Sulfate Attacks on Uncarbonated Fly Ash + Cement Pastes Partially Immersed in Na2SO4 Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparation and Curing
2.3. Partial Sulfate Exposure
2.4. Micro-Analysis
3. Results
3.1. Visual Observation
3.1.1. Effect of Carbonation
3.1.2. Effect of w/c Ratio and Fly Ash Replacement
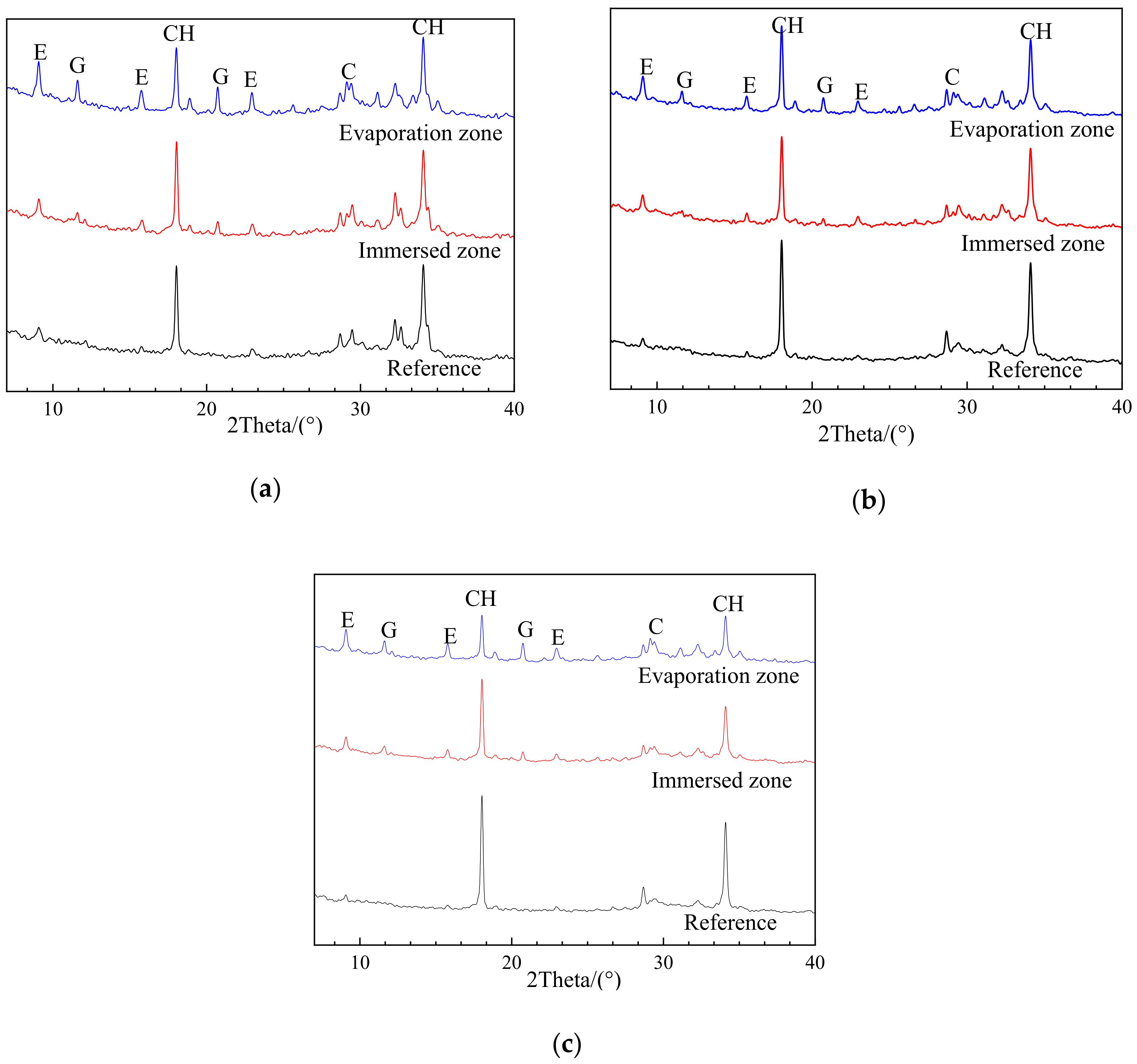
3.2. XRD Characterization on Chemical Components
3.2.1. Effects of Sulfate Exposure
3.2.2. Effects of Exposure Zone
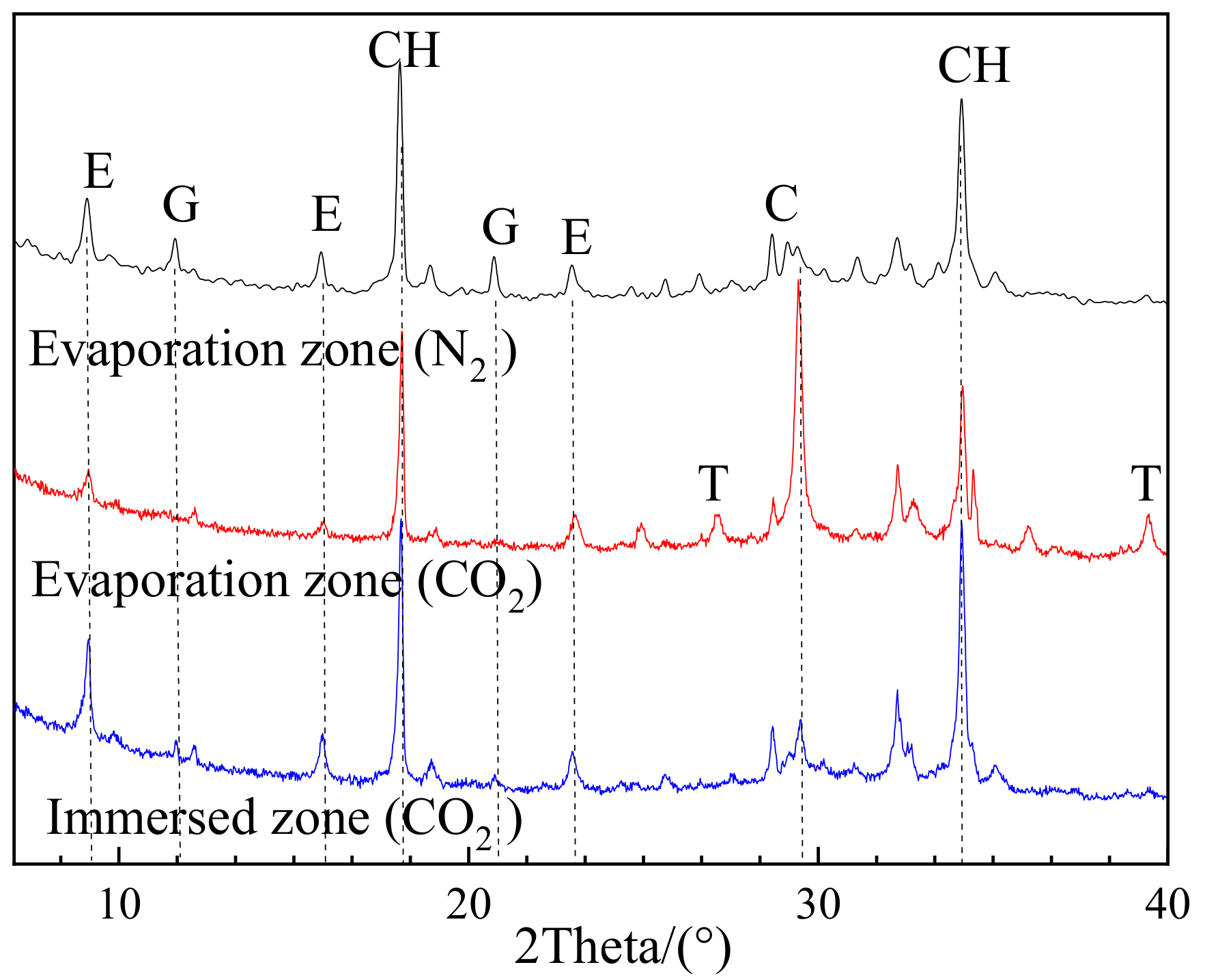
3.2.3. Effects of Carbonation on Deterioration Regime
