Alanates, a Comprehensive Review
Abstract
1. Introduction
2. General Syntheses Procedures
2.1. Syntheses in Organic Solvents
2.1.1. Direct Synthesis
2.1.2. Reaction of Metal Hydrides and Aluminum Salts
2.1.3. Metathesis of Alanates
2.2. Syntheses Assisted by Mechanical Milling
2.2.1. Direct Synthesis by Mechanical Milling
2.2.2. Reaction of Metal Hydrides and Aluminum Salts under Mechanical Milling
2.2.3. Reaction of Metal Hydrides and Alane under Mechanical Milling
2.2.4. Metathesis of Alanates under Mechanical Milling
3. The “Single Metal” Alanates
3.1. AlH3
3.2. Alanates of Group 1
3.2.1. Lithium Alanate
3.2.2. Reactive Mixtures (Composites) with LiAlH4
Composites of LiAlH4-MgH2
Composites of LiAlH4-LiBH4
Composites of LiAlH4-LiNH2
3.2.3. Sodium Alanate
Role of Catalyst
- Morphology/particle size effects. Beattie et al. demonstrated that Ti-doped NaAlH4 particles presented few morphological changes as compared with un-doped materials [135]. By-products of the addition of materials, such as TiCl3, i.e., Ti-Al alloys, and NaCl, can act as grain refiners for Al and NaH phases, keeping particle sizes small [136]. In general, much effort is put to reduce particle sizes and to avoid the sintering of particles, and thus maintaining the hydriding/dehydriding performance.
- Location of Ti and substitution of atoms. The Ti atoms can be located in the bulk, in interstitial positions, at the subsurface, or the surface. The Ti preferred position depends on the doping level and synthesis technique (impregnation vs. ball milling), or in theoretical calculations, the choice of reference states. The Ti atoms can be located in NaH, Al, Na3AlH6, or NaAlH4 phases. Theoretical studies have been performed basically to include all of these possibilities. Some studies have unraveled the interactions of Ti (or Ti-compounds) with NaH and Al. Other reports indicated interactions of Ti (or Ti-compounds) with Na3AlH6 and NaAlH4. Contradictory results/conclusions frequently come across.Additionally, many studies point to atom substitution and formation of defects. The replacement of Al by Ti in NaAlH4 could be possible, yet this configuration is metastable [137,138]. Løvvik situates the substitution in the second metal layer from the surface [137,138]. Other DFT calculations suggest that the most frequent Ti-defect in NaAlH4 is a defect that is formed by the substitution of Al by Ti and the addition of two hydrogen ions; this defect has a −1 charge [139].The substitution of Na by Ti and other metal atoms also has been investigated. Marashdeh et al. classified the catalysts as “good” (Sc, Ti, Zr) and “bad” (Pt, Pd) according to their ability to exchange places with a Na atom on a (001) surface of NaAlH4 [140]. In the “zipper model”, Ti-species, at the surface or at a grain-boundary, displace subsurface Na atoms and eject them to the NaAlH4 surface. Subsequently, the Na atoms react quickly with other species and destabilize the surface, which returns the Ti-species to a surface location [134,140]. For Na3AlH6, Michel et al. found a competition between Ti substitution on the Na sites (+1 charge defect) and Ti substitution on the Al site, with an additional bound to H atom (neutral site) [139].For the hydrogenation reaction, the reports indicate that Ti near an Al surface (subsurface) promotes H2 dissociation and H spillover on the Al surface [141]. Wang et al. remind us, in favor of this role of subsurface Ti, that metallic aluminum does not absorb diatomic hydrogen from the gas phase by itself. Meanwhile, atomic hydrogen strongly reacts with aluminum surfaces to form alanes [142]. Thus, subsurface Ti would promote H2 dissociation and enhance H mobility and adsorption [142]. These effects constitute essentially the “hydrogen pump” action mechanism that was proposed for Ti [134]. Theoretical calculations of subsurface Sc, V or Nb substitution of Al indicate that these materials could also perform as a catalyst [143]. Wang et al. also remind us that Ti, Zr, V, Fe, Ni, Nb, Y, La, Ce, Pr, Nd, and Sm are expected to be good catalysts based on their ability to “mix” well with Al [142].
- Progressive changes of the oxidation state of Ti-species. While Ti+3 species is the most recurrent initial oxidation state of the Ti-catalyst, several reports conclude that the oxidation state changes to Ti0, followed by the formation of Tix-Aly alloys, and finally the formation of Al3Ti [134,144,145,146]. However, Al3Ti seems to be an inefficient catalyst, as compared to other Ti or Ti-compounds [134,147]. Perhaps the formation of Tix-Aly alloys and Al3Ti is the reason for the long-term (after hydriding/dehydriding cycling) decay of hydrogen storage capacity [148].
- Formation of Ti-Al-H complexes. Theoretical calculations suggest that the replacement of Na by Ti near o connected with [AlH4]− would lead to the formation of Ti-Al-H complexes that can help during the dehydrogenation/rehydrogenation reactions [149,150,151]. TiAl2H7 and TiAl2H2 are two optimized structures of the Ti-Al-H complexes [150]. The effect of the Ti-Al-H complexes would be to reduce the desorption energy of hydrogen [149,151] and to break H-H and Al-H bonds as a result of balanced electron-accepting/back-donation [151].
- Additional effects. Other effects, such as the formation of mobile species or vacancies, the changes in the Fermi level of reacting species, or the destabilization of Al–H bonds, can also influence the hydrogenation/dehydrogenation reactions [134].
3.2.4. Reactive Mixtures (Composites) with NaAlH4
Composites of NaAlH4-MgH2
Other Composites with NaAlH4
3.2.5. Potassium Alanate
3.2.6. Rubidium Alanate
3.2.7. Cesium Alanate
- 210–229 °C: polymorphic transition, the material gets an intense yellow color.
- 280–302: hydrogen evolution due to the proposed reaction:3CsAlH4 → 2CsH + CsAl3H8 + H2
- 454–485 °C: further decomposition reaction of 2CsH + CsAl3H8:2CsH + CsAl3H8 → 3Cs + 5H2 + 3Al
- 666–672 °C: melting of Al. This reaction pathway does not follow the same decomposition and formation of intermediaries as the rest of the alanates of group 1. In-situ diffraction data is missing for further confirmation of this proposed decomposition pathway. Krech et al. [183] demonstrated a reversible polymorphic transformation between orthorhombic and tetragonal CsAlH4; the transformation can be activated by ball-milling or by thermal treatment:
3.3. Alanates of Group 2
3.3.1. Beryllium-Alanate
3.3.2. Magnesium Alanate
3.3.3. Reactive Mixtures (Composites) with Mg(AlH4)2
- Despite the reduction in dehydrogenation temperatures, the “ideal” dehydrogenation temperature—compatible with PEM fuel cells—is not attained.
- Re-hydrogenation is only partially achieved through the formation of MgH2, not Mg(AlH4)2.
3.3.4. Calcium Alanate
3.3.5. Reactive Mixtures (Composites) with Ca(AlH4)2
3.3.6. Strontium Alanates
3.3.7. Barium Alanates
3.4. Alanates of Transition Metals
3.4.1. Scandium Alanate
3.4.2. Yttrium Alanate
3.4.3. Titanium Alanate
3.4.4. Zirconium Alanate
3.4.5. Vanadium Alanate
3.4.6. Niobium Alanates
3.4.7. Tantalum Alanates
3.4.8. Manganese Alanate
3.4.9. Iron Alanate
3.4.10. Copper Alanate
3.4.11. Silver Alanate
3.4.12. Zinc Alanate
3.5. Alanates of the Main Group
3.5.1. Gallium Alanate
3.5.2. Indium Alanate
3.5.3. Thallium Alanate
3.5.4. Tin Alanate
3.6. Alanates of Lanthanides and Actinides
3.6.1. Lanthanum, Cerium, Praseodymium and Neodymium Alanates
3.6.2. Europium Alanate
3.6.3. Ytterbium Alanate
3.6.4. Thorium-Aluminum Hydride
4. Cation-Mixed Alanates
4.1. Li-Na Alanates
4.2. Li-K Alanates
4.3. Li-Mg Alanates
4.4. Li-Ca Alanates
4.5. Na-K Alanates
5. Anion Substitution
6. Techniques of Characterization of Alanates
Fourier Transformed Infrared Spectroscopy (IR) and Raman Spectroscopy
7. Thermodynamics
8. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandrock, G.A. Panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 1999, 293–295, 877–888. [Google Scholar] [CrossRef]
- Wiswall, R.H., Jr.; Reilly, J.J. Metal Hydrides for Energy Storage. Office of Scientific & Technical Information Technical Reports, Brookhaven National Laboratory, Upton, N.Y. 1972. Available online: https://www.osti.gov/servlets/purl/6051964 (accessed on 2 July 2019).
- Vigeholm, B.; Kjøller, J.; Larsen, B. Magnesium for hydrogen storage. J. Less Common Met. 1980, 74, 341–350. [Google Scholar] [CrossRef]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Yu, X.; Tang, T.; Sun, D.; Ouyang, L.; Zhu, O. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Gross, K.; Majzoub, E. Direct Synthesis of Catalyzed Hydride Compounds. U.S. Patent No. US2003 0143154 A1, 31 July 2003. [Google Scholar]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid state hydrogen storage in alanates and alanate-based compounds: A review. Metals 2018, 8, 567. [Google Scholar] [CrossRef]
- Na Ranong, C.; Hoehne, M.; Franzen, J.; Hapke, J.; Fieg, G.; Dornheim, M.; Eigen, N.; Bellosta von Colbe, J.M.; Metz, O. Concept, design and manufacture of a prototype hydrogen storage tank based on sodium alanate. Chem. Eng. Technol. 2009, 32, 1154–1163. [Google Scholar] [CrossRef]
- Ley, M.B.; Meggouh, M.; Moury, R.; Peinecke, K.; Felderhoff, M. Development of hydrogen storage tank systems based on complex metal hydrides. Materials 2015, 8, 5891–5921. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jorgensen, S.W.; Dedrick, D.E. Performance of a full-scale hydrogen-storage tank based on complex hydrides. Faraday Discuss. 2011, 151, 327–352. [Google Scholar] [CrossRef]
- Reid, W.E.; Bish, J.M.; Brenner, A. Electrodeposition of metals from organic solutions III. Preparation and electrolysis of titanium and zirconium compounds in nonaqueous media. J. Electrochem. Soc. 1957, 104, 21–29. [Google Scholar] [CrossRef]
- Wiberg, E.; Usón, R. Zur Kenntnis eines Titan-aluminium-wassertsoffs Ti(AlH4)4. Z. Nat. 1951, 6, 392–393. [Google Scholar]
- DOE technical targets for onboard hydrogen storage for ligh-duty vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 12 August 2019).
