Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes
Abstract
1. Introduction
2. Materials and Methods
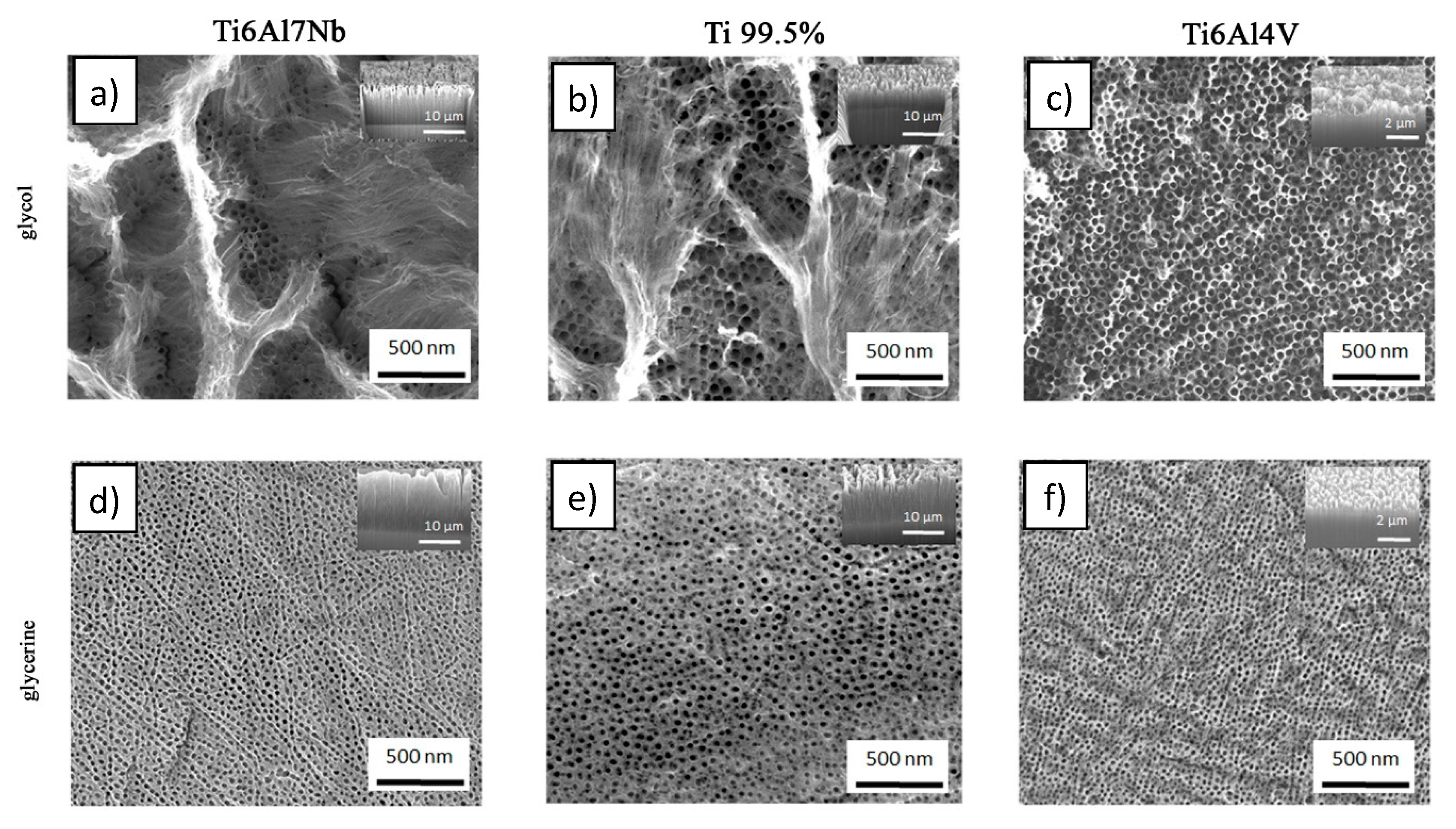
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, N.; Li, D.; Zou, J.; Xie, R.; Wang, Z.; Tang, B. Surface Texture-Based Surface Treatments on Ti6Al4V Titanium Alloys for Tribological and Biological Applications: A Mini Review. Materials 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskuła, M. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Gulati, K.; Sinn Aw, M.; Findlay, D.; Losic, D. Local drug delivery to the bone by drug-releasing implants: Perspectives of nano-engineered titania nanotube arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Sukiman, N.L.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Dabbagh, A.; Abu Kasim, N.H.; Basirun, W.J. In vitro bioactivity and corrosion resistance enhancement of Ti-6Al-4V by highly ordered TiO2 nanotube arrays. J. Aust. Ceram. Soc. 2019, 55, 187–200. [Google Scholar] [CrossRef]
- Luz, A.R.; Santos, L.S.; Lepienski, C.M.; Kuroda, P.B.; Kuromoto, N.K. Characterization of the morphology, structure and wettability of phase dependent lamellar and nanotube oxides on anodized Ti-10Nb alloy. Appl. Surf. Sci. 2018, 448, 30–40. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Q.; Tang, H.; Li, G.; Chi, Y. Fabrication, characterization and biocompatibility of TiO2 nanotubes via anodization of Ti6Al7Nb. Compos. Interfaces 2016, 23, 223–230. