A Mini Review on Bismuth-Based Z-Scheme Photocatalysts
Abstract
1. Introduction
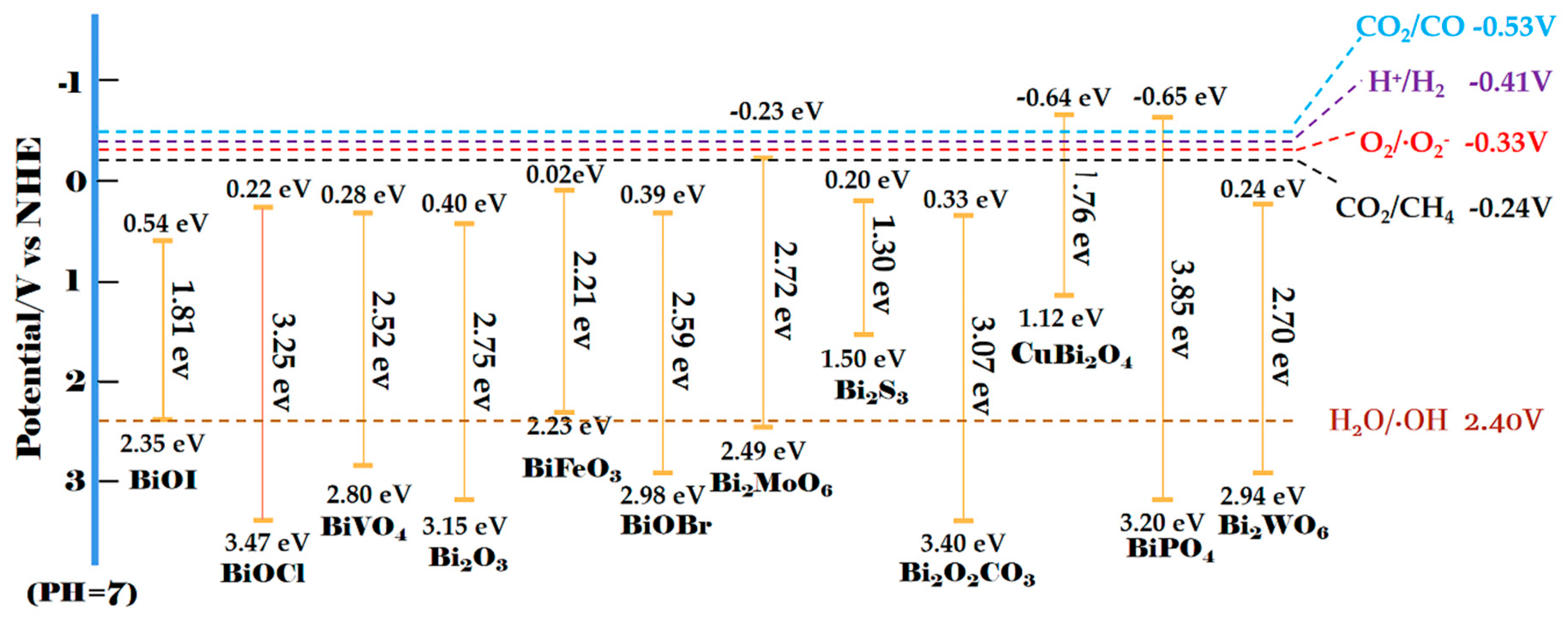
2. Synthesis Methods of Bi-Based Direct Z-Scheme Photocatalysts
3. Applications of Bi-Based Z-Scheme Photocatalysts
3.1. Degradation of Pollutants in Water
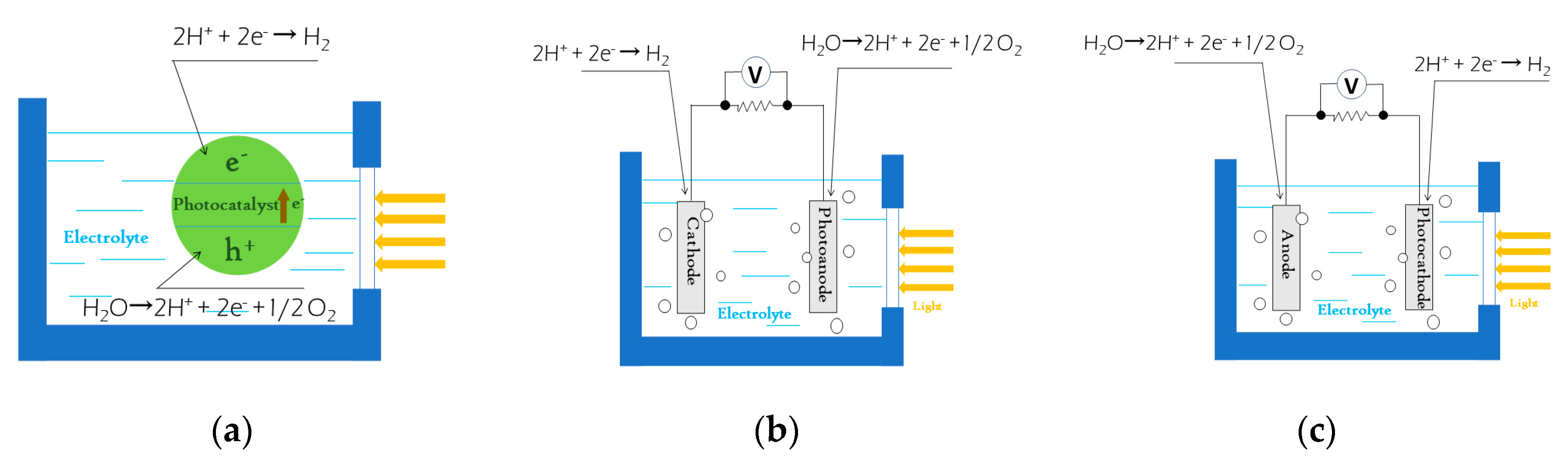
3.2. Water Splitting
3.3. CO2 Reduction
3.4. Removal of Gas Phase Pollutants and Other Applications
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Xiang, Q.J.; Cheng, B.; Yu, J.G. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Rev. 2015, 54, 11350–11360. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Liao, Y.H.; Xia, D.H.; Zhang, Q.; He, H.J.W.; Yang, J.L.; Huang, Y.J.; Liu, H.D.; Zhang, F.; He, C.; et al. In-situ fabrication of AgI-BiOI nanoflake arrays film photoelectrode for efficient wastewater treatment, electricity production and enhanced recovery of copper in photocatalytic fuel cell. Catal. Today 2020, 339, 379–390. [Google Scholar] [CrossRef]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chemseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive Evaluation of CuBi2O4 as a Photocathode Material for Photoelectrochemical Water Splitting. Chem. Mater. 2016, 28, 4231–4242. [Google Scholar] [CrossRef]
- Liu, A.J.; Zhu, Y.C.; Li, K.Z.; Chu, D.M.; Huang, J.; Li, X.; Zhang, C.Y.; Yang, P.; Du, Y.K. A high performance p-type nickel oxide/cuprous oxide nanocomposite with heterojunction as the photocathodic catalyst for water splitting to produce hydrogen. Chem. Phys. Lett. 2018, 703, 56–62. [Google Scholar] [CrossRef]
- Hampel, B.; Pap, Z.; Sapi, A.; Szamosvolgyi, A.; Baia, L.; Hernadi, K. Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production. Catalysts 2020, 10, 85. [Google Scholar] [CrossRef]
- Sun, N.; Qu, Y.; Yang, C.H.; Yang, Z.D.; Yan, R.; Wenyu, E.; Zhang, Z.Q.; Li, Z.J.; Li, H.N.; Khan, I.; et al. Efficiently photocatalytic degradation of monochlorophenol on in-situ fabricated BiPO4/β-Bi2O3 heterojunction microspheres and O2-free hole-induced selective dechloridation conversion with H2 evolution. Appl. Catal. B Environ. 2020, 263, 118313. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujii, Y.; Ichihashi, Y.; Zhang, S.G.; Ikeue, K.; Park, D.R.; Koyano, K.; Tatsumi, T.; Anpo, M. Selective formation of CH3OH in the photocatalytic reduction of CO2 with H2O on titanium oxides highly dispersed within zeolites and mesoporous molecular sieves. Catal. Today 1998, 45, 221–227. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Kondo, Y.; Yoshikawa, H.; Awaga, K.; Murayama, M.; Mori, T.; Sunada, K.; Bandow, S.; Iijima, S. Preparation, Photocatalytic Activities, and Dye-Sensitized Solar-Cell Performance of Submicron-Scale TiO2 Hollow Spheres. Langmuir 2008, 24, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.T.; Yu, A.; Pan, H.Y.; Gao, X.F.; Chen, Q.; Peng, L.M. CdS quantum dots sensitized TiO2 nanotube-array photoelectrodes. J. Am. Chem. Soc. 2009, 130, 1124–1125. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.W.; Zhu, B.C.; Jiang, C.J.; Cheng, B.; You, W.; Yu, J.G. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Sorcar, S.; Hwang, Y.; Grimes, C.A.; In, S.-I. Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO2 reduction into CH4. Mater. Today 2017, 20, 507–515. [Google Scholar] [CrossRef]
- Xu, M.; Zada, A.; Rui, Y.; Li, H.N.; Sun, N.; Qu, Y. Ti2O3/TiO2 heterophase junction with enhanced charge separation and spatially separated active sites for photocatalytic CO2 reduction. Phys. Chem. Chem. Phys. 2020, 22, 4526–4532. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.Y.; Ye, L.Q.; Ma, T.Y.; Zhang, T.R.; Zhang, Y.i.; Huang, H.W. Macroscopic Spontaneous Polarization and Surface Oxygen Vacancies Collaboratively Boosting CO2 Photoreduction on BiOIO3 Single Crystals. Adv. Mater. 2020, 32, 1908350. [Google Scholar] [CrossRef]
- Jin, J.P.; Chen, S.T.; Wang, J.M.; Chen, C.; Peng, T.Y. One-pot hydrothermal preparation of PbO-decorated brookite/anatase TiO2 composites with remarkably enhanced CO2 photoreduction activity. Appl. Catal. B Environ. 2020, 263, 118353. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Fabrication of nanocomposite photocatalyst CuBi2O4/Bi3ClO4 for removal of acid brown 14 as water pollutant under visible light irradiation. J. Hazard. Mater. 2019, 361, 210–220. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, M.Y.; He, Z.T.; Zhong, Y.; Ding, H.; Chen, D.M. Preparation of a p-n heterojunction 2D BiOI nanosheet/1DBiPO4 nanorod composite electrode for enhanced visible light photoelectrocatalysis. Chin. J. Catal. 2019, 40, 446–457. [Google Scholar] [CrossRef]
- Liang, M.J.; Yang, Z.Y.; Yang, Y.; Mei, Y.; Zhou, H.R.; Yang, S.J. One-step introduction of metallic Bi and non-metallic C in Bi2WO6 with enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 30, 1310–1321. [Google Scholar] [CrossRef]
- Li, W.F.; Yu, R.; Li, M.; Guo, N.; Yu, H.W.; Yu, Y. Photocatalytical degradation of diclofenac by Ag-BiOI-rGO: Kinetics, mechanisms and pathways. Chemosphere 2019, 218, 966–973. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.Y.; Ren, H.J.; Huang, X.L.; Hou, W.X.; Wang, C.; Shi, W.L.; Lu, C.Y. Fabrication of p-n CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl. Surf. Sci. 2019, 491, 88–94. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.-K. BiFeO3/BiVO4 p−n heterojunction for efficient and stable photocatalytic and photoelectrochemical water splitting under visible-light irradiation. Catal. Today 2020, 340, 188–196. [Google Scholar] [CrossRef]
