Vanadium Chemical Compounds Forms in Wastes of Vanadium Pentoxide Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. X-ray Fluorescence Analysis
2.1.2. Synthesis of Model Vanadium Containing Sludges
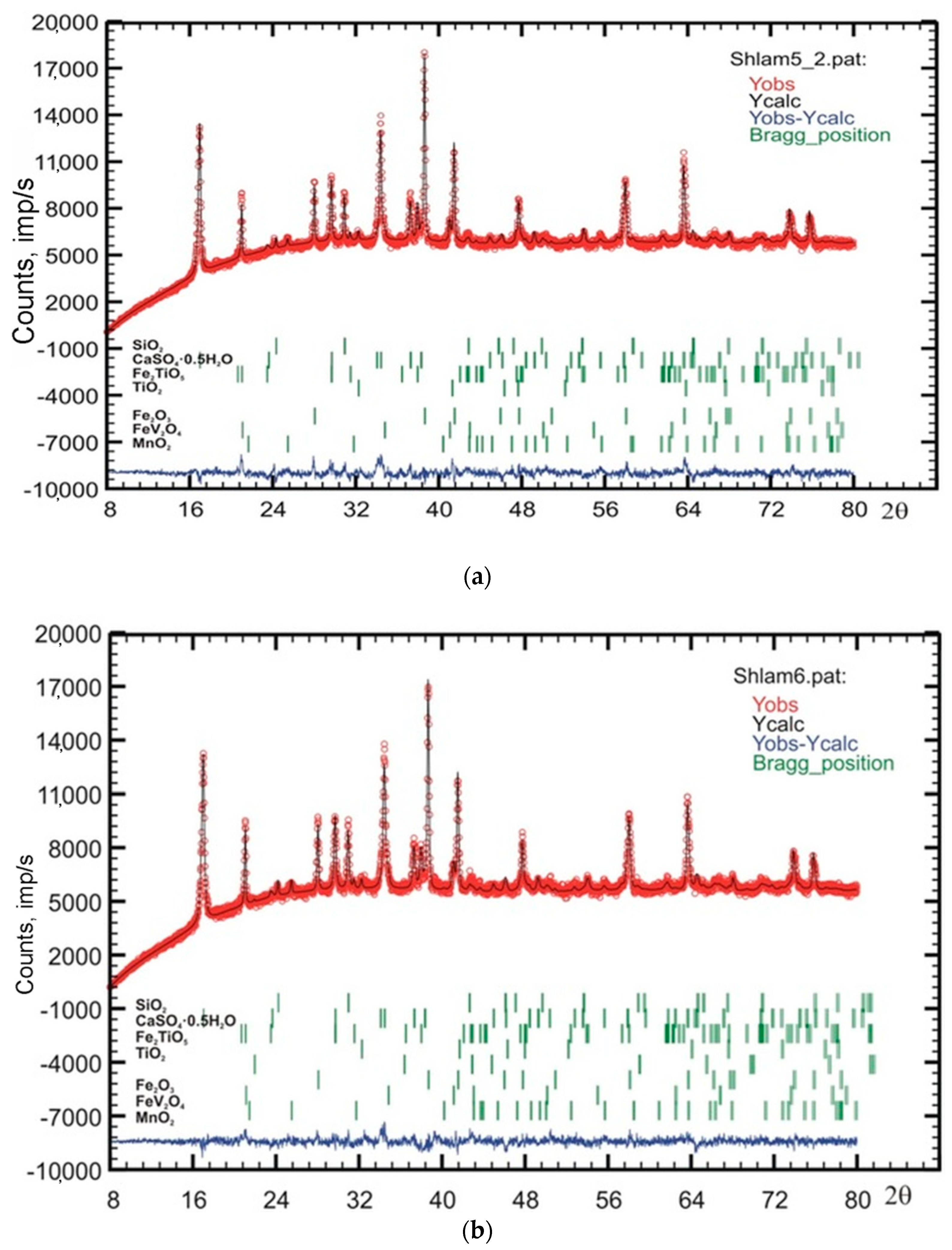
2.1.3. X-ray Diffraction Analysis
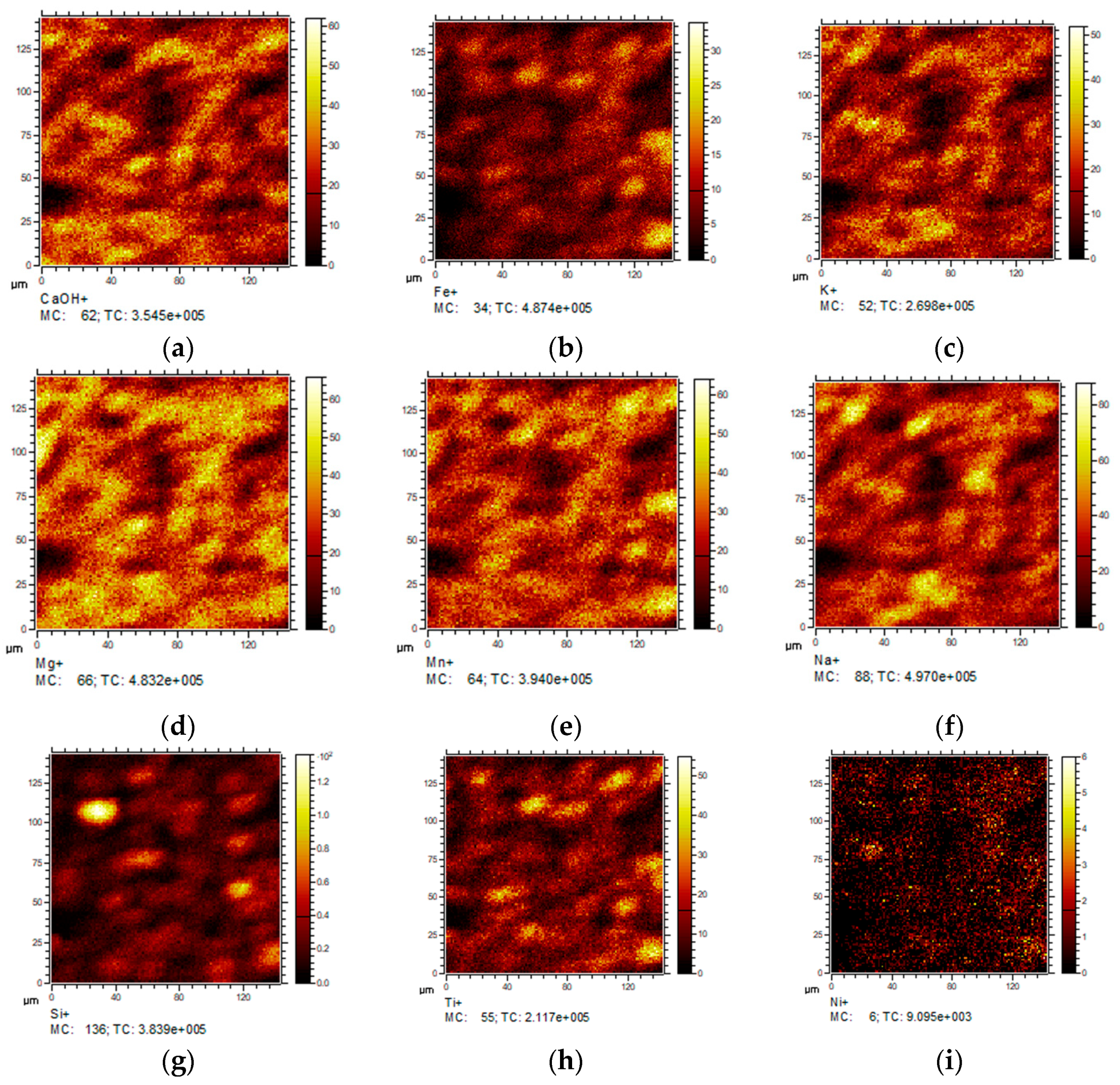
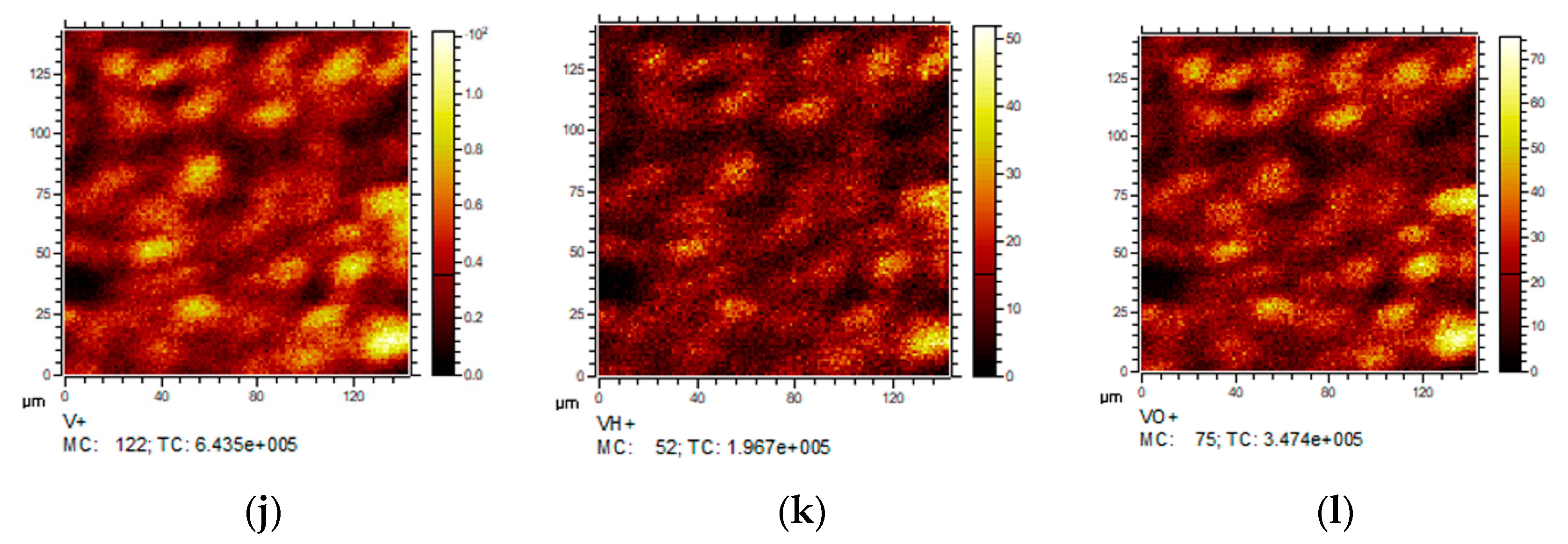
2.1.4. Secondary Ions Mass Spectroscopy
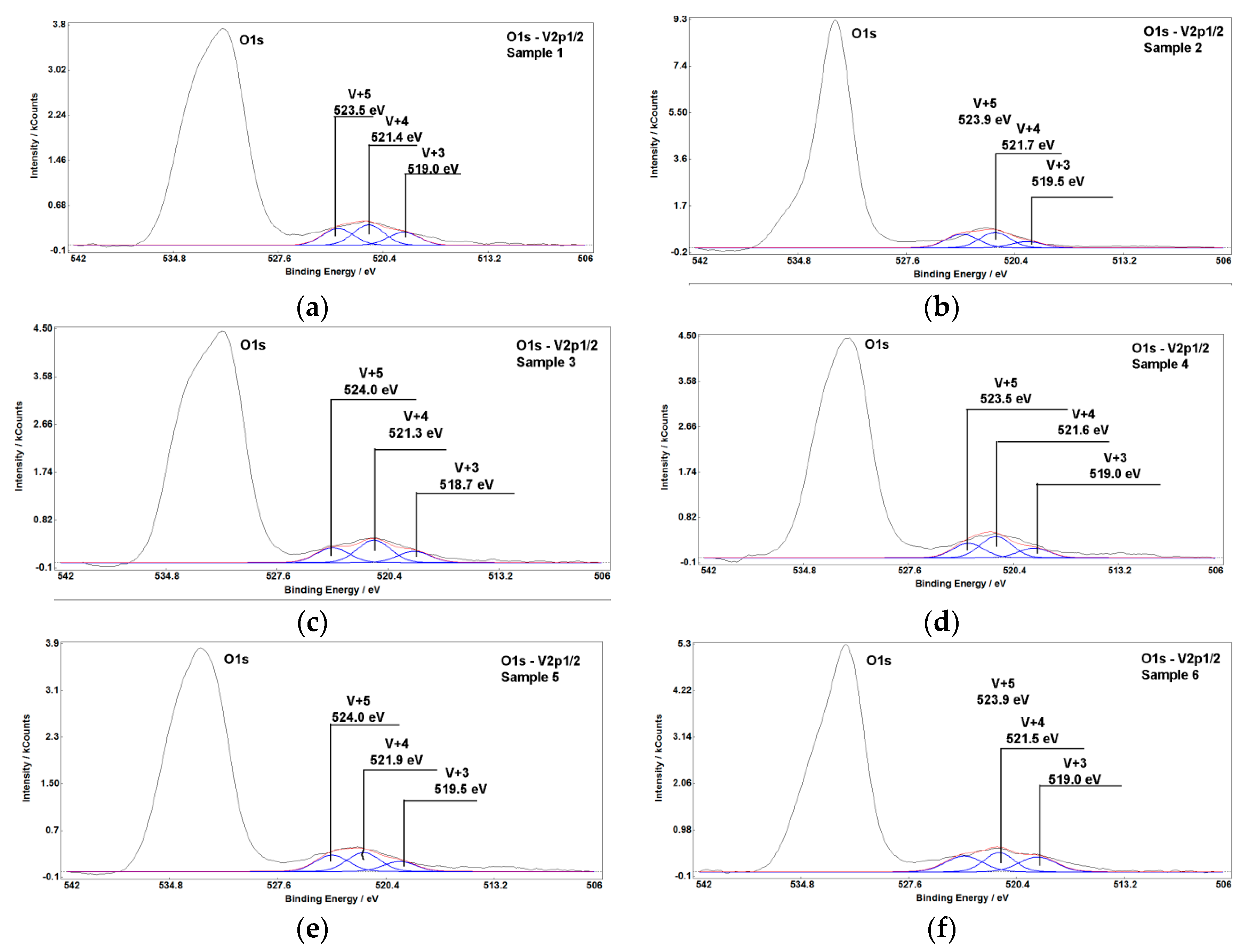
2.1.5. X-ray Photoelectron Spectroscopy
2.2. Materials
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, G.; Zhang, Y.; Bao, S.; Yuan, Y.; Jian, X.; Li, R. Selective vanadium extraction from vanadium bearing ferro-phosphorus via roasting and pressure hydrogen reduction. Sep. Purif. Technol. 2019, 220, 293–299. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Huang, J.; Liu, T.; Yuan, Y.Z.; Xue, N.N. Eco-friendly leaching and separation of vanadium over iron impurity from vanadium-bearing shale using oxalic acid as a leachant. ACS Sustain. Chem. Eng. 2018, 6, 1900–1908. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Liu, T.; Huang, J.; Yuna, Y.Z.; Xue, N.N. Source separation of vanadium over iron from roasted vanadium-bearing shale during acid leaching via ferric fluoride surface coating. J. Clean. Prod. 2018, 181, 399–407. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.B.; Liu, J. Comparison of direct acid leaching process and blank roasting acid leaching process in extracting vanadium from stone coal. Int. J. Miner. Process. 2014, 128, 40–47. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coordin. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef]
- Lam, T.-N.; Trinh, M.-G.; Huang, C.-C.; Kung, P.-C.; Huang, W.-C.; Chang, W.; Amalia, L.; Chin, H.-H.; Tsou, N.-T.; Shih, S.-J.; et al. Investigation of Bone Growth in Additive-Manufactured Pedicle Screw Implant by Using Ti-6Al-4V and Bioactive Glass Powder Composite. Int. J. Mol. Sci. 2020, 21, 7438. [Google Scholar] [CrossRef]
- Zaki, Z.I.; El-Sadek, M.H.; Ali, H.H.; Ahmed, H. Synthesis of Vanadium Carbide by Mechanical Activation Assisted Carbothermic Reduction. Materials 2020, 13, 4408. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Lin, Q.; Mu, D.; Lu, J.; Huang, H.; Huang, H. Reactive Infiltration and Microstructural Characteristics of Sn-V Active Solder Alloys on Porous Graphite. Materials 2020, 13, 1532. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved Osteoblast and Chondrocyte Adhesion and Viability by Surface-Modified Ti6Al4V Alloy with Anodized TiO2 Nanotubes Using a Super-Oxidative Solution. Materials 2015, 8, 867–883. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Soriano, M.D.; Natoli, A.; Rodríguez-Castellón, E.; López Nieto, J.M. Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania. Materials 2018, 11, 1562. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 169, 793–805. [Google Scholar] [CrossRef]
- Lyakishev, N.P.; Slotvinsky-Sidak, N.P.; Pliner, Y.L.; Lappo, S.I. Vanadium in Ferrous Metallurgy; Metallurgiya: Moscow, Russia, 1983; p. 192. (In Russian) [Google Scholar]
- Zaiko, V.P.; Zhuchkov, V.I.; Leontiev, L.I.; Karnaukhov, V.N.; Voronov, Y.I. Technology of Vanadium-Containing Ferroalloys; Akademkniga: Moscow, Russia, 2004; p. 515. (In Russian) [Google Scholar]
- Zhu, X.; Li, W.; Guan, X. Vanadium extraction from titano-magnetite by hydrofluoric acid. Int. J. Miner. Proc. 2016, 157, 55–59. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Zheng, F.; Chen, F.; Yang, L.; Jiang, T.; Qiu, G. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets. Trans. Nonferr. Met. Soc. China 2020, 30, 1687–1696. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Mnichowicz, B.; Harinath, A.V.; Li, H.; Bahrami, B. Chemical deactivation of commercial vanadium SCR catalysts in diesel emission control application. Chem. Eng. J. 2016, 287, 680–690. [Google Scholar] [CrossRef]
- Xiao, Q.G.; Chen, Y.; Gao, Y.Y.; Xu, H.B.; Zhang, Y. Leaching of silica from vanadium bearing steel slag in sodium hydroxide solution. Hydrometallurgy 2010, 102, 216–221. [Google Scholar] [CrossRef]