- Bergemann, N.; Pistidda, C.; Milanese, C.; Girella, A.; Hanse, B.R.S.; Wurr, J.; Bellosta von Colbe, J.M.; Jepsen, J.; Jensen, T.R.; Marini, A.; et al. NaAlH4 production from waste aluminum by reactive ball milling. Int. J. Hydrogen Energy 2014, 39, 9877–9882. [Google Scholar] [CrossRef]
- Guerrero-Ortiz, R.; Tena-Garcia, J.R.; Flores-Jacobo, A.; Suarez-Alcantara, K. From the can to the tank: NaAlH4 from recycled aluminum. Int. J. Hydrogen Energy 2019, 44, 20183–20190. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossaina, S.; Nisfindya, O.B.; Azad, A.T.; Dawood, M.; Azada, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Sanjeev, L. Process for Preparing Dry Sodium Aluminum Hydride. U.S. Patent No. 5,295,581, 22.03, 22 March 1994. [Google Scholar]
- Ashby, E.C.; Brendel, G.J.; Redman, H.E. Direct synthesis of complex metal hydrides. Inorg. Chem. 1963, 2, 499–504. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.W.; Jensen, T.J. Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Finholt, A.E.; Bond, A.C., Jr.; Schlesinger, H.I. Lithium aluminum hydride, aluminum hydride and lithium gallium hydride, and some of their applications in organic and inorganic chemistry. J. Am. Chem. Soc. 1947, 69, 1199–1203. [Google Scholar] [CrossRef]
- Barton, L. 1.7.4.2. of Compounds of Aluminum. In Inorganic Reactions and Methods: The Formation of the Bond to Hydrogen (Part 2), Volume 2; Zuckerman, J.J., Hagen, A.P., Eds.; VCH Publishers, Inc.: Oklahoma, OK, USA, 1987; pp. 136–137. [Google Scholar]
- Wiberg, E.; Bauer, R. Zur Kenntnis eines Magnesium-aluminium-wasserstoffs Mg(AlH4)2. Z. Nat. 1950, 5, 397–398. [Google Scholar]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Wiberg, E.; Bauer, R. Neues zur Kenntnis des Magnesium-aluminium-wasserstoffs Mg(AlH4)2. Z. Nat. 1952, 7, 131–132. [Google Scholar] [CrossRef]
- Schwab, W.; Wintersberger, K. Über Darstellung und Eigenschaften von Calciumaluminiumhydrid Ca(AlH4)2. Z. Nat. 1953, 8, 690–691. [Google Scholar] [CrossRef]
- Schwarz, M.; Haiduc, A.; Stil, H.; Paulus, P.; Geerlings, H. The use of complex metal hydrides as hydrogen storage materials: Synthesis and XRD-studies of Ca(AlH4)2 and Mg(AlH4)2. J. Alloys Compd. 2005, 404–406, 762–765. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O. Synthesis and structures of magnesium alanate and two solvent adducts. J. Alloys Compd. 2002, 345, 286–296. [Google Scholar] [CrossRef]
- Komiya, K.; Morisaku, N.; Shinzato, Y.; Ikeda, K.; Orimo, S.; Ohki, Y.; Tatsumi, K.; Yukawa, H.; Morinaga, M. Synthesis and dehydrogenation of M(AlH4)2 (M = Mg, Ca). J. Alloys Compd. 2007, 446–447, 237–241. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Felderhoff, M.; Bogdanović, B.; Schüth, F.; Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem. Commun. 2005, 41, 4732–4734. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, B.; Schwickardi, M. Method for Reversibly Storing Hydrogen on the Basis of Alkali Metals and Aluminum. U.S. Patent No. US 2003/0053948 A1, 20 March 2003. [Google Scholar]
- Huot, J.; Ravnsbæk, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Eigen, N.; Gosch, F.; Dornheim, M.; Klassen, T.; Bormann, R. Improved hydrogen sorption of sodium alanate by optimized processing. J. Alloys Compd. 2008, 465, 310–316. [Google Scholar] [CrossRef]
- Hlova, I.; Goldston, J.F.; Gupta, S.; Kobayashi, T.; Pruski, M. A benign synthesis of alane by the composition-controlled mechanochemical reaction of sodium hydride and aluminum chloride. J. Mater. Sci. 2017, 52, 11900–11910. [Google Scholar] [CrossRef]
- Hlova, I.; Gupta, S.; Goldston, J.E.; Kobayashi, T.; Pruski, M.; Pecharsky, V.K. Dry mechanochemical synthesis of alane from LiH and AlCl3. Faraday Discuss. 2014, 170, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Hlova, I. Mechanochemical Synthesis of Hydrogen-Storage Materials Based on Aluminum, Magnesium and Their Compounds. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2015. [Google Scholar]
- Dymova, T.N.; Mal’Tseva, N.N.; Konoplev, V.N.; Golovanova, A.I.; Aleksandrov, D.P.; Sizareva, A.S. Solid-phase solvate-free formation of magnesium hydroaluminates Mg(AlH4)2 and MgAlH5 upon mechanochemical activation or heating of magnesium hydride and aluminum chloride mixtures. Russ. J. Coord. Chem. 2003, 29, 385–398. [Google Scholar] [CrossRef]
- Kabbour, H.; Ahn, C.C.; Hwang, S.-J.; Bowman, C.R.; Graetz, J. Direct synthesis and NMR characterization of calcium alanate. J. Alloys Compd. 2007, 445–447, 264–266. [Google Scholar] [CrossRef]
- Iosub, V.; Matsunaga, T.; Tange, K.; Ishikiriyama, M. Direct synthesis of Mg(AlH4)2 and CaAlH5 crystalline compounds by ball milling and their potential as hydrogen storage materials. Int. J. Hydrogen Energy 2009, 34, 906–912. [Google Scholar] [CrossRef]
- Sato, T.; Ikeda, K.; Li, H.-W.; Yukawa, H.; Morinaga, M.; Orimo, S. Direct dry syntheses and thermal analyses of a series of aluminum complex hydrides. Mater. Trans. 2009, 50, 182–196. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.-K.; Shim, J.-H.; Cho, Y.W.; Yoon, K.B. Mechanochemical synthesis and thermal decomposition of Mg(AlH4)2. J. Alloys Compd. 2006, 422, 283–287. [Google Scholar] [CrossRef]
- Pommerin, A.; Wosylus, A.; Felderhoff, M.; Schüth, F.; Weidenthaler, C. Synthesis, crystal structures, and hydrogen-storage properties of Eu(AlH4)2 and Sr(AlH4)2 and their decomposition intermediates, EuAlH5 and SrAlH5. Inorg. Chem. 2012, 51, 4143–4150. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloys Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and properties of aluminum hydride. J. Am. Chem. Soc. 1976, 98, 2450–2454. [Google Scholar] [CrossRef]
- Brinks, H.W.; Istad-Lem, A.; Hauback, B.C. Mechanochemical synthesis and crystal structure of α’-AlD3 and α-AlD3. J. Phys. Chem. B 2006, 110, 25833–25837. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Characterization of mechanochemically synthesized alane (AlH3) nanoparticles. J. Alloys Compd. 2009, 487, 370–376. [Google Scholar] [CrossRef]
- Gupta, S.; Kobayashi, T.; Hlova, I.Z.; Goldston, J.F.; Pruski, M.; Pecharsky, V.K. Solvent-free mechanochemical synthesis of alane, AlH3: Effect of pressure on the reaction pathway. Green Chem. 2014, 16, 4378–4388. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.J.; García-Díaz, B.L.; Teprovich, J.A., Jr.; Knight, D.A.; Zidan, R. Advances in the electrochemical regeneration of aluminum hydride. Appl. Phys. A 2012, 106, 545–550. [Google Scholar] [CrossRef]
- Zidan, R. Electrochemical Process and Production of Novel Complex Hydrides. U.S. Patent No. US 8,470,156 B2, 25 June 2013. [Google Scholar]
- Zidan, R. Enhancing Electrochemical Methods for Producing and Regenerating Alane by Using Electrochemical Catalytic Additive. U.S. patent No. US 9, 850,585 B1, 26 December 2017. [Google Scholar]
- Graetz, J.; Reilly, J.J. Thermodynamics of the α, β and γ polymorphs of AlH3. J. Alloys Compd. 2006, 424, 262–265. [Google Scholar] [CrossRef]
- Graetz, J.; Hauback, B.C. Recent developments in aluminum-based hydrides for hydrogen storage. MRS Bull. 2013, 38, 473–479. [Google Scholar] [CrossRef]
- Graetz, J.; Chaudhuri, S.; Lee, Y.; Vogt, T.; Muckerman, J.T.; Reilly, J.J. Pressure-induced structural and electronic changes in α-AlH3. Phys. Rev. B 2006, 74, 214114. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Aguey-Zinsou, K.F. Hydrogen storage properties of nanoconfined aluminium hydride (AlH3). Chem. Eng. Sci. 2019, 194, 64–70. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kato, T.; Brown, C.; Jensen, C.M. Intrinsic and mechanical modified thermal stabilities of α-, β- and γ-aluminum trihydries AlH3. Appl. Phys. A 2006, 83, 5–8. [Google Scholar] [CrossRef]
- Sandrock, G.; Reilly, J.; Graetz, J.; Zhou, W.-M.; Johnson, J.; Wegrzyn, J. Alkali metal hydride doping of α-AlH3 for enhanced H2 desorption kinetics. J. Alloys Compd. 2006, 421, 185–189. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J. Decomposition kinetics of the AlH3 polymorphs. J. Phys. Chem. B 2005, 47, 22181. [Google Scholar] [CrossRef]
- Ikeda, K.; Muto, S.; Tatsumi, K.; Menjo, M.; Kato, S.; Bielmann, M.; Züttel, A.; Jensen, C.M.; Orimo, S. Dehydriding reaction of AlH3: In situ microscopic observations combined with thermal and surface analyses. Nanotechnology 2009, 20, 204004. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Dong, Z.; Cao, G.; Liu, Y.; Chen, L.; Yan, M. Dehydriding properties of γ-AlH3. Int. J. Hydrogen Energy 2013, 38, 10851–10865. [Google Scholar] [CrossRef]
- Sartori, S.; Opalka, S.M.; Løvvik, O.M.; Guzik, M.N.; Tang, X.; Hauback, B.C. Experimental studies of α-AlD3 and α’-AlD3 versus first-principles modeling of the alane isomorphs. J. Mater. Chem. 2008, 18, 2361–2370. [Google Scholar] [CrossRef]
- Sandrock, G.; Reilly, J.; Graetz, J.; Zhou, W.-M.; Johnson, J.; Wegrzyn, J. Accelerated thermal decomposition of AlH3 for hydrogen-fueled vehicles. Appl. Phys. A. 2005, 80, 687–690. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liu, Y.; Dong, Z.; Ge, H.; Li, S.; Yan, M. Hydrogen desorption properties of the MgH2–AlH3 composites. J. Phys. Chem. C 2014, 118, 37–45. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Zhou, H.; Gao, S.; Ge, H.; Li, S.; Yan, M. Improved hydrogen desorption properties of LiBH4 by AlH3 addition. Int. J. Hydrogen Energy 2016, 41, 22118–22127. [Google Scholar] [CrossRef]
