Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
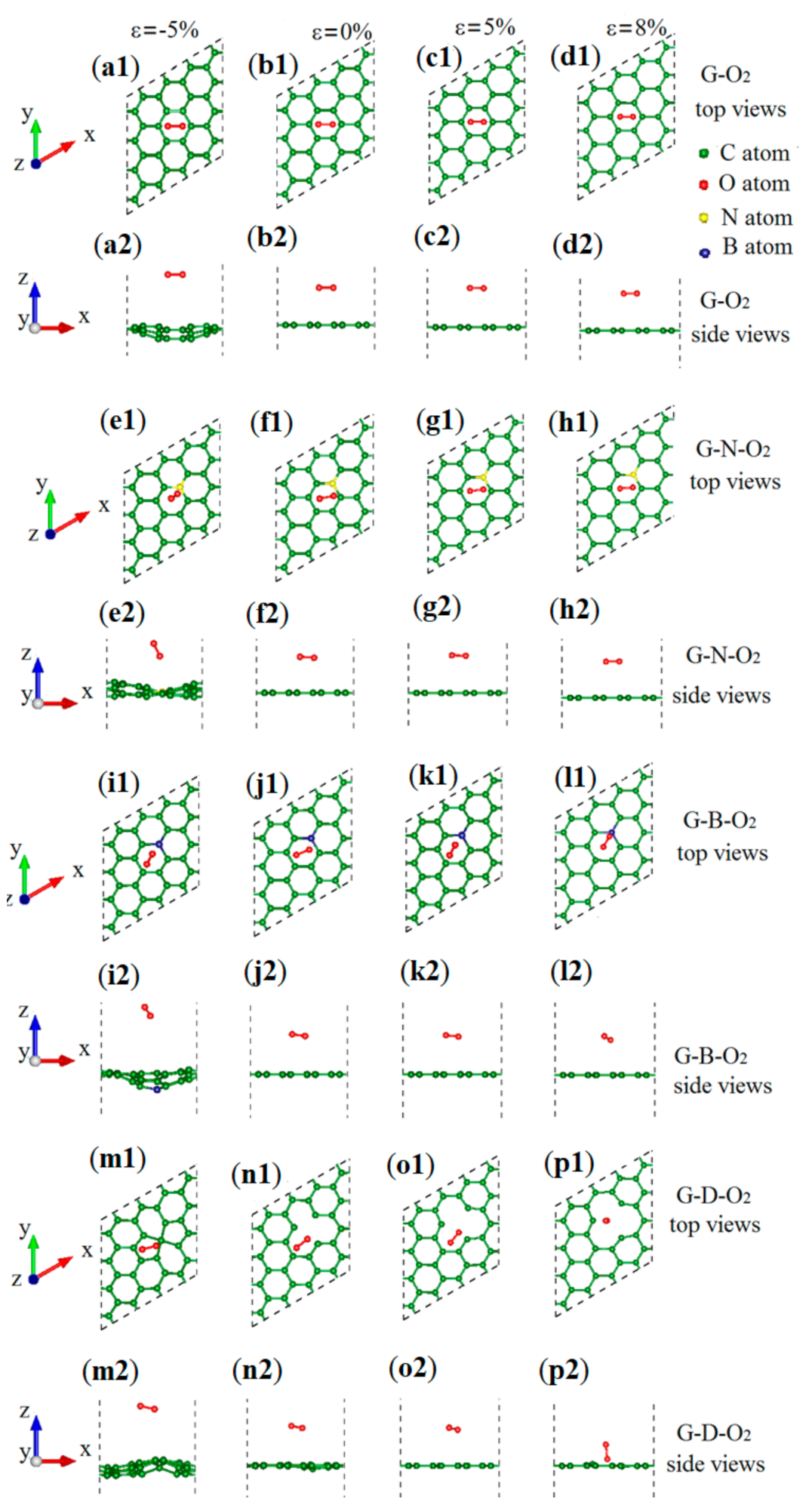
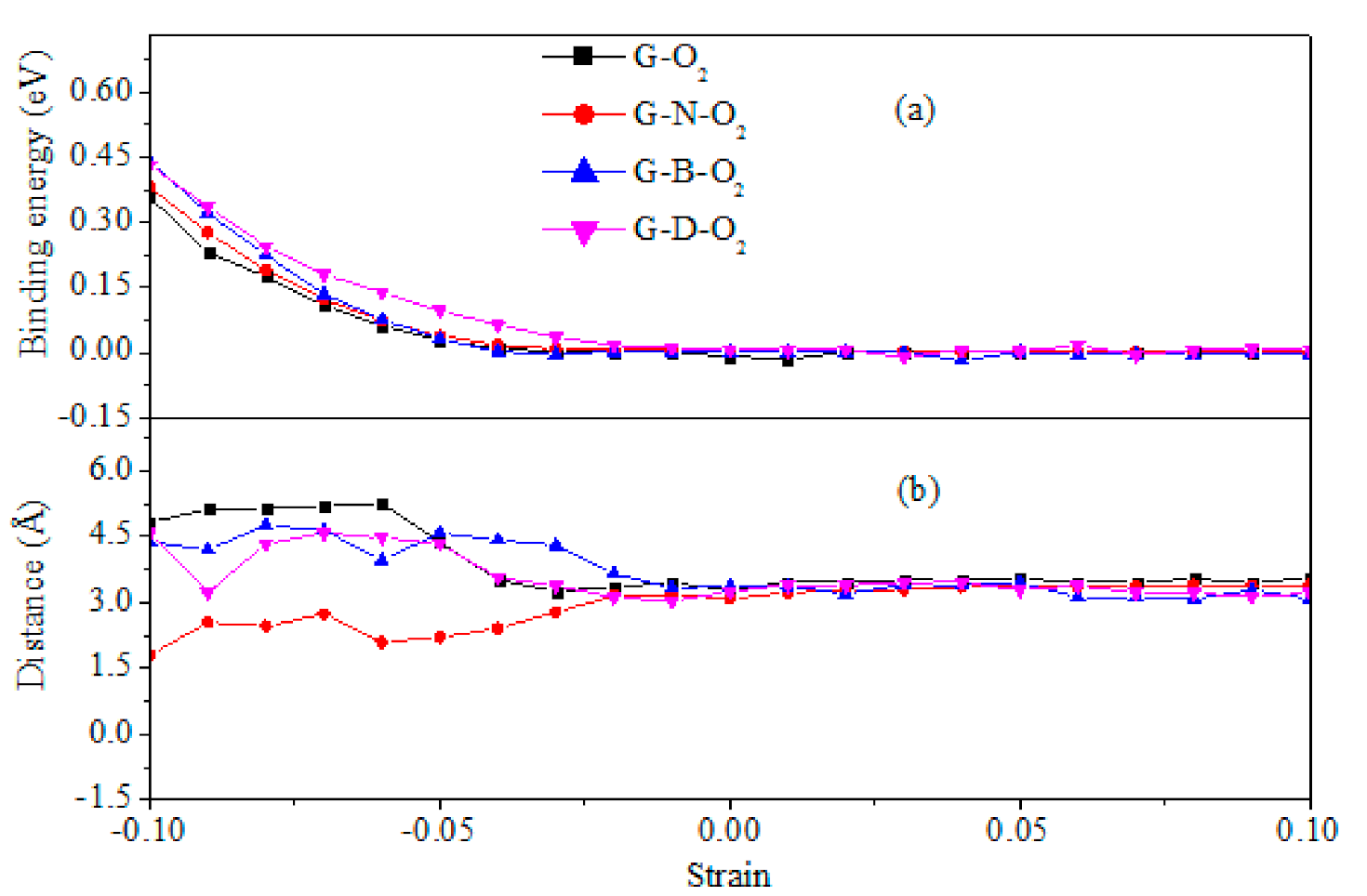
3.1. Geometric Structures and Stability
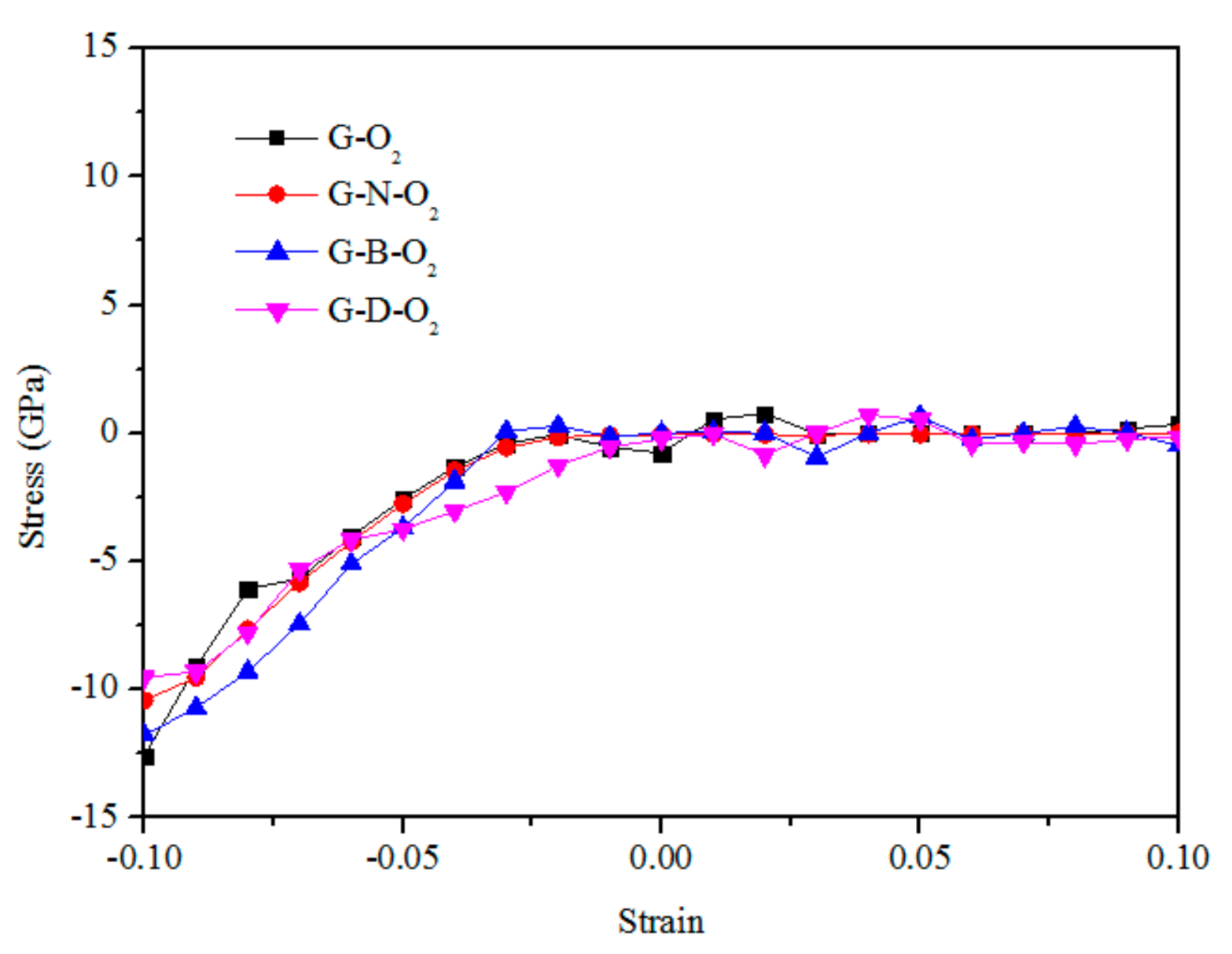
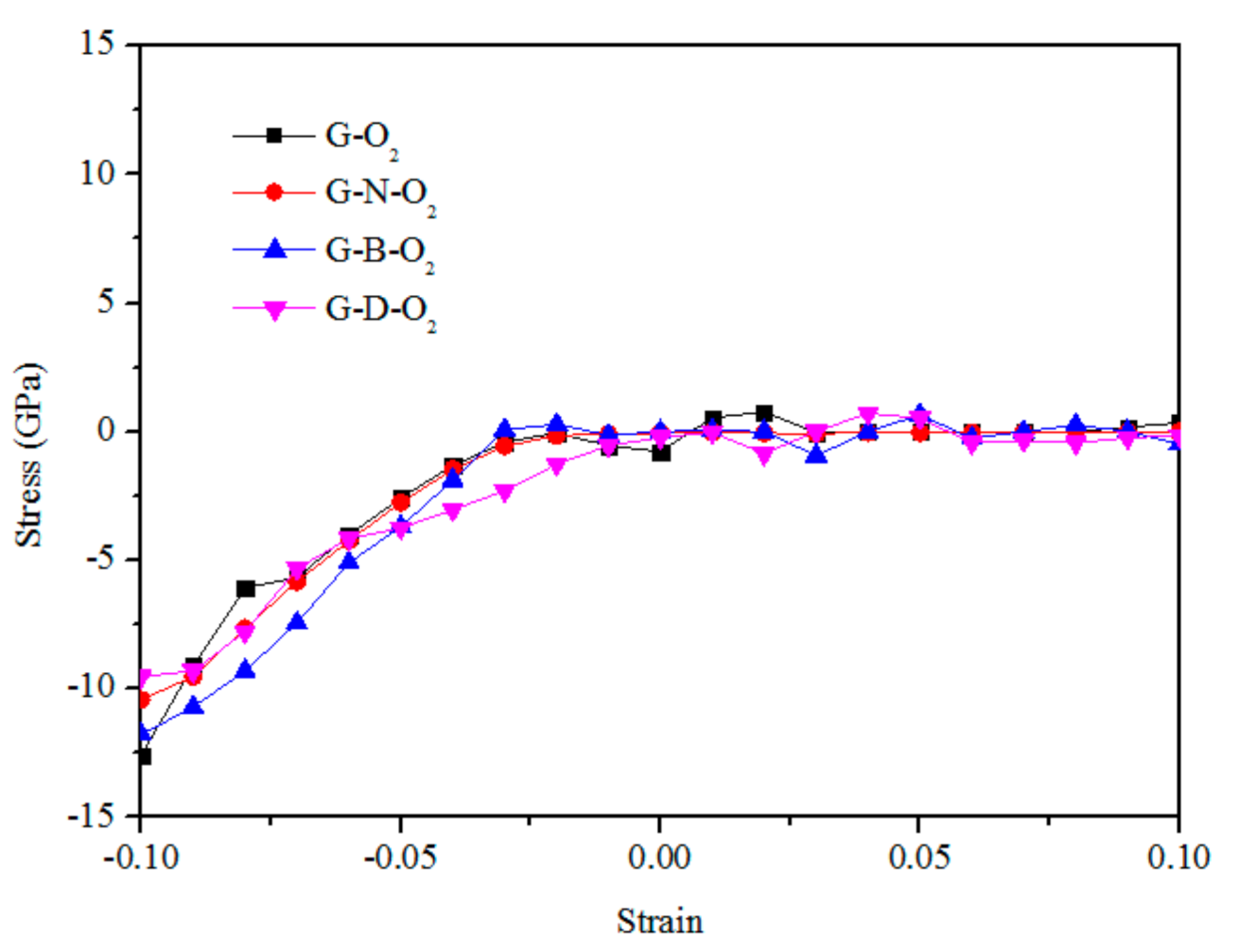
3.2. Mechanical Properties
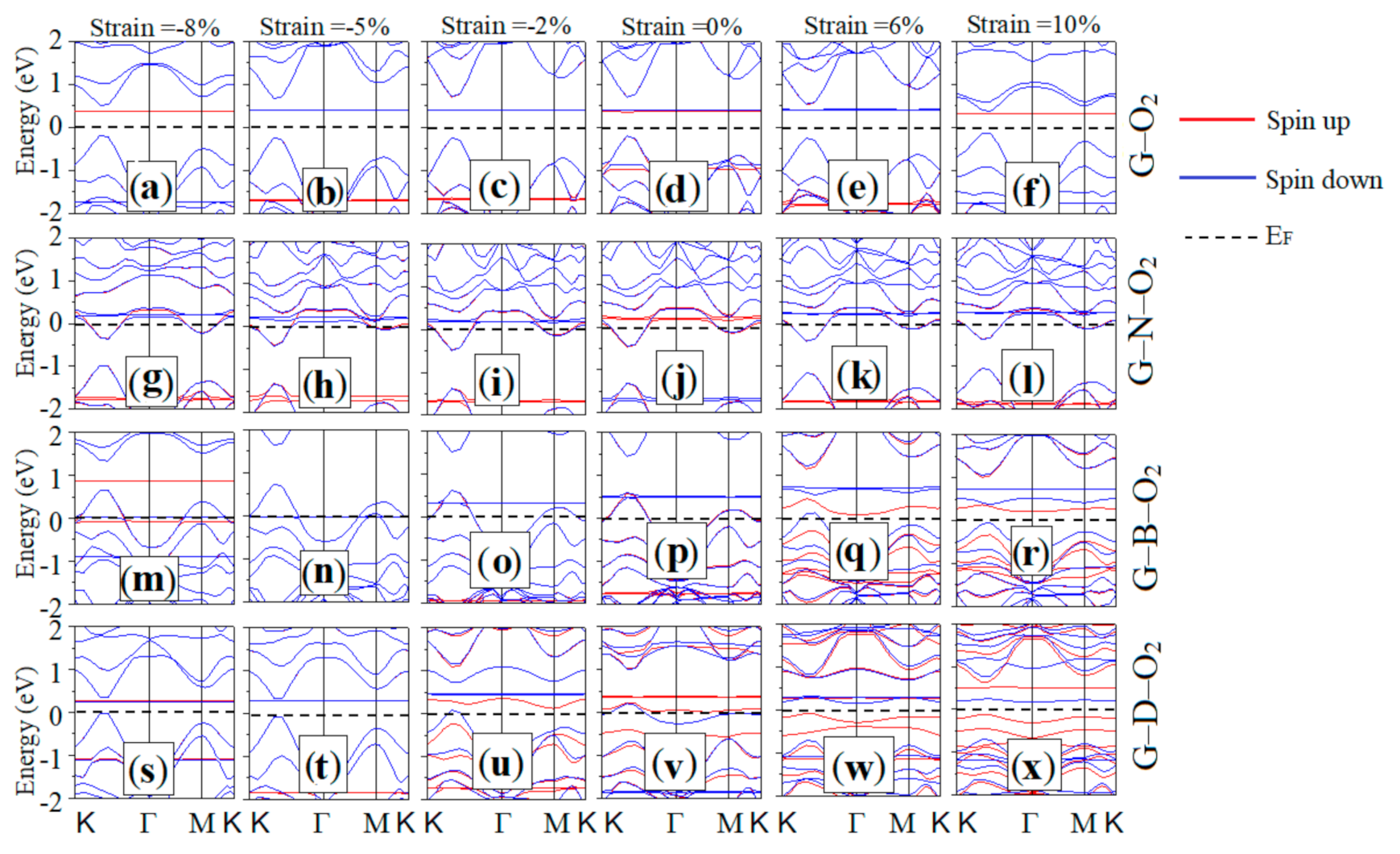
3.3. Electronic Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, S.; Kim, H.; Kim, J.-H.; Yeo, W.-H. Advanced Nanomaterials, Printing Processes, and Applications for Flexible Hybrid Electronics. Materials 2020, 13, 3587. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Bulavskiy, M.O.; Pinakov, D.V.; Chekhova, G.N.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L.G.; Okotrub, A.V. Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials 2020, 13, 3538. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, W.-T.; Cao, S.; Zhou, A.-N.; Zhang, J.-M. First-principles study of transition metal monatomic chains intercalated AA-stacked bilayer graphene nanoribbons. Phys. E Low Dimens. Syst. Nanostruct. 2019, 106, 114–120. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Li, B.; Shao, Z.-G. First-principle investigation of CO and CO2 adsorption on Fe-doped penta-graphene. Appl. Surf. Sci. 2019, 469, 641–646. [Google Scholar] [CrossRef]
- Long, X.J.; Cai, Y.; Jian, W.R.; Wang, L.; Luo, S.N. Acoustic and double elastic shock waves in single-crystal graphene. J. Appl. Phys. 2020, 127, 055101. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.-W. Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials 2020, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Cao, C.; Zhang, B.; Duan, H.; Long, M. First-principles study on the effects of doping and adsorption on the electronic and magnetic properties of diamond nanothreads. Phys. E Low Dimens. Syst. Nanostruct. 2020, 118, 113949. [Google Scholar] [CrossRef]
- Coros, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.-O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Phys. E Low Dimens. Syst. Nanostruct. 2020, 119, 113971. [Google Scholar] [CrossRef]
- Cinchetti, M.; Sturmeit, H.M.; Zamborlini, G.; Cossaro, A.; Verdini, A.; Floreano, L.; D’Incecco, E.; Stredansky, M.; Jugovac, M.; Jugovac, M.; et al. Evaluation of molecular orbital symmetry via oxygen-induced charge transfer quenching at a metal-organic interface. Appl. Surf. Sci. 2020, 504, 144343. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Q.; Cui, Y.; Tan, D.; Bao, X. Growth Mechanism of Graphene on Ru(0001) and O2 Adsorption on the Graphene/Ru(0001) Surface. J. Phys. Chem. C 2009, 113, 8296–8301. [Google Scholar] [CrossRef]
- Kuang, H.; Cheng, Y.; Cui, C.Q.; Jiang, S.P. Carbon Nanotubes-Supported Pt Electrocatalysts for O2 Reduction Reaction—Effect of Number of Nanotube Walls. J. Nanosci. Nanotechnol. 2020, 20, 2736–2745. [Google Scholar] [CrossRef]
- Wang, L.; Zihlmann, S.; Baumgartner, A.; Overbeck, J.; Watanabe, K.; Taniguchi, T.; Makk, P.; Schönenberger, C. In Situ Strain Tuning in hBN-Encapsulated Graphene Electronic Devices. Nano Lett. 2019, 19, 4097–4102. [Google Scholar] [CrossRef]