- Dai, S.F.; Zheng, X.; Wang, X.B.; Finkelman, R.B.; Jiang, Y.F.; Ren, D.Y.; Yan, X.Y.; Zhou, Y.P. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Mishra, D.; Chaudhury, G.R.; Kim, D.J.; Ahn, J.G. Recovery of metal values from spent petroleum catalyst using leaching-solvent extraction technique. Hydrometallurgy 2010, 101, 35–40. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Qiu, H.D.; Zhang, H.; Zhao, B.; Zhu, J.F.; Liu, D.R. Dynamics study on vanadium extraction technology from chloride leaching steel slag. Rare Metal Mater. Eng. 2013, 42, 696–699. [Google Scholar] [CrossRef]
- Silin, I.; Hahn, K.M.; Gürsel, D.; Kremer, D.; Gronen, L.; Stopić, S.; Friedrich, B.; Wotruba, H. Mineral Processing and Metallurgical Treatment of Lead Vanadate Ores. Minerals 2020, 10, 197. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Xie, B.; Zhang, B.; Zhao, X.; Wang, M.; He, Z.; Li, W.; Chen, J. A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal. Minerals 2019, 9, 594. [Google Scholar] [CrossRef]
- Qu, Y.; Li, H.; Wang, X.; Tian, W.; Shi, B.; Yao, M.; Cao, L.; Yue, L. Selective Parameters and Bioleaching Kinetics for Leaching Vanadium from Red Mud Using Aspergillus niger and Penicillium tricolor. Minerals 2019, 9, 697. [Google Scholar] [CrossRef]
