Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material
Abstract
1. Introduction
2. Materials and Methods
3. Results
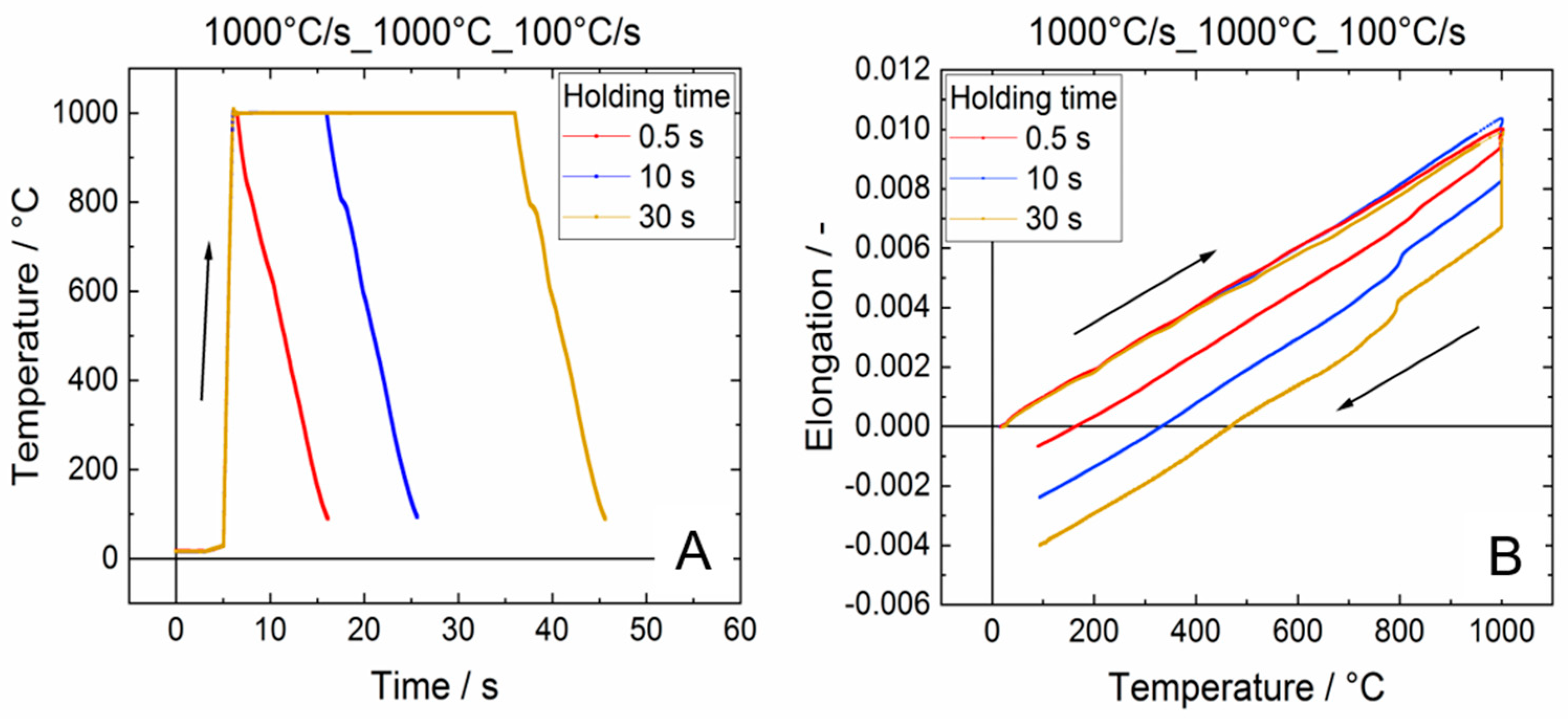
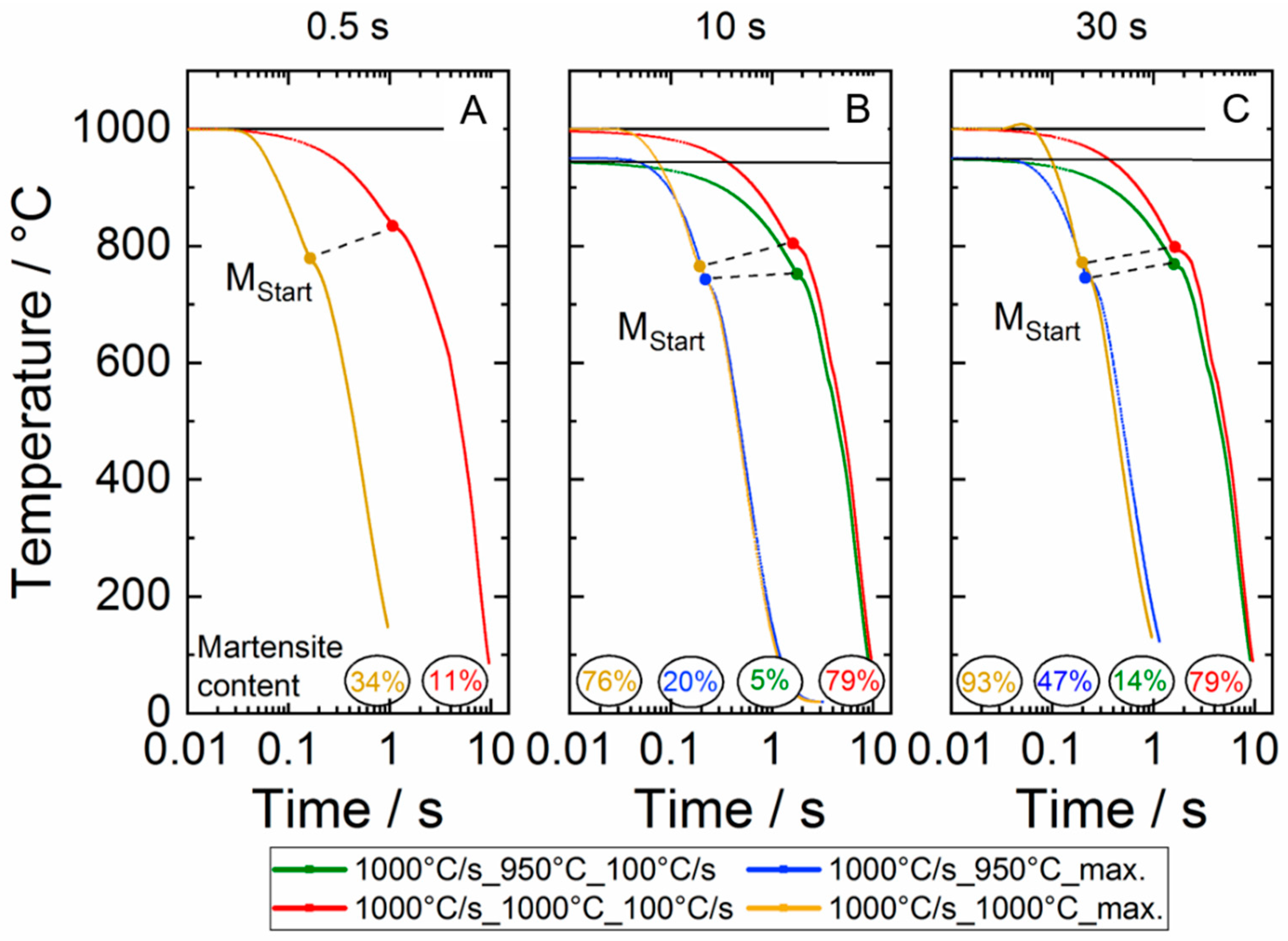
3.1. Dilatometer Curves
Influence of the Target Temperature on the Martensite Start Temperature
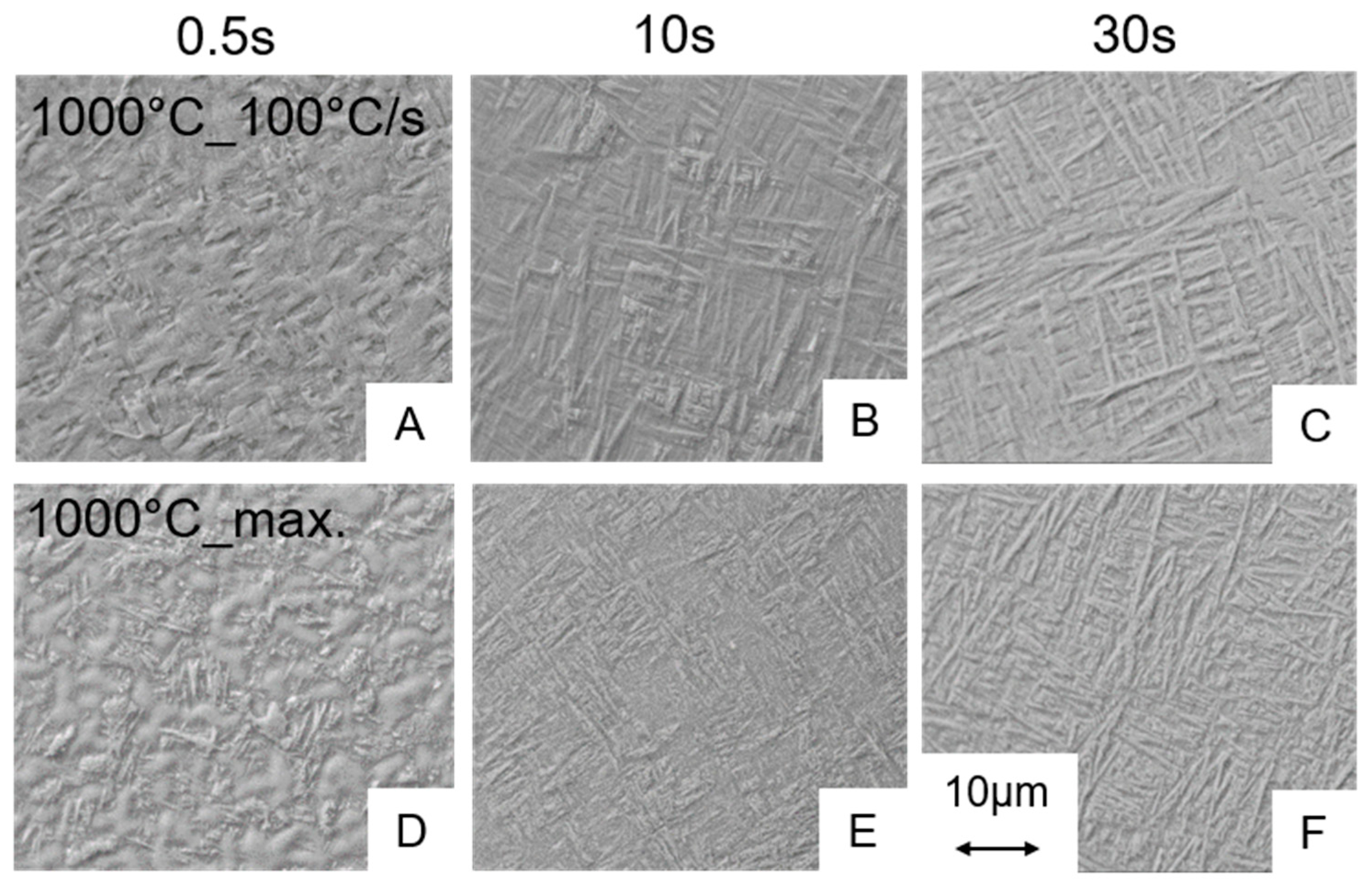
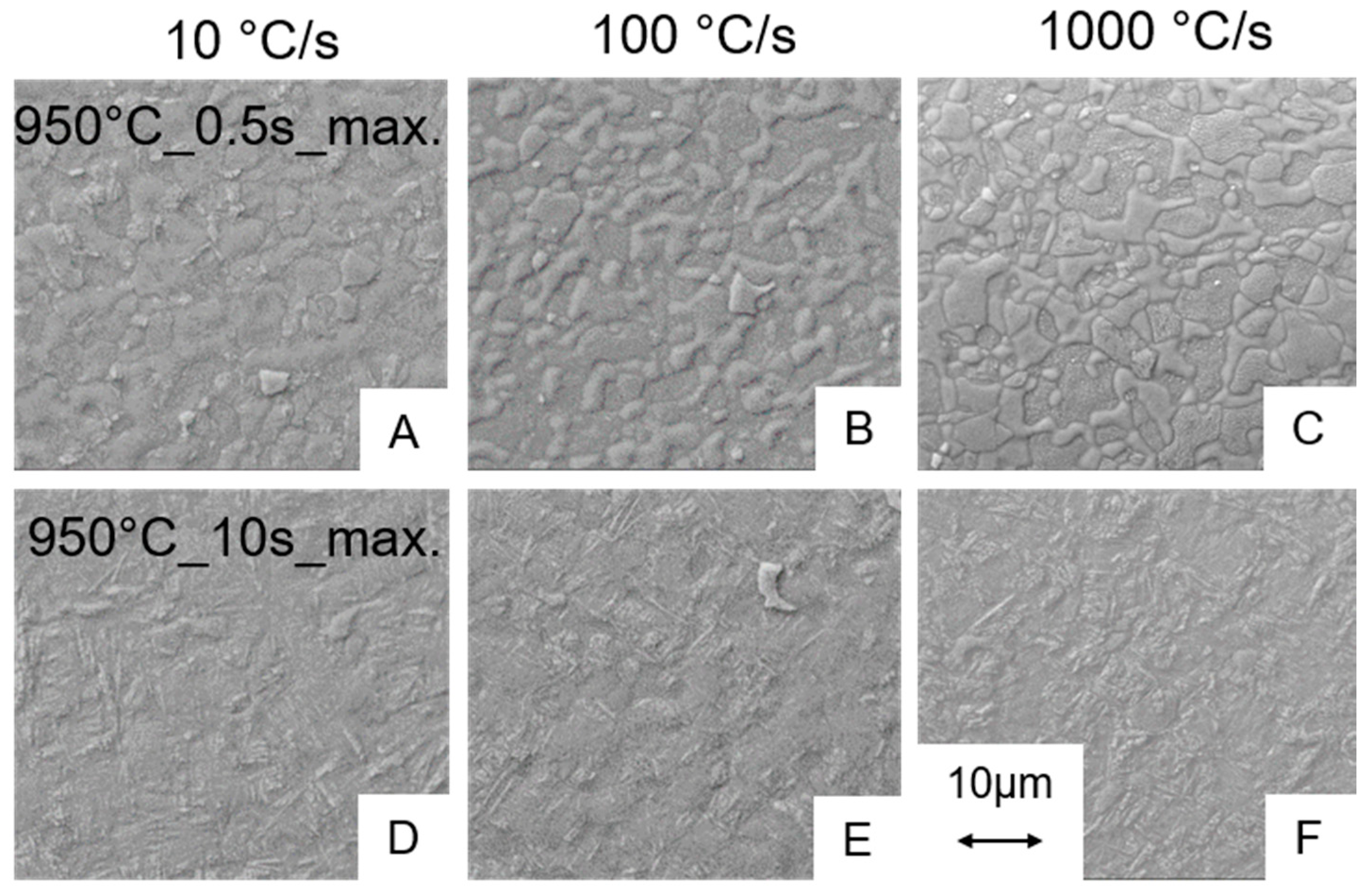
3.2. Metallography
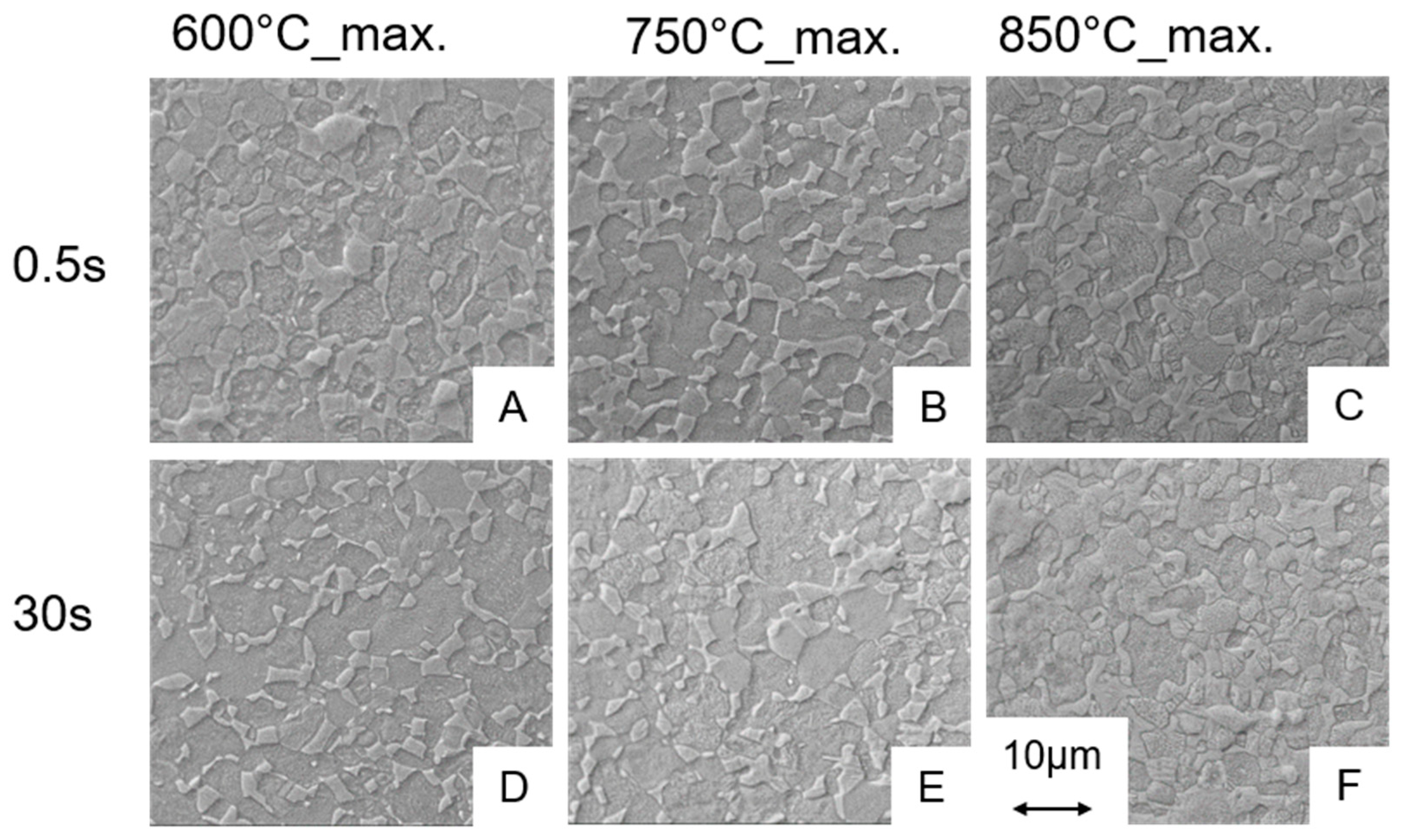
3.2.1. Influence of the Heating Rate on the Microstructure
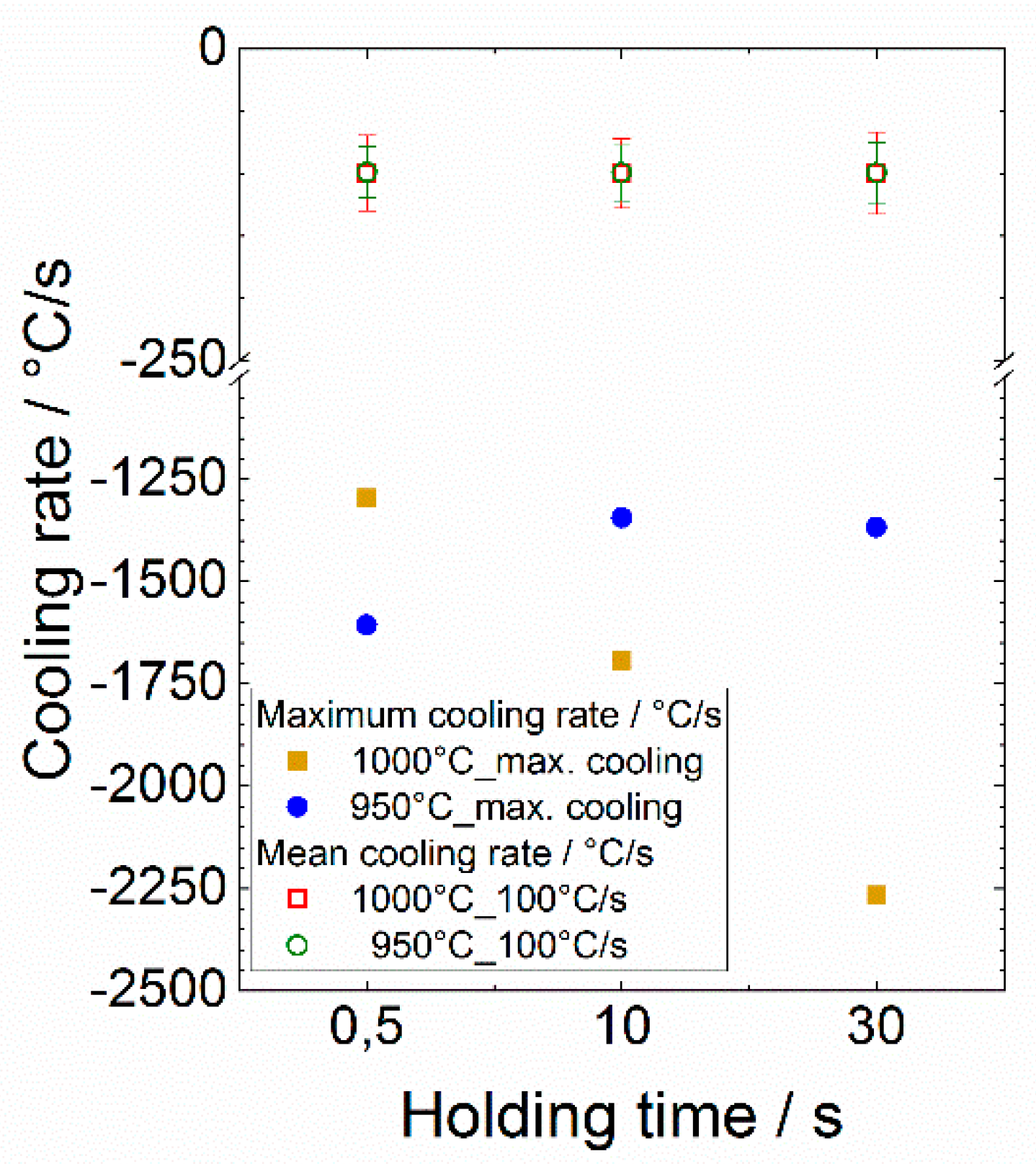
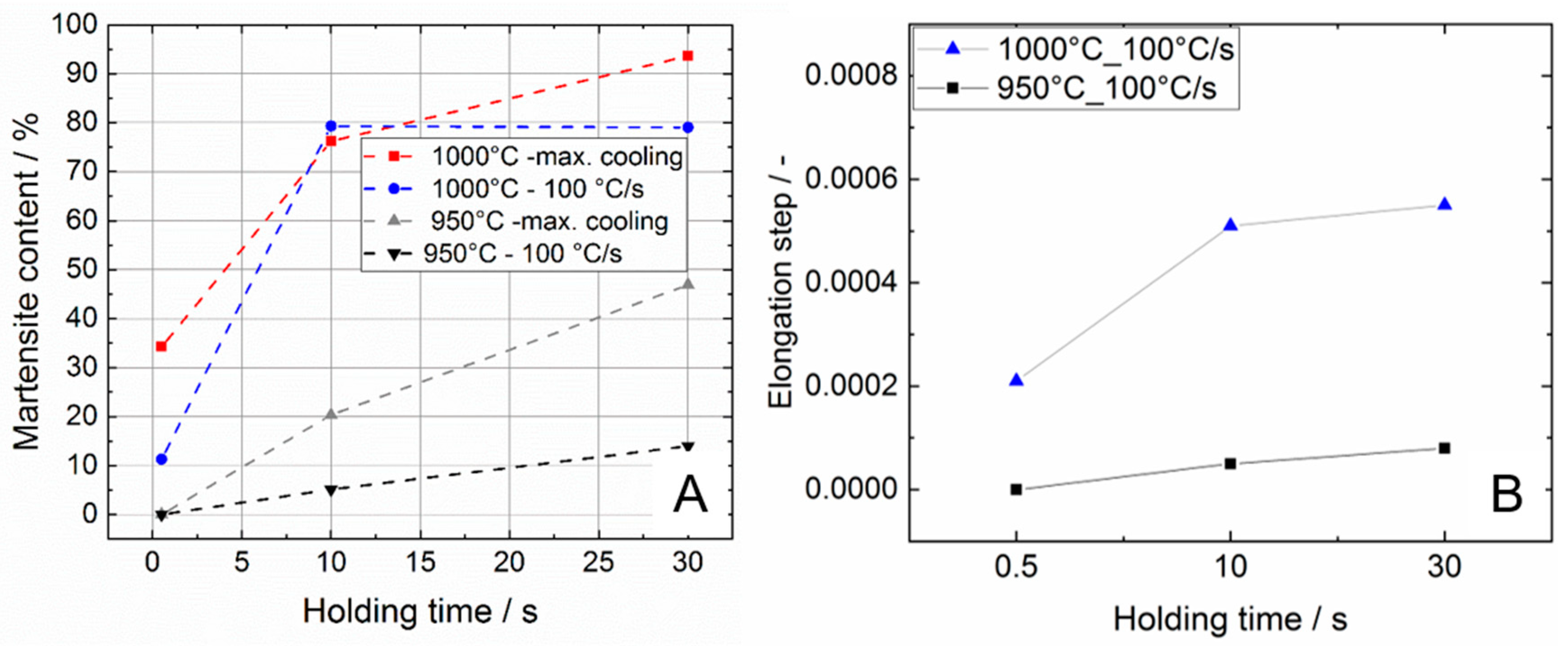
3.2.2. Influence of the Holding Time and Cooling Rate on the Martensite Content
4. Conclusions
