Characterization of Glass-Ceramic Sealant for Solid Oxide Fuel Cells at Operating Conditions by Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
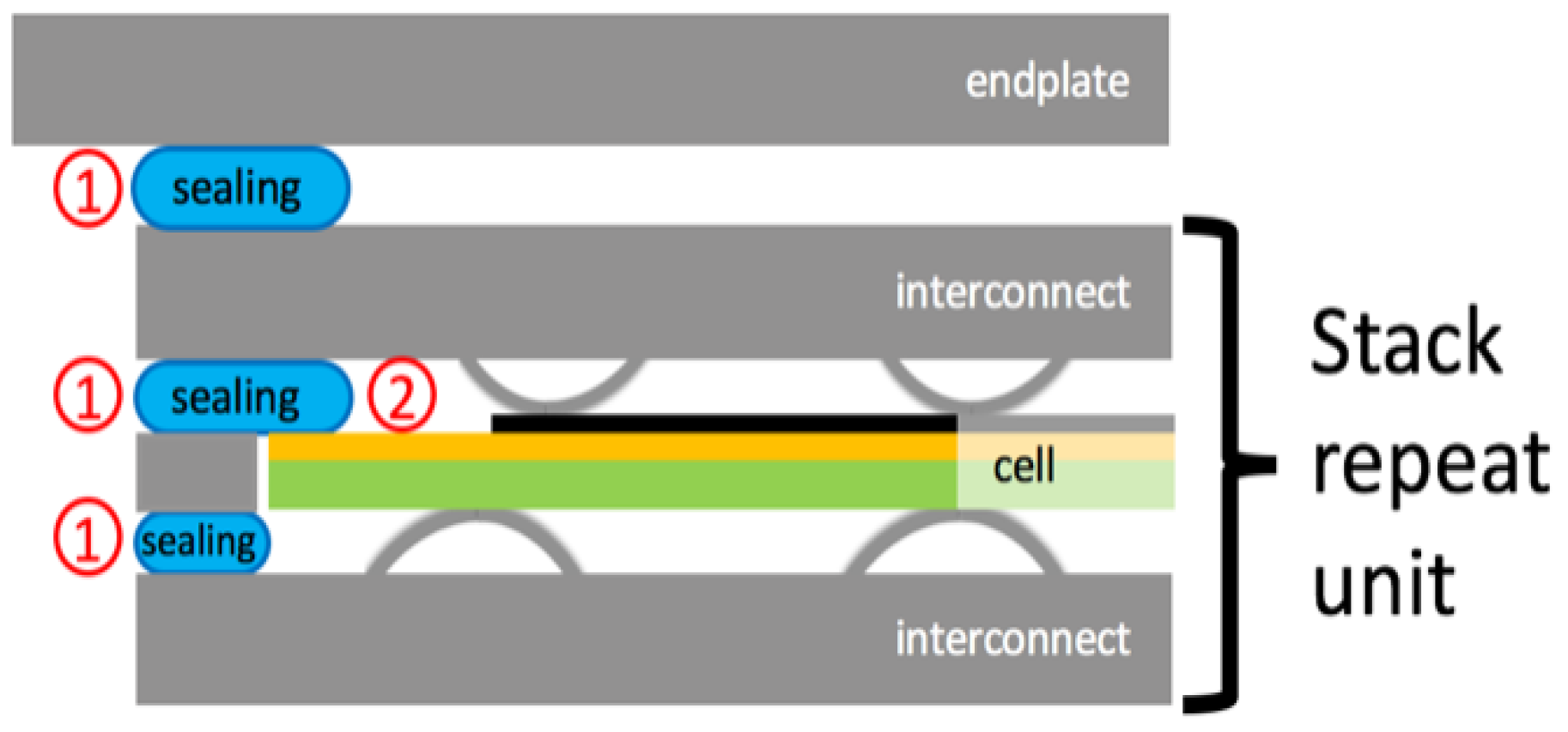
2.1. Preparation of Samples
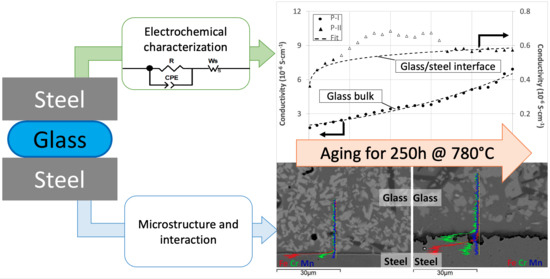
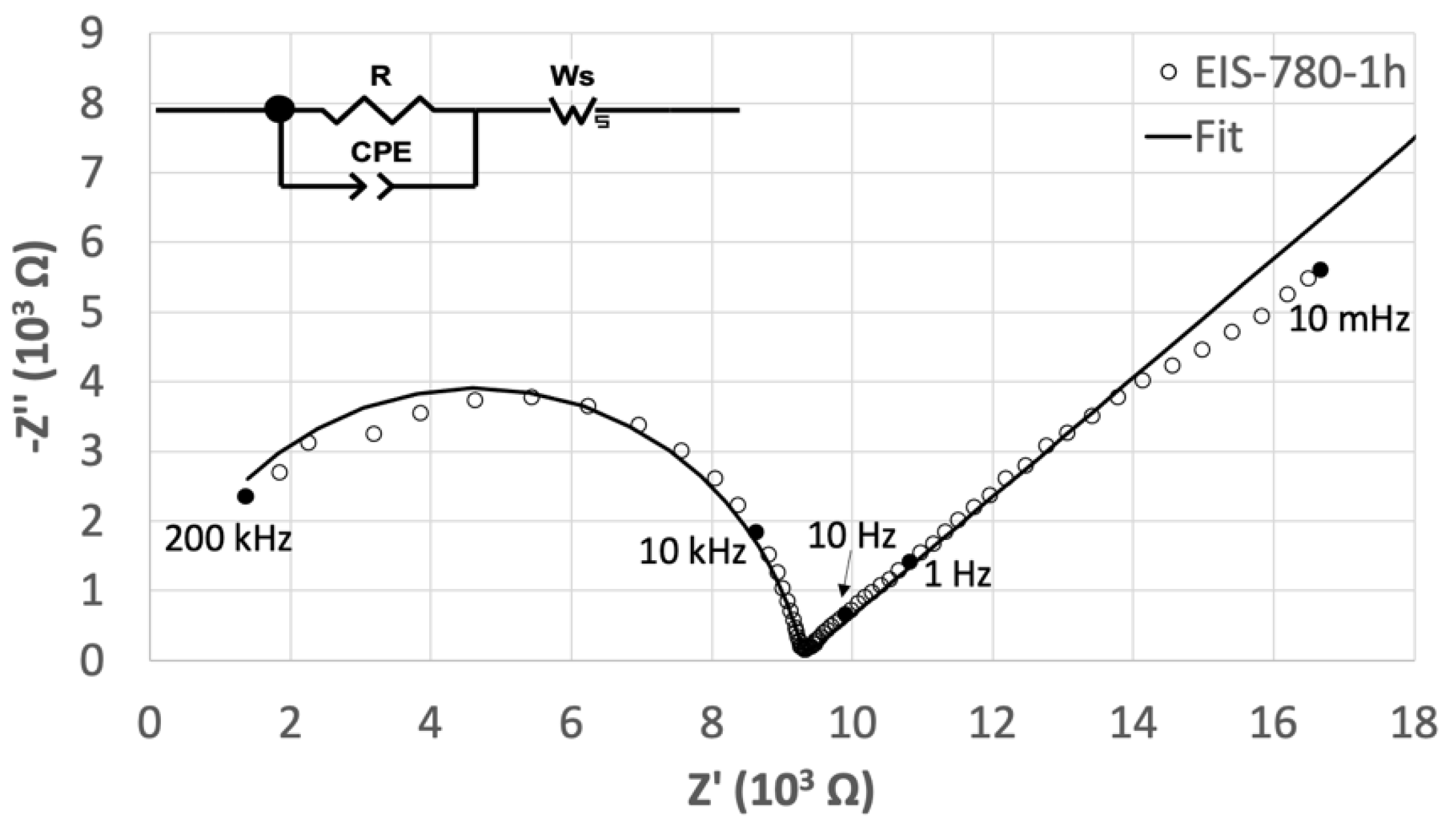
2.2. Electrochemical Characterization
2.3. Post-Experiment Characterization
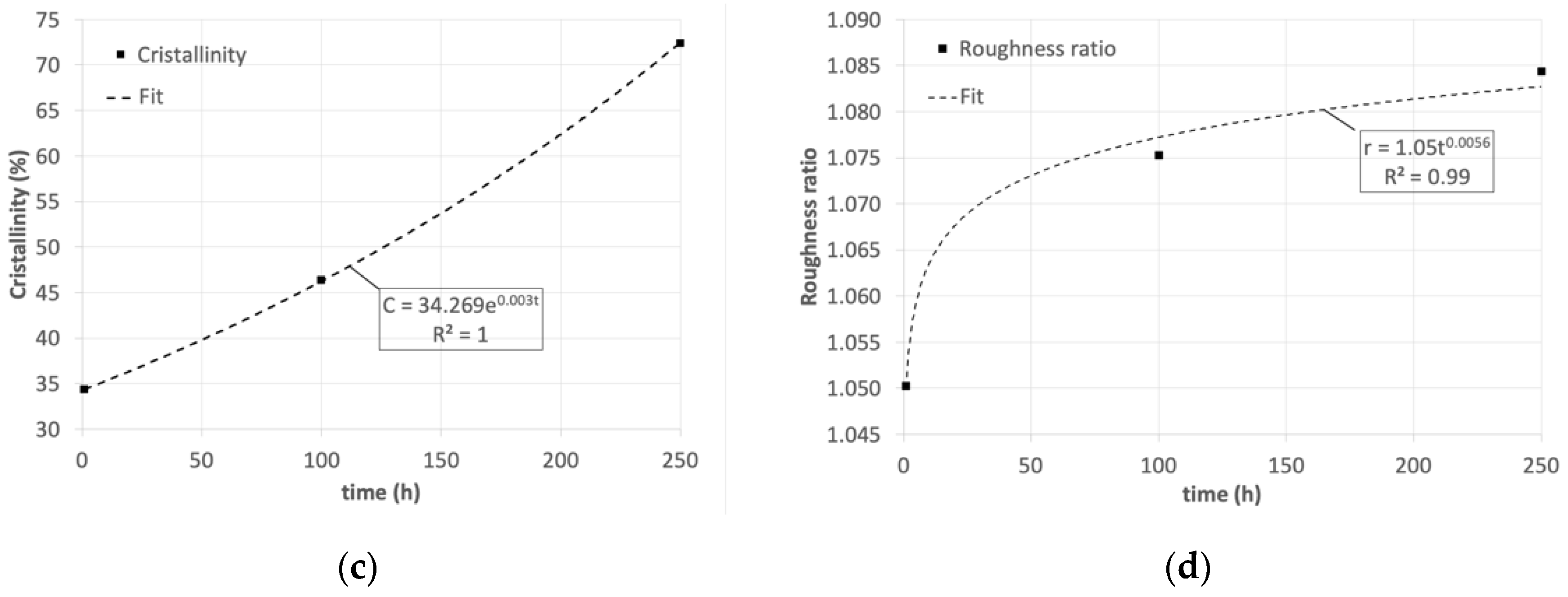
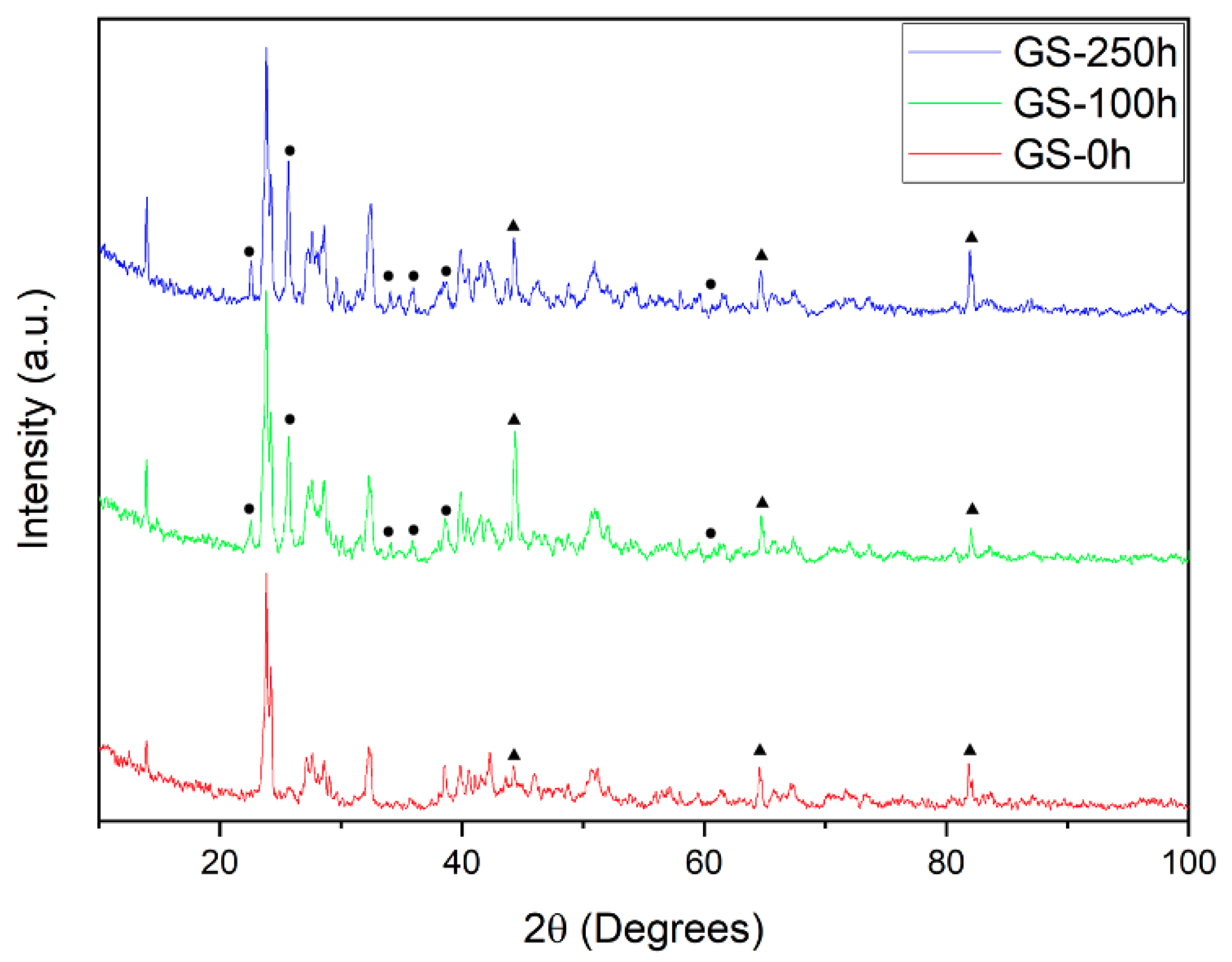
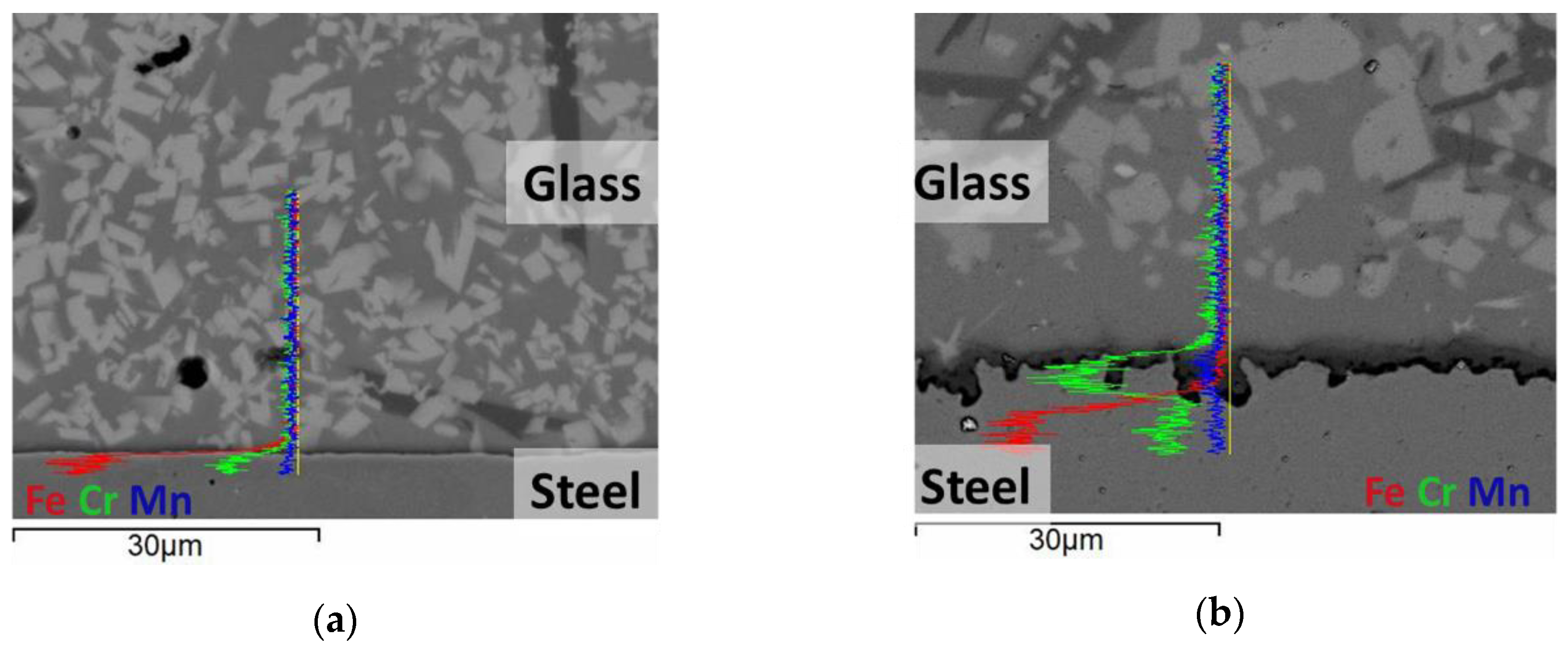
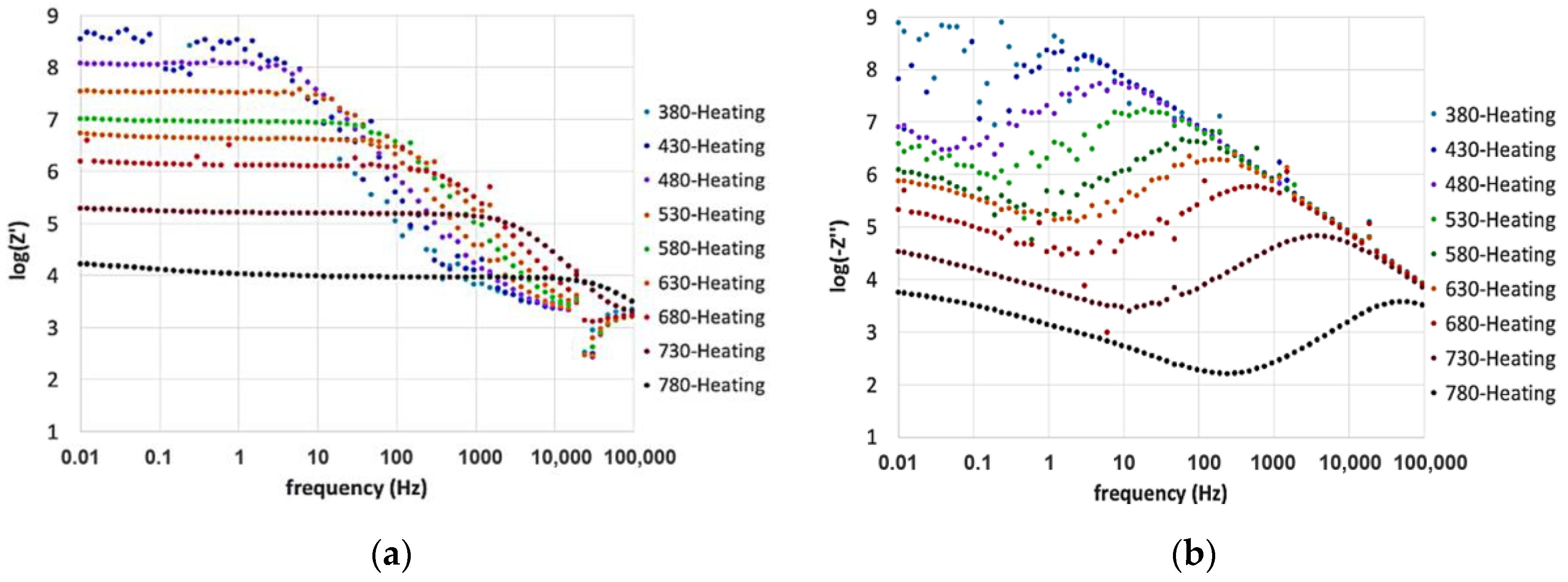
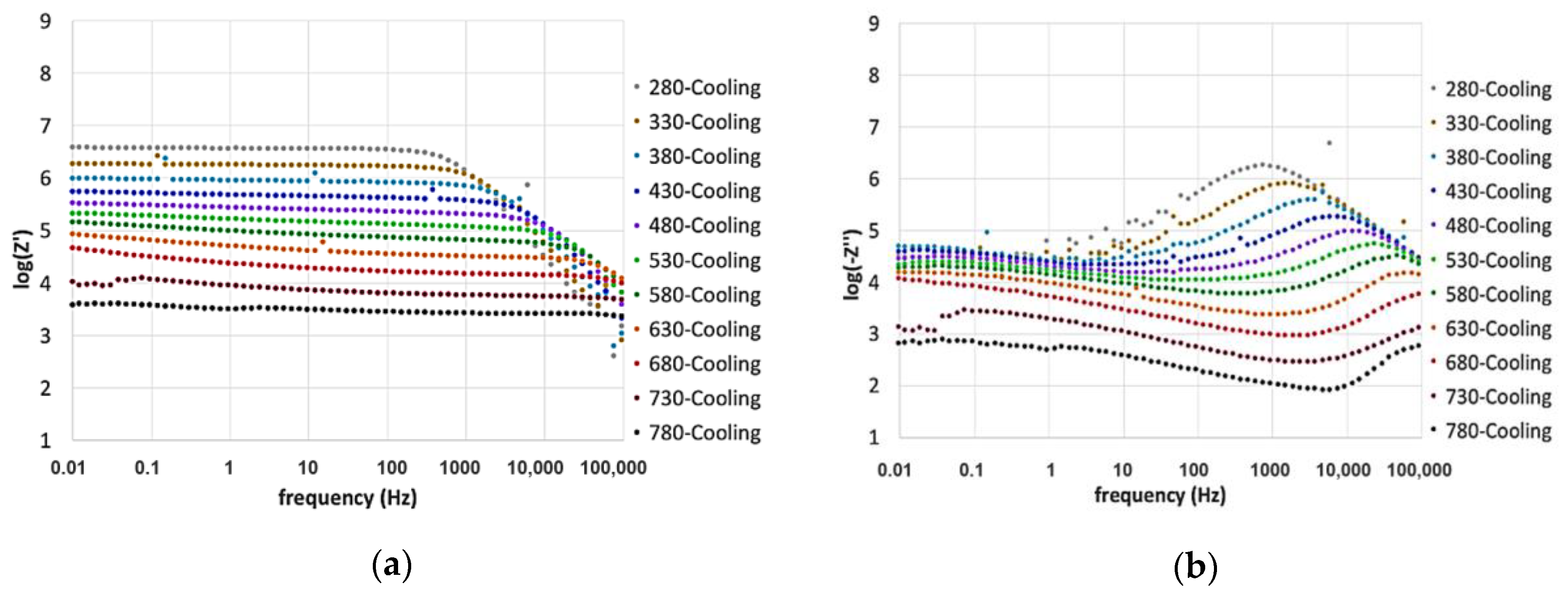
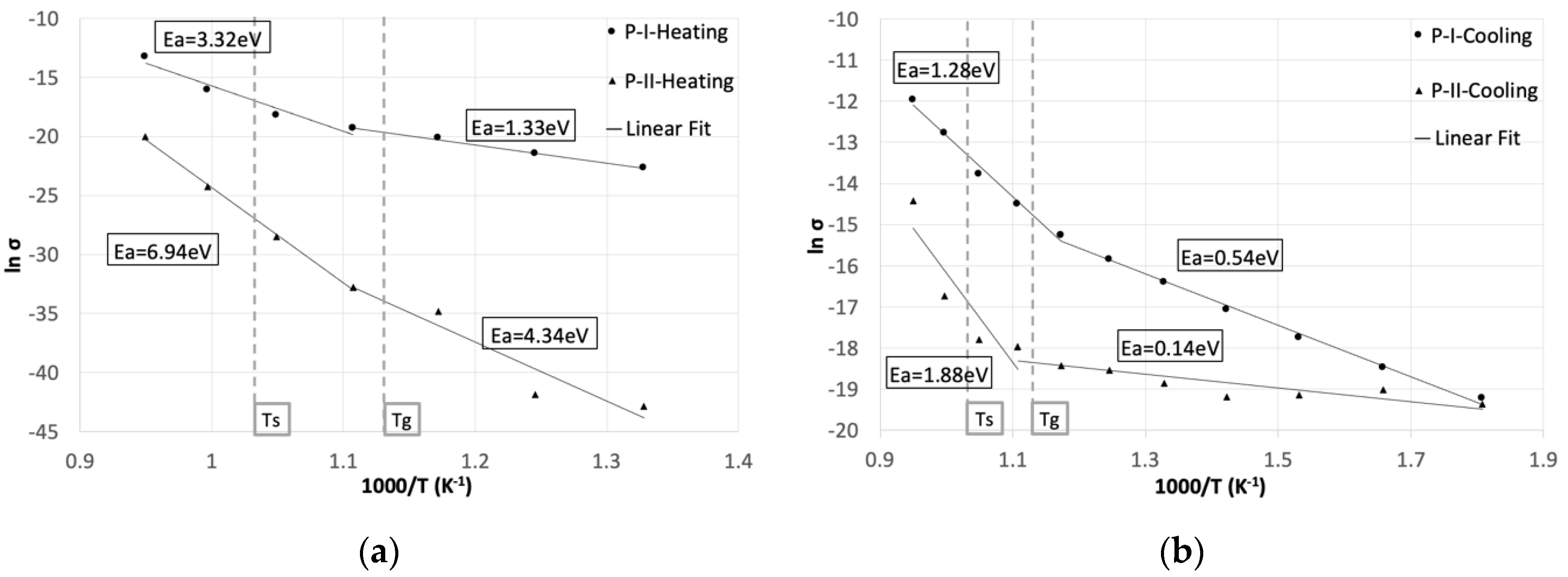
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; John Wiley & Sons Inc.: Chichester, UK, 2003; Volume 2. [Google Scholar]
- Singhal, S.C.; Kendall, K. High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design, and Applications; Elsevier Advanced Technology: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Chiodelli, G.; Malavasi, L. Electrochemical open circuit voltage (OCV) characterization of SOFC materials. Ionics 2013, 19, 1135–1144. [Google Scholar] [CrossRef]