- Jalaei, M.; Civalek, Ö. A nonlocal strain gradient refined plate theory for dynamic instability of embedded graphene sheet including thermal effects. Compos. Struct. 2019, 220, 209–220. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Sun, F.; Tian, M.; Sun, X.; Xu, T.; Liu, X.; Zhu, S.; Zhang, X.; Qu, L. Stretchable Conductive Fibers of Ultrahigh Tensile Strain and Stable Conductance Enabled by a Worm-Shaped Graphene Microlayer. Nano Lett. 2019, 19, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ng, S.-P.; Ding, N.; Wu, C.-M.L. Strain-induced switch for hydrogen storage in cobalt-decorated nitrogen-doped graphene. Appl. Surf. Sci. 2019, 473, 174–181. [Google Scholar] [CrossRef]
- Lee, U.; Han, Y.; Lee, S.; Kim, J.S.; Lee, Y.H.; Kim, U.J.; Son, H. Time Evolution Studies on Strain and Doping of Graphene Grown on a Copper Substrate Using Raman Spectroscopy. ACS Nano 2019, 14, 919–926. [Google Scholar] [CrossRef]
- Kresse, G. Ab initio molecular dynamics for liquid metals. J. Non-Crystalline Solids 1995, 47, 222–229. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Qu, L.-H.; Yu, J.; Mu, Y.-L.; Fu, X.-L.; Zhong, C.-G.; Min, Y.; Zhou, P.-X.; Zhang, J.