Magnetic Particles for Advanced Molecular Diagnosis
Abstract
1. Introduction
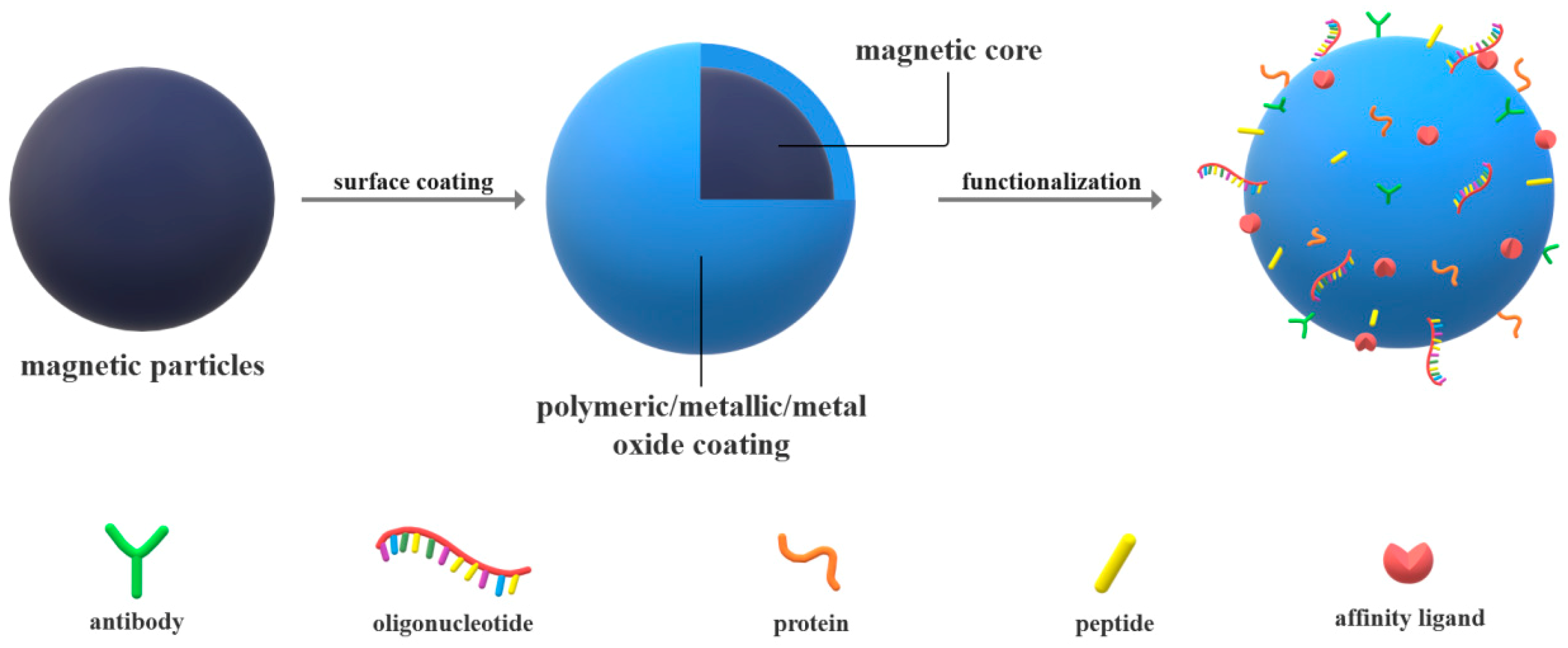
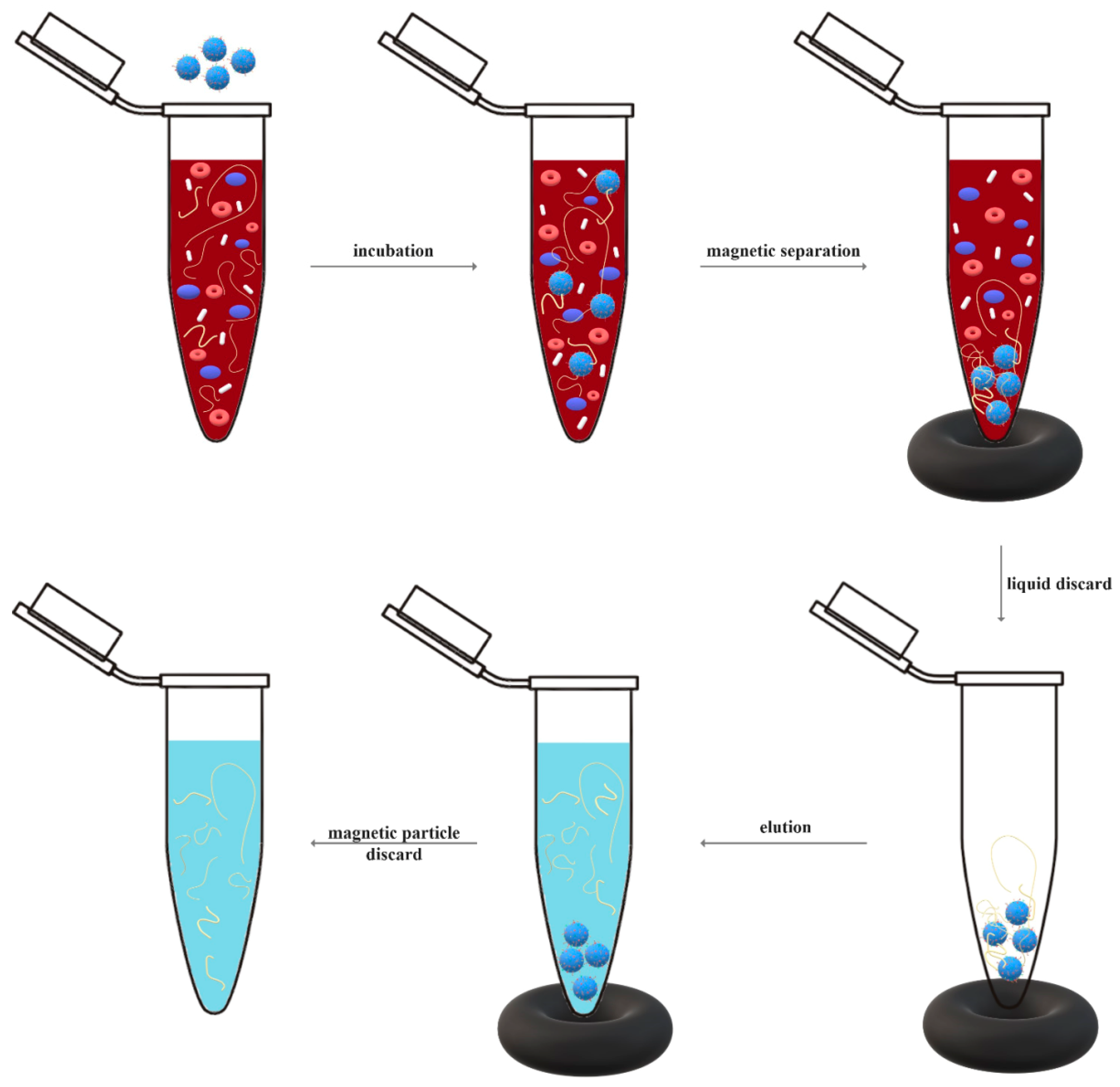
2. Magnetic Particles in Diagnosis
3. Cancer Diagnosis
4. Diagnosis of Infectious Diseases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.; Zoëga Andreasen, S.; Wolff, A.; Duong Bang, D. From Lab on a Chip to Point of Care Devices: The Role of Open Source Microcontrollers. Micromachines 2018, 9, 403. [Google Scholar] [CrossRef]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Contrast Agents Delivery: An Up-to-Date Review of Nanodiagnostics in Neuroimaging. Nanomaterials 2019, 9, 542. [Google Scholar] [CrossRef]
- Spataro, N.; Rodríguez, J.A.; Navarro, A.; Bosch, E. Properties of human disease genes and the role of genes linked to Mendelian disorders in complex disease aetiology. Hum. Mol. Genet. 2017, 26, 489–500. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Chapter 1—Cellular and Molecular Diagnostics: An Introduction. In Advances in Cell and Molecular Diagnostics; Raghavendra, P., Pullaiah, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–32. [Google Scholar]
- Jackson, M.; Marks, L.; May, G.H.W.; Wilson, J.B. The genetic basis of disease. Essays Biochem. 2018, 62, 643–723. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Chapter 3—Advancements in Genetic Applications for Cellular and Molecular Diagnostics. In Advances in Cell and Molecular Diagnostics; Raghavendra, P., Pullaiah, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 57–84. [Google Scholar]
- Patrinos, G.P.; Danielson, P.B.; Ansorge, W.J. Chapter 1—Molecular Diagnostics: Past, Present, and Future. In Molecular Diagnostics, 3rd ed.; Patrinos, G.P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–11. [Google Scholar]
- Turner, S.A.; Tsongalis, G.J. Chapter 4—Automation of the Molecular Diagnostic Laboratory. In Diagnostic Molecular Pathology; Coleman, W.B., Tsongalis, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 35–46. [Google Scholar]
- Wanger, A.; Chavez, V.; Huang, R.S.P.; Wahed, A.; Actor, J.K.; Dasgupta, A. Chapter 12—Overview of Molecular Diagnostics Principles. In Microbiology and Molecular Diagnosis in Pathology; Wanger, A., Chavez, V., Huang, R., Wahed, A., Dasgupta, A., Actor, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–257. [Google Scholar]
- Williams, E.S.; Silverman, L.M. Chapter 30—Molecular Diagnosis of Human Disease. In Molecular Pathology, 2nd ed.; Coleman, W.B., Tsongalis, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 691–707. [Google Scholar]
- Li, T.; Zhang, Z.-j.; Ma, X.; Lv, X.; Xiao, H.; Guo, Q.-n.; Liu, H.-y.; Wang, H.-d.; Wu, D.; Lou, G.-y.; et al. Prenatal diagnosis for a Chinese family with a de novo DMD gene mutation: A case report. Medicine 2017, 96, e8814. [Google Scholar] [CrossRef]
- Raghavendra, P.; Pullaiah, T. Chapter 8—Future of Cellular and Molecular Diagnostics: Bench to Bedside. In Advances in Cell and Molecular Diagnostics; Raghavendra, P., Pullaiah, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 203–270. [Google Scholar]
- Galkin, O.Y.; Besarab, O.B.; Pysmenna, M.O.; Gorshunov, Y.V.; Dugan, O.M. Modern magnetic immunoassay: Biophysical and biochemical aspects. Regul. Mech. Biosyst. 2017, 9, 47–55. [Google Scholar] [CrossRef]