- Coralli, A.; Sarruf, B.J.M.; de Miranda, P.E.V.; Osmieri, L.; Specchia, S.; Minh, N.Q. Chapter 2-Fuel Cells. In Science and Engineering of Hydrogen-Based Energy Technologies; de Miranda, P.E.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 39–122. [Google Scholar]
- Spotorno, R.; Piccardo, P.; Schiller, G. Effect of Cathode Contacting on Anode Supported Cell Performances. J. Electrochem. Soc. 2016, 163, F872–F876. [Google Scholar] [CrossRef]
- Spotorno, R.; Piccardo, P.; Perrozzi, F.; Valente, S.; Viviani, M.; Ansar, A. Microstructural and Electrical Characterization of Plasma Sprayed Cu-Mn Oxide Spinels as Coating on Metallic Interconnects for Stacking Solid Oxide Fuel Cells. Fuel Cells 2015, 15, 728–734. [Google Scholar] [CrossRef]
- Bongiorno, V.; Piccardo, P.; Anelli, S.; Spotorno, R. Influence of Surface Finishing on High-Temperature Oxidation of AISI Type 444 Ferritic Stainless Steel Used in SOFC Stacks. ACTA Metall. Sin. 2017, 697–711. [Google Scholar] [CrossRef]
- Haanappel, V.A.C.; Shemet, V.; Gross, S.M.; Koppitz, T.; Menzler, N.H.; Zahid, M.; Quadakkers, W.J. Behaviour of various glass–ceramic sealants with ferritic steels under simulated SOFC stack conditions. J. Power Sources 2005, 150, 86–100. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Lu, K. Seal glass for solid oxide fuel cells. J. Power Sources 2010, 195, 7129–7139. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Reddy, A.A.; Kharton, V.V.; Ferreira, J.M.F. Aluminosilicate-based sealants for SOFCs and other electrochemical applications—A brief review. J. Power Sources 2013, 242, 486–502. [Google Scholar] [CrossRef]
- Ghosh, S.; Sharma, A.D.; Kundu, P.; Basu, R.N. Glass-based Sealants for Application in Planar Solid Oxide Fuel Cell Stack. Trans. Indian Ceram. Soc. 2008, 67, 161–182. [Google Scholar] [CrossRef]
- Batfalsky, P.; Haanappel, V.A.C.; Malzbender, J.; Menzler, N.H.; Shemet, V.; Vinke, I.C.; Steinbrech, R.W. Chemical interaction between glass–ceramic sealants and interconnect steels in SOFC stacks. J. Power Sources 2006, 155, 128–137. [Google Scholar] [CrossRef]
- Sabato, A.G.; Cempura, G.; Montinaro, D.; Chrysanthou, A.; Salvo, M.; Bernardo, E.; Secco, M.; Smeacetto, F. Glass-ceramic sealant for solid oxide fuel cells application: Characterization and performance in dual atmosphere. J. Power Sources 2016, 328, 262–270. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Chang, C.-T.; Jung, G.-B.; Chan, S.-H.; Lee, W.-T.; Chang, S.-W.; Kao, I.-C. Study of sealants for SOFC. Int. J. Hydrogen Energy 2016, 41, 21812–21819. [Google Scholar] [CrossRef]
- Celik, S. Influential parameters and performance of a glass-ceramic sealant for solid oxide fuel cells. Ceram. Int. 2015, 41, 2744–2751. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for solid oxide fuel cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Stoynov, Z.B.; Vladikova, D.E.; Burdin, B.G.; Laurencin, J.; Montinaro, D.; Nakajo, A.; Piccardo, P.; Thorel, A.; Hubert, M.; Spotorno, R.; et al. Differential Resistance Analysis–a New Tool for Evaluation of Solid Oxide Fuel Cells Degradation. MRS Adv. 2017, 2, 3991–4003. [Google Scholar] [CrossRef]
- Piccardo, P.; Pecunia, A.; Bongiorno, V.; Spotorno, R.; Wuillemin, Z.; Ouweltjes, J.P. Ageing of Materials at Inlet and Outlet Fuel Manifolds in a SOFC Stack. ECS Trans. 2015, 68, 2611–2624. [Google Scholar] [CrossRef]
- Spotorno, R.; Piccardo, P.; Perrozzi, F. Interaction between Crofer 22 APU Current Collector and LSCF Cathode Contacting Layer under Cell Operation. ECS Trans. 2015, 68, 1633–1640. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Thomsen, E.C.; Choi, J.-P.; Stevenson, J.W. Compliant alkali silicate sealing glass for solid oxide fuel cell applications: The effect of protective YSZ coating on electrical stability in dual environment. J. Power Sources 2012, 202, 149–156. [Google Scholar] [CrossRef]
- Hong, F.; Holland, D. Studies of interface reactions between glass ceramic coatings and metals. J. Non-Cryst. Solids 1989, 112, 357–363. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ferraris, M.; Cho, J.; Boccaccini, A.R. Glass–ceramic seal to join Crofer 22 APU alloy to YSZ ceramic in planar SOFCs. J. Eur. Ceram. Soc. 2008, 28, 61–68. [Google Scholar] [CrossRef]
- Spotorno, R.; Fracchia, E.; Schiller, G.; Piccardo, P. Effect of YSZ Coatings as Diffusion Barrier between Glass Sealing and Steel. ECS Trans. 2017, 78, 1749–1758. [Google Scholar] [CrossRef]
- Runge, A.; Kahnt, H. Ionic Conduction in Glass Ceramics. Ber. Bunsenges. Phys. Chem. 1996, 100, 1526–1530. [Google Scholar] [CrossRef]