- Wu, X.F.; Cheng, J.S.; Li, X.F.; Li, Y.H.; Lv, K.L. Enhanced visible photocatalytic oxidation of NO by repeated calcination of g-C3N4. Appl. Surf. Sci. 2019, 465, 1037–1046. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H.J.; Sun, Y.J.; Li, J.Y.; Cui, W.; Wang, L.A.; Zhang, W.D.; Yuan, X.Y.; Wang, Z.M.; Zhang, Y.X.; et al. Bi metal prevents the deactivation of oxygen vacancies in Bi2O2CO3 for stable and efficient photocatalytic NO abatement. Appl. Catal. B Environ. 2020, 264, 118545. [Google Scholar] [CrossRef]
- Huo, W.C.; Dong, X.A.; Li, J.Y.; Liu, M.; Liu, X.Y.; Zhang, Y.X.; Dong, F. Synthesis of Bi2WO6 with gradient oxygen vacancies for highly photocatalytic NO oxidation and mechanism study. Chem. Eng. J. 2019, 361, 129–138. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, W.D.; Li, J.Y.; Jiang, G.M.; Zhou, Y.; Lee, S.C.; Dong, F. Transformation pathway and toxic intermediates inhibition of photocatalytic NO removal on designed Bi metal@defective Bi2O2SiO3. Appl. Catal. B Environ. 2019, 241, 187–195. [Google Scholar] [CrossRef]
- Wang, L.; Xu, K.; Cui, W.; Lv, D.D.; Wang, L.; Ren, L.; Xu, X.; Dong, F.; Dou, S.X.; Hao, W.C.; et al. Monolayer Epitaxial Heterostructures for Selective Visible-Light-Driven Photocatalytic NO Oxidation. Adv. Funct. Mater. 2019, 29, 1808084. [Google Scholar] [CrossRef]
- Carbonaro, S.; Sugihara, M.N.; Strathmann, T.J. Continuous-flow photocatalytic treatment of pharmaceutical micropollutants: Activity, inhibition, and deactivation of TiO2 photocatalysts in wastewater effluent. Appl. Catal. B Environ. 2013, 129, 1–12. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.L.; Zhang, Q.X.; Su, Y.H.; Shen, L.Z.; Yao, K.; Chen, Z.F.; Liu, Y.; Cai, Z.W.; Lv, W.Y. Photocatalytic degradation of clofibric acid by g-C3N4/P25 composites under simulated sunlight irradiation: The significant effects of reactive species. Chemosphere 2017, 172, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Guan, Z.H.; Wang, P.; Zhang, Q.Q.; Dai, Y.; Huang, B.B. Amorphous TiO2-modified CuBi2O4 Photocathode with enhanced photoelectrochemical hydrogen production activity. Chin. J. Catal. 2018, 39, 1704–1710. [Google Scholar] [CrossRef]
- Wang, X.N.; Bi, W.L.; Zhai, P.P.; Wang, X.B.; Li, H.J.; Mailhot, G.; Dong, W.B. Adsorption and photocatalytic degradation of pharmaceuticals by BiOCl x I y nanospheres in aqueous solution. Appl. Surf. Sci. 2016, 360, 240–251. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y.J. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Fujishima, A.; Kenichi, H. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sohn, Y.; Huang, W.X.; Taghipour, F. Recent progress and perspectives in the photocatalytic CO2 reduction of Ti-oxide-based nanomaterials. Appl. Surf. Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Joo, J.B.; Zhang, Q.; Dahl, M.; Lee, I.; Goebl, J.; Zaera, F.; Yin, Y.D. Control of the nanoscale crystallinity in mesoporous TiO2 shells for enhanced photocatalytic activity. Energy Environ. Sci. 2012, 5, 6321–6327. [Google Scholar] [CrossRef]
- Qian, R.F.; Zong, H.X.; Schneider, J.; Zhou, G.D.; Zhao, T.; Li, Y.L.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Negrín-Montecelo, Y.; Testa-Anta, M.; Marín-Caba, L.; Pérez-Lorenzo, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Comesaña-Hermo, M. Titanate Nanowires as One-Dimensional Hot Spot Generators for Broadband Au–TiO2 Photocatalysis. Nanomaterials 2019, 9, 990. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhai, C.Y.; Gao, H.F.; Zeng, L.X.; Du, Y.K.; Zhu, M.S. Enhanced photo-assisted ethanol electro-oxidation activity by using broadband visible light absorption of a graphitic C3N4/BiOI carrier. Sustain. Energy Fuels 2019, 3, 439–449. [Google Scholar] [CrossRef]
- Liu, H.H.; Yang, C.; Huang, J.; Chen, J.F.; Zhong, J.B.; Li, J.Z. Ionic liquid-assisted hydrothermal preparation of BiOI/BiOCl heterojunctions with enhanced separation efficiency of photo-generated charge pairs and photocatalytic performance. Inorg. Chem. Commun. 2020, 113, 107806. [Google Scholar] [CrossRef]
- Samran, B.; Lunput, S.; Tonnonchiang, S.; Chaiwichian, S. BiFeO3/BiVO4 nanocomposite photocatalysts with highly enhanced photocatalytic activity for rhodamine B degradation under visible light irradiation. Phys. B Condens. Matter 2019, 561, 23–28. [Google Scholar] [CrossRef]
- Lim, H.; Rawal, S.B. Integrated Bi2O3 nanostructure modified with Au nanoparticles for enhanced photocatalytic activity under visible light irradiation. Prog. Nat. Sci. Mater. Int. 2017, 27, 289–296. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zheng, Y.J.; He, Z.G.; Lu, Z.X.; Wang, L.; Li, C.F.; Jiao, F.P.; Deng, C.Y. Synthesis of Bi2S3 microsphere and its efficient photocatalytic activity under visible-light irradiation. Trans. Nonferrous Met. Soc. China 2018, 28, 2002–2010. [Google Scholar] [CrossRef]