- Short-term heat treatment with a maximum temperature to 950 °C, which is slightly below the β-transus temperature, and very short holding times of 0.5 s, also cause no significant phase transformations. However, heat treatment with temperatures ≥950 °C and comparable heating and cooling rates should not take longer than 0.5 s; otherwise, the resulting microstructure will no longer fulfil the requirements for medical applications;
- Heating rates between 10 and 1000 °C/s showed no significant influence on the resulting microstructure during short-term heat treatments;
- The cooling rate of 100 °C/s is too low to trigger a complete transformation of the β phase into martensite for maximum temperatures of 950 and 1000 °C, respectively;
- The cooling rates obviously do not affect the martensite start temperature in contrast to the maximum control temperature. Here, an increase in the maximum temperature results in a slight increase in the martensite start temperature, which is due to the degree of chemical homogenization;
- The analysis of the elongation kink in the dilatometer curve, which appears when the martensite transformation sets in, can be used to assess the martensite phase fraction that has been transformed during quenching from high maximum temperatures. The results are in good agreement with metallographic analysis.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.R.; Gilbert, J.L.; Jacobs, J.J.; Bauer, T.W.; Paprosky, W.; Leurgans, S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin. Orthop. Relat. Res. 2002. [Google Scholar] [CrossRef]