- Goncharenko, I.N.; Glazkov, V.P.; Irodova, A.V.; Somenkov, V.A. Neutron diffraction study of crystal structure and equation of state AID3 up to the pressure of 7.2 GPa. Physica B 1991, 174, 117–120. [Google Scholar] [CrossRef]
- Brinks, H.W.; Brown, C.; Jensen, C.M.; Graetz, J.; Reilly, J.J.; Hauback, B.C. Synthesis and crystal structure of β-AlD3. J. Alloys Compd. 2007, 433, 180–183. [Google Scholar] [CrossRef]
- Brinks, H.W.; Langley, W.; Jensen, C.M.; Graetz, J.; Reilly, J.J.; Hauback, B.C. The crystal structure of γ-AlD3. J. Alloys Compd. 2007, 441, 364–367. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Haga, T.; Matsumoto, M.; Koiwai, A. Direct formation of LiAlH4 by a mechanochemical reaction. J. Alloys Compd. 2007, 441, 189–191. [Google Scholar] [CrossRef]
- Block, J.; Gray, A.P. The thermal decomposition of lithium aluminum hydride. Inorg. Chem. 1965, 4, 304–305. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Ares, J.R.; Aguey-Zinsou, F.; Porcu, M.; Sykes, J.M.; Dornheim, M.; Klassen, T.; Bormann, R. Thermal and mechanically activated decomposition of LiAlH4. Mater. Res. Bull. 2008, 43, 1263–1275. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible hydrogen storage via titanium-catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Effects of NbF5 addition on the hydrogen storage properties of LiAlH4. Int. J. Hydrogen Energy 2010, 35, 2361–2367. [Google Scholar] [CrossRef]
- Blanchard, D.; Brinks, H.W.; Hauback, B.C.; Norby, P.; Muller, J. Isothermal decomposition of LiAlD4 with and without additives. J. Alloys Compd. 2005, 404–406, 743–747. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Vidya, R.; Fjellvåg, H.; Kjekshus, A. Huge-pressure-induced volume collapse in LiAlH4 and its implications to hydrogen storage. Phys. Rev. B 2003, 68, 212101. [Google Scholar] [CrossRef]
- Sklar, N.; Post, B. The crystal structure of lithium aluminum hydride. Inorg. Chem. 1967, 6, 669–671. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Fjellvåg, H. Accurate structure of LiAlD4 studied by combined powder neutron and X-ray diffraction. J. Alloys Compd. 2002, 346, 184–189. [Google Scholar] [CrossRef]
- Sato, T.; Tomiyasu, K.; Ikeda, K.; Otomo, T.; Feygenson, M.; Neuefeind, J.; Yamada, K.; Orimo, S.I. Local atomic structural investigations of precursory phenomenon of the hydrogen release from LiAlD4. J. Alloys Compd. 2014, 586, 244–247. [Google Scholar] [CrossRef]
- Pitt, M.P.; Blanchard, D.; Hauback, B.C.; Fjellvåg, H.; Marshall, W.G. Pressure-induced phase transitions of the LiAlD4 system. Phys. Rev. B 2005, 72, 214113. [Google Scholar] [CrossRef]
- Løvvik, O.; Opalka, S.; Brinks, W.H.; Hauback, B.C. Crystal structure and thermodynamic stability of the lithium alanates LiAlH4 and Li3AlH6. Phys. Rev. B 2004, 69, 134117. [Google Scholar] [CrossRef]
- Liu, X.; McGrady, G.S.; Langmi, H.W.; Jensen, C.M. Facile cycling of Ti-doped LiAlH4 for high performance hydrogen storage. J. Am. Chem. Soc. 2009, 131, 5032–5033. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Langmi, H.W.; Beattie, S.D.; Azenwi, F.F.; McGradyc, S.; Jensen, C.M. Ti-doped LiAlH4 for hydrogen storage: Synthesis, catalyst loading and cycling performance. J. Am. Chem. Soc. 2011, 133, 15593–15597. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Wegrzyn, J.; Reilly, J.J. Regeneration of lithium aluminum hydride. J. Am. Chem. Soc. 2008, 130, 17790–17794. [Google Scholar] [CrossRef] [PubMed]
- Brinks, H.W.; Fossdal, A.; Fonneløp, J.E.; Hauback, B.C. Crystal structure and stability of LiAlD4 with TiF3 additive. J. Alloys Compd. 2005, 397, 291–295. [Google Scholar] [CrossRef]
- Ke, X.; Chen, C. Thermodynamic functions and pressure-temperature phase diagram of lithium alanates by ab initio calculations. Phys. Rev. B 2007, 76, 024112. [Google Scholar] [CrossRef]
- Jang, J.-W.; Shim, J.-H.; Cho, Y.C.; Lee, B.-J. Thermodynamic calculation of LiH ↔ Li3AlH6 ↔ LiAlH4 reactions. J. Alloys Compd. 2006, 420, 286–290. [Google Scholar] [CrossRef]
- Bogdanović, B.; Brand, R.A.; Marjanović, A.; Schwickardi, M.; Tölle, J. Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J. Alloys Compd. 2000, 302, 36–58. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Yu, X.; Ismail, M.; Liu, H. Enhanced hydrogen storage performance of LiAlH4–MgH2–TiF3 composite. Int. J. Hydrogen Energy 2011, 36, 5369–5374. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhai, F.; Qu, X.; Volinsky, A.V. Improved hydrogen storage performance of MgH2-LiAlH4 composite by the addition of MnFe2O4. J. Phys. Chem. C 2013, 117, 26940–26947. [Google Scholar] [CrossRef]
- Lin, I.C.; Tsai, W.-T. In situ synchrotron X-ray diffraction study on the rehydrogenation behavior of MgH2-LiAlH4 composites. In Proceedings of the 2017 IEEE International Conference on Applied System Innovation: Applied System Innovation for Modern Technology, ICASI, Sapporo, Japan, 13–17 May 2017; Volume 2017, pp. 1918–1921. [Google Scholar]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Effect of different additives on the hydrogen storage properties of the MgH2-LiAlH4 destabilized system. RSC Adv. 2011, 1, 408–414. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.; Xu, L.; Chen, L.; Li, S.; Chen, C. An investigation on the reaction mechanism of LiAlH4–MgH2 hydrogen storage system. Mater. Chem. Phys. 2010, 124, 83–87. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Enhanced hydrogen storage properties of 4MgH2-LiAlH4 composite system by doping with Fe2O3 nanopowder. Int. J. Hydrogen Energy 2014, 39, 7834–7841. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Zhu, Y.; Zhuang, X.; Dong, J.; Qu, Y.; Guo, X.; Chen, J.; Wang, Z.; Li, L. The hydrogen storage performance of a 4MgH2-LiAlH4-TiH2 composite system. J. Alloys Compd. 2016, 676, 557–564. [Google Scholar] [CrossRef]
- Meggouh, M.; Grant, D.M.; Walker, G.S. Optimizing the destabilization of LiBH4 for hydrogen storage and the effect of different Al sources. J. Phys. Chem. C 2011, 115, 22054–22061. [Google Scholar] [CrossRef]
- Jin, A.-S.; Shim, J.-H.; Cho, Y.W.; Yi, K.-W.; Zabara, O.; Fichtner, M. Reversible hydrogen storage in LiBH4–Al–LiH composite powder. Scr. Mater. 2008, 58, 963–965. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Liu, H.K.; Yu, X.B. Reversible hydrogen storage in titanium-catalyzed LiAlH4–LiBH4 system. J. Alloys Compd. 2009, 487, 434–438. [Google Scholar] [CrossRef]
- Sridechprasat, P.; Phuitot, L.; Rangsunvigit, P.; Kitiyanna, B.; Kulprathipanja, S. A revisit to the hydrogen desorption/absorption behaviors of LiAlH4/LiBH4: Effects of catalysts. Energies 2012, 5, 3691–3700. [Google Scholar] [CrossRef]
- Gu, R.; Wang, C.Y.; Liu, D.M.; Gao, C.; Li, Y.T.; Si, T.Z. De-/rehydrogenation properties and reaction mechanisms of 4LiBH4–LiAlH4–MgF2 system. Int. J. Hydrogen Energy 2015, 40, 10536–10541. [Google Scholar] [CrossRef]
- Mao, J.F.; Yu, X.B.; Guo, Z.P.; Poh, C.K.; Liu, H.K.; Wu, Z.; Ni, J. Improvement of the LiAlH4−NaBH4 system for reversible hydrogen storage. J. Phys. Chem. C 2009, 113, 10813–10818. [Google Scholar] [CrossRef]
- Xia, G.; Meng, Q.; Guo, Z.; Gud, Q.; Liu, H.; Liu, Z.; Yu, X. Nanoconfinement significantly improves the thermodynamics and kinetics of co-infiltrated 2LiBH4–LiAlH4 composites: Stable reversibility of hydrogen absorption/resorption. Acta Mater. 2013, 61, 6882–6893. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, G.; Zhang, J.; Guo, Z.; Yu, X. Graphene-tailored molecular bonds for advanced hydrogen and lithium storage performance. Energy Storage Mater. 2019, 17, 178–185. [Google Scholar] [CrossRef]
- Carr, C.L.; Jayawardana, W.; Zou, H.; White, J.L.; Gabaly, F.E.; Corandi, M.S.; Stavila, V.; Allendorf, M.D.; Majzoub, E.H. Anomalous H2 desorption rate of NaAlH4 confined in nitrogen-doped nanoporous carbon frameworks. Chem. Mater. 2018, 30, 2930–2938. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M. Nanoconfinement effects in energy storage materials. Phys. Chem. Chem. Phys. 2011, 13, 21186–21195. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Investigation on chemical reaction between LiAlH4 and LiNH2. J. Power Sources 2006, 159, 167–170. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L.; Jang, M. Mechano-chemical synthesis of nanostructured hydride composites based on Li-Al-N-Mg for solid state hydrogen storage. Eng. Rev. 2011, 31, 111–123. [Google Scholar]
- Chen, R.; Wang, X.; Chen, L.; Li, S.; Ge, H.; Lei, Y.; Chen, C. An investigation on the reaction pathway between LiAlH4 and LiNH2 via gaseous ammonia. J. Alloys Compd. 2010, 495, 17–22. [Google Scholar] [CrossRef]
- Dolotko, O.; Kobayashi, T.; Wiench, J.W.; Pruski, M.; Pecharsky, V. Investigation of the thermochemical transformations in the LiAlH4-LiNH2 system. Int. J. Hydrogen Energy 2011, 36, 10626–10634. [Google Scholar] [CrossRef]