- Shi, H.X.; Fan, J.; Zhao, Y.Y.; Hu, X.Y.; Zhang, X.; Tang, Z.S. Visible light driven CuBi2O4/Bi2MoO6 p-n heterojunction with enhanced photocatalytic inactivation of E. coli and mechanism insight. J. Hazard. Mater. 2020, 381, 121006. [Google Scholar] [CrossRef]
- Qiu, F.Z.; Li, W.J.; Wang, F.Z.; Li, H.D.; Liu, X.T.; Ren, C.J. Preparation of novel p-n heterojunction Bi2O2CO3/BiOBr photocatalysts with enhanced visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2017, 517, 25–32. [Google Scholar] [CrossRef]
- Liu, D.; Cai, W.B.; Wang, Y.G.; Zhu, Y.F. Constructing a novel Bi2SiO5/BiPO4 heterostructure with extended light response range and enhanced photocatalytic performance. Appl. Catal. B Environ. 2018, 236, 205–211. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, S.W.; Jiang, W.; Liu, Y.; Liu, J.S.; Wang, Z.H. Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 501, 156–163. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- He, R.A.; Cao, S.W.; Zhou, P.; Yu, J.G. Recent advances in visible light Bi-based photocatalysts. Chin. J. Catal. 2014, 35, 989–1007. [Google Scholar] [CrossRef]
- Zeng, L.; Zhe, F.; Wang, Y.; Zhang, Q.L.; Zhao, X.Y.; Hu, X.; Wu, Y.; He, Y.M. Preparation of interstitial carbon doped BiOI for enhanced performance in photocatalytic nitrogen fixation and methyl orange degradation. J. Colloid Interface Sci. 2019, 539, 563–574. [Google Scholar] [CrossRef]
- Tian, F.; Li, G.F.; Zhao, H.P.; Chen, F.X.; Li, M.; Liu, Y.L.; Chen, R. Residual Fe enhances the activity of BiOCl hierarchical nanostructure for hydrogen peroxide activation. J. Catal. 2019, 370, 265–273. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.F.; Yu, J.C. Fe Enhanced Visible-Light-Driven Nitrogen Fixation on BiOBr Nanosheets. Chem. Mater. 2020, 32, 1488–1494. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, R.T.; Lu, H.J.; Xie, C.A.; Ao, W.H.; Chen, D.M.; Ma, C.; Yao, W.Q.; Zhu, Y.F. One-pot synthesis of C/Bi/Bi2O3 composite with enhanced photocatalytic activity. Appl. Catal. B Environ. 2017, 219, 63–72. [Google Scholar] [CrossRef]
- Ge, Z.H.; Qin, P.; He, D.S.; Chong, X.; Feng, D.; Ji, Y.H.; Feng, J.; He, J.Q. Highly Enhanced Thermoelectric Properties of Bi/Bi2S3 Nanocomposites. Acs Appl. Mater. Interfaces 2017, 9, 4828–4834. [Google Scholar] [CrossRef]
- Xu, W.C.; Fang, J.Z.; Chen, Y.F.; Lu, S.Y.; Zhou, G.Y.; Zhu, X.M.; Fang, Z.Q. Novel heterostructured Bi2S3/Bi2Sn2O7 with highly visible light photocatalytic activity for the removal of rhodamine B. Mater. Chem. Phys. 2015, 154, 30–37. [Google Scholar] [CrossRef]
- Guo, J.H.; Shi, L.; Zhao, J.Y.; Wang, Y.; Tang, K.B.; Zhang, W.Q.; Xie, C.Z.; Yuan, X.Y. Enhanced visible-light photocatalytic activity of Bi2MoO6 nanoplates with heterogeneous Bi2MoO6-x@Bi2MoO6 core-shell structure. Appl. Catal. B Environ. 2018, 224, 692–704. [Google Scholar] [CrossRef]
- He, R.G.; Xu, D.F.; Cheng, B.; Yu, J.G.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Li, H.J.; Tu, W.G.; Zhou, Y.; Zou, Z.G. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef]
- Qi, K.Z.; Cheng, B.; Yu, J.G.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Yu, J.G.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocataly. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Ding, L.; Wei, R.; Chen, H.; Hu, J.; Li, J. Controllable synthesis of highly active BiOCl hierarchical microsphere self-assembled by nanosheets with tunable thickness. Appl. Catal. B Environ. 2015, 172, 91–99. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.Z.; Xu, H.L.; Sun, S.M.; Shang, M. Bi2O3 Hierarchical Nanostructures: Controllable Synthesis, Growth Mechanism, and their Application in Photocatalysis. Chem. A Eur. J. 2009, 15, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.Q.; Fang, H.B.; Zheng, Y.Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]
- Fu, Y.S.; Li, J.; Li, J.G. Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties. Nanomaterials 2019, 9, 359. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.Y.; Fu, J.W.; Cheng, B.; Yu, J.G. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423. [Google Scholar] [CrossRef]
- Ali, R.; Ma, M.; Kim, S.; Shah, M.S.A.S.; Chung, C.; Park, J.H.; Yoo, P.J. Mediator and Co-Catalysts-Free Direct Z-Scheme Composites of Bi2WO6-Cu3P for Solar-Water Splitting. Nanoscale 2018, 10, 3026–3036. [Google Scholar] [CrossRef]
- Rivest, J.B.; Jain, P.K. Cation exchange on the nanoscale: An emerging technique for new material synthesis, device fabrication, and chemical sensing. Chem. Soc. Rev. 2013, 42, 89–96. [Google Scholar] [CrossRef]
- Yuan, X.J.; Shen, D.Y.; Zhang, Q.; Zou, H.B.; Liu, Z.L.; Peng, F. Z-scheme Bi2WO6/CuBi2O4 heterojunction mediated by interfacial electric field for efficient visible-light photocatalytic degradation of tetracycline. Chem. Eng. J. 2019, 369, 292–301. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, L.L.; Dong, F.Y.; Chen, Q.; Jiang, H.Y.; Xu, M.; Shi, J.S. Non-additional carbon source one-step synthesis of Bi2O2CO3-based ternary composite for efficient Z-scheme photocatalysis. J. Colloid Interface Sci. 2019, 536, 575–585. [Google Scholar] [CrossRef]
- Wang, H.; Ye, H.L.; Zhang, B.H.; Zhao, F.Q.; Zeng, B.Z. Electrostatic interaction mechanism based synthesis of a Z-scheme BiOI–CdS photocatalyst for selective and sensitive detection of Cu2+. J. Mater. Chem. A 2017, 5, 10599–10608. [Google Scholar] [CrossRef]