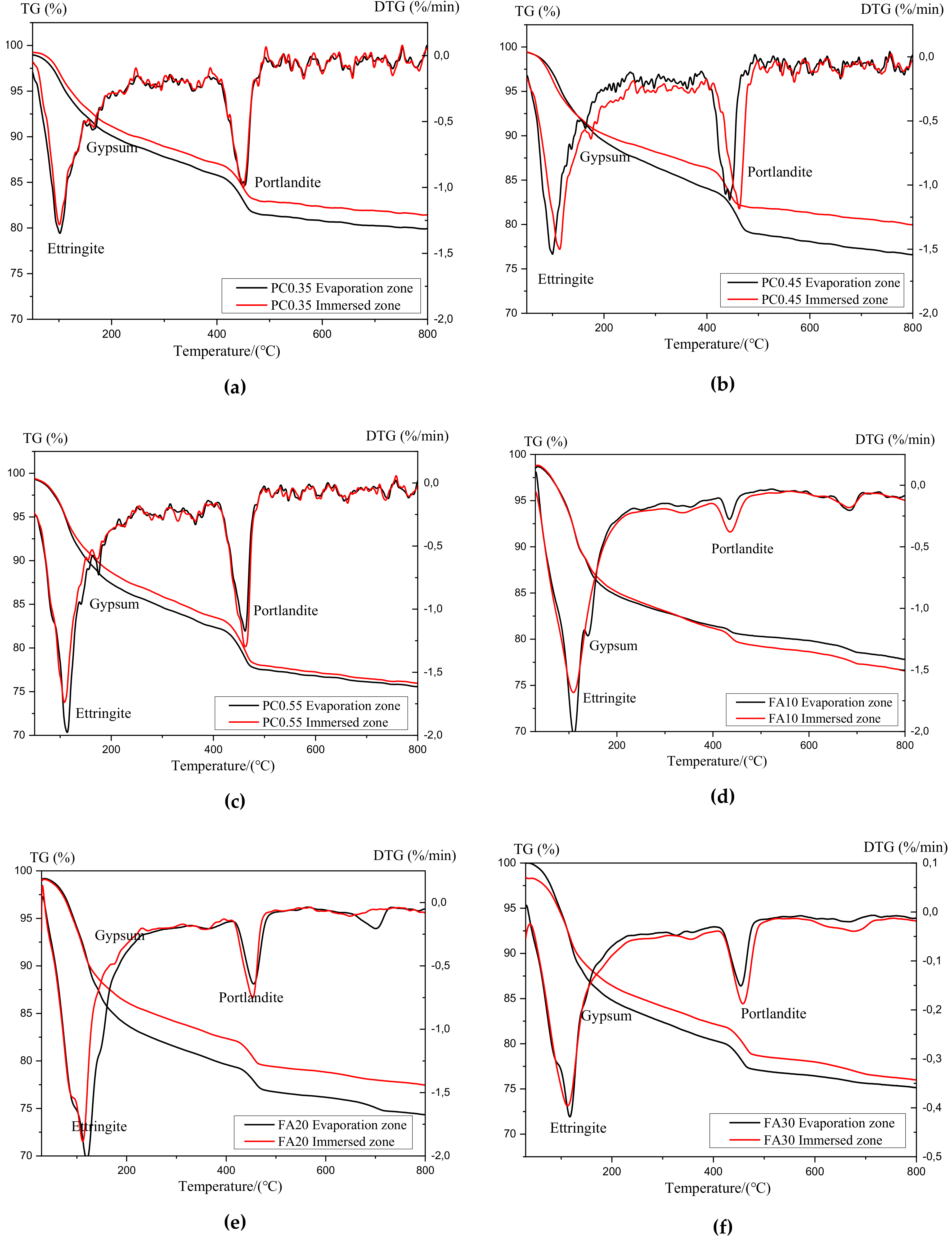
3.3. Thermao-Gravimetric (TG) Analysis of Sulfate Attack Products
3.3.1. Thermogravimetric Curves
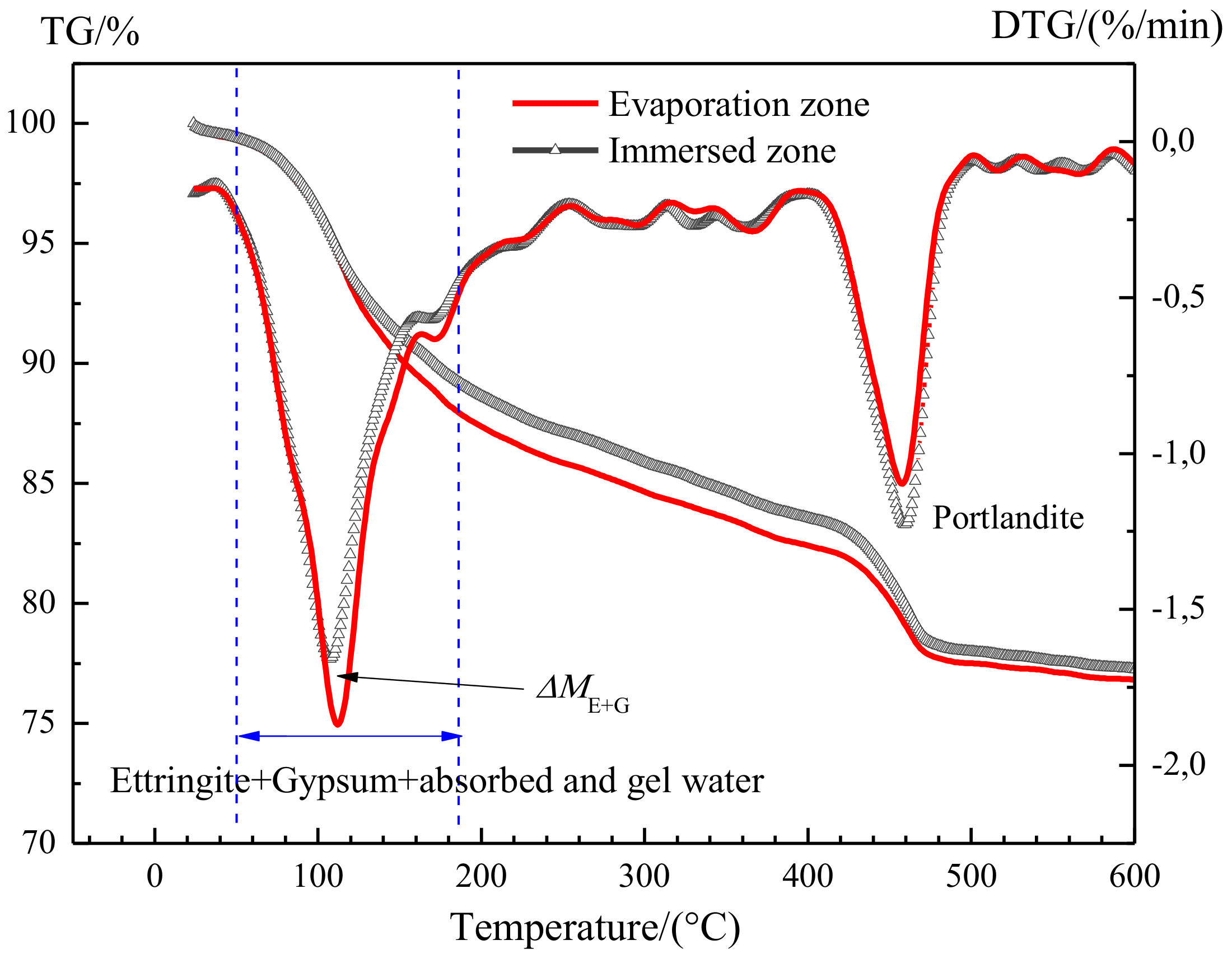
3.3.2. Quantitative Analysis of the Expansive Components
- Before sulfate exposure, all the specimens had been cured in saturated lime water for more than 50 days, thus cement hydration must have been nearly completed. As such, the amount of C-S-H gel in the evaporation and immersion zone should be identical and remain stable, regardless of the following-up curing regime and age.
