Efficacy of a Nickel-Titanium Ultrasonic Instrument for Biofilm Removal in a Simulated Complex Root Canal
Abstract
1. Introduction
2. Materials and Methods
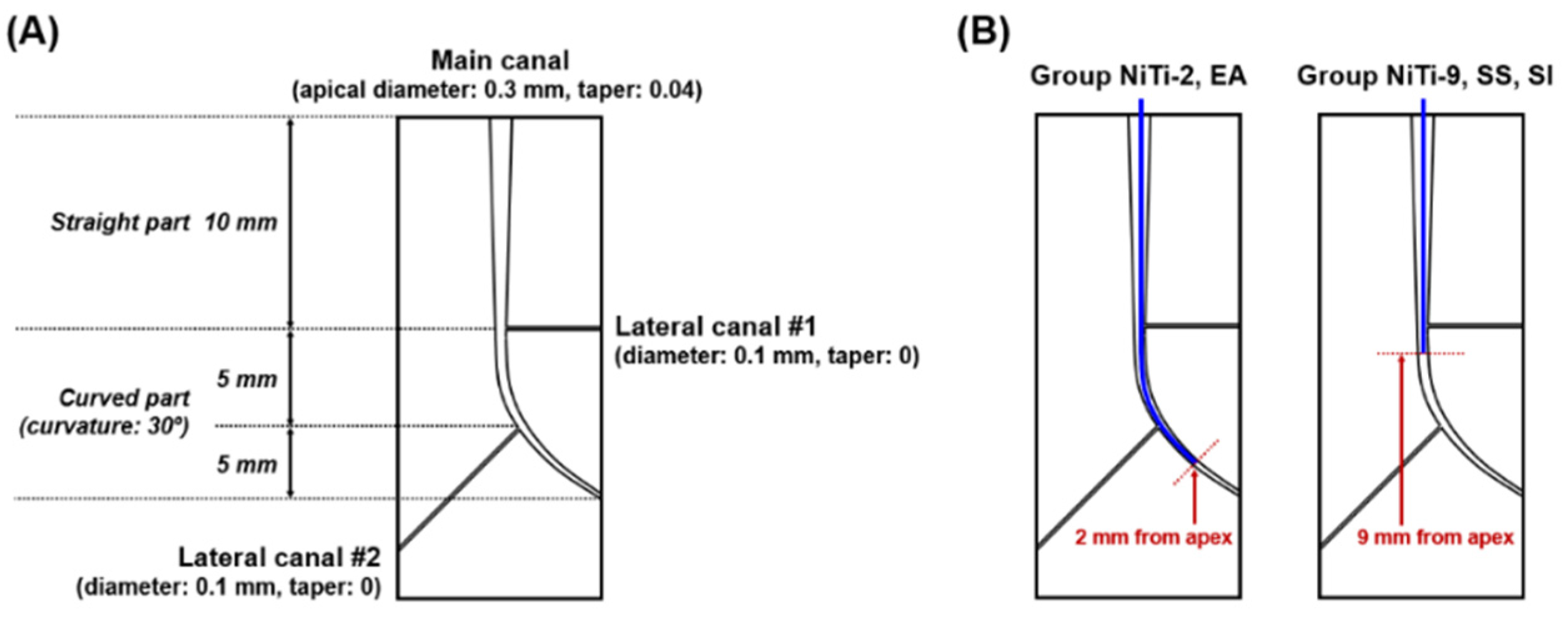
2.1. Fabrication of Root Canal Models
2.2. Formation of E. faecalis Biofilm within the Root Canal Model
2.3. Irrigation Procedures
2.3.1. SI Group (Control)
2.3.2. SS Group
2.3.3. NiTi-9 Group
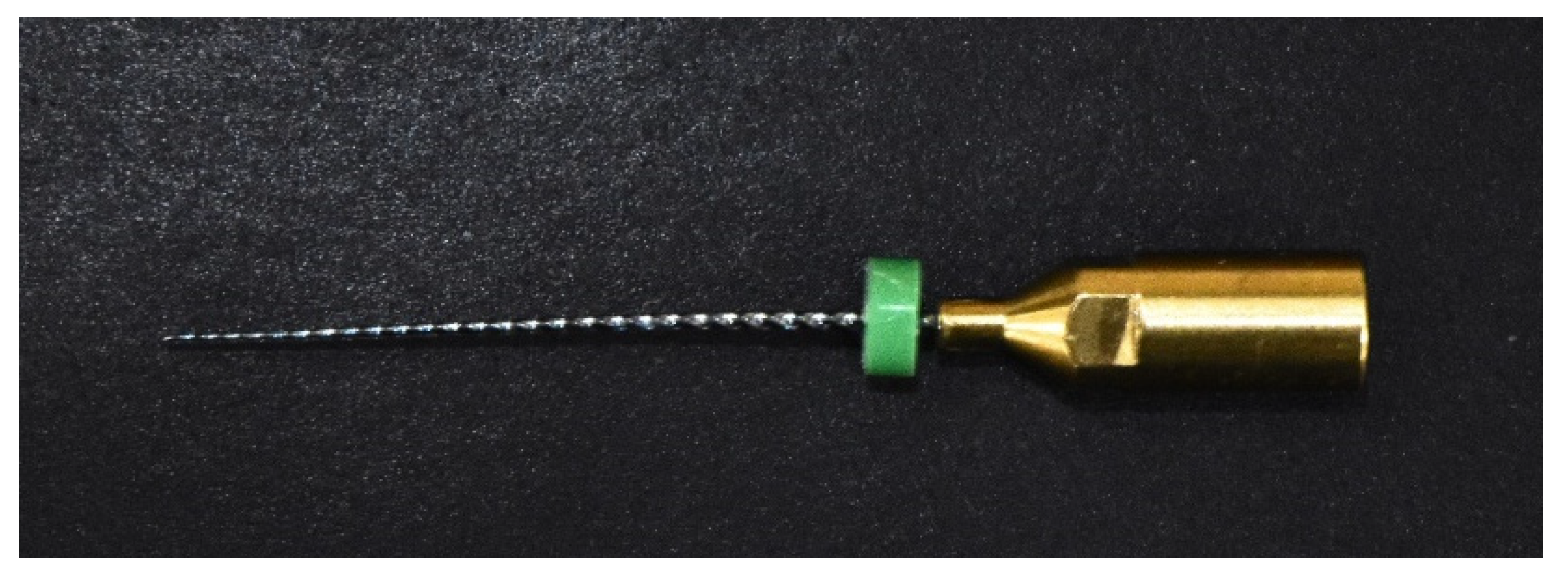
2.3.4. NiTi-2 Group
2.3.5. EA Group
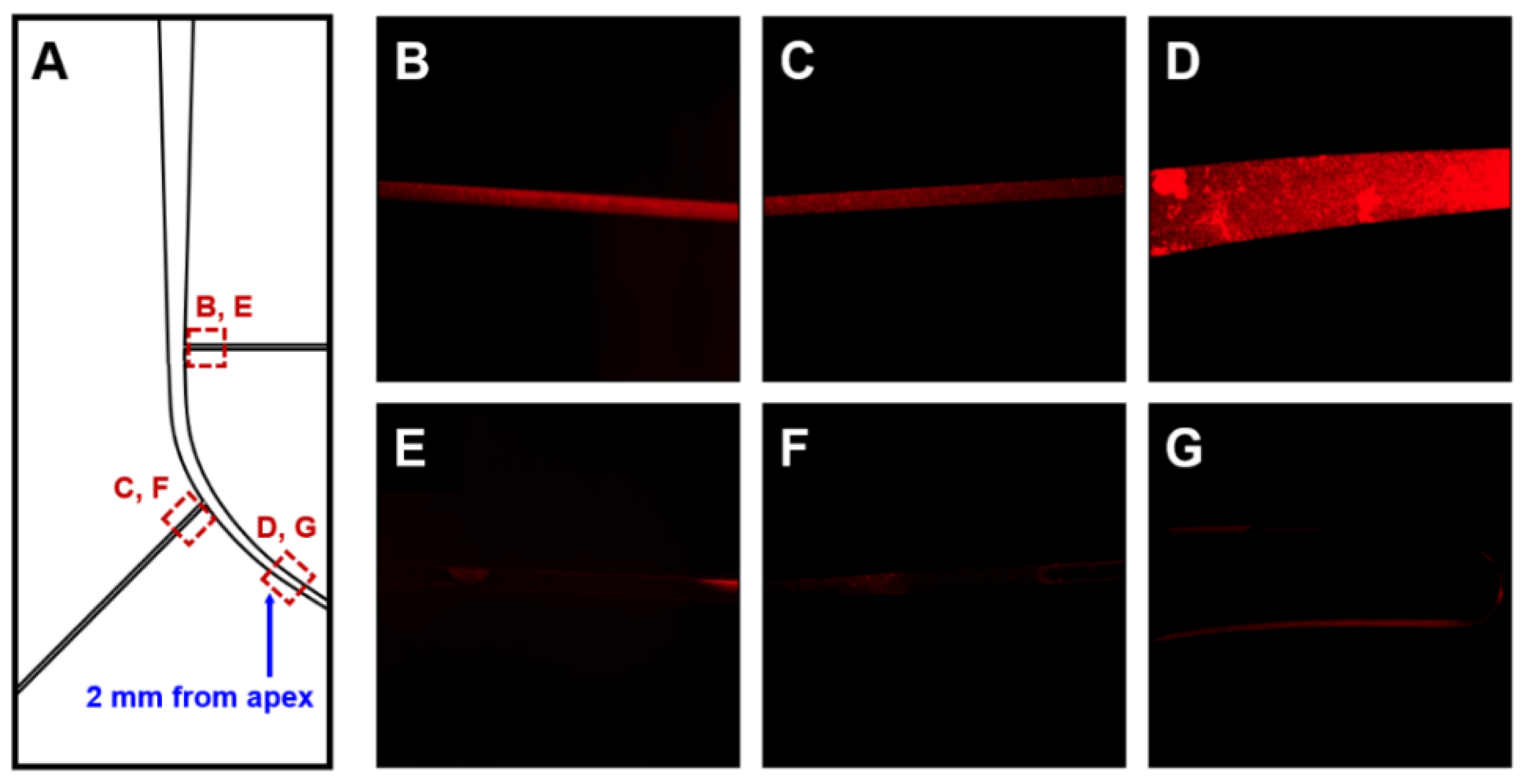
2.4. Effects of Irrigation on Removal of E. faecalis Biofilm
2.5. Statistical Analysis
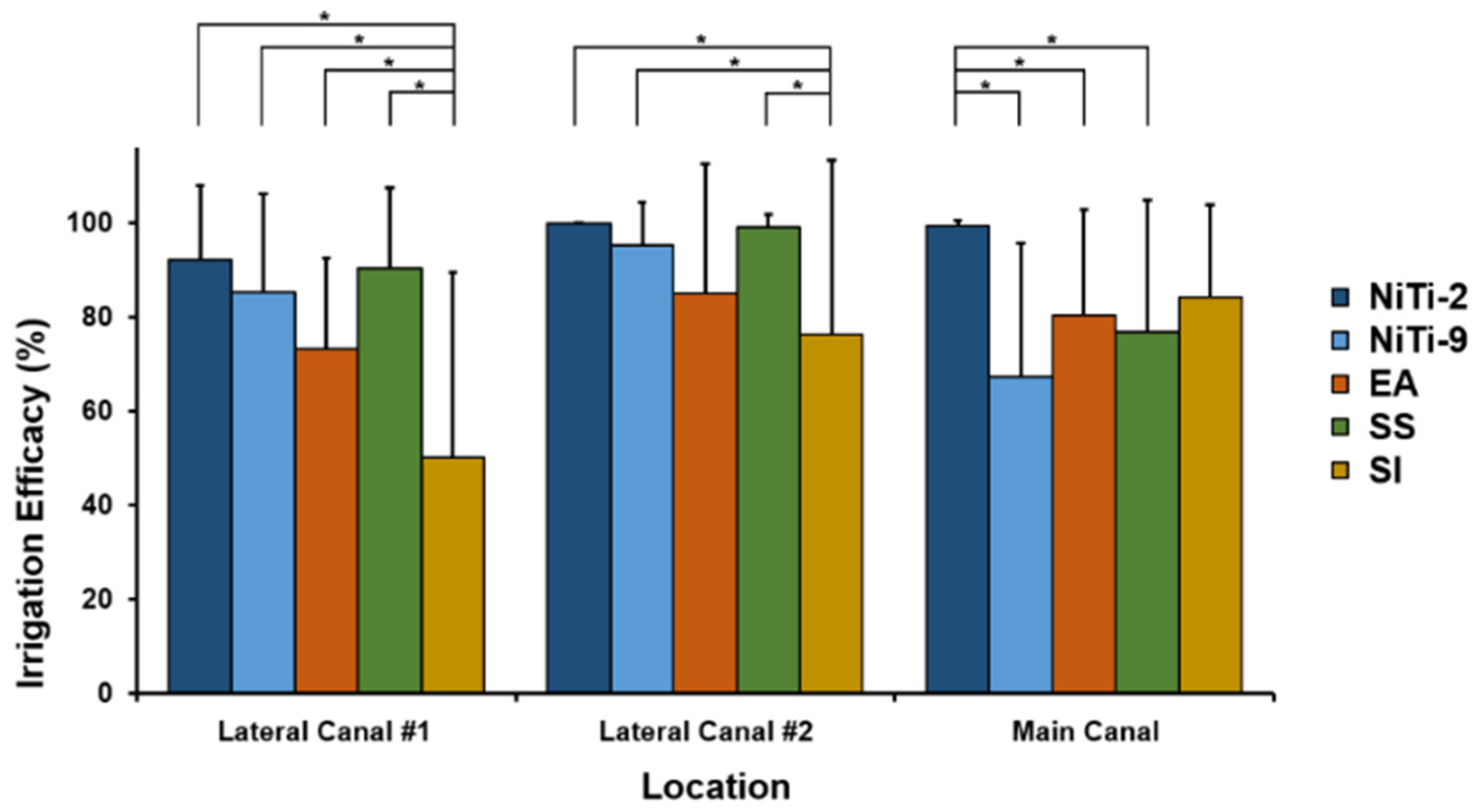
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gutarts, R.; Nusstein, J.; Reader, A.; Beck, M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J. Endod. 2005, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Burleson, A.; Nusstein, J.; Reader, A.; Beck, M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J. Endod. 2007, 33, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.; Maki, J. An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. J. Endod. 2009, 35, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Pacifici, A.; Zanza, A.; Giudice, A.D.; Reda, R.; Testarelli, L.; Gambarini, G.; Pacifici, L. Assessment of Real-Time Operative Torque during Nickel–Titanium Instrumentation with Different Lubricants. Appl. Sci. 2020, 10, 6201. [Google Scholar] [CrossRef]
- Ram, Z. Effectiveness of root canal irrigation. Oral Surg. Oral Med. Oral Pathol. 1977, 44, 306–312. [Google Scholar] [CrossRef]