- Nelan, R.L.; Hayward, M.-K.; Jones, J.L. The growth of molecular diagnostics: Stratified Medicine Programme, the 100,000 Genomes Project and the future. Diagn. Histopathol. 2017, 23, 458–467. [Google Scholar] [CrossRef]
- Morganti, S.; Tarantino, P.; Ferraro, E.; D’Amico, P.; Viale, G.; Trapani, D.; Duso, B.A.; Curigliano, G. Complexity of genome sequencing and reporting: Next generation sequencing (NGS) technologies and implementation of precision medicine in real life. Crit. Rev. Oncol. Hematol. 2019, 133, 171–182. [Google Scholar] [CrossRef]
- Grody, W.W.; Deignan, J.L. 6—Diagnostic Molecular Genetics∗. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics, 7th ed.; Pyeritz, R.E., Korf, B.R., Grody, W.W., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 165–203. [Google Scholar]
- Sgourou, A.; Papachatzopoulou, A.; Katsila, T.; Patrinos, G.P. Chapter 3—Low- and Medium-Throughput Variant Detection Methods: A Historical Perspective. In Molecular Diagnostics, 3rd ed.; Patrinos, G.P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–39. [Google Scholar]
- Dumitru, R.M. Chapter 9—Genetic Susceptibility in Biochemical and Physiological Traits. In Cardiovascular Diseases; Papageorgiou, N., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 177–217. [Google Scholar]
- Dwivedi, S.; Purohit, P.; Misra, R.; Pareek, P.; Goel, A.; Khattri, S.; Pant, K.K.; Misra, S.; Sharma, P. Diseases and Molecular Diagnostics: A Step Closer to Precision Medicine. Indian J. Clin. Biochem. 2017, 32, 374–398. [Google Scholar] [CrossRef]
- Belmont, J.W. 96—Molecular Methods. In Clinical Immunology, 5th ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Content Repository Only: London, UK, 2019; pp. 1297–1310. [Google Scholar]
- Landsverk, M.; Wong, L.-J.C. Clinical Molecular Diagnostic Techniques: A Brief Review. In Next Generation Sequencing; Springer: New York, NY, USA, 2013; pp. 19–36. [Google Scholar]
- Fairfax, M.R.; Bluth, M.H.; Salimnia, H. Diagnostic Molecular Microbiology: A 2018 Snapshot. Clin. Lab. Med. 2018, 38, 253–276. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Makrigiorgos, G.M. 4—Nucleic Acid Techniques. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–86. [Google Scholar]
- Thatcher, S.A. 3—Nucleic Acid Isolation. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–46. [Google Scholar]
- Leong, S.S.; Yeap, S.P.; Lim, J. Working principle and application of magnetic separation for biomedical diagnostic at high- and low-field gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef]
- Xianyu, Y.; Wang, Q.; Chen, Y. Magnetic particles-enabled biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Huang, G.; Lu, C.-H.; Yang, H.-H. Chapter 3—Magnetic Nanomaterials for Magnetic Bioanalysis. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Wang, X., Chen, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–109. [Google Scholar]
- Moro, L.; Turemis, M.; Marini, B.; Ippodrino, R.; Giardi, M.T. Better together: Strategies based on magnetic particles and quantum dots for improved biosensing. Biotechnol. Adv. 2017, 35, 51–63. [Google Scholar] [CrossRef]
- Jamshaid, T.; Tenório Neto, E.; Eissa, M.; Kunita, M.; Errachid, A.; Elaissari, A. Magnetic particles: From preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications. TrAC Trends Anal. Chem. 2016, 79, 344–362. [Google Scholar] [CrossRef]