- During the sulfate exposure, the chemical attack of sodium sulfates would not cause the decomposition of C-S-H gel [13,27]. In fact, sodium sulfates react with Ca(OH)2 to form not only harmful gypsum, but also Na(OH)2. The resulting Na(OH)2 ensures the continuation of high alkalinity in the system, and thus the stability of C-S-H gel. Again, the amount of gel water in the evaporation zone and the immersion zone would remain stable.
- After sulfate exposure, the specimens were immediately moved into a vacuum drying oven containing silica gel to remove any moisture until the start of TG analysis. Therefore, after vacuum drying, the water content in the immersion zone should not be significantly different from that in the evaporation zone.
3.3.3. Effects of w/c Ratios
3.3.4. Effects of Fly Ash Replacements
4. Discussion
5. Conclusions
- For uncarbonated specimens after partial sulfate exposure, chemical sulfate attack products (i.e., ettringite and gypsum) were formed in both the evaporation and immersion zone, while no thenardite was identified in the evaporation zone, implying no physical attack. Moreover, more chemical attack products tend to be generated in the evaporation zone than in the immersed zone. The results confirmed that the chemical attack rather than the physical attack is the deterioration mechanism and is responsible for the more severe damage in the evaporation zone than in the immersed zone;
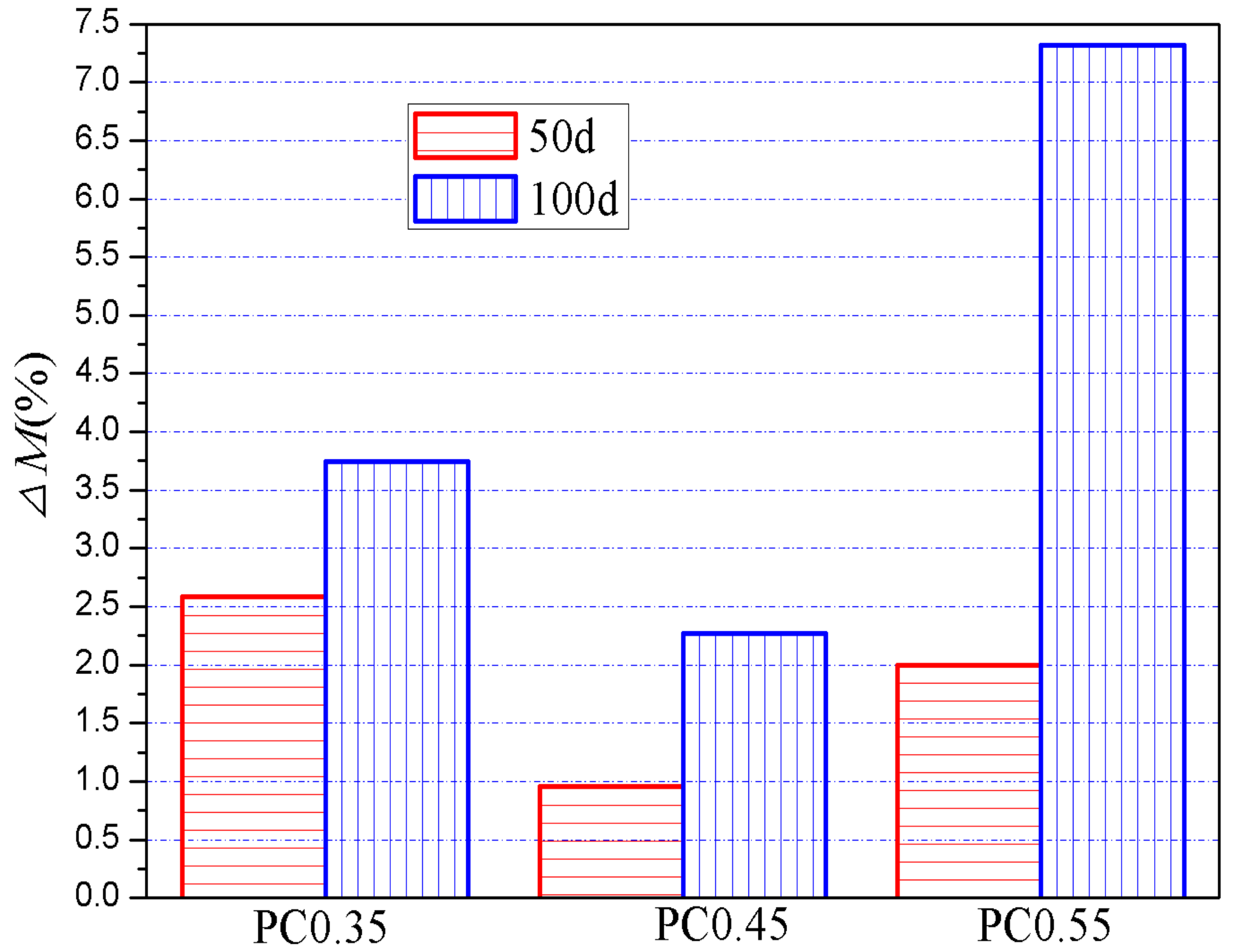
- With the increase in w/c ratios, the sulfate resistance of the evaporation zone first increased and then decreased, and the best performance was achieved by a w/c ratio of 0.45. A possible reason for this phenomenon could be that the pore connectivity dominates the sulfates’ resistance of the evaporation zone when the w/c ratio is higher than 0.45, whereas the pore size governs the sulfate resistance when the w/c is relatively low (e.g., ≤0.45). To verify this viewpoint, it is recommended to further investigate the effects of the w/c on the pore structures and/or absorptivity;