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.; Bai, L.; Tang, B.; Chu, P.K. Electrochemical surface engineering of titanium-based alloys for biomedical application. Electrochim. Acta 2018, 271, 699–718. [Google Scholar] [CrossRef]
- Mello, M.G.; Taipina, M.O.; Rabelo, G.; Cremasco, A.; Caram, R. Production and characterization of TiO2 nanotubes on Ti-Nb-Mo-Sn system for biomedical applications. Surf. Coat. Technol. 2017, 326, 126–133. [Google Scholar] [CrossRef]
- Bhosle, S.M.; Friedrich, C.R. Rapid heat treatment for anatase conversion of titania nanotube orthopedic surfaces. Nanotechnology 2017, 28, 405603. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Hu, R.; Huang, Q.; Wu, K.; Zhang, L.; Tang, P.; Lin, C. Antibacterial and cytocompatible AgNPs constructed with the assistance of Mefp-1 for orthopaedic implants. RSC Adv. 2017, 7, 38434–38443. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Wu, K.; Chen, Z.; Wang, X.; Lin, C.; Lai, Y. Progress in TiO2 nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef] [PubMed]
- Dikicia, T.; Erola, M.; Toparlia, M.; Celika, E. Characterization and photocatalytic properties of nanoporous titanium dioxide layer fabricated on pure titanium substrates by the anodic oxidation process. Ceram. Int. 2014, 40, 1587–1591. [Google Scholar] [CrossRef]