- Lu, J.; Fang, Z.Z. Dehydrogenation of a combined LiAlH4/LiNH2 system. J. Phys. Chem. B 2005, 109, 20830–20834. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, L.H.; Ranvsbæk, D.B.; Grundlach, C.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A novel intermediate in the LiAlH4–LiNH2 hydrogen storage system. Dalton Trans. 2014, 43, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Rather, S.; So, C.S.; Hwang, S.W.; Kim, A.R.; Nahm, K.S. Thermal decomposition of LiAlH4 chemically mixed with lithium amide and transition metal chlorides. Int. J. Hydrogen Energy 2009, 34, 8937–8943. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Kaskel, S.; Pommerin, A.; Schlichte, K.; Schüth, F. Improved hydrogen storage properties of Ti-doped sodium alanate using titanium nanoparticles as doping agents. Adv. Mater. 2003, 15, 1012–1015. [Google Scholar] [CrossRef]
- Gross, K.J.; Guthrie, S.; Takara, S.; Thomas, G. In-situ X-ray diffraction study of the decomposition of NaAlH4. J. Alloys Compd. 2000, 297, 270–281. [Google Scholar] [CrossRef]
- Jensen, C.; Wang, Y.; Chou, M.Y. Alanates as hydrogen storage materials. In Solid-State Hydrogen Storage: Materials and Chemistry; Walker, G., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2008; pp. 381–419. [Google Scholar]
- Lauher, J.W.; Dougherty, D.; Herley, P.J. Sodium tetrahydroaluminate. Acta Crystallogr. Sect. B 1979, 35, 1454–1456. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Jensen, C.M.; Murphy, K.; Maeland, A.J. Neutron diffraction structure determination of NaAlD4. J. Alloys Compd. 2003, 358, 142–145. [Google Scholar] [CrossRef]
- Vajeeston, P.; Fjellvåg, H. Crystal structures of aluminum-based hydrides. Emerg. Mater. Res. 2015, 4, 192–217. [Google Scholar] [CrossRef]
- Canton, P.; Fichtner, M.; Frommen, C.; Léon, A. Synchrotron X-ray studies of Ti-doped NaAlH4. J. Phys. Chem. B 2006, 110, 3051–3054. [Google Scholar] [CrossRef]
- Rönnebro, E.; Noréus, D.; Kadir, K.; Reiser, A.; Bogdanović, B. Investigation of the perovskite related structures of NaMgH3, NaMgF3 and Na3AlH6. J. Alloys Compd. 2000, 299, 101–106. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Sodium alanate system for efficient hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 8798–8812. [Google Scholar] [CrossRef]
- Pitt, M.P.; Vullum, P.E.; Sørby, M.H.; Emerich, H.; Paskevicius, M.; Buckley, C.E.; Walmsley, J.C.; Holmestad, R.; Hauback, B.C. Hydrogen absorption kinetics of the transition-metal-chloride-enhanced NaAlH4 system. J. Phys. Chem. C 2012, 116, 14205–14217. [Google Scholar] [CrossRef]
- Lee, G.-J.; Shim, J.-H.; Whan, Y.C.; Lee, K. Reversible hydrogen storage in NaAlH4 catalyzed with lanthanide oxides. Int. J. Hydrogen Energy 2007, 32, 1911–1915. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, Y.; Liu, G.; Han, Y.; Qiu, F.; Yan, C.; Song, D.; Jiao, L.; Yuan, H. Direct synthesis of sodium alanate with novel catalytic TiB2. J. Alloys Compd. 2011, 509S, S747–S749. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Huang, S.; Wang, P.; Tian, H. Electronic and dehydrogenation properties of TiB2 cluster-doped NaAlH4 (101) surface: A first-principle approach. Int. J. Hydrogen Energy 2014, 39, 14178–14183. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloys Compds. 2013, 566, 137–141. [Google Scholar] [CrossRef]
- Mosher, D.A.; Tang, X.; Brown, R.J.; Saitta, S.; Laube, B.L.; Dold, R.H.; Anton, D.L. High Density Hydrogen Storage System Demonstration Using NaAlH4 Based Complex Compound Hydrides. 1–161; United Technologies Research Center: East Hartford, CT, USA, 2007. [Google Scholar]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N.; Stark, A. Cycling properties of Sc-and Ce-doped NaAlH4 hydrogen storage materials prepared by the one-step direct synthesis method. J. Alloys Compd. 2009, 471, 383–386. [Google Scholar] [CrossRef]
- Berseth, P.A.; Harter, A.G.; Zidan, R.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R.; Jena, P. Carbon nanomaterials as catalysts for hydrogen uptake and release in NaAlH4. Nano Lett. 2009, 4, 1501–1505. [Google Scholar] [CrossRef]
- Rongeat, C.; Scheerbaum, N.; Schultz, L.; Gutfleisch, O. Catalysis of H2 sorption in NaAlH4: General description and new insights. Acta Mater. 2011, 59, 1725–1733. [Google Scholar] [CrossRef]
- Lozano, G.A.; Na Ranong, C.; Bellosta von Colbe, J.M.; Bormann, R.; Fieg, G.; Hapke, J.; Dornheim, M. Empirical kinetic model of sodium alanate reacting system (I). Hydrogen absorption. Int. J. Hydrogen Energy 2010, 35, 6763–6772. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Proposed mechanisms for the catalytic activity of Ti in NaAlH4. Chem. Rev. 2012, 112, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.D.; Mcgrady, G.S. Hydrogen desorption studies of NaAlH4 and LiAlH4 by in situ heating in an ESEM. Int. J. Hydrogen Energy 2009, 34, 9151–9156. [Google Scholar] [CrossRef]
- Singh, S.; Eijt, S.W.H.; Huot, J.; Kockelmann, W.A.; Wagemaker, M.; Mulder, F.M. The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation. Acta Mater. 2007, 55, 5549–5557. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Opalka, S.M. Density functional calculations of Ti-enhanced NaAlH4. Phys. Rev. B 2005, 71, 054103. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Opalka, S.M. Stability of Ti in NaAlH4. Appl. Phys. Lett. 2006, 88, 161917. [Google Scholar] [CrossRef]
- Michel, K.J.; Ozoliņš, V. Site substitution of Ti in NaAlH4 and Na3AlH6. J. Phys. Chem C 2011, 115, 21454–21464. [Google Scholar] [CrossRef]
- Marashdeh, A.; Versluis, J.-W.I.; Valdés, A.; Olsen, R.A.; Løvvik, O.M.; Kroes, G.-J. Effect of transition metal dopants on initial mass transport in the dehydrogenation of NaAlH4: Density functional theory study. J. Phys. Chem. C 2013, 117, 3–14. [Google Scholar] [CrossRef]
- Al-Mahboob, A.; Muller, E.; Karim, A.; Muckerman, J.T.; Ciobanu, C.V.; Sutter, P. Site-dependent activity of atomic Ti catalysts in Al-based hydrogen storage materials. J. Am. Chem. Soc. 2012, 134, 10381–10384. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Stumpf, R.; Lin, P.; Chou, M.Y. Catalytic effect of near-surface alloying on hydrogen interaction on the aluminum surface. Phys. Rev. B 2011, 83, 195419. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Chou, M.Y. Hydrogen interaction with the Al surface promoted by subsurface alloying with transition metals. J. Phys. Chem. C 2012, 116, 18663–18668. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, G.; Zhang, G.; Yan, X.; Li, P.; Yao, Y.; Luo, D.; Chen, C.; Tang, T. Evolution of the active species and catalytic mechanism of Ti doped NaAlH4 for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6088–6095. [Google Scholar] [CrossRef]
- Léon, A.; Kircher, O.; Rothe, J.; Fichtner, M. Chemical state and local structure around titanium atoms in NaAlH4 doped with TiCl3 using X-ray absorption spectroscopy. J. Phys. Chem. B 2004, 108, 16372–16376. [Google Scholar] [CrossRef]
- Haiduc, A.G.; Stil, H.A.; Schwarz, M.A.; Paulus, P.; Geerlings, J.J.C. On the fate of the Ti catalyst during hydrogen cycling of sodium alanate. J. Alloys Compd. 2005, 393, 252–263. [Google Scholar] [CrossRef]
- Kang, X.D.; Wang, P.; Song, X.P.; Yao, X.D.; Lu, G.Q.; Cheng, H.M. Catalytic effect of Al3Ti on the reversible dehydrogenation of NaAlH4. J. Alloys Compd. 2006, 424, 365–369. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C.; Srinivasan, S.S.; Jensen, C.M. Synchrotron X-ray studies of Al1−yTiy formation and re-hydriding inhibition in Ti-enhanced NaAlH4. J. Phys. Chem. B 2005, 109, 15780–15785. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, Q. A precursor state for formation of TiAl3 complex in reversible hydrogen desorption/adsorption from Ti-doped NaAlH4. Chem. Commun. 2006, 42, 1822–1824. [Google Scholar] [CrossRef]
- Dathara, G.K.P.; Mainardi, D.S. Structure and dynamics of Ti-Al-H compounds in Ti-doped NaAlH4. Mol. Simul. 2008, 34, 201–210. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Ge, Q. Effect of doped transition metal on reversible hydrogen release/uptake from NaAlH4. Chem. Eur. J. 2009, 15, 1685–1695. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Mao, J.F.; Dou, S.X. The hydrogen storage properties and reaction mechanism of the MgH2-NaAlH4 composite system. Int. J. Hydrogen Energy 2011, 36, 9045–9050. [Google Scholar] [CrossRef]
- Bendyna, J.K.; Dyjak, S.; Notten, P.H.L. The influence of ball-milling time on the dehydrogenation properties of the NaAlH4-MgH2 composite. Int. J. Hydrogen Energy 2015, 40, 4200–4206. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Improved hydrogen storage performance of MgH2-NaAlH4 composite by the addition of TiF3. Int. J. Hydrogen Energy 2012, 37, 8395–8401. [Google Scholar] [CrossRef]
- Rafi-ud-Din; Qu, X.; Zahid, G.H.; Asghar, Z.; Shahzad, M.; Iqbal, M.; Ahmad, E. Improved hydrogen storage performances of MgH2-NaAlH4 system catalyzed by TiO2 nanoparticles. J. Alloys Compd. 2014, 604, 317–324. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, Y.; Sun, W.; Lou, H.; Liu, Y.; Qi, Q.; Zhang, J.; Liu, J.; Yan, K.; Jin, H.; et al. The enhanced de/re-hydrogenation performance of 4MgH2-NaAlH4 composite by doping with TiH2. J. Alloys Compd. 2017, 698, 1002–1008. [Google Scholar] [CrossRef]