- Dyrkacz, R.M.R.; Brandt, J.-M.; Ojo, O.A.; Turgeon, T.R.; Wyss, U.P. The influence of head size on corrosion and fretting behaviour at the head-neck interface of artificial hip joints. J. Arthroplast. 2013, 28, 1036–1040. [Google Scholar] [CrossRef]
- Cooper, H.J.; Della Valle, C.J.; Berger, R.A.; Tetreault, M.; Paprosky, W.G.; Sporer, S.M.; Jacobs, J.J. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J. Bone Joint Surg. Am. 2012, 94, 1655–1661. [Google Scholar] [CrossRef]
- Inoue, D.; Restrepo, C.; Nourie, B.; Restrepo, S.; Hozack, W.J. Patients with Modular-Neck Total Hip Arthroplasty: A Brief Five-Year Follow-Up Study. J. Arthroplast. 2020, 35, S268–S272. [Google Scholar] [CrossRef]
- Gill, I.P.S.; Webb, J.; Sloan, K.; Beaver, R.J. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J. Bone Joint Surg. Br. 2012, 94, 895–900. [Google Scholar] [CrossRef]
- Steinhilper, W.; Sauer, B. Konstruktionselemente des Maschinenbaus 1; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-24300-4. [Google Scholar]
- Mueller, U.; Braun, S.; Schroeder, S.; Sonntag, R.; Kretzer, J.P. Same Same but Different? 12/14 Stem and Head Tapers in Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Altenberger, I.; Gibmeier, J.; Herzog, R.; Noster, U.; Scholtes, B. Analysis and Assessment of Residual Stress States in Mechanically Surface Treated Materials. Mater. Sci. Res. Int. Spec. Tech. Pub. 2001, 1, 275–284. [Google Scholar]
- Sahin, A.Z. Analysis of surface heating by induction. J. Mater. Eng. Perform. 1996, 5, 657–662. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S.; Specht, E.D. In situ observations of lattice expansion and transformation rates of α and β phases in Ti–6Al–4V. Mater. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Dąbrowski, R. The Kinetics of Phase Transformations during Continuous Cooling of the Ti6Al4V Alloy from the Single-Phase β Range. Arch. Metall. Mater. 2011, 56, 703–707. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α + β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- He, J.; Li, D.; Jiang, W.; Ke, L.; Qin, G.; Ye, Y.; Qin, Q.; Qiu, D. The Martensitic Transformation and Mechanical Properties of Ti6Al4V Prepared via Selective Laser Melting. Materials 2019, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Aristizabal, M.; Kong, W.; Villapun, V.; Cox, S.C.; Grover, L.M.; Attallah, M.M. Selective Laser Melting of Ti-6Al-4V: The Impact of Post-processing on the Tensile, Fatigue and Biological Properties for Medical Implant Applications. Materials 2020, 13, 2813. [Google Scholar] [CrossRef] [PubMed]
- Implants for Surgery-Metallic Materials-Classification of Microstructures for Alpha-Beta Titanium Alloy Bars; ISO20160:2006; ISO: Geneva, Switzerland, 2006.
- Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNSR56401); ASTM F136-13; ASTM International: West Conshohocken, PA, USA, 2018.
- Chirurgische Implantate—Metallische Werkstoffe—Teil 3: Titan 6-Aluminium 4-Vanadium Knetlegierung (ISO 5832-3:2016); DIN EN ISO 5832-3:2012-08; Beuth Verlag GmbH: Berlin, Germany, 2017.
- Titan und Titanlegierungen; Springer-Verlag: Berlin, Germany; New York, NY, USA, 1974; ISBN 3-540-05233-X.
