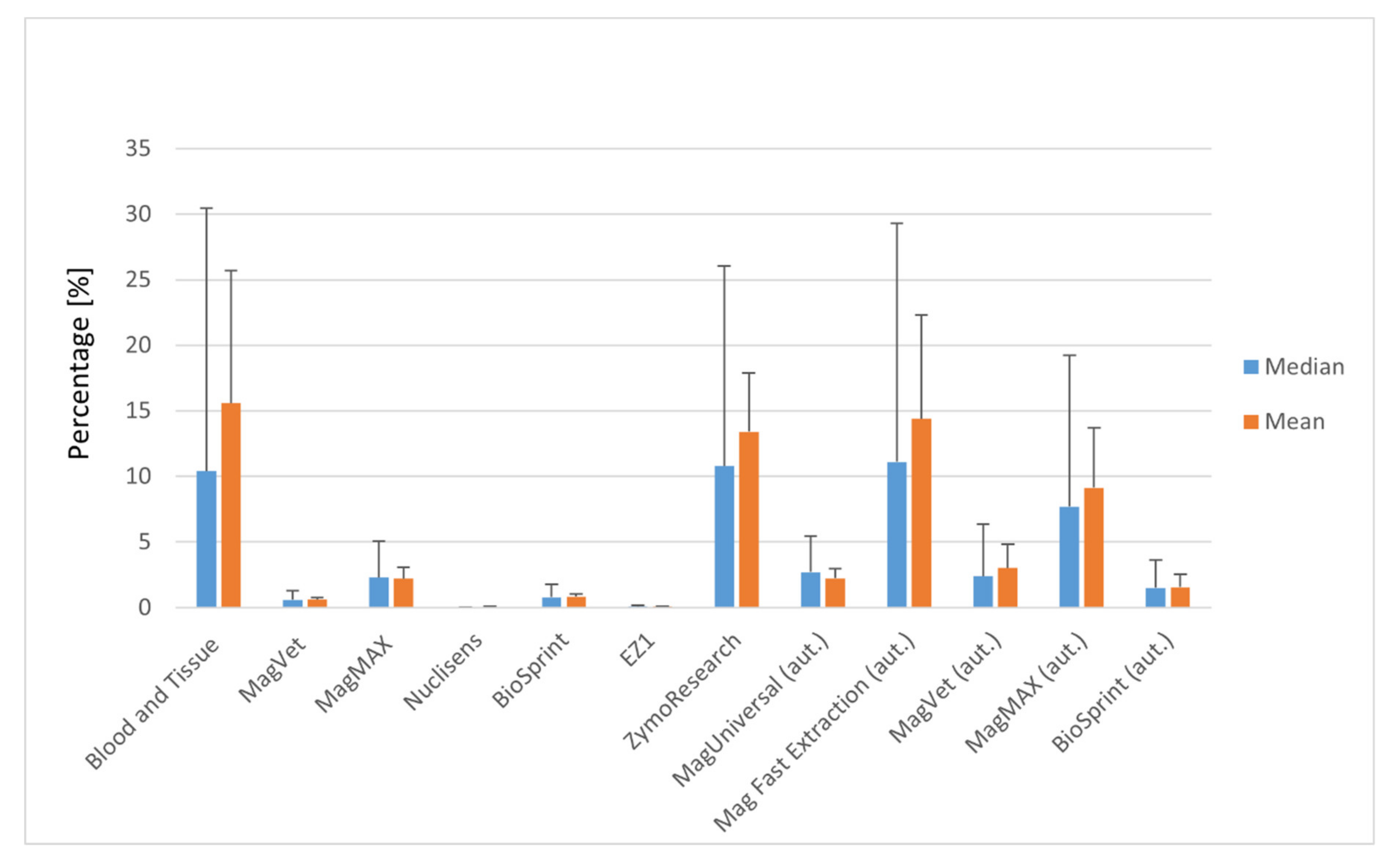
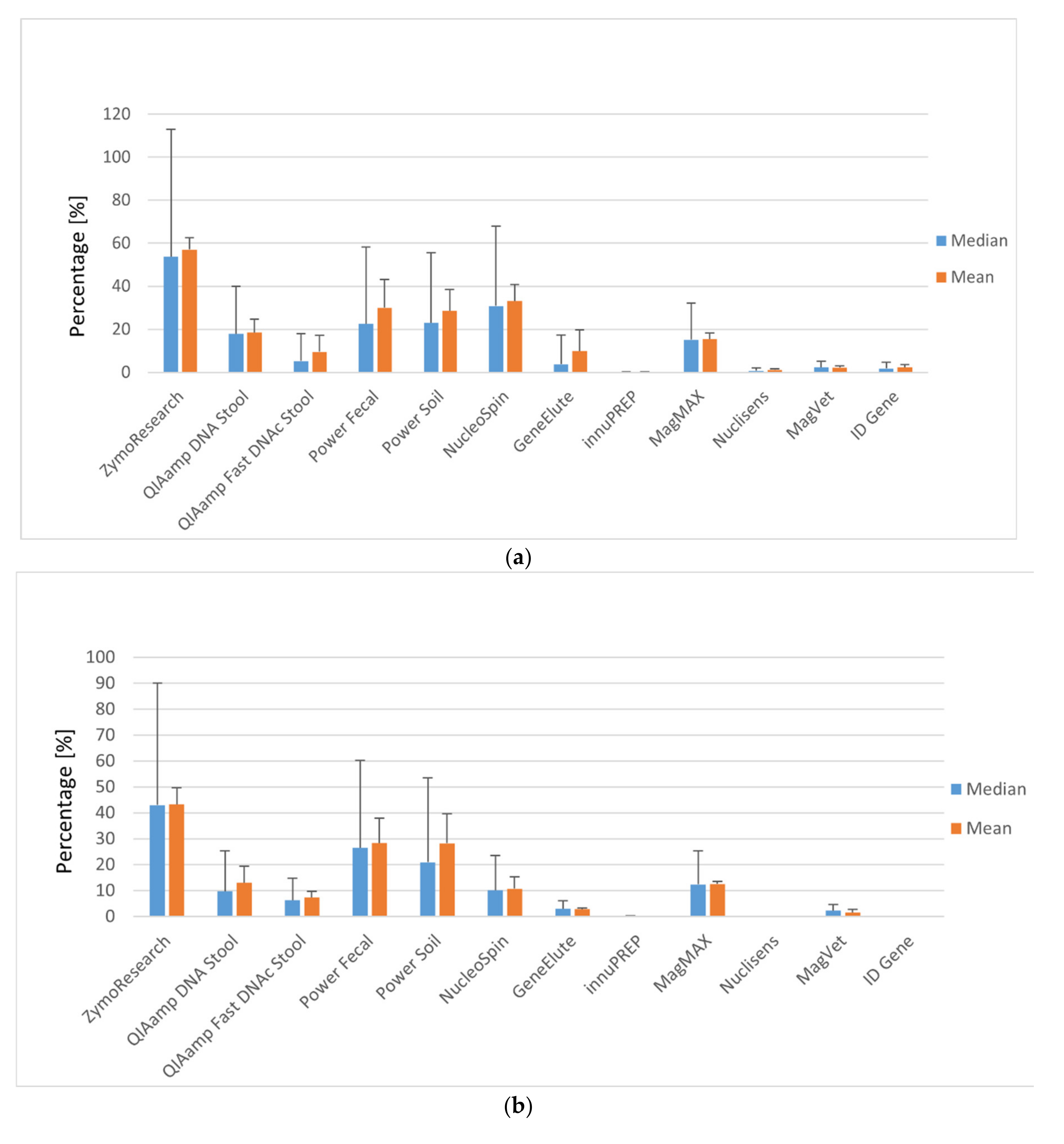
3.1. Efficiency of Manual MAP DNA Isolation
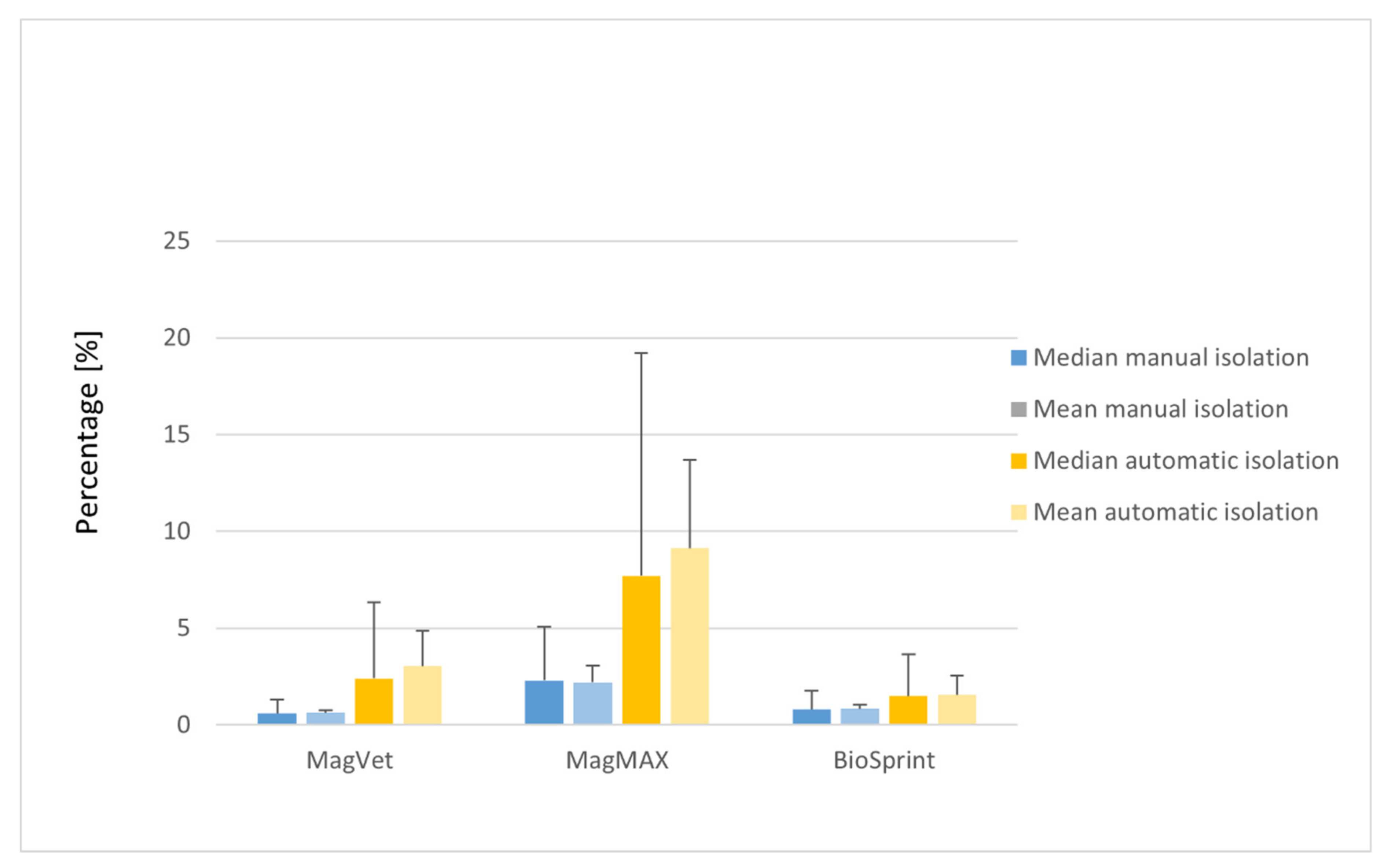
3.2. Manual versus Automatic Magnetic Separation of MAP DNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stabel, J.R. Johne’s disease: A hidden threat. J. Dairy Sci. 1998, 81, 283–288. [Google Scholar] [CrossRef]
- Olsen, I.; Sigurgardottir, G.; Djonne, B. Paratuberculosis with special reference to cattle. A review. Vet. Q. 2002, 24, 12–28. [Google Scholar] [CrossRef]
- Khare, S.; Ficht, T.A.; Santos, R.L.; Romano, J.; Ficht, A.R.; Zhang, S.; Grant, I.R.; Libal, M.; Hunter, D.; Adams, L.G. Rapid and sensitive detection of Mycobacterium avium subsp. paratuberculosis in bovine milk and feces by a combination of immunomagnetic bead separation-conventional PCR and real-time PCR. J. Clin. Microbiol. 2004, 42, 1075–1081. [Google Scholar] [CrossRef]
- Whitlock, R.H.; Wells, S.J.; Sweeney, R.W.; Van Tiem, J. ELISA and fecal culture for paratuberculosis (Johne’s disease): Sensitivity and specificity of each method. Vet. Microbiol. 2000, 77, 387–398. [Google Scholar] [CrossRef]
- Leite, F.L.; Stokes, K.D.; Robbe-Austerman, S.; Stabel, J.R. Comparison of fecal DNA extraction kits for the detection of Mycobacterium avium subsp. paratuberculosis by polymerase chain reaction. J. Vet. Diagn. Investig. 2013, 25, 27–34. [Google Scholar] [CrossRef]
- Whittington, R.J.; Sergeant, E.S.G. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp paratuberculosis in animal populations. Aust. Vet. J. 2001, 79, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Donat, K.; Hahn, N.; Eisenberg, T.; Schlez, K.; Kohler, H.; Wolter, W.; Rohde, M.; Putzschel, R.; Roesler, U.; Failing, K.; et al. Within-herd prevalence thresholds for the detection of Mycobacterium avium subspecies paratuberculosis-positive dairy herds using boot swabs and liquid manure samples. Epidemiol. Infect. 