- Dasa, M.; Ballaa, V.K.; Sampath Kumarb, T.S.; Mannaa, I. Fabrication of Biomedical Implants using Laser Engineered Net Shaping (LENSTM). Trans. Indian Ceram. Soc. 2013, 72, 169–174. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res. Part A 2005, 75, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Indira, K.; Kamachi Mudali, U.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth Anodic TiO2 Nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Nyga, P.; Czerwiński, M. Ethanol-based electrolyte for nanotubular anodic TiO2 formation. Corros. Sci. 2018, 134, 99–102. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Forbot, D.; Norek, M.; Michalska-Domańska, M.; Król, A. The impact of electrolyte’s viscosity on the formation of nanoporous anodic aluminum oxide. Electrochim. Acta 2014, 133, 57–64. [Google Scholar] [CrossRef]
- Michalska-Domanska, M.; Stępniowski, W.J.; Jaroszewicz, L.R. Characterization of nanopores arrangement of anodic alumina layers synthesized on low-(AA1050) and high- purity aluminum by two-step anodizing in sulfuric acid with addition of ethylene glycol at low temperature. J. Porous Mater. 2017, 24, 779–786. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminum—A comparative study with the AAO produced on high purity aluminum. Electrochim. Acta 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Atkins, P.W.; Paula, J.D. Physical Chemistry, 8th ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-Organized Porous Titanium Oxide Prepared in H2SO4/HF Electrolytes. Electrochem. Solid-State Lett. 2003, 6, B12. [Google Scholar] [CrossRef]