- Wan, S.P.; Zhong, Q.; Ou, M.; Zhang, S.l. Synthesis and characterization of direct Z-scheme Bi2MoO6/ZnIn2S4 composite photocatalyst with enhanced photocatalytic oxidation of NO under visible light. J. Mater. Sci. 2017, 52, 11453–11466. [Google Scholar] [CrossRef]
- Li, B.S.; Lai, C.; Zeng, G.M.; Qin, L.; Yi, H.; Huang, D.L.; Zhou, C.Y.; Liu, X.G.; Cheng, M.; Xu, P.; et al. Facile Hydrothermal Synthesis of Z-Scheme Bi2Fe4O9/Bi2WO6 Heterojunction Photocatalyst with Enhanced Visible Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 18824–18836. [Google Scholar] [CrossRef] [PubMed]
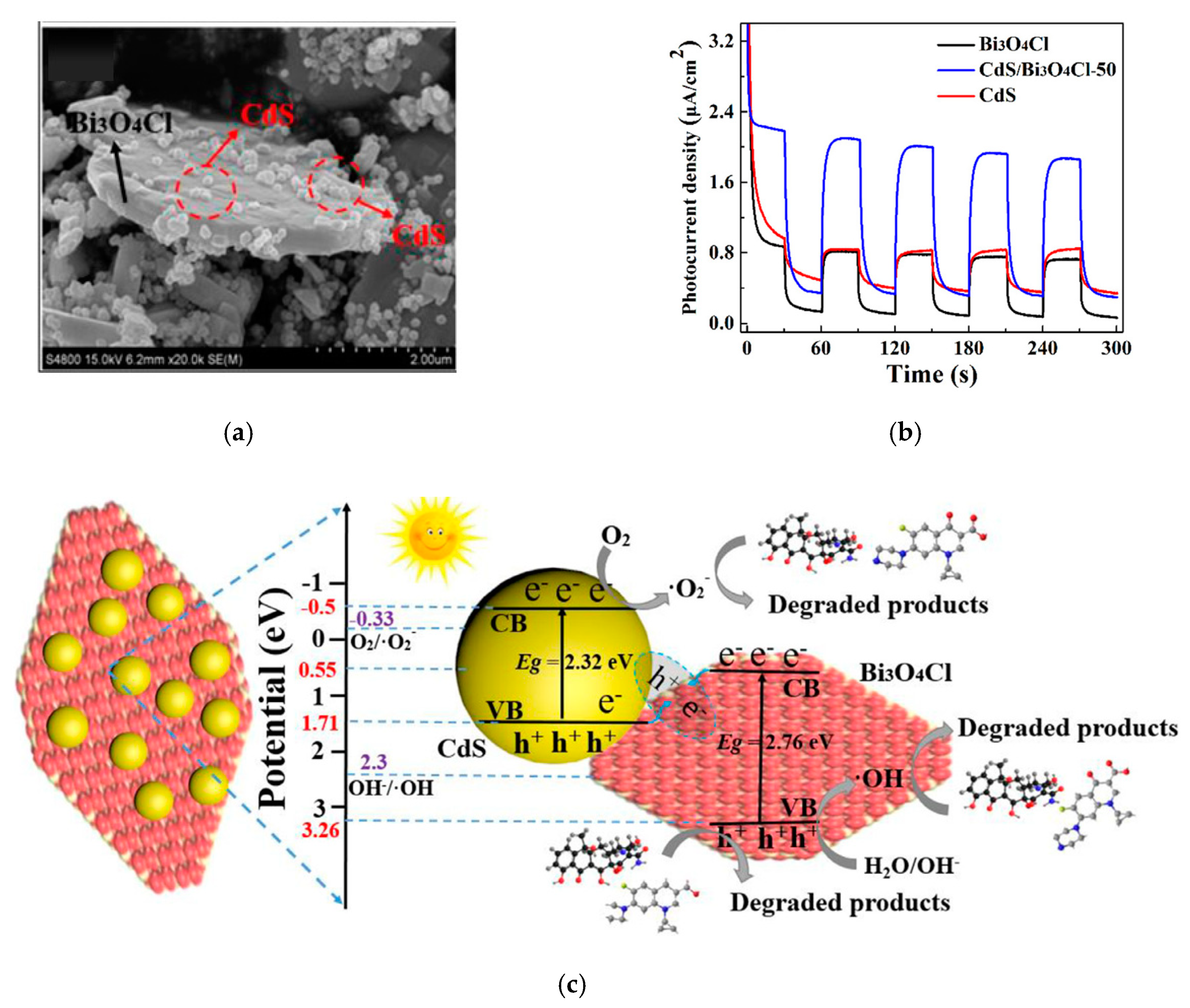
- Che, H.N.; Che, G.B.; Jiang, E.H.; Liu, C.B.; Dong, H.J.; Li, C.M. A novel Z-Scheme CdS/Bi3O4Cl heterostructure for photocatalytic degradation of antibiotics: Mineralization activity, degradation pathways and mechanism insight. J. Taiwan Inst. Chem. Eng. 2018, 91, 224–234. [Google Scholar] [CrossRef]
- He, R.A.; Zhou, J.Q.; Fu, H.Q.; Zhang, S.Y.; Jiang, C.J. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Song, C.J.; Feng, Y.; Shi, W.D.; Liu, C.B. Fabrication and mechanism of a novel direct solid-state Z-scheme photocatalyst CdS/BiOI under visible light. CrystEngComm 2016, 18, 7796–7804. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, X.L.; Zhang, X.H.; Ding, X.; Yang, Z.X.; Dai, K.; Chen, H. A plate-on-plate sandwiched Z-scheme heterojunction photocatalyst: BiOBr-Bi2MoO6 with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 391, 194–201. [Google Scholar] [CrossRef]
- Zhang, J.F.; Hu, Y.F.; Jiang, X.L.; Chen, S.F.; Meng, S.G.; Fu, X.L. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef]
- Cui, M.; Yu, J.X.; Lin, H.J.; Wu, Y.; Zhao, L.H.; He, Y.M. In-situ preparation of Z-scheme AgI/Bi5O7I hybrid and its excellent photocatalytic activity. Appl. Surf. Sci. 2016, 387, 912–920. [Google Scholar] [CrossRef]
- Xue, W.J.; Peng, Z.W.; Huang, D.L.; Zeng, G.M.; Wen, X.J.; Deng, R.; Yang, Y.; Yan, X.L. In situ synthesis of visible-light-driven Z-scheme AgI/Bi2WO6 heterojunction photocatalysts with enhanced photocatalytic activity. Ceram. Int. 2019, 45, 6340–6349. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.L.; Wang, H.B.; Han, M.M.; Guan, W.S.; Huang, H.; Liu, Y.; Kang, Z.H. Study on highly enhanced photocatalytic tetracycline degradation of type II AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Hu, K.; Chen, C.Y.; Zhu, Y.; Zeng, G.M.; Huang, B.B.; Chen, W.Q.; Liu, S.H.; Lei, C.; Li, B.S.; Yang, Y. Ternary Z-scheme heterojunction of Bi2WO6 with reduced graphene oxide (rGO) and meso-tetra (4-carboxyphenyl) porphyrin (TCPP) for enhanced visible-light photocatalysis. J. Colloid Interface Sci. 2019, 540, 115–125. [Google Scholar] [CrossRef]
- Shi, W.L.; Guo, F.; Yuan, S.L. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Zhang, J.F.; Fu, J.W.; Wang, Z.L.; Cheng, B.; Dai, K.; Ho, W. Direct Z-scheme porous g-C3N4/BiOI heterojunction for enhanced visible-light photocatalytic activity. J. Alloy Compd. 2018, 766, 841–850. [Google Scholar] [CrossRef]
- Xia, Y.M.; He, Z.M.; Su, J.B.; Tang, B.; Liu, Y. Enhanced photocatalytic performance of Z-scheme Cu2O/Bi5O7I nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 15271–15281. [Google Scholar] [CrossRef]
- Lu, X.Y.; Che, W.J.; Hu, X.F.; Wang, Y.; Zhang, A.T.; Deng, F.; Luo, S.L.; Dionysiou, D.D. The facile fabrication of novel visible-light-driven Z-scheme CuInS2/Bi2WO6 heterojunction with intimate interface contact by in situ hydrothermal growth strategy for extraordinary photocatalytic performance. Chem. Eng. J. 2019, 356, 819–829. [Google Scholar] [CrossRef]
- Jiang, T.G.; Wang, K.; Guo, T.; Wu, X.Y.; Zhang, G.K. Fabrication of Z-scheme MoO3/Bi2O4 heterojunction photocatalyst with enhanced photocatalytic performance under visible light irradiation. Chin. J. Catal. 2020, 41, 161–169. [Google Scholar] [CrossRef]
- Qin, H.M.; Wang, K.; Jiang, L.S.; Li, J.; Wu, X.Y.; Zhang, G.K. Ultrasonic-assisted fabrication of a direct Z-scheme BiOI/Bi2O4 heterojunction with superior visible light-responsive photocatalytic performance. J. Alloy Compd. 2020, 821, 153417. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, E.; Liu, H.; Lu, Q.F. Engineering of Z-scheme 2D/3D architectures with Bi2MoO6 on TiO2 nanosphere for enhanced photocatalytic 4-nitrophenol degradation. J. Taiwan Inst. Chem. Eng. 2019, 105, 65–74. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Zhang, J.J.; Xu, C.J. 2D/2D Z-scheme Bi2WO6/Porous-g-C3N4 with synergy of adsorption and visible-light-driven photodegradation. Appl. Surf. Sci. 2018, 447, 125–134. [Google Scholar] [CrossRef]
- Chen, J.F.; Hu, C.; Deng, Z.; Gong, X.; Su, Y.; Yang, Q.; Zhong, J.B.; Li, J.Z.; Duan, R. Insight into visible light-driven photocatalytic performance of direct Z-scheme Bi2WO6/BiOI composites constructed in-situ. Chem. Phys. Lett. 2019, 716, 134–141. [Google Scholar] [CrossRef]
- Wang, Z.L.; Hu, T.P.; Dai, K.; Zhang, J.F.; Liang, C.H. Construction of Z-scheme Ag3PO4/Bi2WO6 composite with excellent visible-light photodegradation activity for removal of organic contaminants. Chin. J. Catal. 2017, 38, 2021–2029. [Google Scholar] [CrossRef]