- Tay, F.R.; Gu, L.S.; Schoeffel, G.J.; Wimmer, C.; Susin, L.; Zhang, K.; Arun, S.N.; Kim, J.; Looney, S.W.; Pashley, D.H. Effect of vapor lock on root canal debridement by using a side-vented needle for positive-pressure irrigant delivery. J. Endod. 2010, 36, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Bago, I.; Plečko, V.; Gabrić Pandurić, D.; Schauperl, Z.; Baraba, A.; Anić, I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int. Endod. J. 2013, 46, 339–347. [Google Scholar] [CrossRef]
- Mozo, S.; Llena, C.; Forner, L. Review of ultrasonic irrigation in endodontics: Increasing action of irrigating solutions. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e512. [Google Scholar] [CrossRef]
- Ahmad, M.; Ford, T.R.P.; Crum, L.A. Ultrasonic debridement of root canals: Acoustic streaming and its possible role. J. Endod. 1987, 13, 490–499. [Google Scholar] [CrossRef]
- Ahmad, M.; Ford, T.P.; Crum, L.; Walton, A. Ultrasonic debridement of root canals: Acoustic cavitation and its relevance. J. Endod. 1988, 14, 486–493. [Google Scholar] [CrossRef]
- Roy, R.; Ahmad, M.; Crum, L. Physical mechanisms governing the hydrodynamic response of an oscillating ultrasonic file. Int. Endod. J. 1994, 27, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.N.; Pinto, E.B.; Galo, R.; Falci, S.G.M.; Mesquita, A.T. Passive ultrasonic irrigation in root canal: Systematic review and meta-analysis. Acta Odontol. Scand. 2019, 77, 55–60. [Google Scholar] [CrossRef]
- Merino, A.; Estevez, R.; de Gregorio, C.; Cohenca, N. The effect of different taper preparations on the ability of sonic and passive ultrasonic irrigation to reach the working length in curved canals. Int. Endod. J. 2013, 46, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.M.; Verhaagen, B.; Versluis, M.; van der Sluis, L.W. Evaluation of a sonic device designed to activate irrigant in the root canal. J. Endod. 2010, 36, 143–146. [Google Scholar] [CrossRef]