- Raghava Reddy, K.; Reddy, P.A.; Reddy, C.V.; Shetti, N.P.; Babu, B.; Ravindranadh, K.; Shankar, M.V.; Reddy, M.C.; Soni, S.; Naveen, S. Chapter 10—Functionalized magnetic nanoparticles/biopolymer hybrids: Synthesis methods, properties and biomedical applications. In Methods in Microbiology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 227–254. [Google Scholar]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of Nanoparticles on Brain Health: An Up to Date Overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Afradi, N.; Foroughifar, N.; Qomi, M.; Pasdar, H. Folic acid-supported Fe3O4 magnetic nanoparticles as a new, highly effective heterogeneous biocatalyst for the synthesis of 3,4-dihydropyrimidine thiones and their in vitro investigation as antibacterial active agents. Biointerface Res. Appl. Chem. 2018, 8, 3661–3669. [Google Scholar]
- Davoodi, S.D.; Saghavaz, B.H. Optimal synthesis and characterization of magnetic CuMnFe2O4 nanoparticles coated by PEG for drug delivery. Biointerface Res. Appl. Chem. 2017, 7, 2249–2252. [Google Scholar]
- Elazab, H.A. Laser vaporization and controlled condensation (LVCC) of graphene supported Pd/Fe3O4 nanoparticles as an efficient magnetic catalysts for Suzuki Cross-Coupling. Biointerface Res. Appl. Chem. 2018, 8, 3314–3318. [Google Scholar]
- Mehta, R. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater. Sci. Eng. C 2017, 79, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and development of superparamagnetic nanoparticles in sample pretreatment and immunochromatographic assay. TrAC Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Fatah, S.M. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar] [CrossRef]
- Kovacevic, N. Magnetic Beads Based Nucleic Acid Purification for Molecular Biology Applications. In Sample Preparation Techniques for Soil, Plant, and Animal Samples; Micic, M., Ed.; Springer: New York, NY, USA, 2016; pp. 53–67. [Google Scholar]
- Sobczak-Kupiec, A.; Venkatesan, J.; Alhathal AlAnezi, A.; Walczyk, D.; Farooqi, A.; Malina, D.; Hosseini, S.H.; Tyliszczak, B. Magnetic nanomaterials and sensors for biological detection. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2459–2473. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Sakamoto, S.; Hatakeyama, M.; Yamaguchi, Y.; Suematsu, M.; Itonaga, M.; Handa, H. Application of high-performance magnetic nanobeads to biological sensing devices. Anal. Bioanal. Chem. 2019, 411, 1825–1837. [Google Scholar] [CrossRef]
- Tangchaikeeree, T.; Polpanich, D.; Elaissari, A.; Jangpatarapongsa, K. Magnetic particles for in vitro molecular diagnosis: From sample preparation to integration into microsystems. Colloids Surf B Biointerfaces 2017, 158, 1–8. [Google Scholar] [CrossRef]
- Van Reenen, A.; de Jong, A.M.; den Toonder, J.M.J.; Prins, M.W.J. Integrated lab-on-chip biosensing systems based on magnetic particle actuation – a comprehensive review. Lab A Chip 2014, 14, 1966–1986. [Google Scholar] [CrossRef]
- Moerland, C.P.; van Ijzendoorn, L.J.; Prins, M.W.J. Rotating magnetic particles for lab-on-chip applications-a comprehensive review. Lab A Chip 2019, 19, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Nanosupport bound lipases their stability and applications. Biointerface Res. Appl. Chem. 2017, 7, 2194–2216. [Google Scholar]