- Plerdseanoy, P.; Meethon, S.; Utke, R. Dehydrogenation kinetics, reversibility, and reaction mechanisms of reversible hydrogen storage material based on nanoconfined MgH2-NaAlH4. J. Phys. Chem. Solids 2015, 87, 16–22. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, S.K.; Dixit, V.; Shukla, V.; Shahi, R.R.; Shaz, M.A.; Srivastava, O.N. Catalytic effect of carbon nanostructures on the hydrogen storage properties of MgH2-NaAlH4 composite. Int. J. Hydrogen Energy 2014, 39, 14240–14246. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Dou, S.X. An investigation on the hydrogen storage properties and reaction mechanism of the destabilized MgH2-Na2AlH6 (4:1) system. Int. J. Hydrogen Energy 2013, 38, 1478–1483. [Google Scholar] [CrossRef]
- Ranvsbæk, D.B.; Jensen, T.R. Tuning the hydrogen storage properties and reactivity: Investigation of the LiBH4-NaAlH4 system. J. Phys. Chem. Solids 2010, 71, 144–1149. [Google Scholar] [CrossRef]
- Morioka, H.; Kakizaki, K.; Chung, S.C.; Yamada, A. Reversible hydrogen decomposition of KAlH4. J. Alloys Compd. 2003, 353, 310–314. [Google Scholar] [CrossRef]
- Dymova, T.N.; Eliseeva, N.G.; Bakum, S.I.; Dergachev, Y.M. Direct synthesis of alkali metal aluminum hydrides in the melt. Dokl. Akad. Nauk SSSR 1974, 215, 1369–1372. [Google Scholar]
- Pukazhselvan, D.; Fagg, D.P.; Srivastava, O.N. On step high pressure mechanochemical synthesis of reversible alanates NaAlH4 and KAlH4. Int. J. Hydrogen Energy 2015, 40, 4916–4924. [Google Scholar] [CrossRef]
- Ares, J.R.; Zhang, J.; Charpentier, T.; Cuevas, F.; Latroche, M. Asymmetric reaction path and hydrogen sorption mechanism in mechanochemically synthesized potassium alanate (KAlH4). J. Phys. Chem. C 2016, 120, 21299–21308. [Google Scholar] [CrossRef]
- Mamatha, M.; Weidenhaler, C.; Pommerin, A.; Felderhoff, M.; Schüth, F. Comparative studies of the decomposition of alanates followed by in situ XRD and DSC methods. J. Alloys Compd. 2006, 416, 303–314. [Google Scholar] [CrossRef]
- Matysina, Z.A.; Zaginaichenko, S.Y.; Schur, D.V.; Zolotarenko, A.D.; Zolotarenko, A.D.; Gabdulin, M.T. Hydrogen sorption properties of potassium alanate. Russ. Phys. J. 2018, 61, 253–263. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.-F.; Leardini, F.; Jímenez Ferrer, I.; Fernandez, J.-F.; Guo, Z.-X.; Sánchez, C. Hydrogen absorption/desorption mechanism in potassium alanate (KAlH4) and enhancement by TiCl3 doping. J. Phys. Chem. C 2009, 113, 6845–6851. [Google Scholar] [CrossRef]
- Arroyo y de Dompablo, M.E.; Ceder, G. First principles investigations of complex hydrides AMH4 and A3MH6 (A = Li, Na, K, M = B, Al, Ga) as hydrogen storage systems. J. Alloys Compd. 2004, 364, 6–12. [Google Scholar] [CrossRef]
- Santhanam, R.; McGrady, G.S. Synthesis of alkali metal hexahydroaluminate complexes using dimethyl ether as a reaction medium. Inorg. Chim. Acta 2008, 361, 473–478. [Google Scholar] [CrossRef]
- Dymova, T.N.; Selivokhina, M.S.; Eliseeva, N.G. Thermal stability of potassium alumohydride. Dokl. Akad. Nauk 1963, 153, 1330–1332. [Google Scholar]
- Sorte, E.G.; Emery, S.B.; Majzoub, E.H.; Ellis-Caleo, T.; Ma, Z.L.; Hammann, B.A.; Hayes, S.E.; Bowman, R.C., Jr.; Conradi, M.S. NMR study of anion dynamics in solid KAlH4. J. Phys. Chem. C 2014, 118, 5725–5732. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Jensen, T.R. New compounds in the potassium-aluminium-hydrogen system observed during release and uptake of hydrogen. Int. J. Hydrogen Energy 2012, 37, 345–356. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Heyn, R.H.; Blom, R.; Fjellvag, H. The crystal structure of KAlD4. J. Alloys Compd. 2005, 394, 35–38. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvag, H. Crystal structure of KAlH4 from first principle calculations. J. Alloys Compds. 2004, 363, L7–L11. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekhus, A.; Fjellvåg, H. First-principles investigations of aluminum hydrides: M3AlH6 (M = Na, K). Phys. Rev. B 2005, 71, 092103. [Google Scholar] [CrossRef]
- Weindenthaler, C.; Felderhoff, M.; Bernert, T.; Sørby, M.H.; Hauback, B.C.; Krech, D. Synthesis, crystal structure analysis and decomposition of RbAlH4. Crystals 2018, 8, 103. [Google Scholar] [CrossRef]
- Adkis, T.G.; Gavrilenko, V.V.; Zahartin, I.I.; Ignat’eva, I.A. Study of the infrared spectra of alkali metal aluminum hydrides. Zhural Prikl. Spektrosk. 1967, 6, 806–812. [Google Scholar] [CrossRef]
- Bestide, J.P.; Hajri, J.E.; Claudy, P.; Hajbi, A.E. A new route to alkali metal aluminum hydrides MAlH4 with M = Na, K, Rb, Cs and structural features for the whole family with M = Li to Cs. Synth. React. Inorg. Met. Org. Chem. 1995, 25, 1037–1047. [Google Scholar] [CrossRef]
- Dymova, T.N.; Bakum, S.I.; Mirsaidov, U. Phase States of Alkaly Aluminum Hydrides. Dokl. Akad. Nauk SSSR 1974, 216, 87–90. [Google Scholar]
- Vajeeston, P.; Ravindran, P.; Vidya, R.; Fjellvåg, H.; Kjeshus, A. Design of potential hydrogen-storage materials using first-principle density-functional calculations. Cryst. Growth Des. 2004, 4, 471–477. [Google Scholar] [CrossRef]
- Adimi, S.; Arabi, H.; Ghorbani, S.R.; Pourarin, F. AB-initio study of pressure-induced aluminum hydrides AAlH4 (A = Li, Na, K, Rb, Cs). Int. J. Hydrogen Energy 2017, 42, 25303–25309. [Google Scholar] [CrossRef]
- Ravindan, P.; Vajeeston, P.; Vidya, R.; Fjellvåg, H.; Kjekshus, A. Modeling of hydrogen storage materials by density-functional calculations. J. Power Sources 2006, 159, 88–99. [Google Scholar] [CrossRef]
- Krech, D.; Zibrowius, B.; Weidenthaler, C.; Felderhoff, M. On the preparation and structure of cesium aluminum tetrahydride. Eur. J. Inorg. Chem. 2014, 2014, 5683–5688. [Google Scholar] [CrossRef]
- Bakum, S.I.; Kuznetsova, S.F.; Tarasov, V.P. CsAlH4 thermolysis. Russ. J. Inorg. Chem. 2013, 58, 1547–1549. [Google Scholar] [CrossRef]
- Bernert, T.; Krech, D.; Kockelmann, W.; Felderhoff, M.; Frankcombe, T.J.; Weidenthaler, C. Crystal structure relation between tetragonal and orthorhombic CsAlD4: DFT and time-of-flight neutron powder diffraction studies. Eur. J. Inorg. Chem. 2015, 2015, 5545–5550. [Google Scholar] [CrossRef]
- Graetz, J. Metastable metal hydrides for hydrogen storage. ISRN Mater. Sci. 2012, 2012, 863025. [Google Scholar] [CrossRef]
- Mackay, K.M. Hydrogen Compounds of the Metallic Elements; E. & F. N. Spon Ltd.: London, UK, 1966; p. 177. [Google Scholar]
- Schlesinger, H.I.; Brown, H.C.; Abraham, B.; Bond, A.C.; Davidson, N.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.; Horvitz, L.; Hyde, E.K.; et al. New developments in the chemistry of diborane and the borohydrides. I. General summary. J. Am. Chem. Soc. 1953, 75, 186–190. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Hyde, E.K. The preparation of other borohydrides by metathetical reactions utilizing the alkali metal borohydrides. J. Am. Chem. Soc. 1953, 75, 209–213. [Google Scholar] [CrossRef]
- Ashby, E.C.; Sanders, J.R.; Claudy, P.; Schwartz, R.D. A study of the reactions of lithium aluminum hydride and sodium aluminum hydride with beryllium chloride in diethyl ether and tetrahydrofuran. A report questioning the existence of Be(AlH4)2 in solution. Inorg. Chem. 1973, 12, 2860–2868. [Google Scholar] [CrossRef]
- Wiberg, E.; Bauer, R. Zur Kenntnis eines Berylliumwasserstoffs BeH2. Z. Nat. 1951, 6, 171. [Google Scholar] [CrossRef][Green Version]
- Klaveness, A.; Vajeeston, P.; Ravindran, P.; Fjellvåg, H.; Kjekshus, A. Structure and bonding in BAlH5 (B = Be, Ca, Sr) from first-principle calculations. J. Alloys Compd. 2007, 443, 225–232. [Google Scholar] [CrossRef]
- Santhosh, M.; Rajeswarapalanichamy, R.; Priyanga, G.S.; Kanagaprabha, S.; Cinthia, A.J.; Iyakutti, K. A first principles study of structural stability, electronic structure and mechanical properties of beryllium alanate BeAlH5. AIP Conf. Proc. 2015, 1665, 090032. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Li, Z.; Liu, X.; Jlang, L. Synthesis of magnesium alanate by ball milling MgH2 and AlCl3 mixtures. Rare Met. 2011, 30, 55–58. [Google Scholar] [CrossRef]
- Xiao, X.; Qin, T.; Jiang, Y.; Jiang, F.; Li, M.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Significantly enhanced hydrogen desorption properties of Mg(AlH4)2 nanoparticles synthesized using solvent free strategy. Prog. Nat. Sci. 2017, 27, 112–120. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O. Magnesium alanate—A material for reversible hydrogen storage? J. Alloys Compd. 2003, 356–357, 418–422. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, Y.; Zhang, X.; Zhou, Y.; Gao, M.; Pan, H. Synthesis and hydrogen storage thermodynamics and kinetics of Mg(AlH4)2 submicron rods. Int. J Hydrog. Energy 2012, 37, 18148–18154. [Google Scholar] [CrossRef]
- Fossdal, A.; Brinks, H.W.; Fichtner, M.; Hauback, B.C. Thermal decomposition of Mg(AlH4)2 studied by in situ synchrotron X-Ray diffraction. J. Alloys Compd. 2005, 404–406, 752–756. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Zhang, X.; Gao, M.; Pan, H. Role of particle size, grain size, microstrains and lattice distortion in improved dehydrogenation properties of the ball-milled Mg(AlH4)2. Int. J. Hydrogen Energy 2013, 38, 1460–1468. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Y.; Zhang, X.; Gao, M.; Pan, H. TiF4-doped Mg(AlH4)2 with significantly improved dehydrogenation properties. Int. J. Hydrogen Energy 2013, 38, 13343–13351. [Google Scholar] [CrossRef]