- Hedges, M.; Calder, N. Rapid Manufacture of Perfomance Materials via LENS. In Cost Effective Manufacture via Net-Shape Processing; Meeting Proceedings RTO-MP-AVT-139, Paper 13; RTO: Neuilly-sur-Seine, France, 2006; pp. 13-1–13-14. [Google Scholar]
- Durejko, T.; Ziętala, M.; Polkowski, W.; Czujko, T. Thin wall tubes with Fe3Al/SS316L graded structure obtained by using laser engineered net shaping technology. Mater. Des. 2014, 63, 766–774. [Google Scholar] [CrossRef]
- Łazińska, M.; Durejko, T.; Zasada, D.; Bojar, Z. Microstructure and mechanical properties of a Fe-28%Al-5%Cr-1%Nb-2%B alloy fabricated by Laser Engineered Net Shaping. Mater. Lett. 2017, 196, 87–90. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Durejko, T.; Michalska-Domańska, M.; Łazińska, M.; Aniszewska, J. Characterization of nanoporous anodic aluminum oxide formed on laser pre-treated aluminum. Mater. Charact. 2016, 122, 130–136. [Google Scholar] [CrossRef]
- Sopha, H.; Tesar, K.; Knotek, P.; Jäger, A.; Hromadko, L.; Macak, J.M. TiO2 nanotubes grown on Ti substrates with different microstructure. Mater. Res. Bull. 2018, 103, 197–204. [Google Scholar] [CrossRef]