- Mizin, V.G.; Rabinovich, E.M.; Sirina, T.P. Complex Processing of Vanadium Raw Materials: Chemistry and Technology; Ural Branch of the Russian Academy of Science: Yekaterinburg, Russia, 2005; p. 416. (In Russian) [Google Scholar]
- Volkov, A.I.; Kologrieva, U.A.; Kovalev, A.I.; Vainshtein, D.L. Effect of degree of oxidation and element form in vanadium slag on its treatment production capacity. Metallurgist 2019, 63, 813–818. [Google Scholar] [CrossRef]
- Kovalev, A.; Wainstein, D.; Vakhrushev, V.; Volkov, A.; Kologrieva, U. Features of the Microstructure and Chemical Compositions of Vanadium-Containing Slags Including Determination of Vanadium Oxidation Degrees. Materials 2019, 12, 3578. [Google Scholar] [CrossRef]
- Kologrieva, U.A.; Volkov, A.I.; Kirichenko, A.S.; Ermolov, V.M.; Mirakova, M.G. Development of a Production Scheme for Utilizing Vanadium Pentoxide Hydrometallurgical Production Waste. Metallurgist 2019, 63, 403–408. [Google Scholar] [CrossRef]
- Volkov, A.I.; Osipov, K.B.; Zhdanov, P.A. X-ray fluorescence analysis of vanadium slag after borate melting. Zavod. Lab. Diagnost. Materialov. 2016, 82, 8–15. (In Russian) [Google Scholar]
- UNIFIT for Windows Software. Unifit Scientific Software GmbH. Available online: http://www.unifit-software.de (accessed on 15 September 2020).