- Xu, L.; Qi, X.; Li, X.; Bai, Y.; Liu, H. Recent advances in applications of nanomaterials for sample preparation. Talanta 2016, 146, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Rhee, M.; Singh, A.; Tripathi, A. Microfluidic Sample Preparation for Medical Diagnostics. Annu. Rev. Biomed. Eng. 2015, 17, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nazario, C.E.D.; Fumes, B.H.; da Silva, M.R.; Lanças, F.M. New materials for sample preparation techniques in bioanalysis. J. Chromatogr. B 2017, 1043, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Coen, M.; Hardick, J.; Gaydos, C.A.; Wong, K.-Y.; Smith, C.; Wilson, S.A.; Vayugundla, S.P.; Wong, S. Low-Cost 3D Printers Enable High-Quality and Automated Sample Preparation and Molecular Detection. PLoS ONE 2016, 11, e0158502. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rampazzo, R.C.P.; Costa, A.D.T.; Krieger, M.A. Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics. Biomed Res. Int. 2017, 2017, 9306564. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H. Chapter 6—Extraction and Purification of Nucleic Acids and Proteins. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–166. [Google Scholar]
- Shen, C.-H. Chapter 7—Detection and Analysis of Nucleic Acids. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 167–185. [Google Scholar]
- Bougas, L.; Langenegger, L.D.; Mora, C.A.; Zeltner, M.; Stark, W.J.; Wickenbrock, A.; Blanchard, J.W.; Budker, D. Nondestructive in-line sub-picomolar detection of magnetic nanoparticles in flowing complex fluids. Sci. Rep. 2018, 8, 3491. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, S.; Pelaz, B.; Parak, W.J.; Lentijo-Mozo, S.; Soulantica, K.; Dieckhoff, J.; Ludwig, F.; Guenther, A.; Tschöpe, A.; Schotter, J. Homogeneous Biosensing Based on Magnetic Particle Labels. Sensors 2016, 16, 828. [Google Scholar] [CrossRef] [PubMed]
- Ríos, Á.; Zougagh, M. Recent advances in magnetic nanomaterials for improving analytical processes. TrAC Trends Anal. Chem. 2016, 84, 72–83. [Google Scholar] [CrossRef]
- Hussain, B.; Yüce, M.; Ullah, N.; Budak, H. 3—Bioconjugated nanomaterials for monitoring food contamination. In Nanobiosensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 93–127. [Google Scholar]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Reports on progress in physics. Phys. Soc. 2015, 78, 016601. [Google Scholar]
- Gupta, S.; Ramesh, K.; Ahmed, S.; Kakkar, V. Lab-on-Chip Technology: A Review on Design Trends and Future Scope in Biomedical Applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 311–322. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, R.; Mao, L.; Zhao, Y. Chapter 10—Active colloids: Toward an intelligent micromachine. In Anisotropic Particle Assemblies; Wu, N., Lee, D., Striolo, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–312. [Google Scholar]
- Huang, S.; He, Y.-Q.; Jiao, F. Advances of Particles/Cells Magnetic Manipulation in Microfluidic Chips. Chin. J. Anal. Chem. 2017, 45, 1238–1246. [Google Scholar] [CrossRef]
- Giouroudi, I.; Kokkinis, G. Recent Advances in Magnetic Microfluidic Biosensors. Nanomaterials 2017, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sinha, A.; Puri, I.K. Magnetic-particle-based microfluidics. In Microfluidics and Nanofluidics Handbook: Fabrication, Implementation, and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 433–483. [Google Scholar]
- Kong, L.X.; Perebikovsky, A.; Moebius, J.; Kulinsky, L.; Madou, M. Lab-on-a-CD: A Fully Integrated Molecular Diagnostic System. J. Lab. Autom. 2015, 21, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Parmeshwar, R.; Rajan, S.S.; Shrestha, K. Principles of cancer screening. Surgery 2018, 36, 139–144. [Google Scholar] [CrossRef]