- The incorporation of fly ash resulted in a decrease in sulfate resistance of the evaporation zone. The effect of fly ash on the sulfate attack is related to the w/c used. In this study, a w/c ratio of 0.45 for the control sample was used, with which the pore size dominates the sulfate resistance performance. In this case, the pore size refinement by the fly ash replacement, leading to a higher capillary rise, can be the reason for the decrease in the sulfate resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Deng, D.; De Schutter, G.; Yu, Z. Micro-analysis of “salt weathering” on cement paste. Cem. Concr. Compos. 2011, 33, 179–191. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; De Schutter, G.; Yu, Z. Chemical sulfate attack performance of partially exposed cement and cement+fly ash paste. Constr. Build. Mater. 2012, 28, 230–237. [Google Scholar] [CrossRef]
- Peyvandi, A.; Holmes, D.; Soroushian, P.; Balachandra, A.M. Monitoring of Sulfate Attack in Concrete by Al27 and Si29 MAS NMR Spectroscopy. J. Mater. Civ. Eng. 2015, 27, 04014226. [Google Scholar] [CrossRef]
- Najjar, M.F.; Nehdi, M.; Soliman, A.; Azabi, T. Damage mechanisms of two-stage concrete exposed to chemical and physical sulfate attack. Constr. Build. Mater. 2017, 137, 141–152. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.-P.; Metalssi, O.O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Benavente, D.; Del Cura, M.G.; Bernabéu, A.; Ordóñez, S. Quantification of salt weathering in porous stones using an experimental continuous partial immersion method. Eng. Geol. 2001, 59, 313–325. [Google Scholar] [CrossRef]
- Flatt, R.J.; Caruso, F.; Sanchez, A.M.A.; Scherer, G.W. Chemo-mechanics of salt damage in stone. Nat. Commun. 2014, 5, 4823. [Google Scholar] [CrossRef]
- ACI (American Concrete institute) Committee 201 Physical salt attack. Guide to Durable Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2016. [Google Scholar]
- Irassar, E.; Di Maio, A.; Batic, O. Sulfate attack on concrete with mineral admixtures. Cem. Concr. Res. 1996, 26, 113–123. [Google Scholar] [CrossRef]
- Nehdi, M.; Hayek, M. Behavior of blended cement mortars exposed to sulfate solutions cycling in relative humidity. Cem. Concr. Res. 2005, 35, 731–742. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, X.; Zhao, L.; Zhao, M.; Liu, Y.; Zeng, X. Effect of nanosilica on sulfate resistance of cement mortar under partial immersion. Constr. Build. Mater. 2020, 231, 117180. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Deng, D.; Xie, Y.; Long, G.; Tang, X. Physical sulfate attack on concrete lining–A field case analysis. Case Stud. Constr. Mater. 2017, 6, 206–212. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Deng, D.; De Schutter, G.; Hou, L. The role of Ca(OH)2 in sulfate salt weathering of ordinary concrete. Constr. Build. Mater. 2016, 123, 127–134. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Hou, L.; Deng, D. Effect of carbonation on physical sulfate attack on concrete by Na2SO4. Constr. Build. Mater. 2018, 193, 211–220. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Impact of accelerated carbonation on OPC cement paste blended with fly ash. Cem. Concr. Res. 2015, 67, 226–236. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F. Thermal stability and decomposition mechanisms of ettringite at <120 °C. Cem. Concr. Res. 2001, 31, 1333–1339. [Google Scholar] [CrossRef]
- Elbeyli, I.Y.; Derun, E.M.; Gülen, J.; Pişkin, S. Thermal analysis of borogypsum and its effects on the physical properties of Portland cement. Cem. Concr. Res. 2003, 33, 1729–1735. [Google Scholar] [CrossRef]
- Lothenbach, B.; Wieland, E. A thermodynamic approach to the hydration of sulphate-resisting Portland cement. Waste Manag. 2006, 26, 706–719. [Google Scholar] [CrossRef]
- Stepkowska, E.; Blanes, J.; Franco, F.; Real, C.; Pérez-Rodríguez, J. Phase transformation on heating of an aged cement paste. Thermochim. Acta 2004, 420, 79–87. [Google Scholar] [CrossRef]
- Ye, G.; Liu, X.; De Schutter, G.; Poppe, A.-M.; Taerwe, L. Influence of limestone powder used as filler in SCC on hydration and microstructure of cement pastes. Cem. Concr. Compos. 2007, 29, 94–102. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Rec. J. Transp. Res. Board 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulfate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attack research — Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Ma, X.; Çopuroğlu, O.; Schlangen, E.; Han, N.; Xing, F. Expansion and degradation of cement paste in sodium sulfate solutions. Constr. Build. Mater. 2018, 158, 410–422. [Google Scholar] [CrossRef]
- Beltrame, N.A.M.; Da Luz, C.A.; Perardt, M.; Hooton, R.D. Alkali activated cement made from blast furnace slag generated by charcoal: Resistance to attack by sodium and magnesium sulfates. Constr. Build. Mater. 2020, 238, 117710. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.; Qi, B.; Shen, D. Deterioration mechanism of plain and blended cement mortars partially exposed to sulfate attack. Constr. Build. Mater. 2017, 154, 849–856. [Google Scholar] [CrossRef]