- Li, Z.L.; Jin, C.Y.; Wang, M.; Kang, J.; Wu, Z.M.; Yang, D.E.; Zhu, T. Novel rugby-like g-C3N4/BiVO4 core/shell Z-scheme composites prepared via low-temperature hydrothermal method for enhanced photocatalytic performance. Sep. Purif. Technol. 2020, 232, 115937. [Google Scholar] [CrossRef]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Sheppard, L.R.; Nowotny, J. Materials for photoelectrochemical energy conversion. Adv. Appl. Ceram. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.W.; Han, D.D.; Liu, T.Y.; Wang, G.M.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Chou, X.Y.; Ye, J.; Cui, M.M.; He, Y.Z.; Li, Y.D. Constructing heterojunction of BiPO4/SnS2 nano-flower with sharp-tips effect and bi-functional catalyst as a direct Z-scheme system for high-efficiency photocatalytic performance. Mater. Chem. Phys. 2020, 240, 122241. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.Q.; Ke, S.Q.; Zhang, S.M.; Zhu, M.; Zhang, Y.; Shi, W.Y.; Horike, S.; Tang, L. Construction of heterojunction Bi/Bi5O7I/Sn3O4 for efficient noble-metal-free Z-scheme photocatalytic H2 evolution. Chem. Eng. J. 2020, 382, 122810. [Google Scholar] [CrossRef]
- Zhu, M.S.; Sun, Z.C.; Mamoru, F.; Tetsuro, M. Z-Scheme Photocatalytic Overall Pure-Water Splitting on 2D Heterostructure of Black Phosphorus/BiVO4 under Visible Light. Angew. Chem. 2018, 57, 2160–2164. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Jiang, X.; Sun, R.K.; Yang, J.L.; Jiang, X.L.; Liu, Z.Q.; Xie, M.Z.; Xie, E.Q.; Han, W.H. Z-scheme Bi2O2.33/Bi2S3 heterojunction nanostructures for photocatalytic overall water splitting. Chem. Eng. J. 2020, 382, 123020. [Google Scholar] [CrossRef]
- Sepahvand, H.; Sharifnia, S. Photocatalytic overall water splitting by Z-scheme g-C3N4/BiFeO3 heterojunction. Int. J. Hydrog. Energy 2019, 44, 23658–23668. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hezam, A.; Hendi, A.H.Y.; Qamar, M.; Yamani, Z.H.; Byrappa, K. Ternary Bi2S3/MoS2/TiO2 with double Z-scheme configuration as high performance photocatalyst. Appl. Surf. Sci. 2020, 499, 143938. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Nagi Saeed, A.M.; Ganesh, V.; Neppolian, B.; Byrappa, K. Direct Z-scheme Cs2O–Bi2O3–ZnO heterostructures for photocatalytic overall water splitting. J. Mater. Chem. A 2018, 6, 21379–21388. [Google Scholar] [CrossRef]
- Zhu, R.S.; Tian, F.; Yang, R.J.; He, J.S.; Zhong, J.; Chen, B.Y. Z scheme system ZnIn2S4/RGO/BiVO4 for hydrogen generation from water splitting and simultaneous degradation of organic pollutants under visible light. Renew. Energy 2019, 139, 22–27. [Google Scholar] [CrossRef]
- Shen, H.Q.; Liu, G.W.; Zhao, Y.; Li, D.; Jiang, J.H.; Ding, J.R.; Mao, B.D.; Shen, H.; Kim, K.-S.; Shi, W.D. Artificial all-solid-state system by RGO bridged Cu2O and Bi2WO6 for Z-scheme H2 production and tetracycline degradation. Fuel 2020, 259, 116311. [Google Scholar] [CrossRef]
- Shen, H.Q.; Wang, M.; Zhang, X.Z.; Li, D.; Liu, G.W.; Shi, W.D. 2D/2D/3D architecture Z-scheme system for simultaneous H2 generation and antibiotic degradation. Fuel 2020, 280, 118618. [Google Scholar] [CrossRef]
- Li, J.N.; Pan, Z.W.; Zhou, K.B. Enhanced photocatalytic oxygen evolution activity by formation of Ir@IrOx(OH)y core–shell heterostructure. Nanotechnology 2018, 29, 405705. [Google Scholar] [CrossRef]
- Ran, J.R.; Zhang, J.; Yu, J.G.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Liu, Y.p.; Li, Y.h.; Peng, F.; Lin, Y.; Yang, S.Y.; Zhang, S.S.; Wang, H.J.; Cao, Y.H.; Yu, H. 2H- and 1T- mixed phase few-layer MoS2 as a superior to Pt co-catalyst coated on TiO2 nanorod arrays for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 241, 236–245. [Google Scholar] [CrossRef]
- Crake, A. Metal-organic frameworks based materials for photocatalytic CO2 reduction. Mater. Sci. Technol. 2017, 33, 1737–1749. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Wang, T.; Gong, J.L. Nano-designed semiconductors for electro- and photoelectro-catalytic conversion of carbon dioxide. Chem. Soc. Rev. 2018, 47, 5423–5443. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Li, Y. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Wei, Z.H.; Wang, Y.F.; Li, Y.Y.; Zhang, L.; Yao, H.C.; Li, Z.J. Enhanced photocatalytic CO2 reduction activity of Z-scheme CdS/BiVO4 nanocomposite with thinner BiVO4 nanosheets. J. CO2 Util. 2018, 28, 15–25. [Google Scholar] [CrossRef]
- Wang, J.C.; Yao, H.C.; Fan, Z.Y.; Zhang, L.; Wang, J.S.; Zang, S.Q.; Li, Z.J. Indirect Z-Scheme BiOI/g-C3N4 Photocatalysts with Enhanced Photoreduction CO2 Activity under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 3765–3775. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, K.Q.; Zhang, F.; Fu, X.Z.; Xu, Y.J. Unveiling the interplay between light-driven CO2 photocatalytic reduction and carbonaceous residues decomposition: A case study of Bi2WO6-TiO2 binanosheets. Appl. Catal. B Environ. 2018, 237, 424–431. [Google Scholar] [CrossRef]
- Guo, L.N.; You, Y.; Huang, H.W.; Tian, N.; Ma, T.Y.; Zhang, Y.H. Z-scheme g-C3N4/Bi2O2[BO2(OH)] heterojunction for enhanced photocatalytic CO2 reduction. J. Colloid Interface Sci. 2020, 568, 139–147. [Google Scholar] [CrossRef]