- Brandão, M.; Julião, I.; Carrilho, C.; Fontes, F.; Lunet, N. Cancer in Sub-Saharan Africa. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 212–224. [Google Scholar]
- Pinsky, P.F. Principles of Cancer Screening. Surg. Clin. N. Am. 2015, 95, 953–966. [Google Scholar] [CrossRef]
- Heyn, H. Chapter 29—Personalized Therapy—Epigenetic Profiling as Predictors of Prognosis and Response. In Epigenetic Cancer Therapy; Gray, S.G., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 677–698. [Google Scholar]
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef]
- Shankaran, D.R. Chapter 8—Nano-Enabled Immunosensors for Point-of-Care Cancer Diagnosis. In Applications of Nanomaterials; Woodhead Publishing: Cambridge, UK; Sawston, UK, 2018; pp. 205–250. [Google Scholar]
- Ye, F.; Zhao, Y.; El-Sayed, R.; Muhammed, M.; Hassan, M. Advances in nanotechnology for cancer biomarkers. Nano Today 2018, 18, 103–123. [Google Scholar] [CrossRef]
- Huss, R. Chapter 19—Biomarkers. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 235–241. [Google Scholar]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early detection of cancer: Past, present, and future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.; Sadana, N.; Sadana, R. 7—Detection of Cancer Biomarkers by SPR and Optofluidic Ring Resonance Sensors. In A Fractal Analysis of Chemical Kinetics with Applications to Biological and Biosensor Interfaces; Sadana, A., Sadana, N., Sadana, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 157–169. [Google Scholar]
- Wong, K.-C.; Chen, J.; Zhang, J.; Lin, J.; Yan, S.; Zhang, S.; Li, X.; Liang, C.; Peng, C.; Lin, Q.; et al. Early Cancer Detection from Multianalyte Blood Test Results. iScience 2019, 15, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Davis, J.B.; Couch, R.D.; Fredolini, C.; Zhou, W.; Petricoin, E.; Espina, V. Chapter 9—Clinical Proteomics and Molecular Pathology. In Molecular Pathology, 2nd ed.; Coleman, W.B., Tsongalis, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 183–203. [Google Scholar]
- Cisarovsky, C.; Benetkiewicz, M.; Faivre, S.; Raymond, E.; de Gramont, A. Chapter 5—Biomarker Development in Targeting Cancer Epigenetic. In Drug Discovery in Cancer Epigenetics; Egger, G., Arimondo, P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 123–142. [Google Scholar]
- Javeed, N.; Mukhopadhyay, D. 19—Translational Potential of Tumor Exosomes in Diagnosis and Therapy. In Diagnostic and Therapeutic Applications of Exosomes in Cancer; Amiji, M., Ramesh, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 343–353. [Google Scholar]
- Im, H.; Yang, K.S.; Lee, H.; Castro, C.M. 7—Nanotechnology Platforms for Cancer Exosome Analyses. In Diagnostic and Therapeutic Applications of Exosomes in Cancer; Amiji, M., Ramesh, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 119–128. [Google Scholar]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Teeman, E.; Shasha, C.; Evans, J.E.; Krishnan, K.M. Intracellular dynamics of superparamagnetic iron oxide nanoparticles for magnetic particle imaging. Nanoscale 2019, 11, 7771–7780. [Google Scholar] [CrossRef]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.F.C.; Yang, C.; Tsang, H.L.; Lau, P.M.; Yong, K.T.; Ho, H.P.; Kong, S.K. An Aptamer Bio-barCode (ABC) assay using SPR, RNase H, and probes with RNA and gold-nanorods for anti-cancer drug screening. Analyst 2017, 142, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Merlos Rodrigo, M.A.; Krejcova, L.; Kudr, J.; Cernei, N.; Kopel, P.; Richtera, L.; Moulick, A.; Hynek, D.; Adam, V.; Stiborova, M.; et al. Fully automated two-step assay for detection of metallothionein through magnetic isolation using functionalized γ-Fe2O3 particles. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1039, 17–27. [Google Scholar] [CrossRef]