- Montedo, O.R.K.; Faller, C.A.; Bertan, F.M.; Jiusti, J.; Piva, R.H.; Piva, D.H. Microstructural development and electrical behavior during crystalization of iron-rich glass-ceramics obtained from mill scale. Ceram. Int. 2017, 43, 11864–11873. [Google Scholar] [CrossRef]
- Kundu, S.; Varma, K.B.R. Frequency and Temperature-Independent Electrical Transport Properties of 2BaO-0.5Na2O-2.5Nb2O5-4.5B2O3 Glass-Ceramics. J. Am. Ceram. Soc. 2014, 97, 3582–3588. [Google Scholar] [CrossRef]
- Lara, C.; Pascual, M.J.; Keding, R.; Duràn, A. Electrical behaviour of glass–ceramics in the systems RO–BaO–SiO2 (R = Mg, Zn) for sealing SOFCs. J. Power Sources 2006, 157, 377–384. [Google Scholar] [CrossRef]
- Ojha, P.K.; Chongdar, T.K.; Gokhale, N.M.; Kulkarni, A.R. Investigation of crystallization kinetic of SrO-La2O3-Al2O3-B2O3-SiO2 glass and its suitability for SOFC sealant. Int. J. Hydrogen Energy 2011, 36, 14996–15001. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–34. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Potter, L.D. A flexible procedure for analyzing impedance spectroscopy results: Description and illustrations. Solid State Ion. 1987, 24, 61–79. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-Ray Powder Patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rangarajan, B.; Bharadwaja, S.S.N.; Furman, E.; Shrout, T.; Lanagan, M. Impedance Spectroscopy Studies of Fresnoites in BaO–TiO2–SiO2 System. J. Am. Ceram. Soc. 2010, 93, 522–530. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials, 2nd ed.; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Moguš-Milanković, A.; Šantić, A.; Ličina, V.; Day, D.E. Dielectric behavior and impedance spectroscopy of bismuth iron phosphate glasses. J. Non-Cryst. Solids 2005, 351, 3235–3245. [Google Scholar] [CrossRef]
- Qu, D. Application of a.c. impedance technique to the study of the proton diffusion process in the porous MnO2 electrode. Electrochim. Acta 2003, 48, 1675–1684. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Barsoukov, E. Impedance Spectroscopy Therory, Experiment, and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- ZView® Operating Manual; Scribner Associates, Inc.: Southern Pines, NC, USA, 2015.