2016, 144, 413–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patterson, S.; Bond, K.; Green, M.; van Winden, S.; Guitian, J. Mycobacterium avium paratuberculosis infection of calves—The impact of dam infection status. Prev. Vet. Med. 2019, 181. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Chamberlin, W.M.; Sarosiek, J.; McCallum, R.W. Crohn’s disease and the mycobacterioses: A quarter century later. Causation or simple association? Crit. Rev. Microbiol. 2012, 38, 52–93. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 1992, 30, 166–171. [Google Scholar] [CrossRef]
- Streeter, R.N.; Hoffsis, G.F.; Bech-Nielsen, S.; Shulaw, W.P.; Rings, D.M. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 1995, 56, 1322–1324. [Google Scholar] [PubMed]
- McAloon, C.G.; Roche, S.; Ritter, C.; Barkema, H.W.; Whyte, P.; More, S.J.; O’Grady, L.; Green, M.J.; Doherty, M.L. A review of paratuberculosis in dairy herds—Part 2: On-farm control. Vet. J. 2019, 246, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R.; Hitchings, E.I.; McCartney, A.; Ferguson, F.; Rowe, M.T. Effect of Commercial-Scale High- Temperature, Short-Time Pasteurization on the Viability of Mycobacterium paratuberculosis in Naturally Infected Cows’ Milk. Appl. Environ. Microbiol. 2002, 68, 602. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R.; Ball, H.J.; Rowe, M.T. Incidence of Mycobacterium paratuberculosis in Bulk Raw and Commercially Pasteurized Cows’ Milk from Approved Dairy Processing Establishments in the United Kingdom. Appl. Environ. Microbiol. 2002, 68, 2428. [Google Scholar] [CrossRef]