- Castelo-Baz, P.; Martín-Biedma, B.; Cantatore, G.; Ruíz-Piñón, M.; Bahillo, J.; Rivas-Mundiña, B.; Varela-Patiño, P. In vitro comparison of passive and continuous ultrasonic irrigation in simulated lateral canals of extracted teeth. J. Endod. 2012, 38, 688–691. [Google Scholar] [CrossRef]
- Conde, A.J.; Estevez, R.; Loroño, G.; Valencia de Pablo, Ó.; Rossi-Fedele, G.; Cisneros, R. Effect of sonic and ultrasonic activation on organic tissue dissolution from simulated grooves in root canals using sodium hypochlorite and EDTA. Int. Endod. J. 2017, 50, 976–982. [Google Scholar] [CrossRef]
- Duque, J.A.; Duarte, M.A.; Canali, L.C.; Zancan, R.F.; Vivan, R.R.; Bernardes, R.A.; Bramante, C.M. Comparative Effectiveness of New Mechanical Irrigant Agitating Devices for Debris Removal from the Canal and Isthmus of Mesial Roots of Mandibular Molars. J. Endod. 2017, 43, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Verhaagen, B.; Walmsley, A.D.; Versluis, M.; van der Sluis, L.W. Measurement and visualization of file-to-wall contact during ultrasonically activated irrigation in simulated canals. Int. Endod. J. 2013, 46, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.S.; Cunha, R.S.; da Silveira Bueno, C.E.; Pelegrine, R.A.; Fontana, C.E.; de Martin, A.S. Investigation of the Efficacy of Passive Ultrasonic Irrigation Versus Irrigation with Reciprocating Activation: An Environmental Scanning Electron Microscopic Study. J. Endod. 2016, 42, 659–663. [Google Scholar] [CrossRef]
- de Gregorio, C.; Estevez, R.; Cisneros, R.; Heilborn, C.; Cohenca, N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: An in vitro study. J. Endod. 2009, 35, 891–895. [Google Scholar] [CrossRef]