- Li, M.L.; Zhang, L.X.; Fan, X.Q.; Zhou, Y.J.; Wu, M.Y.; Shi, J.L. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Yan, J.Y.; Wang, C.H.; Ma, H.; Li, Y.Y.; Liu, Y.C.; Suzuki, N.; Terashima, C.; Fujishima, A.; Zhang, X.T. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118401. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.Q.; Wang, L.; Ye, L.Q.; Shi, X.; Bai, W. Size-dependent role of gold in g-C3N4/BiOBr/Au system for photocatalytic CO2 reduction and dye degradation. Sol. Energy Mater. Sol. Cells 2016, 157, 406–414. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B Environ. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Wang, L.; Shi, X.; Wang, P.; Bai, W.; Wong, P.K. g-C3N4/Bi4O5I2 heterojunction with I3− /I− redox mediator for enhanced photocatalytic CO2 conversion. Appl. Catal. B Environ. 2016, 194, 98–104. [Google Scholar] [CrossRef]
- Kim, C.; Cho, K.M.; Al-Saggaf, A.; Gereige, I.; Jung, H.T. Z-scheme Photocatalytic CO2 Conversion on Three-Dimensional BiVO4/Carbon-Coated Cu2O Nanowire Arrays under Visible Light. ACS Catal. 2018, 8, 4170–4177. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.H.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.M.; Ding, H.; Feng, J.J.; Zhang, J.Z.; Zhu, Y.F.; Hamid, S.; Bahnemann, D.W. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency. Appl. Catal. B Environ. 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Di, T.M.; Zhu, B.C.; Cheng, B.; Yu, J.G.; Xu, J.S. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, Z.H.; Heil, T.; Meng, A.Y.; Cheng, B.; Cao, S.W.; Yu, J.G.; Antonietti, M. Highly Selective CO2 Capture and Its Direct Photochemical Conversion on Ordered 2D/1D Heterojunctions. Joule 2019, 3, 2792–2805. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Jaroniec, M.; Chen, X.B. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Fang, R.M.; Huang, H.B.; Ji, J.; He, M.; Feng, Q.Y.; Zhan, Y.J.; Leung, D.Y.C. Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature. Chem. Eng. J. 2018, 334, 2050–2057. [Google Scholar] [CrossRef]
- Stohl, A.; Aamaas, B.; Amann, M.; Baker, L.H.; Bellouin, N.; Berntsen, T.K.; Boucher, O.; Cherian, R.; Collins, W.; Daskalakis, N.; et al. Evaluating the climate and air quality impacts of short-lived pollutants. Atmos. Chem. Phys. 2015, 15, 10529–10566. [Google Scholar] [CrossRef]
- Peng, M.Q.; Zhao, R.; Xia, M.; Li, C.J.; Gong, X.L.; Wang, D.; Xia, D.S. Study on the mechanism of NO removal by plasma-adsorption catalytic process. Fuel 2017, 200, 290–298. [Google Scholar] [CrossRef]
- Hermawan, A.A.; Chang, J.W.; Pasbakhsh, P.; Hart, F.; Talei, A. Halloysite nanotubes as a fine grained material for heavy metal ions removal in tropical biofiltration systems. Appl. Clay Sci. 2018, 160, 106–115. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.Z.; Zhang, X.Y.; Guo, Y.H.; Zhang, Y.S.; Wang, Y.; Sun, B.M. A plasma-assisted catalytic system for NO removal over CuCe/ZSM-5 catalysts at ambient temperature. Fuel Process. Technol. 2017, 158, 199–205. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Li, S.P.; Gao, J.Z.; Zhang, F.C.; Liu, C.L.; Wang, Q.Z.; Hojamberdiev, M. Constructing a 2D/2D Bi2O2CO3/Bi4O5Br2 heterostructure as a direct Z-scheme photocatalyst with enhanced photocatalytic activity for NOx removal. Appl. Surf. Sci. 2019, 493, 913–925. [Google Scholar] [CrossRef]
- Shin, S.H.; Jo, W.K. Longitudinal variations in indoor VOC concentrations after moving into new apartments and indoor source characterization. Environ. Sci. Pollut. Res. 2012, 20, 3696–3707. [Google Scholar] [CrossRef]
- Sun, R.Z.; Shi, Q.M.; Zhang, M.; Xie, L.H.; Chen, J.S.; Yang, X.M.; Chen, M.X.; Zhao, W.R. Enhanced photocatalytic oxidation of toluene with a coral-like direct Z-scheme BiVO4 /g-C3N4 photocatalyst. J. Alloy Compd. 2017, 714, 619–626. [Google Scholar] [CrossRef]
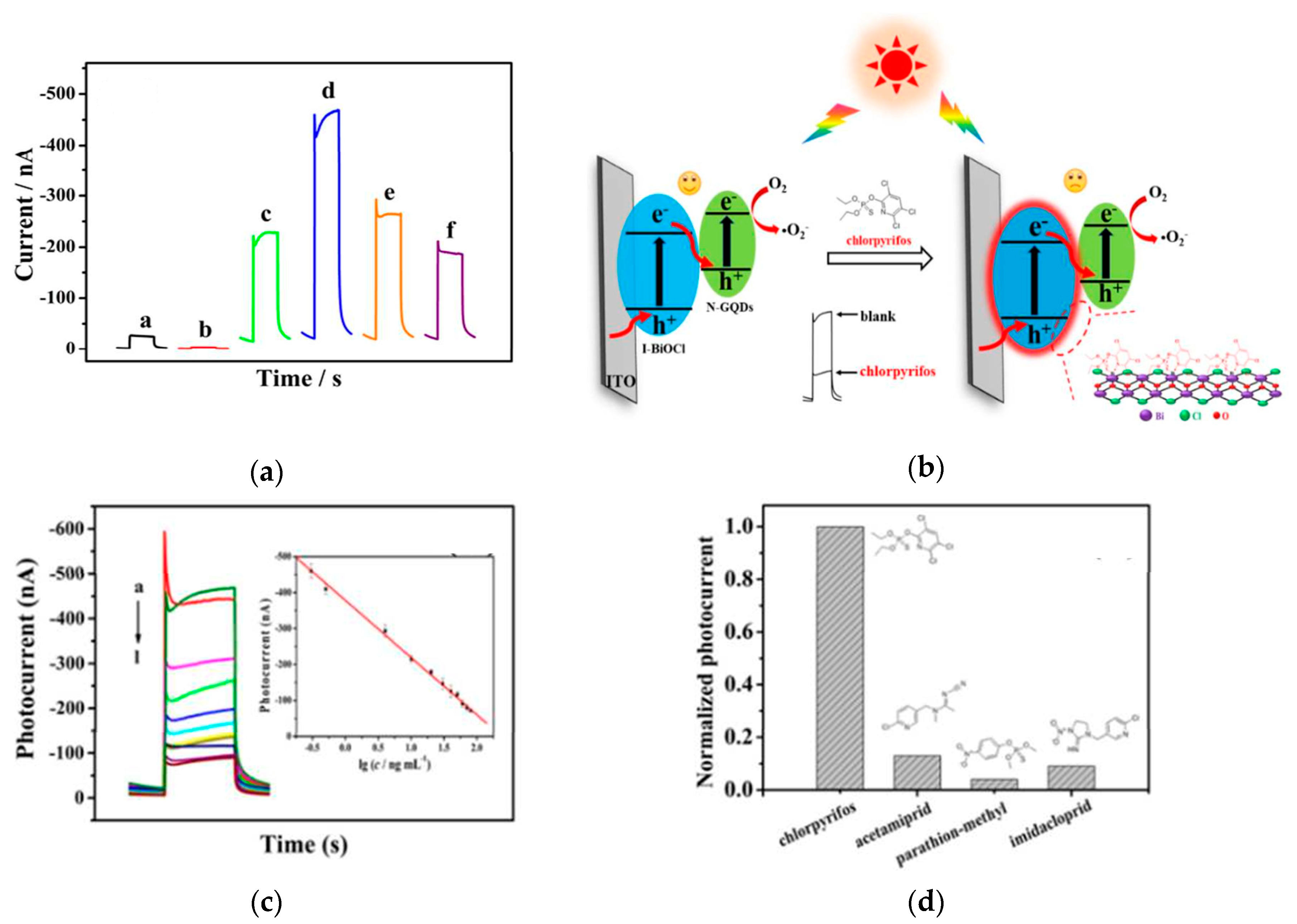
- Wang, H.; Zhang, B.H.; Zhao, F.Q.; Zeng, B.Z. One-Pot Synthesis of N-GQDs Functionalized I-BiOCl Z-Scheme Cathodic Materials for “Signal-Off“ Photoelectrochemical Sensing of Chlorpyrifos. ACS Appl. Mater. Interfaces 2018, 10, 35281–35288. [Google Scholar] [CrossRef]
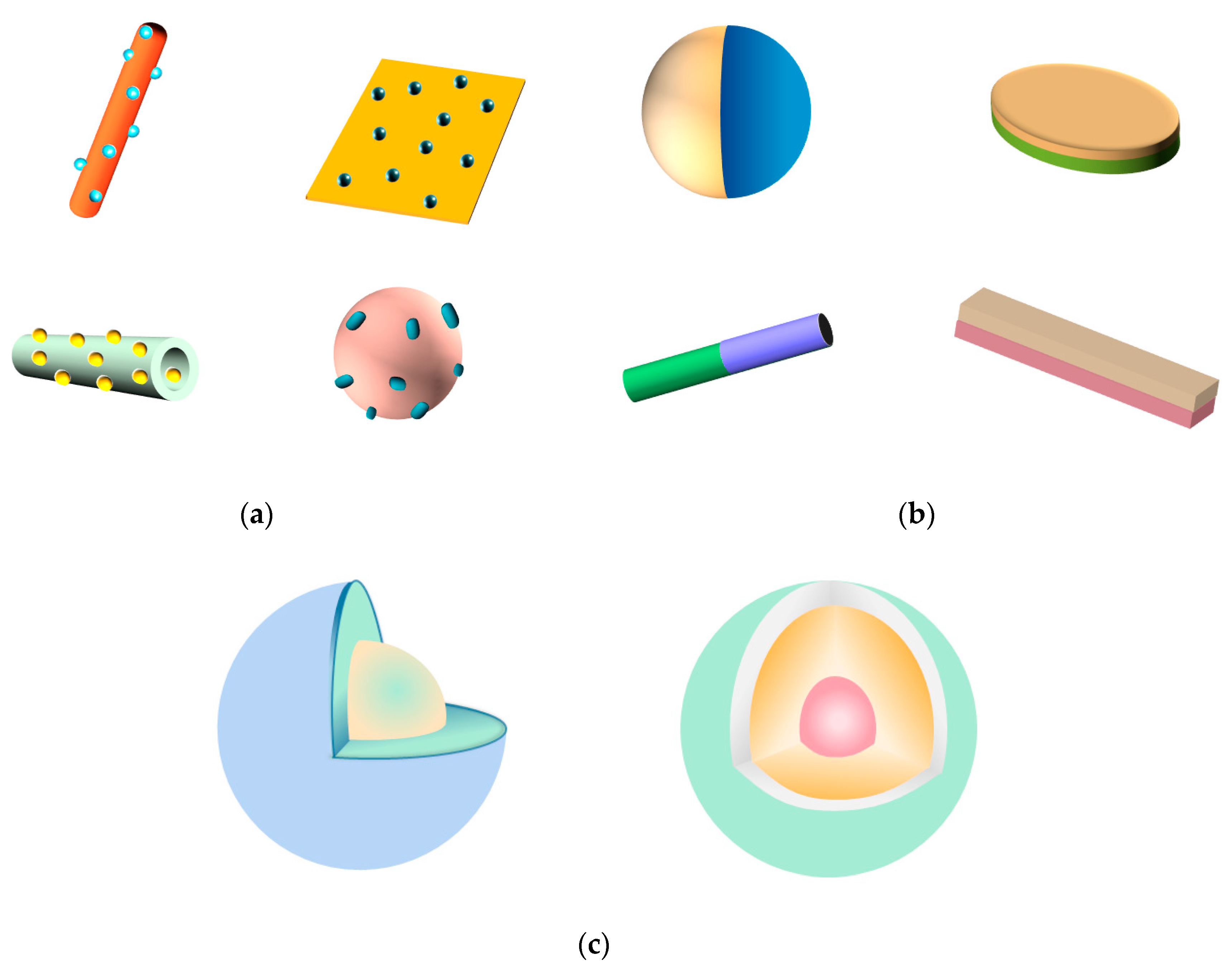

| Synthesis Method | Advantages | Shortcomings | Interfacial Properties of the Prepared Z-Scheme Hetero- Junction | Geometrical Configurations of the Prepared Z-Scheme Heterojunction |
|---|---|---|---|---|
| Hydrothermal and Solvothermal Method | Controllable Size, High Crystallinity, Low Cost, Simple Operation, One-Pot Synthesis without Need of Post Annealing | High Requirements in Temperature, Pressure and Corrosion Resistance for Equipment, Required High Temperature | Strong Interaction and Intimate Interface | Surface-Decorated Structure |
| Solid-State Synthesis | High Synthetic Efficiency, Simple and Solvent-Free Synthetic Process | High Energy Consumption, High Cost, Required High Temperature | Strong Interaction and Tight-Contact Interface | Surface-Decorated Structure |
| Deposition- Precipitation Method | Narrow Size Distributions of Products, Good Thermal Stability of Products | Poor Reproducibility, Uncontrollable Deposition Location and Nucleation Site | Strong Interaction and Intimate Interface | Surface-Decorated Structure |