- Gremaud, R.; Borgschulte, A.; Lohstroh, W.; Schreuders, H.; Züttel, A.; Dam, B.; Griessen, R. Ti-Catalyzed Mg(AlH4)2: A reversible hydrogen storage material. J. Alloys Compd. 2005, 404–406, 775–778. [Google Scholar] [CrossRef]
- Fossdal, A.; Brinks, H.W.; Fichtner, M.; Hauback, B.C. Determination of the crystal structure of Mg(AlH4)2 by combined X-ray and neutron diffraction. J. Alloys Compd. 2005, 387, 47–51. [Google Scholar] [CrossRef]
- Fichtner, M.; Engel, J.; Fuhr, O.; Glöss Rubner, O.; Ahlrichs, R. The structure of magnesium alanate. Inorg. Chem. 2003, 42, 7060–7066. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvag, H. High hydrogen content complex hydrides: A density-functional study. Appl. Phys. Lett. 2006, 89, 071906. [Google Scholar] [CrossRef]
- Van Setten, M.J.; de Wijs, G.A.; Popa, V.A.; Brocks, G. Ab initio study of Mg(AlH4)2. Phys. Rev. B 2005, 72, 073107. [Google Scholar] [CrossRef]
- Akbarzadeh, A.R.; Wolverton, C.; Ozolins, V. First-principles determination of crystal structures, phase stability and reaction thermodynamics in the Li-Mg-Al-H hydrogen storage system. Phys. Rev. B 2009, 79, 184102. [Google Scholar] [CrossRef]
- Klaveness, A.; Vajeeston, P.; Ravindran, P.; Fjellvåg, H.; Kjekshus, A. Structural phase stability and bonding behavior of BAlH5 (B = Mg, Ba) from first-principles calculations. Phys. Rev. B 2006, 73, 094122. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Q. Insight into the kinetic mechanism of the first-step dehydrogenation of Mg(AlH4)2. Scr. Mater. 2017, 130, 223–228. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Molin, P.N. Density-functional band-structure calculations of magnesium alanate Mg(AlH4)2. Phys. Rev. B 2005, 72, 073201. [Google Scholar] [CrossRef]
- Spanò, E.; Bernasconi, M. Ab initio of the vibrational properties of Mg(AlH4)2. Phys. Rev. B 2005, 71, 174301. [Google Scholar] [CrossRef]
- Yang, C.H.; Chen, T.T.; Tsai, W.T.; Liu, B.H. In situ synchrotron X-ray diffraction study on the improved dehydrogenation performance of NaAlH4-Mg(AlH4)2 mixture. J. Alloys Compd. 2013, 577, 6–10. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Pukazhselvan, D.; Scheeja, I.G.; Srivastava, O.N. Studies on synthesis and dehydrogenation behavior of magnesium alanate and magnesium-sodium alanate mixture. Int. J. Hydrogen Energy 2007, 32, 4933–4938. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, X.; Zhang, H.; Jiao, L.; Yuan, H. Destabilization effects of Mg(AlH4)2 on MgH2: Improved desorption performance and its reaction mechanism. Int. J. Hydrogen Energy 2014, 39, 17747–17753. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Q.; Si, T.; Zhang, Q.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Superior hydrogen storage properties of LiBH4 catalyzed by Mg(AlH4)2. Chem. Commun. 2011, 47, 5741–5743. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, Y.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. New insights into the effects of NaCl and LiCl on the hydrogen storage behaviors of a 6LiBH4-Mg(AlH4)2 composite. RSC Adv. 2015, 5, 12144–12151. [Google Scholar] [CrossRef]
- Huang, J.; Gao, M.; Li, Z.; Cheng, X.; Gu, J.; Liu, Y. Destabilization of combined Ca(BH4)2 and Mg(AlH4)2 for improved hydrogen storage properties. J. Alloys Compd. 2016, 670, 135–143. [Google Scholar] [CrossRef]
- Finholt, E.; Barbaras, G.D.; Barbaras, G.K.; Urry, G.; Wartik, T.; Schlesinger, H.I. The preparation of sodium and calcium aluminum hydrides. J. Inorg. Nucl. Chem. 1955, 1, 317–325. [Google Scholar] [CrossRef]
- Fichtner, M.; Frommen, C.; Fuhr, O. Synthesis and properties of calcium alanate and two solvent adducts. Inorg. Chem. 2005, 44, 3479–3484. [Google Scholar] [CrossRef]
- Li, C.; Xiao, X.; Ge, P.; Xue, J.; Li, S.; Ge, H. Investigation on synthesis, structure and catalytic modification of Ca(AlH4)2 complex hydride. Int. J. Hydrogen Energy 2012, 37, 936–941. [Google Scholar] [CrossRef]
- Mamatha, M.; Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schmidt, W.; Schüth, F.; Weidenthaler, C. Mechanochemical preparation and investigation of properties of magnesium, calcium and lithium-magnesium alanates. J. Alloys Compds. 2006, 407, 78–86. [Google Scholar] [CrossRef]
- Sato, T.; Sørby, M.H.; Ikeda, K.; Sato, S.; Hauback, B.C.; Orimo, S. Syntheses, crystal structures, and thermal analyses of solvent-free Ca(AlD4)2 and CaAlD5. J. Alloys Compd. 2009, 487, 472–478. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Frankcombe, T.J.; Felderhoff, M. First crystal structure studies of CaAlH5. Inorg. Chem. 2006, 45, 3849–3851. [Google Scholar] [CrossRef]
- Løvvik, O.M. Crystal structure of Ca(AlH4)2 predicted from density-functional band-structure calculations. Phys. Rev. B 2005, 71, 144111. [Google Scholar] [CrossRef]
- Wolverton, C.; Ozoliņš, V. Hydrogen storage in calcium alanate: First-principles thermodynamics and crystal structures. Phys. Rev. B 2007, 75, 064101. [Google Scholar] [CrossRef]
- Liu, D.M.; Gao, C.; Qian, Z.X.; Si, T.Z.; Zhang, Q.A. Reversible hydrogen storage in LiBH4/Ca(AlH4)2 systems. Int. J. Hydrogen Energy 2013, 38, 3291–3295. [Google Scholar] [CrossRef]
- Hanada, N.; Lohstroh, W.; Fichtner, M. Comparison of the calculated and experimental scenarios for solid-state reactions involving Ca(AlH4)2. J. Phys. Chem. C 2008, 112, 131–138. [Google Scholar] [CrossRef]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Large-scale screening of metal hydride mixtures for high-capacity hydrogen storage from first-principles calculations. J. Phys. Chem. C 2008, 112, 5258–5262. [Google Scholar] [CrossRef]
- Dymova, T.N.; Aleksandrov, D.P.; Konoplev, V.N.; Silina, T.A.; Sizareva, A.S. Peculiarities of the solid-phase formation of strontium pentahydroaluminate from binary hydrides by mechanochemical activation and modeling of theprocess on the basis of thermo gasovolumetry data. Russ. J. Coord. Chem. 2000, 26, 531–537. [Google Scholar]
- Sato, T.; Takagi, S.; Sørby, M.H.; Deledda, S.; Hauback, B.C.; Orimo, S.I. Crystal structural determination of SrAlD5 with corner-sharing AlD6 octahedron chains by X-ray and neutron diffraction. Crystals 2018, 8, 89. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Makamura, Y.; Oikawa, K.I.; Kamiyama, T.; Akiba, E. Synthesis and crystal structure of Sr2AlH7: A new structural type of alkaline earth aluminum hydride. Inorg. Chem. 2002, 41, 6547–6549. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Akiba, E. Synthesis of Sr2AlH7 by ball milling followed by hydrogenation. J. Alloys Compd. 2005, 394, 308–311. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Enoki, H.; Akiba, E. Formation mechanism of Sr2AlH7. J. Alloys Compd. 2007, 427, 153–159. [Google Scholar] [CrossRef]
- Gingl, F.; Vogt, T.; Akiba, E. Trigonal SrAl2H2: The first zintl phase hydride. J. Alloys Compod. 2000, 306, 127–132. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Nakamura, Y.; Oikawa, K.I.; Kamiyama, T.; Akiba, E. New alkaline earth aluminum hydride with one-dimensional zigzag chains of [AlH6]: Synthesis and crystal structure of BaAlH5. Inorg. Chem. 2002, 41, 6941–6943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Nakamura, Y.; Oikawa, K.; Kamiyama, T.; Akiba, E. Hydrogen-induced phase decomposition of Ba7Al13 and the crystal structure of Ba2AlH7. J. Alloys Compd. 2003, 316, 180–186. [Google Scholar] [CrossRef]
- Liu, X.; Asano, K.; Sakaki, K.; Nakamura, Y.; Enoki, H.; Akiba, E. Investigations on the formation and decomposition behaviors of BaAlH5 and Ba2AlH7. J. Phys. Chem. C 2008, 112, 17423–17426. [Google Scholar] [CrossRef]
- Liu, X.; Asano, K.; Akiba, E. Direct synthesis of BaAlH5 and Ba2AlH7 from BaH2 and Al system and their hydriding/dehydriding characteristics. J. Alloys Compd. 2009, 477, 744–748. [Google Scholar] [CrossRef]
- Chahrkin, O.P. Theoretical study of metal tetrahydroborates and alanates L(MH4)3, HL(MH4)2, and H2L(MH4) (L = Be, Mg, Al, Sc, Ti, V, Zn, M = B, Al). Russ. J. Inorg. Chem. 2008, 53, 1910–1919. [Google Scholar] [CrossRef]
- Kost, M.E.; Golovanova, A.I. Interaction of LiH and Al with transition metal halogens. Izv. Akad. Nauk SSSR 1978, 14, 1732–1734. [Google Scholar]
- Cao, Z.; Ouyang, L.; Wang, H.; Liu, J.; Felderhoff, M.; Zhu, M. Reversible hydrogen storage in yttrium aluminum hydride. J. Mater. Chem. A 2017, 5, 6042–6046. [Google Scholar] [CrossRef]
- Kost, M.E.; Golovanova, A.L. Interaction of titanium and iron halides with lithium aluminum hydride in diethyl ether. Translated from Izv. Akad. Nauk SSSR 1975, 5, 991–994. [Google Scholar] [CrossRef]
- Ramzan, M.; Ahuja, R. MN+1AXN (M = Ti, A = Al. X = H) phase class materials with hydrogen: Ti4AlH3 and Ti3AlH2. Appl. Phys. Lett. 2010, 96, 261906. [Google Scholar] [CrossRef]
- Maeland, A.J.; Hauback, B.C.; Fjellvåg, H.; Sørby, M.H. The structures of hydride phases in the Ti3Al/H system. Int. J. Hydrogen Energy 1999, 24, 163–168. [Google Scholar] [CrossRef]