- Ratner, B.; Castner, D. Chapter 3. Electron Spectroscopy for Chemical Analysis. In Surface Analysis—The Principal Techniques, 2nd ed.; Vickerman, J.C., Gilmore, I.S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 47–112. [Google Scholar]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 212. [Google Scholar]
- Kovalev, A.I.; Wainstein, D.L. Surface Analysis Techniques for Investigation of Modified Surfaces, Nanocomposites, Chemical, and Structure Transformations. In Self-Organization during Friction. Advanced Surface Engineered Materials; Fox-Rabinovich, G.S., Totten, G.E., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 81–120. [Google Scholar]
- WebElements Periodic Table >> Titanium >> Radii of Atoms and Ions. Available online: https://www.webelements.com/titanium/atom_sizes.html (accessed on 21 October 2020).
- WebElements Periodic Table >> Vanadium >> Radii of Atoms and Ions. Available online: https://www.webelements.com/vanadium/atom_sizes.html (accessed on 21 October 2020).
- Volkov, A.I.; Ossipov, K.B.; Seregin, A.N.; Zhdanov, P.A.; Seregina, I.F.; Bolshov, M.A. Determination of Degree of Oxidation and Forms of Manganese Compounds in the Ulu-Telyak Oxidized Ore. Inorg. Mater. 2015, 51, 1394–1403. [Google Scholar] [CrossRef]
| Slag No. | V2O5 | MnO | SiO2 | CaO | MgO | TiO2 | Cr2O3 | P2O5 | Al2O3 | Fe | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24.3 | 14.98 | 13.1 | 3.98 | 1.63 | 9.95 | 4.20 | 0.049 | 1.66 | 23.2 | 0.013 |
| 2 | 23.6 | 13.73 | 11.2 | 2.17 | 3.03 | 12.91 | 4.08 | 0.036 | 2.41 | 24.0 | 0.006 |
| 3 | 20.7 | 11.05 | 10.0 | 1.68 | 4.12 | 11.27 | 3.47 | 0.092 | 3.52 | 29.1 | 0.005 |
| 4 | 21.4 | 11.99 | 17.2 | 2.76 | 2.95 | 8.40 | 3.58 | 0.063 | 4.29 | 22.4 | 0.013 |
| Component | Sample | |||||
|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | |
| Na2O | 0.11 | n/d * | n/d | 0.19 | 0.18 | 0.13 |
| MgO | 1.01 | 2.48 | 2.53 | 2.45 | 0.84 | 1.53 |
| Al2O3 | 1.21 | 1.85 | 2.12 | 3.38 | 1.34 | 2.10 |
| SiO2 | 13.09 | 9.09 | 8.46 | 16.81 | 11.0 | 11.20 |
| P2O5 | 0.01 | 0.01 | n/d | 0.02 | 0.03 | 0.04 |
| K2O | 0.054 | 0.022 | 0.018 | 0.310 | 0.107 | n/d |
| CaO | 11.9 | 9.8 | 9.1 | 10.1 | 11.9 | 10.0 |
| TiO2 | 9.7 | 12.2 | 11.25 | 8.8 | 7.5 | 7.69 |
| V2O5 | 1.67 | 1.93 | 1.77 | 1.33 | 3.67 | 2.78 |
| V2O5a.s. | 0.03 | 0.03 | 0.19 | 0.09 | 1.4 | 1.14 |
| Cr2O3 | 4.26 | 4.07 | 3.93 | 3.81 | 3.34 | 3.02 |
| MnO | 9.90 | 7.70 | 6.94 | 7.37 | 6.64 | 6.68 |
| Fe2O3 | 32.9 | 37.3 | 40.8 | 33.0 | 36.5 | 38.7 |