- Botsaris, G.; Swift, B.M.C.; Slana, I.; Liapi, M.; Christodoulou, M.; Hatzitofi, M.; Christodoulou, V.; Rees, C.E.D. Detection of viable Mycobacterium avium subspecies paratuberculosis in powdered infant formula by phage-PCR and confirmed by culture. Int. J. Food Microbiol. 2016, 216, 91–94. [Google Scholar] [CrossRef]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A.; Hermon-Taylor, J.; Hermon-Taylor, A.; Dow, C.T.; Thayer, W.; et al. The Consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) Conference 2017. Front. Public Health 2017, 5, 208. [Google Scholar] [CrossRef]
- Bögli-Stuber, K.; Kohler, C.; Seitert, G.; Glanemann, B.; Antognoli, M.C.; Salman, M.D.; Wittenbrink, M.M.; Wittwer, M.; Wassenaar, T.; Jemmi, T.; et al. Detection of Mycobacterium avium subspecies paratuberculosis in Swiss dairy cattle by real-time PCR and culture: A comparison of the two assays. J. Appl. Microbiol. 2005, 99, 587–597. [Google Scholar] [CrossRef]
- Douarre, P.E.; Cashman, W.; Buckley, J.; Coffey, A.; O’Mahony, J.M. Isolation and detection of Mycobacterium avium subsp. paratuberculosis (MAP) from cattle in Ireland using both traditional culture and molecular based methods. Gut. Pathog. 2010, 2, 11. [Google Scholar] [CrossRef][Green Version]
- Kreader, C.A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol. 1996, 62, 1102. [Google Scholar] [CrossRef]
- Monteiro, L.; Bonnemaison, D.; Vekris, A.; Petry, K.G.; Bonnet, J.; Vidal, R.; Cabrita, J.; Mégraud, F. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 1997, 35, 995. [Google Scholar] [CrossRef]