- Crozeta, B.M.; Chaves de Souza, L.; Correa Silva-Sousa, Y.T.; Sousa-Neto, M.D.; Jaramillo, D.E.; Silva, R.M. Evaluation of Passive Ultrasonic Irrigation and GentleWave System as Adjuvants in Endodontic Retreatment. J. Endod. 2020, 46, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Mohmmed, S.A.; Vianna, M.E.; Penny, M.R.; Hilton, S.T.; Mordan, N.; Knowles, J.C. A novel experimental approach to investigate the effect of different agitation methods using sodium hypochlorite as an irrigant on the rate of bacterial biofilm removal from the wall of a simulated root canal model. Dent. Mater. 2016, 32, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, S.; Wang, S.; Luo, C.; Hou, B. The evaluation of E. faecalis colonies dissolution ability of sodium hypochlorite in microenvironment by a novel device. Biomed. Microdevices 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Mohmmed, S.A.; Vianna, M.E.; Penny, M.R.; Hilton, S.T.; Mordan, N.J.; Knowles, J.C. Investigations into in situ Enterococcus faecalis biofilm removal by passive and active sodium hypochlorite irrigation delivered into the lateral canal of a simulated root canal model. Int. Endod. J. 2018, 51, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Swimberghe, R.; Coenye, T.; De Moor, R.; Meire, M. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef]
- Neelakantan, P.; Cheng, C.; Mohanraj, R.; Sriraman, P.; Subbarao, C.; Sharma, S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er: YAG laser in vitro. Int. Endod. J. 2015, 48, 602–610. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Awan, K.H.; Javed, F. Bactericidal efficacy of photodynamic therapy against Enterococcus faecalis in infected root canals: A systematic literature review. Photodiagn. Photodyn. Ther. 2013, 10, 632–643. [Google Scholar] [CrossRef]