- Ribeiro, J.R.; Gaudet, H.M.; Khan, M.; Schorl, C.; James, N.E.; Oliver, M.T.; DiSilvestro, P.A.; Moore, R.G.; Yano, N. Human Epididymis Protein 4 Promotes Events Associated with Metastatic Ovarian Cancer via Regulation of the Extracelluar Matrix. Front. Oncol. 2018, 7, 332. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Qiu, R.; Foda, M.; Zhang, Y.; Wang, T.; Li, J. The fabrication of magnetic particle-based chemiluminescence immunoassay for human epididymis protein-4 detection in ovarian cancer. Biochem. Biophys. Rep. 2018, 13, 73–77. [Google Scholar] [CrossRef]
- Liu, W.; Nie, L.; Li, F.; Aguilar, Z.P.; Xu, H.; Xiong, Y.; Fu, F.; Xu, H. Folic acid conjugated magnetic iron oxide nanoparticles for nondestructive separation and detection of ovarian cancer cells from whole blood. Biomater. Sci. 2016, 4, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Liu, C.; Yang, W.F.; Zhang, H.T.; Li, Y.F.; Zeng, F.; Cao, J.Q. Using a novel CD133+ immune magnetic particle to separate gastric adenocarcinoma stem cells from peripheral blood and the pluripotency study about the separated cells. J. Biomed. Nanotechnol. 2017, 13, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Chieh, J.J.; Huang, K.W.; Chuang, C.P.; Wei, W.C.; Dong, J.J.; Lee, Y.Y. Immunomagnetic reduction assay on des-gamma-carboxy prothrombin for screening of hepatocellular carcinoma. IEEE Trans. Biomed. Eng. 2016, 63, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Nie, Y.; Tadimety, A.; Shen, T.; Zhang, J.X.J. Microfluidics-enabled rapid manufacturing of hierarchical silica-magnetic microflower toward enhanced circulating tumor cell screening. Biomater. Sci. 2018, 6, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef]
- Natesan, S.; Ponnusamy, C.; Sugumaran, A.; Chelladurai, S.; Shanmugam Palaniappan, S.; Palanichamy, R. Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int. J. Biol. Macromol. 2017, 104, 1853–1859. [Google Scholar] [CrossRef]
- Nosrati, H.; Adibtabar, M.; Sharafi, A.; Danafar, H.; Hamidreza Kheiri, M. PAMAM-modified citric acid-coated magnetic nanoparticles as pH sensitive biocompatible carrier against human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1377–1384. [Google Scholar] [CrossRef]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4 @Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef]
- Dey, C.; Ghosh, A.; Ahir, M.; Ghosh, A.; Goswami, M.M. Improvement of Anticancer Drug Release by Cobalt Ferrite Magnetic Nanoparticles through Combined pH and Temperature Responsive Technique. ChemPhysChem 2018, 19, 2872–2878. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Zhou, G.; Tian, A.; Sun, N. Magnetic gold nanoparticle-mediated small interference RNA silencing Bag-1 gene for colon cancer therapy. Oncol. Rep. 2016, 35, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Fierer, J.; Looney, D.; Pechère, J.-C. 2—Nature and Pathogenicity of Micro-organisms. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 4–25. [Google Scholar]