| Cation Exchange Method | Relatively Rapid Reaction Rate, Well-Preserved Initial Morphology, Size and Compositional Interfaces, High-Quality Nanocrystal, Simple and Flexible Method | Required Post Calcination Treatment | Strong Interaction, High-Quality and Atomic- Precision Contact Interface | Janus, Surface-Decorated or more Complex Custom Structure Including Multicomponent Z-Scheme Heterojunction Structure |
| Electro- Spinning Method | Facile and Simple Method, Simple Setup, Large Surface Area of Products | Low Synthetic Efficiency, High Cost, Required Post-Heating Treatment | Strong Interaction and Intimate Interface | Surface-Decorated Structure |
| Self- Assembly Method | Mild Operation Conditions, Controllable Morphology and Size, Highly Ordered and Dispersive Products | Low Yield, Poor Stability of Products | Moderate Interaction | Core-Shell, Surface-Decorated Structure |
| Mechanical Agitation Method | Simple Setup, Straightforward Method, Avoiding the Use of Complex and Tedious Chemical and Thermal Treatments | Wide Size Distributions of Products, Poor Reproducibility, Uncontrollable Size | No Intimate Interface, Having Easily Detachable Components of Heterojunction, Low Crystallinity | Surface-Decorated Structure |
| Ultrasonic Chemical Method | Narrow Size Distributions of Products, Rapid Reaction Rate, Controllable Morphology and Size | High Cost, Hard to Scaling Up | Strong Interaction and Intimate Interface | Surface-Decorated or Core-Shell Structure |
| Photo- Catalyst | Synthesis Method | Light Source | Catalyst Dose | Pollutants | Photocatalytic Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Bi2WO6/CuBi2O4 | Hydro- Thermal | 300 W Xe Lamp (λ ≥ 400 nm) | 0.5 mg/mL | Tetracycline (15 mg/L, 100 mL) | 0.0393 min−1 (CuBi2O4 0.0054 min−1) | [70] |
| BiOI /g–C3N4 | In situ Reduction and Oxidiza- tion | 60 W LED (Light Emitting Diode) Lamp (λ > 400 nm) | 3.33 mg/mL | Phenol (100 mg/L, 15 mL) | 60% (BiOI 20%) | [76] |
| CdS/BiOI | Hydro- Thermal | 300 W Xe Lamp (λ > 420 nm) | 0.2 mg/mL | RhB (20 mg/L, 100 mL) | 0.03945 min−1 (BiOI 0.00398 min−1) | [77] |
| BiOBr/ Bi2MoO6 | Co-Precipitation | 300 W Xe Lamp (λ ≥ 420 nm) | 0.2 mg/mL | Cipro- Floxacin (10 mg/L 50 mL). RhB (10−5 mol/L, 50 mL) | 84.63% (Bi2MoO6 15.21%); 0.37613 min−1 (Bi2MoO6 0.00689 min−1) | [78] |
| Bi2O3/g–C3N4 | Solid- State Synthesis | 500 W Xe Lamp (λ > 400 nm) | 1.0 mg/mL | MB (1.1 × 10−5 mol/L, 300 mL); RhB (1.0 × 10−5 mol/L, 300 mL) | 0.0253 min−1 (g–C3N4 0.0074 min−1); 0.0101 min−1 (g–C3N4 0.002 min−1) | [79] |
| Bi2Fe4O9/Bi2WO6 | Hydro- Thermal | 300 W Xe lamp (λ ≥ 420 nm) | 0.3 mg/mL | RhB (10 mg/L, 100 mL) | 0.0380 min−1 (Bi2Fe4O9 0.0015 min−1) | [74] |
| AgI/Bi5O7I | Ion Exchange | 350 W Xe lamp (cut off UV and IR light) | 1.0 mg/mL | RhB (10 mg/L, 100 mL) | 0.046 min−1 (Bi5O7I 0.012 min−1) | [80] |
| AgI/Bi2WO6 | Precipitation | 300 W Xe lamp (λ ≥ 420 nm) | 0.3 mg/mL | Tetracycline (20 mg/L, 100 mL) | 0.075 min−1 (Bi2WO6 0.014 min−1) | [81] |
| AgBr/CuBi2O4 | Precipitation | 300 W Xe lamp (λ ≥ 420 nm) | 0.5 mg/mL | Tetracycline (10 mg/L, 100 mL) | 0.03551 min−1 (CuBi2O4 0.00238 min−1) | [82] |
| TCPP/rGO/Bi2WO6 | Ultrasonic Chemical | 300 W Xe lamp (λ > 420 nm) | 0.3 mg/mL | Tetracycline (15 mg/L, 100 mL) | 83.60% (Bi2WO6 48.61%) | [83] |
| Ag3PO4/CuBi2O4 | Precipitation | 300 W Xe lamp (λ > 420 nm) | 0.5 mg/mL | Tetracycline (10 mg/L, 100 mL) | 0.0201 min−1 (CuBi2O4 0.0072 min−1) | [84] |
| Porous g–C3N4/BiOI | Hydro- Thermal | 50 W 410 nm LED light arrays | 1 mg/mL | MB (20 mg/L, 30 mL) | 0.0160 min−1 (BiOI 0.0041 min−1) | [85] |
| CdS/Bi3O4Cl | Hydro- Thermal | 250 W Xe lamp (λ > 420 nm) | 0.5 mg/mL | Tetracycline (10 mg/L, 100 mL). Cipro-Floxacin (10 mg/L, 100 mL) | 0.0643 min−1 (Bi3O4Cl 0.0148 min−1). 0.0151 min−1 (Bi3O4Cl 0.00142 min−1) | [75] |
| Cu2O/Bi5O7I | Glucose Reduction Reaction | 500 W Xe lamp | 1 mg/mL | RhB (10 mg/L, 100 mL) | 0.0233 min−1 (Bi5O7I 0.00736 min−1) | [86] |
| CuInS2/Bi2WO6 | Hydro- Thermal | 300 W Xe lamp (λ ≥ 420 nm) | 0.3 mg/mL | Tetracycline Hydrochloride (10 mg/L, 100 mL) | 0.0176 min−1 (Bi2WO6 0.01473 min−1) | [87] |
| MoO3/Bi2O4 | Hydro- Thermal | 100 W LED lamp (λ = 420 nm) | 0.5 mg/mL | RhB (10 mg/L, 100 mL) | 99.6% (Bi2O4 73%) | [88] |
| BiOI/Bi2O4 | Ultrasonic Chemical | 100 W LED lamp | 0.5 mg/mL | RhB (10 mg/L, 100 mL) | 0.090 min−1 (BiOI 0.003 min−1) | [89] |
| Bi2MoO6/TiO2 | Hydro- Thermal | 800 W Xe lamp | 0.6 mg/mL | 4-Nitrophenol (50 mg/L, 100 mL) | 95.3% (Bi2MoO6 32.7%) | [90] |
| Bi2WO6 /Porous g–C3N4 | Ultrasonic Chemical | 500 W Wolfram lamp (λ ≥ 420 nm) | 0.5 mg/mL | RhB (10 mg/L, 100 mL) | 0.043 min−1 (Bi2WO6 0.013 min−1) | [91] |
| Bi2WO6/BiOI | Hydrothermal | 500 W Xe lamp (λ > 420 nm) | 1 mg/mL | RhB (10 mg/L, 40 mL) | 0.03 min−1 (BiOI 0.002 min−1) | [92] |
| Ag3PO4/Bi2WO6 | Precipitation | 50 W LED lamp (λ = 410 nm) | 1 mg/mL | MB (20 mg/L, 30 mL) | 0.61 min−1 (Bi2WO6 0.10 min−1) | [93] |
| g–C3N4/BiVO4 | Hydrothermal | 250 W Xe lamp (λ > 420 nm) | 0.2 mg/mL | MO (20 mg/L, 50 mL) | 0.09672 min−1 (BiVO4 0.01101 min−1) | [94] |
| Photo- Catalyst | Co- Catalyst | Synthesis Method | Experimental Conditions | Products and Yields | AQY | Ref. |
|---|---|---|---|---|---|---|
| BiPO4/SnS2 | No | Hydrothermal | Visible light irradiation (λ > 380 nm). Pure Water | H2: 303 μmol h−1·g−1 | – | [98] |