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and mechanical behaviour of Ti-6Al-7Nb alloy produced by selective laser melting. Mater. Charact. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Torrescano-Alvarez, J.M.; Curioni, M.; Habazaki, H.; Hashimoto, T.; Skeldon, P.; Zhou, X. Incorporation of alloying elements into porous anodic films on aluminium alloys: The role of cell diameter. Electrochim. Acta 2019, 296, 783–789. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X. Effects of Alloying Elements in Anodizing of Aluminium. Trans. IMF 1997, 75, 18–23. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. The composition of the alloy/film interface during anodic oxidation of Al-W alloys. J. Electrochem. Soc. 1996, 143, 2465–2470. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. The co-enrichment of alloying elements in the substrate by anodic oxidation of Al-W-Cu alloys. Corros. Sci. 1997, 39, 339–354. [Google Scholar] [CrossRef]
- Molchan, I.S.; Molchan, T.V.; Gaponeneko, N.V.; Skeldon, P.; Thompson, G.E. Impurity-driven defect generation in porous anodic alumina. Electrochem. Commun. 2010, 12, 693–696. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.; Choi, J. Fabrication of ruthenium-doped TiO2 electrodes by one-step anodization for electrolysis applications. Electrochem. Commun. 2013, 36, 88–91. [Google Scholar] [CrossRef]
- Gim, Y.; Seong, M.; Choi, Y.W.; Choi, J. RuO2-doping into high-aspect-ratio anodic TiO2 nanotubes by electrochemical potential shock for water oxidation. Electrochem. Commun. 2015, 52, 37–40. [Google Scholar] [CrossRef]