- Thornton, C.G.; Passen, S. Inhibition of PCR amplification by phytic acid, and treatment of bovine fecal specimens with phytase to reduce inhibition. J. Microbiol. Methods 2004, 59, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, M.D.; Vaughan, J.A.; Shiell, B.J.; Beddome, G.J.; Michalski, W.P. Mycobacterial proteome extraction: Comparison of disruption methods. Proteomics 2004, 4, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Grant, I.R.; Ball, H.J.; Rowe, M.T. Isolation of Mycobacterium paratuberculosis from milk by immunomagnetic separation. Appl. Environ. Microbiol. 1998, 64, 3153–3158. [Google Scholar] [CrossRef]
- Foddai, A.; Elliott, C.T.; Grant, I.R. Maximizing capture efficiency and specificity of magnetic separation for Mycobacterium avium subsp. paratuberculosis cells. Appl. Environ. Microbiol. 2010, 76, 7550–7558. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.D.; Foddai, A.; Elliott, C.T.; Grant, I.R. Development of a novel phage-mediated immunoassay for the rapid detection of viable Mycobacterium avium subsp. paratuberculosis. J. Appl. Microbiol. 2013, 115, 808–817. [Google Scholar] [CrossRef]
- Husakova, M.; Dziedzinska, R.; Slana, I. Magnetic Separation Methods for the Detection of Mycobacterium avium subsp. paratuberculosis in Various Types of Matrices: A Review. Biomed. Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Gülçin, G.; Çiğdem, K.S.; Eda, Ö.; Hasan, İ.; Güneş, K.; Ali, T. Comparative DNA isolation behaviours of silica and polymer based sorbents in batch fashion: Monodisperse silica microspheres with bimodal pore size distribution as a new sorbent for DNA isolation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 178–184. [Google Scholar] [CrossRef]
- Kralik, P.; Slana, I.; Kralova, A.; Babak, V.; Whitlock, R.H.; Pavlik, I. Development of a predictive model for detection of Mycobacterium avium subsp. paratuberculosis in faeces by quantitative real time PCR. Vet. Microbiol. 2011, 149, 133–138. [Google Scholar] [CrossRef]