| Bi/Bi5O7I/Sn3O4 | Bi | Hydrothermal | 300 W Xe Lamp (λ > 400 nm). 20% CH3OH Solution | H2: 325.9 μmol h−1·g−1 | – | [99] |
| Cu3P/Bi2WO6 | No | Mechanical Agitation | Xe lamp (AM (air mass) 1.5); 0.5 M Na2HPO4/NaH2PO4 Solution | H2: 4.65 μmol h−1·g−1 O2: 2.3 μmol h−1·g−1 | – | [68] |
| BiVO4 /Black phosphorus | 5 wt% Co3O4 | Self-Assembly | 320 W Xenon Lamp (λ > 420 nm). Pure Water | H2: 160 μmol h−1·g−1 O2: 102 μmol h−1·g−1 | 0.89% at 420 nm | [100] |
| Bi2O2.33/Bi2S3 | 1 wt% Pt | Wet Chemistry | 500 W Xenon Lamp; 0.1 M Na2S/ Na2SO3 Solution | H2: 62.61 μmol h−1 | – | [101] |
| g–C3N4 /BiFeO3 | No | Solid-State Synthesis | Three 125 W Medium Pressure Hg Lamps (UV). Pure Water | H2: 160.75 μmol h−1·g−1 O2: 80.12 μmol h−1·g−1 | – | [102] |
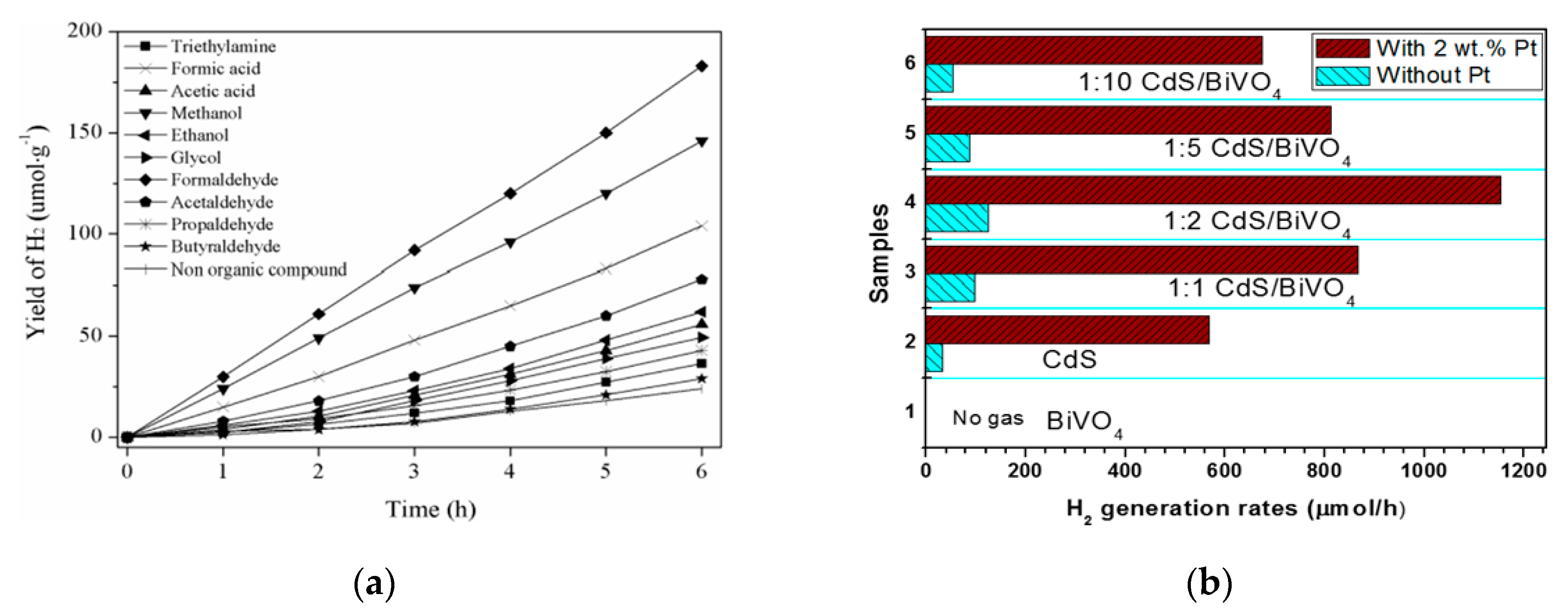
| CdS/BiVO4 | 2 wt% Pt | Solvothermal | 300 W Xe Lamp (λ ≥ 420 nm); 20 vol.% Lactic Acid Solution | H2: 1153 μmol h−1 | – | [103] |
| Bi2S3/MoS2/TiO2 | No | Microwave- Assisted Hydrothermal | 250 W Xe Lamp (λ ≥ 420 nm); 0.35 M Na2S and 0.25 M Na2SO3 Solution | H2: 2195 μmol h−1·g−1 | – | [104] |
| Cs2O/Bi2O3/ZnO | No | Solution Combustion Method | Xe Lamp (AM 1.5 G); Pure Water | H2: 149.5 μmol h−1·g−1 O2: 73.2 μmol h−1·g−1 | 1.68% at 365 nm 0.92% at 420 nm | [105] |
| ZnIn2S4/RGO/BiVO4 | 1 wt% Pt | Hydrothermal | 350 W Xe Lamp (λ > 420 nm); 5 mol·L−1 HCHO | H2: 1687 μmol h−1·g−1 | 22.91% | [106] |
| RGO–Cu2O/Bi2WO6 | No | Solvothermal | Xe Lamp (λ > 420 nm); Pure Water | H2:1.80 μmol h−1·g−1 | – | [107] |
| Cu2O/RGO/BiVO4 | – | Solvothermal | 300 W Xenon Arc Lamp (λ > 420 nm); TC Solution | H2: 5.90 μmol h−1·g−1 | – | [108] |
| Photo- Catalyst | Co- Catalyst | Synthesis Method | Conditions | Products and Yields | Ref. |
|---|---|---|---|---|---|
| CdS/BiVO4 | No | Deposition | 300 W Xenon Arc Lamp (λ > 400 nm). 20 mg Photocatalyst in 180 mL Stainless Steel Reactor with Quartz Window; Filled with CO2 (0.3 MPa). | CH4: 1.75 μmol h−1·g−1 CO: 0.39 μmol h−1·g−1 | [115] |
| BiOI/g–C3N4 | No | Deposition | 300 W Xenon Arc Lamp (λ > 400 nm); 0.1 g Photocatalyst in 180 mL Stainless Steel Cylindrical Vessel with Quartz Window; Introducing CO2 and H2O Vapor by Bubbling Approach. | CH4: 1.76 μmol h−1·g−1 CO: 22.21 μmol h−1·g−1 H2: 2.06 μmol h−1·g−1 O2: 10.81 μmol h−1·g−1 | [116] |
| Bi2WO6/TiO2 | No | Electrostatic Self- Assembly | 300 W Xenon Arc Lamp (780 nm > λ > 320 nm); 20 mg Photocatalyst in 25 mL Quartz Reactor; CO2 was Evacuated by a Mechanical Pump. | CH4: 10.8 μmol h−1·g−1 CO: 25.8 μmol h−1·g−1 | [117] |
| g–C3N4/ Bi2O2[BO2(OH)] | No | Solid-State Synthesis | 300 W Xe Lamp; 20 mg Photocatalyst; 1.7 g Na2CO3 Treated with 15 mL H2SO4 (0.1 mol/L) to in situ Generate CO2. | CO: 6.09 μmol h−1 | [118] |
| Bi2WO6/g–C3N4 | No | Hydrothermal | 300 W Xenon Arc Lamp (λ > 420 nm); 100 mg Catalyst in 500 mL Reactor; Introducing CO2 and H2O Vapor by Bubbling Approach. | CO: 5.19 μmol h−1·g−1 | [119] |
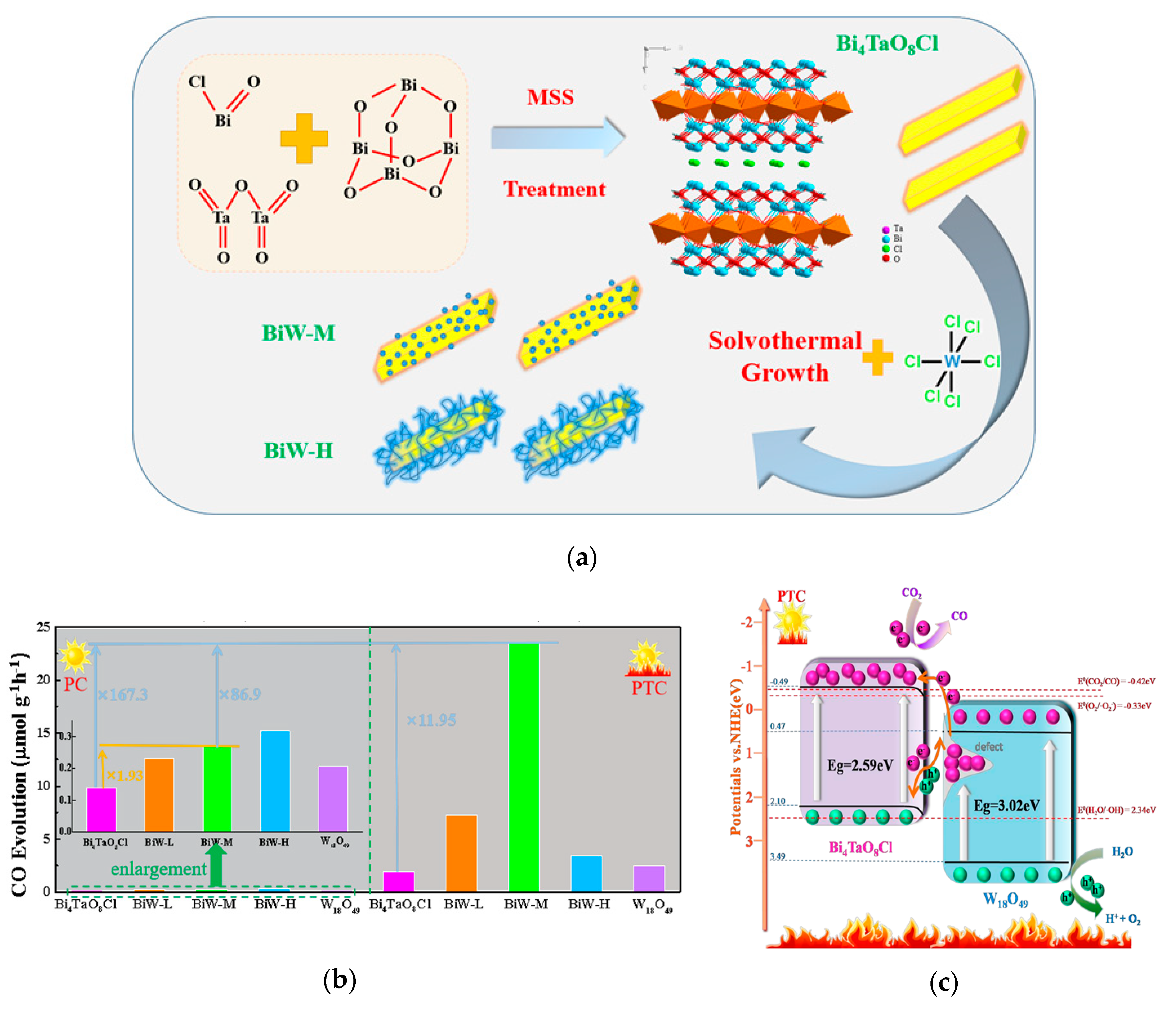
| Bi4TaO8Cl /W18O49 | No | Solvothermal | 180 mW/cm2 Solar Light (λ < 780 nm); 0.02 g Photocatalyst and 2 mL H2O in Reactor; Filled with CO2. The Reactor was Heated to 393 K. | CO: 23.42 μmol h−1·g−1 | [120] |
| Bi2O2CO3/Bi/ Bi2WO6 | Bi | Solvothermal | 300 W Xe Lamp; 0.1 g Photocatalyst and 100 mL H2O in Reactor; CO2 was Inflated into the Reactor (80 kPa). | CH4: 2.54 μmol h−1·g−1 CO: 0.82 μmol h−1·g−1 | [71] |
| g–C3N4/BiOBr | Au | Water Bath | 300 W High Pressure Xenon Lamp. 0.1 g Samples were Uniformly Dispersed onto a Glass Sheet put in 350 mL Reactor; 1.3 g NaHCO3 reacted with 5 mL H2SO4 (4M) to in situ Generate CO2. | CH4: 0.92 μmol h−1·g−1 CO: 6.67 μmol h−1·g−1 | [121] |
| Bi2WO6 /RGO /g–C3N4 | No | Hydrothermal | 300 W Xe Arc Lamp with a UV cut-off Filter of 420 nm; 50 mg of the Catalyst was Uniformly Distributed in the Photoreactor (250 mL); A Water Bubbler to generate a Mixture of CO2 and Water Vapor. | CO: 15.96 μmol h−1·g−1 CH4: 2.51 μmol h−1·g−1 | [122] |
| g–C3N4/Bi4O5I2 | No | Complex Precursor Method | 300 W High Pressure Xenon Lamp (λ > 400 nm); 0.1 g Samples were Uniformly dispersed onto a Glass Sheet, put in 350 mL Reactor; NaHCO3 reacted with 5 mL H2SO4 (4M) to achieve 1 atm CO2. | CO: 45.6 μmol h−1·g−1 | [123] |
| BiVO4/C/Cu2O | No | SILAR | 300 W Xe Lamp (λ > 420 nm); A 1 cm2 Specimen of the Sample was placed at 50 mL Reactor which charged with 5 mL of H2O; The Reactor was purged with CO2. | CO: 3.01 μmol h−1·g−1 | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Chen, H.; Xiong, J.; Xu, X.; Cheng, J.; Liu, X.; Liu, G. A Mini Review on Bismuth-Based Z-Scheme Photocatalysts. Materials 2020, 13, 5057. https://doi.org/10.3390/ma13225057
Li R, Chen H, Xiong J, Xu X, Cheng J, Liu X, Liu G. A Mini Review on Bismuth-Based Z-Scheme Photocatalysts. Materials. 2020; 13(22):5057. https://doi.org/10.3390/ma13225057
Chicago/Turabian StyleLi, Ruizhen, Hanyang Chen, Jianrong Xiong, Xiaoying Xu, Jiajia Cheng, Xingyong Liu, and Guo Liu. 2020. "A Mini Review on Bismuth-Based Z-Scheme Photocatalysts" Materials 13, no. 22: 5057. https://doi.org/10.3390/ma13225057
APA StyleLi, R., Chen, H., Xiong, J., Xu, X., Cheng, J., Liu, X., & Liu, G. (2020). A Mini Review on Bismuth-Based Z-Scheme Photocatalysts. Materials, 13(22), 5057. https://doi.org/10.3390/ma13225057