- Stępniowski, W.; Norek, M.; Budner, B.; Michalska-Domańska, M.; Nowak-Stępniowska, A.; Mostek, A.; Thorat, S.; Salerno, M.; Giersig, M.; Bojar, Z. In-situ electrochemical doping of nanoporous anodic aluminum oxide with indigo carmine organic dye. Thin Solid Films. 2016, 598, 60–64. [Google Scholar] [CrossRef]
- Majchrowicz, A.; Roguska, A.; Pisarek, M.; Lewandowska, M. Nanotubular oxide layers formation on Ti-24Nb-4Zr-8Sn and Ti-13Zr-13Nb alloys in the ethylene glycol-based electrolyte: The role of alloying elements and phase composition. Thin Solid Films. 2019, 692, 137635. [Google Scholar] [CrossRef]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.H.; Wang, Y.-M.; Kuo, H.-H.; Kia, S. Effect of alloy types on the anodizing process of aluminium. Surf. Coat. Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C. 2016, 59, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, A.; Roguska, A.; Pisarek, M.; Lewandowska, M. Tailoring the morphology of nanotubular oxide layers on Ti-24Nb-4Zr-8Sn β-phase titanium alloy. Thin Solid Films. 2019, 679, 15–21. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F.; Wang, X. Synthesis and morphology of TiO2 nanotube arrays by anodic oxidation using modified glycerol-based electrolytes. J. Am. Ceram. Soc. 2007, 90, 2384–2389. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Naduvath, J.; Bhargava, P.; Mallick, S. Mechanism of titania nanograss formation during anodization. Chem. Phys. Lett. 2015, 626, 15–19. [Google Scholar] [CrossRef]