- Epshteyn, A.; Miller, J.B.; Pettigrew, K.A.; Stroud, R.M.; Purdy, A.P. Surface passivated air and moisture stable mixed zirconium aluminum metal-hydride nanoparticles. Mater. Res. Soc. Symp. Proc. 2008, 1056, HH03–HH16. [Google Scholar] [CrossRef]
- Matsubara, E.; Waseda, Y.; Li, X.G.; Aoki, K.; Masumoto, T. X-Ray diffraction study of amorphous Zr3InH4 and Zr3AlH4 alloys produced by hydrogen-induced amorphization method. J. Mater. Sci. Lett. 1990, 9, 1017–1019. [Google Scholar] [CrossRef]
- Wiberg, E.; Neumaier, H. Zur Kenntnis von Doppel-und Tripelhydriden der Übergangsmetalle. I. Über die Umsetzung von Niob(v)-chlorid mit Lithiumalanat. Z. Anorg. Allg. Chem. 1965, 340, 189–200. [Google Scholar] [CrossRef]
- Aubry, J.; Monnier, G.; Hackspill, M.J. Sur la preparation de quelques aluminohydrures métalliques. Comptes Rendus 1954, 238, 2534–2535. [Google Scholar]
- Monnier, G. Contribution a l’étude de l´éther oxyde d´éthyle, milieu réactionel, en chimie minérale. Ann. Chim. 1957, 13, 14–57. [Google Scholar]
- Neumaier, H.; Büchel, D.; Ziegelmaier, G. Über die Umsetzung von Eisen(III)-chlorid mit Lithiumalanat bei tiefen Temperaturen. Z. Anorg. Allg. Chem. 1996, 345, 46–51. [Google Scholar] [CrossRef]
- Scheaffer, G.W.; Roscoe, J.S.; Stewart, A.C. The reduction of iron(III) chloride with lithium aluminohydride and lithium borohydride: Iron(II) borohydride. J. Am. Chem. Soc. 1956, 78, 729–733. [Google Scholar] [CrossRef]
- Ashby, E.C.; Kovar, R.A. Reaction of lithium aluminum hydride with copper(I) and mercury(II) salts. Nature of the reactive species in the conjugate reducing agent LiAlH4-CuI. Inorg. Chem. 1977, 16, 1437–1440. [Google Scholar] [CrossRef]
- Wiberg, E.; Henle, W. Über eine neue Darstellungsweise des Kupferwasserstoffs CuH. Zeitschrift für Naturforschung B 1952, 7, 250. [Google Scholar] [CrossRef]
- Wiberg, E.; Henle, W. Zur Kenntnis eines Silber-aluminium-wasserstoffs AgAlH4. Z. Nat. 1952, 7, 250–251. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Mal´tseva, N.N.; Buzanov, G.A.; Kunetsov, N.T. Hydride compounds of zinc. Russ. J. Inorg. Chem. 2014, 59, 1665–1678. [Google Scholar] [CrossRef]
- Wiberg, E.; Schmidt, M. Zur Kenntnis eines Gallium-aluminium-wasserstoffs Ga(AlH4)3 und eines Galliumwasserstoff-Ätherats GaH3*OR2. Z. Nat. 1951, 6, 172. [Google Scholar]
- Wiberg, E. Neuere Ergebnisse der präparativen Hydrid-Forschung. Angew. Chem. 1953, 65, 16–34. [Google Scholar] [CrossRef]
- Wiberg, E.; Dittmann, O.; Nöth, H.; Schmidt, M. Über Wasserstoff-Verbindungen des Thalliums, V. Zur Kenntnis eines Thallium(I)-boranats TlBH4 und Thallium(I)-alanats TlAlH4. Z. Nat. 1957, 12, 62–63. [Google Scholar]
- Wiberg, E.; Nöth, H. Über Wasserstoff-Verbindungen des Thalliums VI. Zur Kenntnis eines Thallium(III)-boranats TlCl(BH4)2. Z. Nat. 1957, 12, 63–65. [Google Scholar]
- Wiberg, E.; Schmidt, M. Zur Kenntnis eines Thallium-aluminium-wasserstoffs TlCl(AlH4)2. Z. Nat. 1951, 6, 334–335. [Google Scholar] [CrossRef]
- Wiberg, E.; Bauer, R. Zur Kenntnis eines Zinn-aluminium-wasserstoffs Sn(AlH4)4. Z. Nat. 1951, 6, 392. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Pommerin, A.; Felderhoff, M.; Sun, W.; Wolverton, C.; Bogdanović, B.; Schüth, F. Complex rare-earth aluminum hydrides: Mechanochemical preparation, crystal structure and potential for hydrogen storage. J. Am. Chem. Soc. 2009, 131, 16735–16743. [Google Scholar] [CrossRef]
- Mueller, W.M.; Blackledge, J.P.; Libowitz, G.G. Metal Hydrides; Academic Press: Moscow, Russia, 1968; pp. 435–436. [Google Scholar]
- Bergsma, J.; Goedkoop, J.A.; Vucht, J.H.N. Neutron diffraction investigation of solid solutions AlTh2Dn. Acta Cryst. 1961, 14, 223–228. [Google Scholar] [CrossRef]
- Sørby, M.H.; Fjellvåg, H.; Hauback, B.C.; Maeland, A.J.; Yartys, V.A. Crystal structure of Th2Al deuterides. J. Alloys Compd. 2000, 309, 154–164. [Google Scholar] [CrossRef]
- Vajeeston, P.; Vidya, R.; Ravindran, P.; Fjellvåg, H.; Kjekshus, A.; Skjeltorp, A. Electronic structure, phase stability, and chemical bonding in Th2Al and Th2AlH4. Phys. Rev. B 2002, 65, 075101. [Google Scholar] [CrossRef]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi A 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Graetz, J.; Lee, Y.; Reilly, J.J.; Park, S.; Vogt, T. Structures and thermodynamics of the mixed alkali alanates. Phys. Rev. B 2005, 71, 184115. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Swang, O.; Opalka, S.M. Modeling alkali alanates for hydrogen storage by density-functional band-structure calculations. J. Mater. Res. 2005, 20, 3199–3213. [Google Scholar] [CrossRef]
- Majzoub, E.H.; Ozoliņš, V. Prototype electrostatic ground state approach to predicting crystal structures of ionic compounds: Application to hydrogen storage materials. Phys. Rev. B 2008, 77, 104115. [Google Scholar] [CrossRef]
- Claudy, P.; Bonnetot, B.; Bastide, J.P.; Letoffe, J.M. Reactions of lithium and sodium aluminum hydride with sodium or lithium hydride. Preparation of a new alumino-hydride of lithium and sodium LiN2AlH6. Mater. Res. Bull. 1982, 17, 1499–1504. [Google Scholar] [CrossRef]
- Huot, J.; Boily, S.; Güther, V.; Schulz, R. Synthesis of Na3AlH6 and Na2LiAlH6 by mechanical alloying. J. Alloys Compd. 1999, 383, 304–306. [Google Scholar] [CrossRef]
- Okada, N.; Genma, R.; Nishi, Y.; Uchida, H.H. RE-oxide doped alkaline hydrogen storage materials prepared by mechanical activation. J. Mater. Sci. 2004, 39, 5503–5506. [Google Scholar] [CrossRef]
- Genma, R.; Uchida, H.H.; Okada, N.; Nishi, Y. Hydrogen reactivity of Li-containing hydrogen storage materials. J. Alloys Compd. 2003, 356, 358–362. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Gao, M.; Luo, K.; Pan, H.; Wang, Q. Formation reactions and the thermodynamics and kinetics of dehydrogenation reaction of mixed alanate Na2LiAlH6. J. Phys. Chem. C 2009, 113, 7978–7984. [Google Scholar] [CrossRef]
- Genma, R.; Okada, N.; Sobue, T.; Uchida, H.H. Mechanically milled alanates as hydrogen storage materials. Int. J. Hydrogen Energy 2006, 31, 309–311. [Google Scholar] [CrossRef]
- Fossdal, A.; Brinks, H.W.; Fonneløp, J.E.; Hauback, B.C. Pressure-composition isotherms and thermodynamic properties of TiF3-enhanced Na2LiAlH6. J. Alloys Compd. 2005, 397, 135–139. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Li, S.; Ge, H.; Wang, Q. Direct synthesis and hydrogen storage behaviors of nanocrystalline Na2LiAlH6. J. Mater. Sci. 2011, 46, 3314–3318. [Google Scholar] [CrossRef]
- Teprovich, J.A., Jr.; Zhang, J.; Colón-Mercado, H.; Cuevas, F.; Peters, B.; Greenway, S.; Zidan, R.; Latroche, M. Li-driven electrochemical conversion reaction of AlH3, LiAlH4, and NaAlH4. J. Phys. Chem. C 2015, 119, 4666–4674. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fossdal, A.; Brinks, H.W.; Hauback, B.C. Characterization of Al–Ti phases in cycled TiF3-enhanced Na2LiAlH6. J. Alloys Compd. 2006, 416, 274–278. [Google Scholar] [CrossRef]
- Ma, X.Z.; Martinez-Franco, E.; Dornheim, M.; Klassen, T.; Bormann, R. Catalyzed Na2LiAlH6 for hydrogen storage. J. Alloys Compd. 2005, 404–406, 771–774. [Google Scholar] [CrossRef]
- Fonneløp, J.E.; Løvvik, O.M.; Sørby, M.H.; Brinks, H.W.; Hauback, B.C. Adjustment of the decomposition path for Na2LiAlH6 by TiF3 addition. Int. J. Hydrogen Energy 2011, 36, 12279–12285. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Swang, O. Crystal structures and electronic structures of alkali aluminohexahydrides from density functional calculations. J. Alloys Compd. 2005, 404–406, 757–761. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Swang, O. Structure and stability of possible new alanates. Europhys. Lett. 2004, 67, 607–613. [Google Scholar] [CrossRef]
- Brinks, H.W.; Huback, B.C.; Jensen, C.M.; Zidan, R. Synthesis and crystal structure of Na2LiAlD6. J. Alloys Compd. 2005, 392, 27–30. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Cao, Y.; Gao, M.; Pan, H.; Wang, Q. Mechanisms for the enhanced hydrogen desorption performance of the TiF4-catalyzed Na2LiAlH6 used for hydrogen storage. Energy Environ. Sci. 2010, 3, 645–653. [Google Scholar] [CrossRef]
- Rönnebro, E.; Majzoub, E.H. Crystal structure, Raman spectroscopy and Ab initio calculations of a new bialkali alanate K2LiAlH6. J. Phys. Chem. B 2006, 110, 25686–25691. [Google Scholar] [CrossRef] [PubMed]
- Bulychev, B.M.; Semenenko, K.N.; Bitcoev, K.B. Synthesis and investigation of complex compounds of magnesium alanate. Koord Khim 1978, 4, 374–380. [Google Scholar]
- Grove, H.; Brinks, H.W.; Heyn, R.H.; Wu, F.-J.; Opalka, S.M.; Tang, X.; Laube, B.L.; Hauback, B.C. The structure of LiMg(AlD4)3. J. Alloys Compd. 2008, 455, 249–254. [Google Scholar] [CrossRef]
- Grove, H.; Løvvik, O.M.; Huang, W.; Opalka, S.M.; Heyn, R.H.; Hauback, B.C. Decomposition of lithium magnesium aluminum hydride. Int. J. Hydrogen Energy 2011, 36, 7602–7611. [Google Scholar] [CrossRef]