- Zupanc, J.; Vahdat-Pajouh, N.; Schäfer, E. New thermomechanically treated NiTi alloys–a review. Int. Endod. J. 2018, 51, 1088–1103. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Di Nardo, D.; Gambarini, G.; Miccoli, G.; Di Carlo, S.; Iannarilli, G.; Lauria, G.; Seracchiani, M.; Khrenova, T.; Bossù, M.; Testarelli, L. Sonic vs Ultrasonic activation of sodium hypoclorite for root canal treatments. In vitro assessment of debris removal from main and lateral canals. G. Ital. Endod. 2020, 34. [Google Scholar] [CrossRef]
- Ahmad, M.; Roy, R.A. Some observations on the breakage of ultrasonic files driven piezoelectrically. Endod. Dent. Traumatol. 1994, 10, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kwak, S.W.; Ha, J.-H.; Kim, H.-C. Ex-Vivo Comparison of Torsional Stress on Nickel–Titanium Instruments Activated by Continuous Rotation or Adaptive Motion. Materials 2020, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-H.; Kim, S.K.; Cohenca, N.; Kim, H.-C. Effect of R-phase heat treatment on torsional resistance and cyclic fatigue fracture. J. Endod. 2013, 39, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Vanoni-Heineken, I.; Hecker, H.; Weiger, R. Curved versus straight root canals: The benefit of activated irrigation techniques on dentin debris removal. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 529–534. [Google Scholar] [CrossRef]
- De Gregorio, C.; Estevez, R.; Cisneros, R.; Paranjpe, A.; Cohenca, N. Efficacy of different irrigation and activation systems on the penetration of sodium hypochlorite into simulated lateral canals and up to working length: An in vitro study. J. Endod. 2010, 36, 1216–1221. [Google Scholar] [CrossRef]