| CaO | SiO2 | Fe2O3 | MgO | Al2O3 | SO3 | TiO2 | Na2O | K2O | |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 62.68 | 19.62 | 2.961 | 1.89 | 4.37 | 2.06 | 0.236 | 0.11 | 0.711 |
| Fly ash | 3.59 | 53.76 | 6.371 | 0.568 | 31.58 | 0.761 | 1.09 | 0.499 | 1.26 |
| Mixture Code | P·I Cement | Fly Ash | W/C | Exposure Days |
|---|---|---|---|---|
| PC0.35 | 100 | - | 0.35 | 50, 100 |
| PC0.45 | 100 | - | 0.45 | 50, 100 |
| PC0.55 | 100 | - | 0.55 | 50, 100 |
| FA10% | 90 | 10 | 0.45 | 50, 100 |
| FA20% | 80 | 20 | 0.45 | 50, 100 |
| FA30% | 70 | 30 | 0.45 | 50, 100 |
| Specimen | Zone | ME + G+ W (%) | MCH (%) | ΔME + G (%) |
|---|---|---|---|---|
| PC0.35 | EZ | 7.34 | 9.70 | 2.57 |
| IZ | 4.77 | 12.60 | ||
| PC0.45 | EZ | 5.91 | 8.10 | 0.96 |
| IZ | 4.95 | 18.60 | ||
| PC0.55 | EZ | 10.5 | 7.82 | 1.93 |
| IZ | 8.57 | 11.75 | ||
| FA10 | EZ | 11.11 | 7.02 | 1.21 |
| IZ | 9.9 | 9.95 | ||
| FA20 | EZ | 11.73 | 7.11 | 1.71 |
| IZ | 10.02 | 7.36 | ||
| FA30 | EZ | 9.94 | 11.90 | 2.00 |
| IZ | 7.94 | 15.80 |
| Specimen | Zone | ME + G + W (%) | MCH (%) | ΔME + G (%) |
|---|---|---|---|---|
| PC0.35 | EZ | 7.60 | 7.70 | 3.75 |
| IZ | 3.85 | 10.10 | ||
| PC0.45 | EZ | 6.43 | 7.80 | 2.28 |
| IZ | 4.15 | 12.10 | ||
| PC0.55 | EZ | 12.30 | 5.78 | 3.09 |
| IZ | 9.21 | 6.88 | ||
| FA10 | EZ | 12.12 | 3.81 | 3.26 |
| IZ | 8.86 | 5.42 | ||
| FA20 | EZ | 13.57 | 3.36 | 4.19 |
| IZ | 9.38 | 5.14 | ||
| FA30 | EZ | 13.57 | 8.10 | 7.32 |
| IZ | 6.25 | 15.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Pei, M.; Li, Y.; Yuan, Q. Sulfate Attacks on Uncarbonated Fly Ash + Cement Pastes Partially Immersed in Na2SO4 Solution. Materials 2020, 13, 4920. https://doi.org/10.3390/ma13214920
Liu Z, Pei M, Li Y, Yuan Q. Sulfate Attacks on Uncarbonated Fly Ash + Cement Pastes Partially Immersed in Na2SO4 Solution. Materials. 2020; 13(21):4920. https://doi.org/10.3390/ma13214920
Chicago/Turabian StyleLiu, Zanqun, Min Pei, Yuelin Li, and Qiang Yuan. 2020. "Sulfate Attacks on Uncarbonated Fly Ash + Cement Pastes Partially Immersed in Na2SO4 Solution" Materials 13, no. 21: 4920. https://doi.org/10.3390/ma13214920
APA StyleLiu, Z., Pei, M., Li, Y., & Yuan, Q. (2020). Sulfate Attacks on Uncarbonated Fly Ash + Cement Pastes Partially Immersed in Na2SO4 Solution. Materials, 13(21), 4920. https://doi.org/10.3390/ma13214920