- Kim, K.T.; Yang, H.C. Densification behavior of titanium alloy powder during hot pressing. Mater. Sci. Eng. A 2001, 313, 46–52. [Google Scholar] [CrossRef]
- Sha, W.; Malinov, S. Titanium Alloys: Modelling of Microstructure, Properties and Applications; Woodhead Publishing Limited: Oxford, UK; Cambridge, UK; New Delhi, India, 2009; ISBN 978-1-84569-586.-6. [Google Scholar]

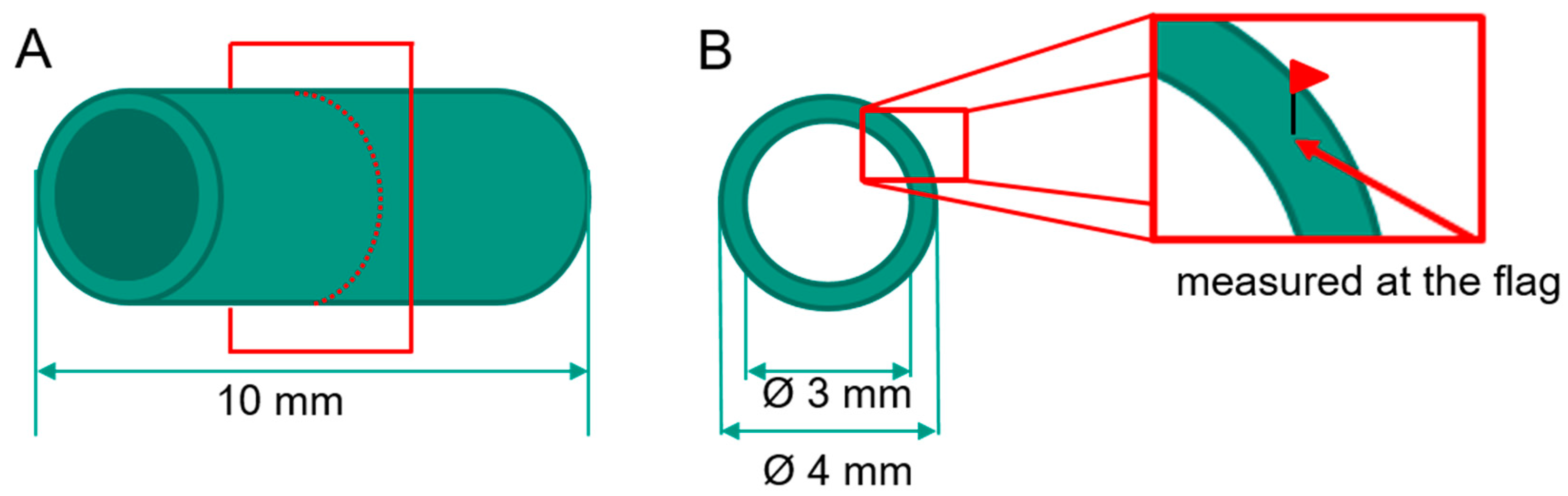


| Young’s Modulus | Yield Strength | Tensile Strength | Vickers Hardness |
|---|---|---|---|
| 106 ± 0.8 GPa | 910 ± 5 MPa | 967 ± 5 MPa | 321 ± 8 HV0.1 |
| Target Temperatures | Heating Rates | Holding Times | Cooling Rates |
|---|---|---|---|
| 600, 750, 850, 950, 1000 °C | 10, 100, 1000 °C/s | 0.5, 10, 30 s | 100 °C/s, max. cooling |
| Maximum Control Temperature | Martensite Start Temperature |
|---|---|
| 950 °C | 756 ± 17 °C |
| 1000 °C | 805 ± 13 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, P.T.; Bormann, T.; Sonntag, R.; Kretzer, J.P.; Gibmeier, J. Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material. Materials 2020, 13, 4948. https://doi.org/10.3390/ma13214948
Mai PT, Bormann T, Sonntag R, Kretzer JP, Gibmeier J. Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material. Materials. 2020; 13(21):4948. https://doi.org/10.3390/ma13214948
Chicago/Turabian StyleMai, Phuong Thao, Therese Bormann, Robert Sonntag, Jan Philippe Kretzer, and Jens Gibmeier. 2020. "Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material" Materials 13, no. 21: 4948. https://doi.org/10.3390/ma13214948
APA StyleMai, P. T., Bormann, T., Sonntag, R., Kretzer, J. P., & Gibmeier, J. (2020). Short-Term Heat Treatment of Ti6Al4V ELI as Implant Material. Materials, 13(21), 4948. https://doi.org/10.3390/ma13214948