- Ahmad, M.; Pitt Ford, T.R.; Crum, L.A. Ultrasonic debridement of root canals: An insight into the mechanisms involved. J. Endod. 1987, 13, 93–101. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61-62, 907–914. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Lopes, H.P. Patterns of microbial colonization in primary root canal infections. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 174–178. [Google Scholar] [CrossRef]
- Layton, G.; Wu, W.I.; Selvaganapathy, P.R.; Friedman, S.; Kishen, A. Fluid Dynamics and Biofilm Removal Generated by Syringe-delivered and 2 Ultrasonic-assisted Irrigation Methods: A Novel Experimental Approach. J. Endod. 2015, 41, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Mohmmed, S.A.; Vianna, M.E.; Penny, M.R.; Hilton, S.T.; Knowles, J.C. The effect of sodium hypochlorite concentration and irrigation needle extension on biofilm removal from a simulated root canal model. Aust. Endod. J. 2017, 43, 102–109. [Google Scholar] [CrossRef]
- Pereira, T.C.; Dijkstra, R.J.B.; Petridis, X.; van der Meer, W.J.; Sharma, P.K.; de Andrade, F.B.; van der Sluis, L.W.M. The influence of time and irrigant refreshment on biofilm removal from lateral morphological features of simulated root canals. Int. Endod. J. 2020. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Senia, E.S. A three-dimensional study of canal curvatures in the mesial roots of mandibular molars. J. Endod. 1992, 18, 294–300. [Google Scholar] [CrossRef]
- Pedullà, E.; Genovese, C.; Messina, R.; La Rosa, G.R.M.; Corsentino, G.; Rapisarda, S.; Arias-Moliz, M.T.; Tempera, G.; Grandini, S. Antimicrobial efficacy of cordless sonic or ultrasonic devices on Enterococcus faecalis-infected root canals. J. Investig. Clin. Dent. 2019, 10, e12434. [Google Scholar] [CrossRef]
- Al-Jadaa, A.; Paqué, F.; Attin, T.; Zehnder, M. Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: Impact of canal location and angulation. Int. Endod. J. 2009, 42, 59–65. [Google Scholar] [CrossRef] [PubMed]