- Gui, Q.; Yu, D.; Li, D.; Song, Y.; Zhu, X.; Cao, L.; Zhang, S.; Ma, W.; You, S. Efficient suppression of nanograss during porous anodic TiO2 nanotubes growth. Appl. Surf. Sci. 2014, 314, 505–509. [Google Scholar] [CrossRef]
- Le Coz, F.; Arurault, F.; Datas, L. Chemical analysis of a single basic cell of porous anodic aluminium oxide templates. Mater. Charact. 2010, 61, 283–288. [Google Scholar] [CrossRef]
- Parkhutik, V.P.; Albella, J.M.; Makushok, Y.E.; Montero, I.; Martinez-Duart, J.M.; Shershulskii, V.I. Study of aluminium anodization in sulphuric and chromic acid solutions—I. Kinetics of growth and composition of oxides. Electrochim. Acta 1990, 35, 955–960. [Google Scholar] [CrossRef]
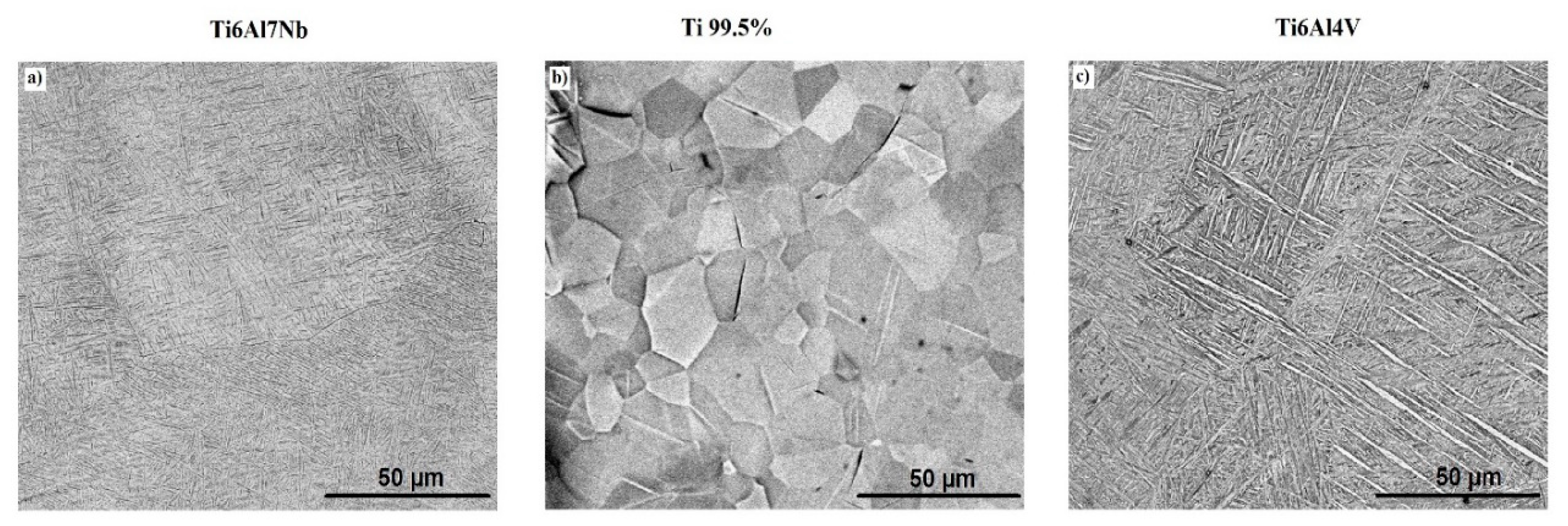
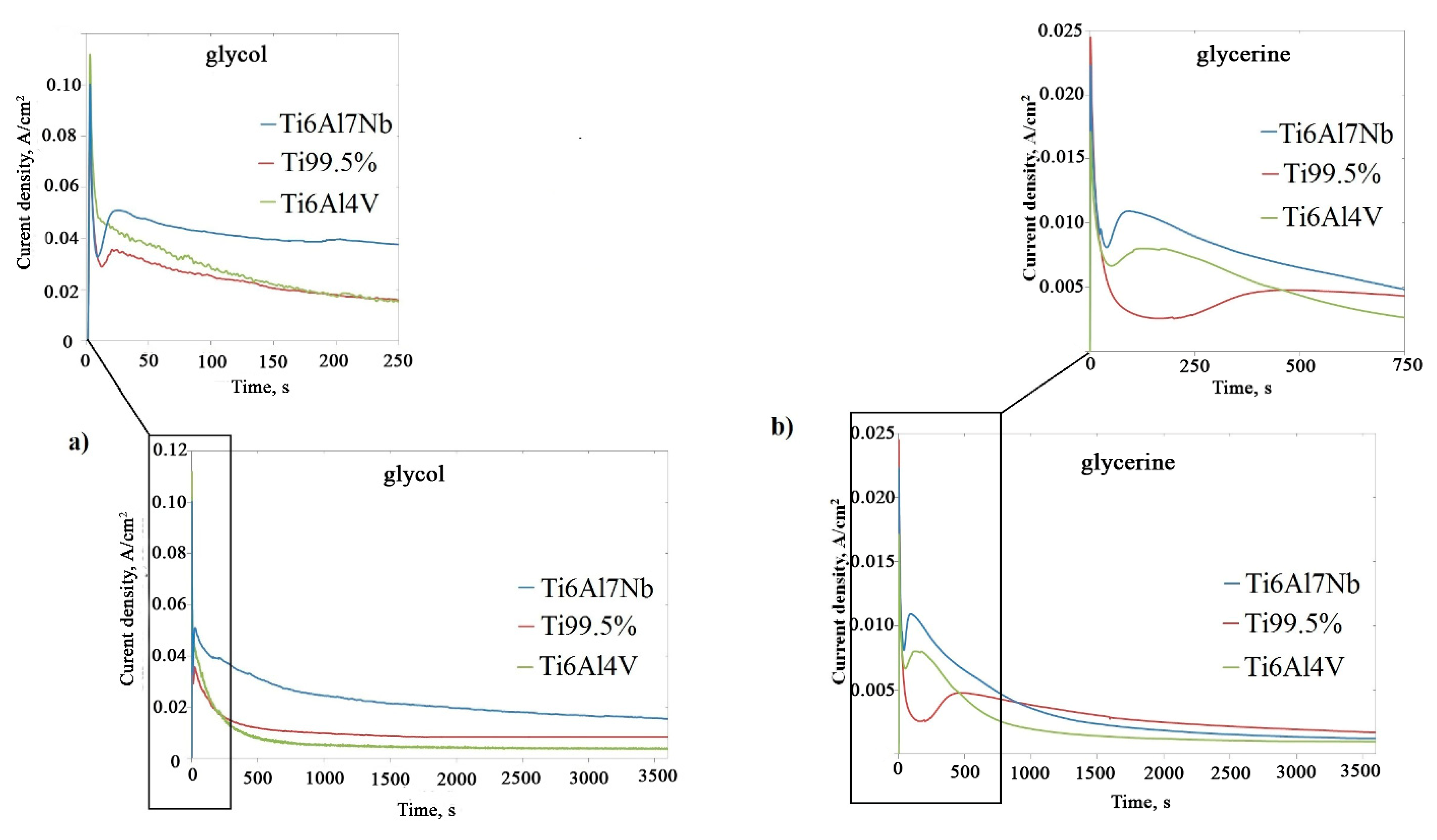
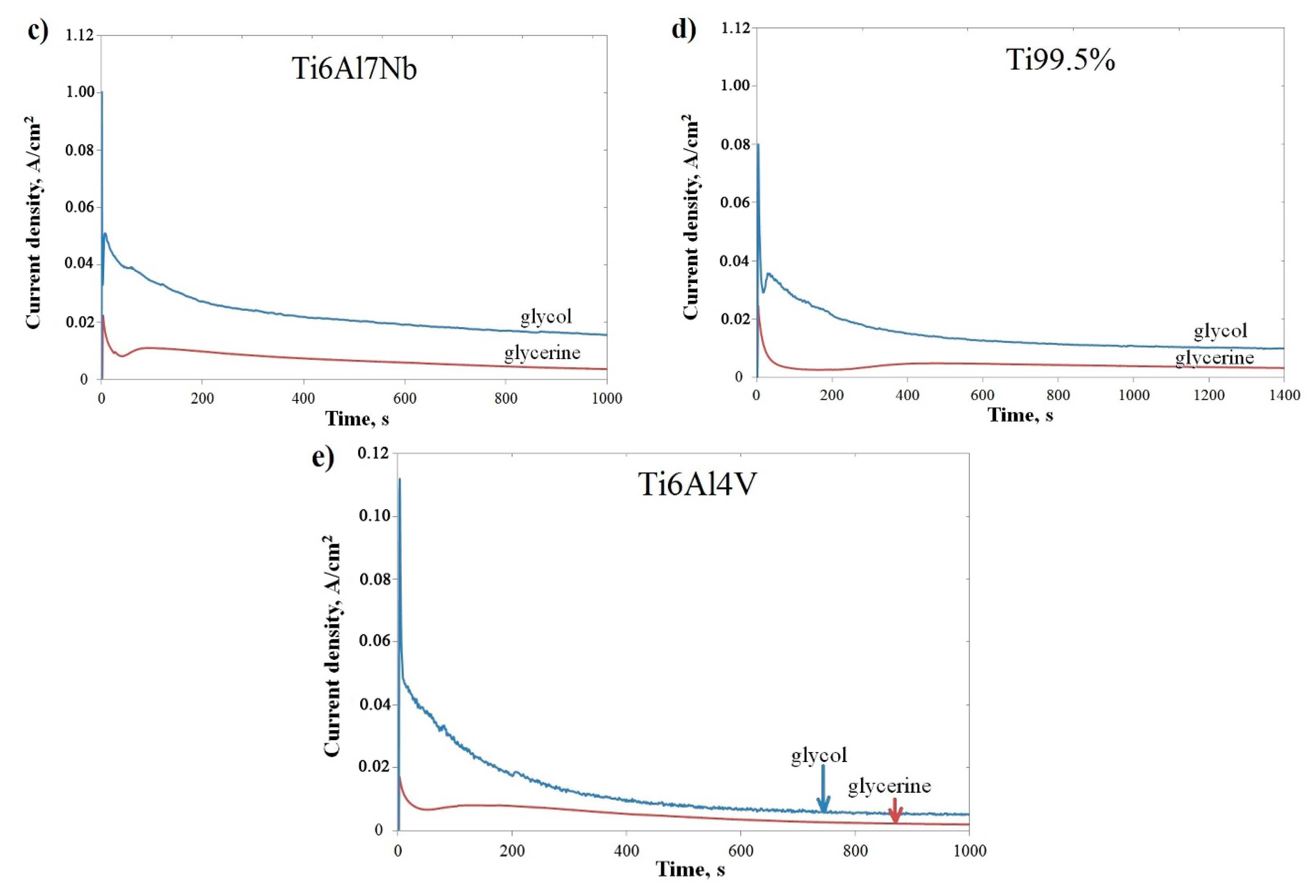
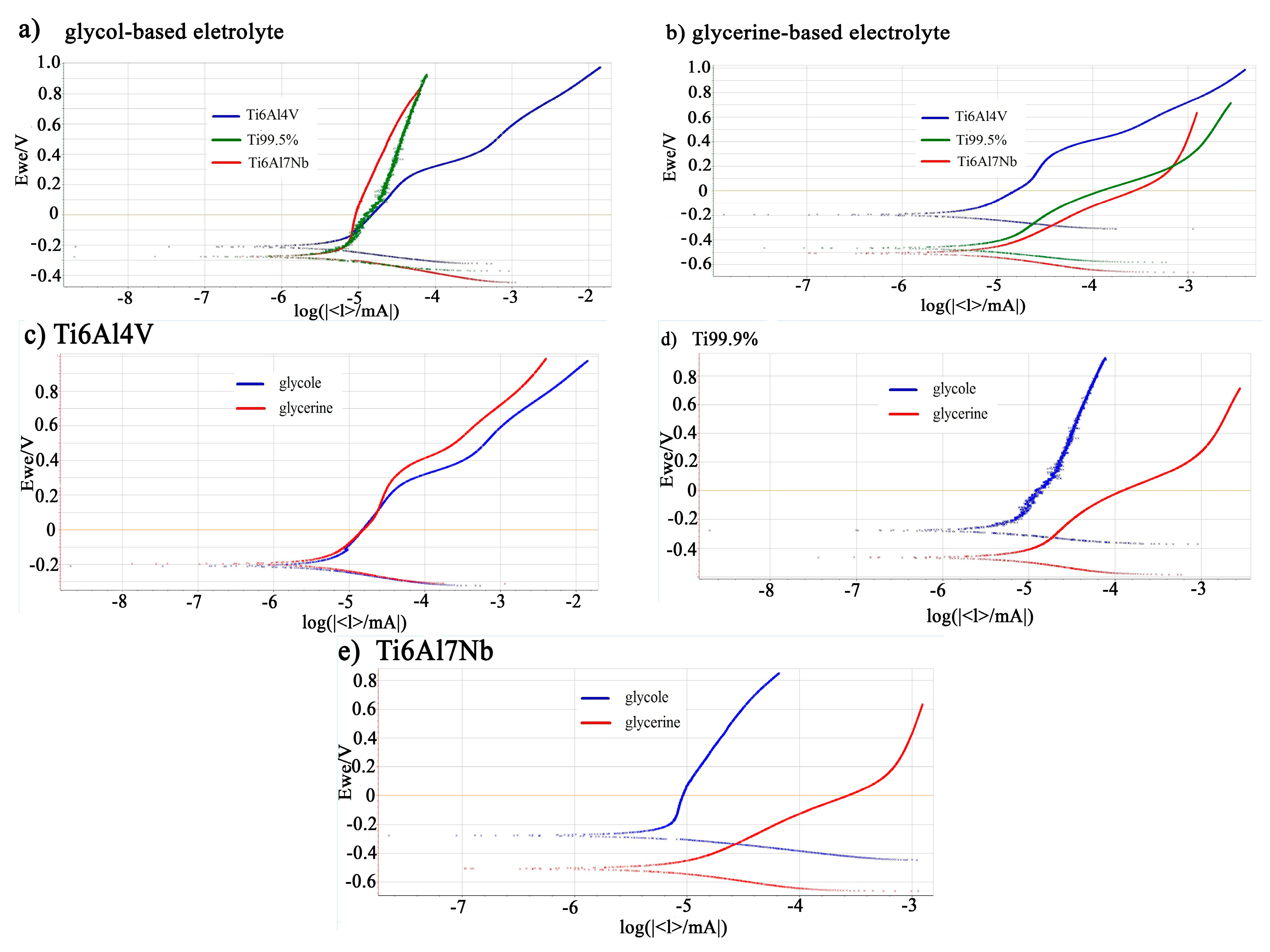
| Ti6Al7Nb | Ti 99.5% | Ti6Al4V | ||||
|---|---|---|---|---|---|---|
| Glycol | Glycerine | Glycol | Glycerine | Glycol | Glycerine | |
| Maximum Current Density [mA/cm2] | 98.3 | 22.1 | 78.1 | 24.3 | 108.9 | 17.0 |
| Average Current Density [mA/cm2] | 21.9 | 3.1 | 10.1 | 2.8 | 6.4 | 2.2 |
| Oxide Thickness [µm] | 10–11 | 3 | 7–8 | 2 | 1 | >1 |
| Nanotube/Nanopore Diameter [nm] | 48 | 27 | 52 | 41 | 37 | 25 |
| Ti6Al7Nb | Ti 99.5% | Ti6Al4V | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Anodization | After Anodization | Before Anodization | After Anodization | Before Anodization | After Anodization | ||||