-M.; Zou, Y.-Q.; Lu, T.-S. Strain tunable structural, mechanical and electronic properties of monolayer tin dioxides and dichalcogenides SnX2 (X O, S, Se, Te). Mater. Res. Bull. 2019, 119. [Google Scholar] [CrossRef]
- Yang, K.; Zaffran, J.; Yang, B. Fast prediction of oxygen reduction reaction activity on carbon nanotubes with a localized geometric descriptor. Phys. Chem. Chem. Phys. 2020, 22, 890–895. [Google Scholar] [CrossRef]
- Marszalek, P.E.; Greenleaf, W.J.; Li, H.; Oberhauser, A.F.; Fernandez, J.M. Atomic force microscopy captures quantized plastic deformation in gold nanowires. Proc. Natl. Acad. Sci. USA 2000, 97, 6282–6286. [Google Scholar] [CrossRef]
- Radchenko, T.; Tatarenko, V. A statistical-thermodynamic analysis of stably ordered substitutional structures in graphene. Phys. E Low Dimens. Syst. Nanostruct. 2010, 42, 2047–2054. [Google Scholar] [CrossRef]
- Radchenko, T.; Tatarenko, V. Kinetics of atomic ordering in metal-doped graphene. Solid State Sci. 2010, 12, 204–209. [Google Scholar] [CrossRef]