- Slana, I.; Kralik, P.; Kralova, A.; Pavlik, I. On-farm spread of Mycobacterium avium subsp. paratuberculosis in raw milk studied by IS900 and F57 competitive real time quantitative PCR and culture examination. Int. J. Food Microbiol. 2008, 128, 250–257. [Google Scholar] [CrossRef]
- Okwumabua, O.; Shull, E.; O’Connor, M.; Moua, T.V.; Danz, T.; Strelow, K. Comparison of three methods for extraction of Mycobacterium avium subspecies paratuberculosis DNA for polymerase chain reaction from broth-based culture systems. J. Vet. Diagn. Investing. 2010, 22, 67–69. [Google Scholar] [CrossRef]
- Plain, K.M.; Waldron, A.M.; Begg, D.J.; de Silva, K.; Purdie, A.C.; Whittington, R.J. Efficient, Validated Method for Detection of Mycobacterial Growth in Liquid Culture Media by Use of Bead Beating, Magnetic-Particle- Based Nucleic Acid Isolation, and Quantitative PCR. J. Clin. Microbiol. 2015, 53, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Herthnek, D.; Nielsen, S.S.; Lindberg, A.; Bölske, G. A robust method for bacterial lysis and DNA purification to be used with real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in milk. J. Microbiol. Methods 2008, 75, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bickley, J.; Short, J.K.; McDowell, D.G.; Parkes, H.C. Polymerase chain reaction (PCR) detection of Listeria monocytogenes in diluted milk and reversal of PCR inhibition caused by calcium ions. Lett. Appl. Microbiol. 1996, 22, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Chui, L.W.; King, R.; Lu, P.; Manninen, K.; Sim, J. Evaluation of four DNA extraction methods for the detection of Mycobacterium avium subsp. paratuberculosis by polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 2004, 48, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Hanrahan, D.P.F.; Pertea, G.; Tassell, C.P.V.T.; Yorke, J.A.; Salzberg, S.L.; et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009, 10, R42. [Google Scholar] [CrossRef]