| Location | Mean Irrigation Efficacy (SE) | p | ||||
|---|---|---|---|---|---|---|
| NiTi-2 | NiTi-9 | SS | EA | SI | ||
| Lateral canal #1 | 92.10 (6.80) | 85.36 (7.40) | 90.38 (7.08) | 73.28 (7.40) | 50.04 (6.80) | Method: 0.0001 Location: 0.0018 Method*Location: 0.0195 |
| Lateral canal #2 | 99.86 (5.93) | 95.14 (6.44) | 99.14 (6.17) | 84.95 (6.44) | 76.19 (5.93) | |
| Main canal apex | 99.40 (6.06) | 67.29 (6.58) | 76.93 (6.30) | 80.38 (6.58) | 84.25 (6.06) | |
| A linear mixed model analysis of the effect of location and method on irrigation efficacy in each root canal according to the type of irrigation method used. NiTi, nickel titanium; SE, standard error | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.-R.; Ku, H.-M.; Kim, D.; Shin, S.-J.; Jung, I.-Y. Efficacy of a Nickel-Titanium Ultrasonic Instrument for Biofilm Removal in a Simulated Complex Root Canal. Materials 2020, 13, 4914. https://doi.org/10.3390/ma13214914
Oh Y-R, Ku H-M, Kim D, Shin S-J, Jung I-Y. Efficacy of a Nickel-Titanium Ultrasonic Instrument for Biofilm Removal in a Simulated Complex Root Canal. Materials. 2020; 13(21):4914. https://doi.org/10.3390/ma13214914
Chicago/Turabian StyleOh, Young-Ryul, Hye-Min Ku, Dohyun Kim, Su-Jung Shin, and Il-Young Jung. 2020. "Efficacy of a Nickel-Titanium Ultrasonic Instrument for Biofilm Removal in a Simulated Complex Root Canal" Materials 13, no. 21: 4914. https://doi.org/10.3390/ma13214914
APA StyleOh, Y.-R., Ku, H.-M., Kim, D., Shin, S.-J., & Jung, I.-Y. (2020). Efficacy of a Nickel-Titanium Ultrasonic Instrument for Biofilm Removal in a Simulated Complex Root Canal. Materials, 13(21), 4914. https://doi.org/10.3390/ma13214914