- Radchenko, T.M.; Tatarenko, V.A. Statistical Thermodynamics and Kinetics of Long-Range Order in Metal-Doped Graphene. Solid State Phenom. 2009, 150, 43–72. [Google Scholar] [CrossRef]
- Jia, T.-T.; Fan, X.-Y.; Zheng, M.-M.; Chen, G. Silicene nanomeshes: Bandgap opening by bond symmetry breaking and uniaxial strain. Sci. Rep. 2016, 6, 20971. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Pereira, V.M.; Peres, N.M.R.; Briddon, P.R.; Neto, A.H.C. Strained graphene: Tight-binding and density functional calculations. New J. Phys. 2009, 11, 115002. [Google Scholar] [CrossRef]
- Gong, L.; Xiu, S.L.; Zheng, M.M.; Zhao, P.; Zhang, Z.; Liang, Y.; Chen, G.; Kawazoe, Y. Electronic properties of silicene superlattices: Roles of degenerate perturbation and inversion symmetry breaking. J. Mater. Chem. C 2014, 2, 8773–8779. [Google Scholar] [CrossRef]
- Xiu, S.; Zheng, M.; Zhao, P.; Zhang, Y.; Liu, H.; Li, S.; Chen, G.; Kawazoe, Y. An effective method of tuning conducting properties: First-principles studies on electronic structures of graphene nanomeshes. Carbon 2014, 79, 646–653. [Google Scholar] [CrossRef]
- Marchon, B.; Carrazza, J.; Heinemann, H.; Somorjai, G. TPD and XPS studies of O2, CO2, and H2O adsorption on clean polycrystalline graphite. Carbon 1988, 26, 507–514. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.-H.; Fu, X.-L.; Zhong, C.-G.; Zhou, P.-X.; Zhang, J.-M. Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene. Materials 2020, 13, 4945. https://doi.org/10.3390/ma13214945
Qu L-H, Fu X-L, Zhong C-G, Zhou P-X, Zhang J-M. Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene. Materials. 2020; 13(21):4945. https://doi.org/10.3390/ma13214945
Chicago/Turabian StyleQu, Li-Hua, Xiao-Long Fu, Chong-Gui Zhong, Peng-Xia Zhou, and Jian-Min Zhang. 2020. "Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene" Materials 13, no. 21: 4945. https://doi.org/10.3390/ma13214945
APA StyleQu, L.-H., Fu, X.-L., Zhong, C.-G., Zhou, P.-X., & Zhang, J.-M. (2020). Equibiaxial Strained Oxygen Adsorption on Pristine Graphene, Nitrogen/Boron Doped Graphene, and Defected Graphene. Materials, 13(21), 4945. https://doi.org/10.3390/ma13214945