| Glycol | Glycerine | Glycol | Glycerine | Glycol | Glycerine | ||||
| Ti | 84.6 | 32.1 | 33.9 | 89.0 | 33.7 | 41.2 | 84.9 | 44.4 | 41.7 |
| Al | 11.7 | 3. 5 | 3.8 | – | – | – | 12.5 | 5.7 | 5.4 |
| Nb | 3.8 | 2.2 | 1.9 | – | – | – | – | – | – |
| V | – | – | – | – | – | – | 2. 6 | 1.4 | 1.5 |
| O | – | 50.3 | 44. 3 | – | 52.4 | 42.7 | – | 35.0 | 35.6 |
| F | – | 11.0 | 15.1 | – | 9.2 | 10.5 | – | 5.8 | 10.3 |
| C | – | 0.9 | 1.0 | – | 4.7 | 5.6 | – | 7.7 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska-Domańska, M.; Łazińska, M.; Łukasiewicz, J.; Mol, J.M.C.; Durejko, T. Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes. Materials 2020, 13, 4743. https://doi.org/10.3390/ma13214743
Michalska-Domańska M, Łazińska M, Łukasiewicz J, Mol JMC, Durejko T. Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes. Materials. 2020; 13(21):4743. https://doi.org/10.3390/ma13214743
Chicago/Turabian StyleMichalska-Domańska, Marta, Magdalena Łazińska, Justyna Łukasiewicz, Johannes M. C. Mol, and Tomasz Durejko. 2020. "Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes" Materials 13, no. 21: 4743. https://doi.org/10.3390/ma13214743
APA StyleMichalska-Domańska, M., Łazińska, M., Łukasiewicz, J., Mol, J. M. C., & Durejko, T. (2020). Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes. Materials, 13(21), 4743. https://doi.org/10.3390/ma13214743