- Wilson, C.C.; Schooley, R.T. 3—Host Responses to Infection. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 26–39. [Google Scholar]
- Tripathi, L.P.; Chen, Y.-A.; Mizuguchi, K.; Morita, E. Network-Based Analysis of Host-Pathogen Interactions. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 932–937. [Google Scholar]
- Wellehan, J.F.X.; Lierz, M.; Phalen, D.; Raidal, S.; Styles, D.K.; Crosta, L.; Melillo, A.; Schnitzer, P.; Lennox, A.; Lumeij, J.T. CHAPTER2—Infectious disease. In Current Therapy in Avian Medicine and Surgery; Speer, B.L., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 22–106. [Google Scholar]
- Carinelli, S.; Martí, M.; Alegret, S.; Pividori, M.I. Biomarker detection of global infectious diseases based on magnetic particles. New Biotechnol. 2015, 32, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Healthc. Mater. 2018, 7, e1701392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mohamed, M.A.A.; Zagorovsky, K.; Chan, W.C.W. State of diagnosing infectious pathogens using colloidal nanomaterials. Biomaterials 2017, 146, 97–114. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y. Development of a fast and efficient method for hepatitis A virus concentration from green onion. J. Virol. Methods 2017, 249, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kanitthamniyom, P.; Zhang, Y. Magnetic digital microfluidics on a bioinspired surface for point-of-care diagnostics of infectious disease. Electrophoresis 2019, 40, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Fang, Z.S.; Chen, Y.T.; Chen, Y.I.; Yao, B.Y.; Cheng, J.Y.; Chien, C.Y.; Chang, Y.C.; Hu, C.M.J. Targeting and Enrichment of Viral Pathogen by Cell Membrane Cloaked Magnetic Nanoparticles for Enhanced Detection. ACS Appl. Mater. Interfaces 2017, 9, 39953–39961. [Google Scholar] [CrossRef]
- Singhal, C.; Dubey, A.; Mathur, A.; Pundir, C.S.; Narang, J. Paper based DNA biosensor for detection of chikungunya virus using gold shells coated magnetic nanocubes. Process Biochem. 2018, 74, 35–42. [Google Scholar] [CrossRef]
- Ricks, K.M.; Shoemaker, C.J.; Dupuy, L.C.; Flusin, O.; Voorhees, M.A.; Fulmer, A.N.; Badger, C.V.; Schmaljohn, C.S.; Schoepp, R.J. Development of a bead-based immunoassay using virus-like particles for detection of alphaviral humoral response. J. Virol. Methods 2019, 270, 12–17. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.; Chen, Z.; Hu, Z.; Fang, Y.; Liao, P.; Deng, Y.; He, N. Performance evaluation of a novel sample in-answer out (SIAO) system based on magnetic nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 1619–1630. [Google Scholar] [CrossRef]
- Villamizar-Gallardo, R.A.; Osma, J.F.; Ortíz, O.O. New technique for direct fluoroimmunomagnetic detection of rotavirus in water samples. J. Water Health 2017, 15, 932–941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meng, X.; Li, F.; Li, F.; Xiong, Y.; Xu, H. Vancomycin modified PEGylated-magnetic nanoparticles combined with PCR for efficient enrichment and detection of Listeria monocytogenes. Sens. Actuatorsb Chem. 2017, 247, 546–555. [Google Scholar] [CrossRef]