- Sting, R.; Hrubenja, M.; Mandl, J.; Seemann, G.; Salditt, A.; Waibel, S. Detection of Mycobacterium avium subsp. paratuberculosis in faeces using different procedures of pre-treatment for real-time PCR in comparison to culture. Vet. J. 2014, 199, 138–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husakova, M.; Kralik, P.; Babak, V.; Slana, I. Efficiency of DNA Isolation Methods Based on Silica Columns and Magnetic Separation Tested for the Detection of Mycobacterium avium Subsp. Paratuberculosis in Milk and Faeces. Materials 2020, 13, 5112. https://doi.org/10.3390/ma13225112
Husakova M, Kralik P, Babak V, Slana I. Efficiency of DNA Isolation Methods Based on Silica Columns and Magnetic Separation Tested for the Detection of Mycobacterium avium Subsp. Paratuberculosis in Milk and Faeces. Materials. 2020; 13(22):5112. https://doi.org/10.3390/ma13225112
Chicago/Turabian StyleHusakova, Marketa, Petr Kralik, Vladimir Babak, and Iva Slana. 2020. "Efficiency of DNA Isolation Methods Based on Silica Columns and Magnetic Separation Tested for the Detection of Mycobacterium avium Subsp. Paratuberculosis in Milk and Faeces" Materials 13, no. 22: 5112. https://doi.org/10.3390/ma13225112
APA StyleHusakova, M., Kralik, P., Babak, V., & Slana, I. (2020). Efficiency of DNA Isolation Methods Based on Silica Columns and Magnetic Separation Tested for the Detection of Mycobacterium avium Subsp. Paratuberculosis in Milk and Faeces. Materials, 13(22), 5112. https://doi.org/10.3390/ma13225112