- Ravagnani, C.; Keding, R.; Russel, C. High temperature impedance spectroscopy of homogeneous and phase separated melts and glasses of the composition 48.5SiO2 48.5B2O3 3Na2O. J. Non-Cryst. Solids 2003, 328, 164–173. [Google Scholar] [CrossRef]
- Dutta, A.; Sinha, T.P.; Jena, P.; Adak, S. Ac conductivity and dielectric relaxation in ionically conducting soda–lime–silicate glasses. J. Non-Cryst. Solids 2008, 354, 3952–3957. [Google Scholar] [CrossRef]
- Maier, J. Space Charge Regions in Solid Two Phase Systems and Their Conduction Contribution—II Contact Equilibrium at the Interface of Two Ionic Conductors and the Related Conductivity Effect. Ber. Bunsenges. Phys. Chem. 1985, 89, 355–362. [Google Scholar] [CrossRef]
- Hunter, C.C.; Ingram, M.D. Na+-ion conducting glasses. Solid State Ion. 1984, 14, 31–36. [Google Scholar] [CrossRef]
- Redwine, R.H.; Field, M.B. Effect of microstructure on transport in glasses. J. Mater. Sci. 1969, 4, 713–721. [Google Scholar] [CrossRef]
- Donald, I.W.; Mallinson, P.M.; Metcalfe, B.L.; Gerrard, L.A.; Fernie, J.A. Recent developments in the preparation, characterization and applications of glass- and glass–ceramic-to-metal seals and coatings. J. Mater. Sci. 2011, 46, 1975–2000. [Google Scholar] [CrossRef]
- Nisino, T.; Moteki, K.; Sikano, H. Thermal decomposition of magnesium chromate. J. Ceram. Assoc. 1963, 71, 159–163. [Google Scholar] [CrossRef][Green Version]
- Zhu, W.Z.; Deevi, S.C. Opportunity of metallic interconnects for solid oxide fuel cells: A status on contact resistance. Mater. Res. Bull. 2003, 38, 957–972. [Google Scholar] [CrossRef]
- Righter, K.; Campbell, A.J.; Humayun, M. Diffusion of trace elements in FeNi metal: Application to zoned metal grains in chondrites. Geochim. Cosmochim. Acta 2005, 69, 3145–3158. [Google Scholar] [CrossRef]
- Harizanova, R.; Keding, R.; Russel, C. Electric conductivity of glasses in the system Na2O/CaO/SiO2/Fe2O3. J. Non-Cryst. Solids 2008, 354, 65–71. [Google Scholar] [CrossRef]
- Qiu, H.H.; Kudo, M.; Sakata, H. Synthesis and electrical properties of Fe2O3-MoO3-TeO2 glasses. Mater. Chem. Phys. 1997, 51, 233–238. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Rao, G.V.S. Electrical Conduction in Metal Oxides. Phys. Status Solidi 1970, 1, 597. [Google Scholar] [CrossRef]
- Goebel, C.; Berger, R.; Bernuy-Lopez, C.; Westlinder, J.; Svensson, J.E.; Froitzheim, J. Long-term (4 year) degradation behavior of coated stainless steel 441 used for solid oxide fuel cell interconnect applications. J. Power Sources 2020, 449, 227480. [Google Scholar] [CrossRef]
- Malki, M.; Echegut, P. Electrical conductivity of the CaO–SiO2 system in the solid and the molten states. J. Non-Cryst. Solids 2003, 323, 131–136. [Google Scholar] [CrossRef]
- Grandjean, A.; Malki, M.; Simonnet, C. Effect of composition on ionic transport in SiO2–B2O3–Na2O glasses. J. Non-Cryst. Solids 2006, 352, 2731–2736. [Google Scholar] [CrossRef]
- Feltri, A.; Grandi, S.; Mustarelli, P.; Cutroni, M.; Mandanici, A. GeO2-doped silica glasses: An ac conductivity study. Solid State Ion. 2002, 154–155, 217–221. [Google Scholar] [CrossRef]
- Prado, M.O.; Campos, A.A.; Soares, P.C.; Rodrigues, A.C.M.; Zanotto, E.D. Liquid–liquid phase separation in alkali-borosilicate glass: An impedance spectroscopy study. J. Non-Cryst. Solids 2003, 332, 166–172. [Google Scholar] [CrossRef]

| Element | Fe | C | Si | Mn | Cr | Ti + Nb |
| Concentration (wt.%) | Balance | 0.015 | 0.60 | 0.30 | 17.80 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spotorno, R.; Ostrowska, M.; Delsante, S.; Dahlmann, U.; Piccardo, P. Characterization of Glass-Ceramic Sealant for Solid Oxide Fuel Cells at Operating Conditions by Electrochemical Impedance Spectroscopy. Materials 2020, 13, 4702. https://doi.org/10.3390/ma13214702
Spotorno R, Ostrowska M, Delsante S, Dahlmann U, Piccardo P. Characterization of Glass-Ceramic Sealant for Solid Oxide Fuel Cells at Operating Conditions by Electrochemical Impedance Spectroscopy. Materials. 2020; 13(21):4702. https://doi.org/10.3390/ma13214702
Chicago/Turabian StyleSpotorno, Roberto, Marlena Ostrowska, Simona Delsante, Ulf Dahlmann, and Paolo Piccardo. 2020. "Characterization of Glass-Ceramic Sealant for Solid Oxide Fuel Cells at Operating Conditions by Electrochemical Impedance Spectroscopy" Materials 13, no. 21: 4702. https://doi.org/10.3390/ma13214702
APA StyleSpotorno, R., Ostrowska, M., Delsante, S., Dahlmann, U., & Piccardo, P. (2020). Characterization of Glass-Ceramic Sealant for Solid Oxide Fuel Cells at Operating Conditions by Electrochemical Impedance Spectroscopy. Materials, 13(21), 4702. https://doi.org/10.3390/ma13214702