- Cihalova, K.; Hegerova, D.; Jimenez, A.M.; Milosavljevic, V.; Kudr, J.; Skalickova, S.; Hynek, D.; Kopel, P.; Vaculovicova, M.; Adam, V. Antibody-free detection of infectious bacteria using quantum dots-based barcode assay. J. Pharm. Biomed. Anal. 2017, 134, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tung, L.M.; Cong, N.X.; Huy, L.T.; Lan, N.T.; Phan, V.N.; Hoa, N.Q.; Vinh, L.K.; Thinh, N.V.; Tai, L.T.; Ngo, D.T.; et al. Synthesis, characterizations of superparamagnetic Fe3O4-Ag hybrid nanoparticles and their application for highly effective bacteria inactivation. J. Nanosci. Nanotechnol. 2016, 16, 5902–5912. [Google Scholar] [CrossRef]
- Bezdekova, J.; Hutarova, J.; Vaculovicova, M. Magnetic molecularly imprinted polymers used for staphylococcus aureus isolation and detection. In Proceedings of the NANOCON 2018 Conference 10th Anniversary International Conference on Nanomaterials-Research and Application, Brno, Czech Republic, 17–19 October 2018. [Google Scholar]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual Recognition Strategy for Specific and Sensitive Detection of Bacteria Using Aptamer-Coated Magnetic Beads and Antibiotic-Capped Gold Nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, H.; Rao, X.; Liu, Z.; Zhu, H.; Xu, Y. Point-of-Care Testing of Pathogenic Bacteria at the Single-Colony Level via Gas Pressure Readout Using Aptamer-Coated Magnetic CuFe2O4 and Vancomycin-Capped Platinum Nanoparticles. Anal. Chem. 2019, 91, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Kearns, H.; Goodacre, R.; Jamieson, L.E.; Graham, D.; Faulds, K. SERS Detection of Multiple Antimicrobial-Resistant Pathogens Using Nanosensors. Anal. Chem. 2017, 89, 12666–12673. [Google Scholar] [CrossRef] [PubMed]
- Halouane, F.; Jijie, R.; Meziane, D.; Li, C.; Singh, S.K.; Bouckaert, J.; Jurazek, J.; Kurungot, S.; Barras, A.; Li, M.; et al. Selective isolation and eradication of: E. coli associated with urinary tract infections using anti-fimbrial modified magnetic reduced graphene oxide nanoheaters. J. Mater. Chem. B 2017, 5, 8133–8142. [Google Scholar] [CrossRef]
- Fang, W.; Han, C.; Zhang, H.; Wei, W.; Liu, R.; Shen, Y. Preparation of amino-functionalized magnetic nanoparticles for enhancement of bacterial capture efficiency. RSC Adv. 2016, 6, 67875–67882. [Google Scholar] [CrossRef]
- De Alcântara Sica de Toledo, L.; et al. Thermal Magnetic Field Activated Propolis Release From Liquid Crystalline System Based on Magnetic Nanoparticles. AAPS Pharmscitech 2018, 19, 3258–3271. [Google Scholar] [CrossRef] [PubMed]
- Mass, M.; Roberti, M.; Salomón, F.; Tropea, S.; Lloret, M.; Brengi, D.; Malatto, L.; Fraigi, L.; Longinotti, G.; Ybarra, G.; et al. Development of a point-of-care platform for diagnosis of infectious diseases. In Proceedings of the Smart Systems Integration 2016-International Conference and Exhibition on Integration Issues of Miniaturized Systems, SSI 2016, Munich, Germany, 9–10 March 2016. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetic Particles for Advanced Molecular Diagnosis. Materials 2019, 12, 2158. https://doi.org/10.3390/ma12132158
Chircov C, Grumezescu AM, Holban AM. Magnetic Particles for Advanced Molecular Diagnosis. Materials. 2019; 12(13):2158. https://doi.org/10.3390/ma12132158
Chicago/Turabian StyleChircov, Cristina, Alexandru Mihai Grumezescu, and Alina Maria Holban. 2019. "Magnetic Particles for Advanced Molecular Diagnosis" Materials 12, no. 13: 2158. https://doi.org/10.3390/ma12132158
APA StyleChircov, C., Grumezescu, A. M., & Holban, A. M. (2019). Magnetic Particles for Advanced Molecular Diagnosis. Materials, 12(13), 2158. https://doi.org/10.3390/ma12132158