| SO3 | 14.30 | ~12.3 | ~11.3 | ~11.3 | 15.1 | 4.90 |
| LOI | 11.25 | n/d | n/d | n/d | n/d | 11.3 |
| Humidity | 4.19 | n/d | n/d | n/d | 6.2 | 22.3 |
| Phase | Sample | |||||
|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | |
| bassanite (CaSO4·0.5H2O) | 21.9 | 17.0 | 16.6 | 16.0 | 17.3 | 17.4 |
| hematite (Fe2O3) | 38.6 | 32.5 | 39.5 | 33.4 | 30.6 | 30.8 |
| pseudobrookite-armalcolite (Fe0.5Mg0.5Ti2O5) | 16.2 | - | - | - | - | - |
| armalcolite (Fe2TiO5) | - | 21.4 | 18.8 | 14.5 | 13.5 | 16.5 |
| spinel (FeV2O4) | 4.0 | 3.8 | 2.2 | 2.6 | 3.0 | 4.4 |
| Spinel unit cell parameter, Å | 8.457 | 8.433 | 8.428 | 8.437 | 8.452 | 8.451 |
| quartz (SiO2) | 5.6 | 3.1 | 2.9 | 2.8 | 4.5 | 6.0 |
| Rutile (TiO2) | 2.1 | 1.1 | 1.9 | 2.5 | 1.2 | 1.2 |
| pyrochroite (Mn(OH)2) | 1.5 | - | - | - | - | - |
| ramsdellite MnO2 | 3.5 | 1.5 | 1.5 | 2.7 | 0.8 | 1.3 |
| grossular Ca3Al2Si3O12 | 1.9 | 1.8 | 1.1 | 0.7 | 0.0 | 0.0 |
| Amorphous phase | 5 | 18 | 15 | 25 | 29 | 22 |
| Sample | Binding Energy, eV | Oxidation Degree | Fraction, % |
|---|---|---|---|
| #1 | 523.5 | V3+ | 33.6 |
| 521.4 | V4+ | 40.5 | |
| 519.0 | V5+ | 25.9 | |
| #2 | 523.9 | V3+ | 39.9 |
| 521.7 | V4+ | 41.7 | |
| 519.5 | V5+ | 18.4 | |
| #3 | 524.0 | V3+ | 30.2 |
| 521.3 | V4+ | 46.2 | |
| 518.7 | V5+ | 23.6 | |
| #4 | 523.5 | V3+ | 31.7 |
| 521.6 | V4+ | 46.8 | |
| 519.0 | V5+ | 21.5 | |
| #5 | 524.0 | V3+ | 36.3 |
| 521.9 | V4+ | 41.6 | |
| 519.5 | V5+ | 22.1 | |
| #6 | 523.9 | V3+ | 34.0 |
| 521.5 | V4+ | 33.9 | |
| 519.0 | V5+ | 32.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, A.; Kologrieva, U.; Kovalev, A.; Wainstein, D.; Vakhrushev, V. Vanadium Chemical Compounds Forms in Wastes of Vanadium Pentoxide Production. Materials 2020, 13, 4889. https://doi.org/10.3390/ma13214889
Volkov A, Kologrieva U, Kovalev A, Wainstein D, Vakhrushev V. Vanadium Chemical Compounds Forms in Wastes of Vanadium Pentoxide Production. Materials. 2020; 13(21):4889. https://doi.org/10.3390/ma13214889
Chicago/Turabian StyleVolkov, Anton, Ulyana Kologrieva, Anatoly Kovalev, Dmitry Wainstein, and Vladimir Vakhrushev. 2020. "Vanadium Chemical Compounds Forms in Wastes of Vanadium Pentoxide Production" Materials 13, no. 21: 4889. https://doi.org/10.3390/ma13214889
APA StyleVolkov, A., Kologrieva, U., Kovalev, A., Wainstein, D., & Vakhrushev, V. (2020). Vanadium Chemical Compounds Forms in Wastes of Vanadium Pentoxide Production. Materials, 13(21), 4889. https://doi.org/10.3390/ma13214889