- Grove, H.; Brinks, H.W.; Løvvik, O.M.; Heyn, R.H.; Hauback, B.C. The crystal structure of LiMgAlD6 from combined neutron and synchrotron X-ray powder diffraction. J. Alloys Compds. 2008, 460, 64–68. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Raghubanshi, H.; Pukazhselvan, D.; Srivastava, O.N. Effects of helical GNF on improving the dehydrogenation behavior of LiMg(AlH4)3 and LiAlH4. Int. J. Hydrogen Energy 2010, 35, 2083–2090. [Google Scholar] [CrossRef]
- Liu, D.M.; Qian, Z.X.; Si, T.Z.; Zhang, Q.A. Synthesis, crystal structure and thermal decomposition of LiCa(AlH4)3. J. Alloys. Compd. 2012, 520, 202–206. [Google Scholar] [CrossRef]
- Wang, H.C.; Zheng, J.; Wu, D.H.; Wei, L.T.; Tang, B.Y. Crystal feature and electronic structure of novel mixed alanate LiCa(AlH4)3: A density functional theory investigation. RSC Adv. 2015, 5, 16439–16445. [Google Scholar] [CrossRef]
- Sato, T.; Takagi, S.; Deledda, S.; Hauback, B.C.; Orimo, S.I. Extending the applicability of the Goldschmidt tolerance factor to arbitrary ionic compounds. Sci. Rep. 2016, 6, 23592. [Google Scholar] [CrossRef]
- Sørby, M.H.; Brinks, H.W.; Fossdal, A.; Thorshaung, K.; Hauback, B.C. The crystal structure and stability of K2NaAlH6. J. Alloys Compd. 2006, 415, 284–287. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Pandey, S.K.; Shahi, R.R.; Hudson, M.S.L.; Shaz, M.A.; Srivastava, O.N. Synthesis, characterization and hydrogen sorption studies of mixed sodium-potassium alanate. Cryst. Res. Technol. 2013, 48, 520–531. [Google Scholar] [CrossRef]
- Pan, R.K.; Yao, J.G.; Ji, R.L.; Liu, W.W.; Yin, D.F. First principles study on elastic and electronic properties of bialkali alanates M2M’AlH6. Int. J. Hydrogen Energy 2018, 43, 3862–3870. [Google Scholar] [CrossRef]
- Fonneløp, J.E.; Corno, M.; Pinatel, E.R.; Sørby, M.H.; Ugliengo, P.; Baricco, M.; Hauback, B.C. Experimental and computational investigations on the AlH3/AlF3 system. J. Alloys Compd. 2011, 509, 10–14. [Google Scholar] [CrossRef]
- Yin, L.C.; Wang, P.; Kang, X.D.; Sun, C.H.; Cheng, H.M. Functional anion concept: Effect of fluorine anion on hydrogen storage of sodium alanate. Phys. Chem. Chem. Phys. 2007, 9, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Eigen, N.; Bösenberg, U.; Bellosta von Colbe, J.; Jensen, T.R.; Cerenius, Y.; Dornheim, M.; Klassen, T.; Bormann, R. Reversible hydrogen storage in NaF-Al composites. J. Alloys Compd. 2009, 477, 76–80. [Google Scholar] [CrossRef]
- Brinks, H.W.; Fossdal, A.; Hauback, B.C. Adjustment of the stability of complex hydrides by anion substitution. J. Phys. Chem. C 2008, 112, 5658–5661. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 192–236. [Google Scholar]
- Biliskov, N. Infrared spectroscopy as a convenient tool for investigation of hydrogen sorption mechanisms and bonding in complex hydrides. In Infrared Spectroscopy: Theory, Advances and Development; Cozzolino, D., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 1–42. [Google Scholar]
- Zavorotynska, O.; Corno, M.; Damin, A.; Spoto, G.; Ugliengo, P.; Barico, M. Vibrational properties of MBH4 and MBF4 crystals (M = Li, Na, K): A combined DFT, infrared, and Raman study. J. Phys. Chem. C 2011, 115, 18890–18900. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Shishidou, T.; Oguchi, T. Ab initio study on the electronic structure and vibration modes of alkali and alkaline-earth amides and alanates. J. Phys. 2009, 21, 185501. [Google Scholar] [CrossRef]
- Parker, S.F. Spectroscopy and bonding in ternary metal hydride complexes-potential hydrogen storage media. Coord. Chem. Rev. 2010, 254, 215–234. [Google Scholar] [CrossRef]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry. Principles of Structure and Reactivity; Harper Collins: New York, NY, USA, 1993; pp. 187–190. [Google Scholar]
- Li, L.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Liu, G.; Jiao, L.; Yuan, H. Enhanced hydrogen storage properties of TiN–LiAlH4 composite. Int. J. Hydrogen Energy 2013, 38, 3695–3701. [Google Scholar] [CrossRef]
- Zang, L.; Cai, J.; Zhao, L.; Gao, W.; Liu, J.; Wang, Y. Improved hydrogen storage properties of LiAlH4 by mechanical milling with TiF3. J. Alloys Compd. 2015, 647, 756–762. [Google Scholar] [CrossRef]
- Ares Fernandez, J.R.; Aguey-Zinsou, F.; Elsaesser, M.; Ma, X.Z.; Dornheim, M.; Klassen, T.; Bormann, R. Mechanical and thermal decomposition of LiAlH4 with metal halides. Int. J. Hydrogen Energy 2007, 32, 1033–1040. [Google Scholar] [CrossRef]
- Liu, S.S.; Sun, L.-X.; Zhang, Y.; Xu, F.; Zhang, J.; Chu, H.-L.; Fan, M.-Q.; Zhang, T.; Song, X.-Y.; Grolier, J.P.E. Effect of ball milling time on the hydrogen storage properties of TiF3-doped LiAlH4. Int. J. Hydrogen Energy 2009, 34, 8079–8085. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinsky, A.A.; Qu, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef]
- Li, Z.; Li, P.; Wan, Q.; Zhai, F.; Liu, Z.; Zhao, K.; Wang, L.; Lü, S.; Zou, L.; Qu, X.; et al. Dehydrogenation improvement of LiAlH4 catalyzed by Fe2O3 and Co2O3 nanoparticles. J. Phys. Chem. C 2013, 117, 18343–18352. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, L.; Fan, X.; Huang, X.; Liu, J.; Yan, K.; Zhang, Y. Synergistic effects played by CMK-3 and NbF5 Co-additives on de/re-hydrogenation performances of NaAlH4. Int. J. Hydrogen Energy 2018, 43, 9705–9712. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, X.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloys Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Kumar, S.; Kain, V.; Kojima, Y. Remarkably improved dehydrogenation of ZrCl4 doped NaAlH4 for hydrogen storage application. Int. J. Hydrogen Energy 2017, 42, 15299–15307. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhao, K.; Liu, Z.; Wang, L.; Zhai, F.; Qu, X.; Volinsay, A.A. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles. J. Power Sources 2014, 248, 388–395. [Google Scholar] [CrossRef]
- Gomes, S.; Renaudin, G.; Hagemann, H.; Yvon, K.; Sulic, M.P.; Jensen, C.M. Effects of milling, doping and cycling of NaAlH4 studied by vibrational spectroscopy and X-ray diffraction. J. Alloys Compd. 2005, 390, 305–313. [Google Scholar] [CrossRef]
- Rafi-Ud-Din; Qu, X.; Li, P. Superior catalytic effects of Nb2O5, TiO2, and Cr2O3 nanoparticles in improving the hydrogen sorption properties of NaAlH4. J. Phys. Chem. 2012, 116, 11924–11938. [Google Scholar] [CrossRef]
- Franke, I.; Flick, T.; Bauer, H. Hydrogen desorption kinetics of CeCl3-doped sodium aluminum hydride compacts measured by parallel in-situ FTIR-ATR-spectroscopy and gravimetry. Int. J. Hydrogen Energy 2015, 40, 4175–4183. [Google Scholar] [CrossRef]
- Bureau, J.C.; Amri, Z.; Létoffé, J.M. Etude comparative des hexahydrido-et des hexadeuteridoaluminates de lithium et de sodium. I-spectres Raman et infrarouge de Li3-et Na3AlH6, et Li3AlD6. Mat. Res. Bull. 1989, 24, 23–31. [Google Scholar] [CrossRef]
- Amama, P.B.; Grant, J.T.; Shamberger, P.J.; Voevodin, A.A.; Fisher, T.S. Improved dehydrogenation properties of Ti-doped LiAlH4: Role of Ti precursors. J. Phys. Chem. C 2012, 116, 21886–21894. [Google Scholar] [CrossRef]
- Ismail, N.; Aboud, A.A.; Hamdel-Din, A.; Farghali, A.A.; Khedr, M.H. Influence of LiH and Ti metal additives on milling LiAlH4 compound. Int. J. Adv. Res. 2014, 2, 307–316. [Google Scholar]
- Wu, X.; Wang, X.; Cao, G.; Li, S.; Ge, H.; Chen, L.; Yan, M. Hydrogen storage properties of LiBH4–Li3AlH6 composites. J. Alloys Compd. 2012, 517, 127–131. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.; Dou, S.X. Improved hydrogen storage property of LiAlH4 by milling with carbon based additives. Int. J. Electroact. Mater. 2013, 1, 13–22. [Google Scholar]
- Halim Yap, F.A.; Ali, N.A.; Idris, N.H.; Ismail, M. Catalytic effect of MgFe2O4 on the hydrogen storage properties of Na3AlH6–LiBH4 composite system. Int. J. Hydrogen Energy 2018, 43, 20882–20891. [Google Scholar] [CrossRef]
- Franke, I.; Bauer, H.D.; Scheppat, B. OCM 2013. Optical Characterization of Materials. Conference Proceedings; Beyerer, J., Puente León, F., Längle, T., Eds.; KIT Scientific Publishing: Karlsruhe, Germany, 2013; pp. 78–88. [Google Scholar]
- Züttel, A. Hydrogen Storage Methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Yoshino, M.; Komiya, K.; Takahasi, Y.; Shinzato, Y.; Yukawa, H.; Morinaga, M. Nature of the chemical bond in complex hydrides, NaAlH4, LiAlH4, LiBH4 and LiNH2. J. Alloys Compds. 2005, 404–406, 185–190. [Google Scholar] [CrossRef]
- Claudy, P.; Bonnetot, B.; Letoffe, M.J.; Turk, G. Determination des constantes thermodynamiques des hydrures simples et complexes de l’aluminium. IV. Enthalpie de formation de LiAIH2 et Li3AIH6. Thermochim. Acta. 1978, 27, 213–221. [Google Scholar] [CrossRef]
- Smith, M.B.; Bass, G.E., Jr. Heats and free energies of formation of the alkali aluminum hydrides and of cesium hydride. J. Chem. Eng. Data 1963, 8, 342–346. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Ismail, M. Catalytic effect of SrFe12O19 on the hydrogen storage properties of LiAlH4. Int. J. Hydrogen Energy 2017, 42, 19126–19134. [Google Scholar] [CrossRef]
- Cai, J.; Zang, L.; Zhao, L.; Liu, J.; Wang, Y. Dehydrogenation characteristics of LiAlH4 improved by in-situ formed catalysts. J. Energy Chem. 2016, 25, 868–873. [Google Scholar] [CrossRef]
- Opalka, S.M.; Anton, D.L. First principles study of sodium–aluminum–hydrogen phases. J. Alloys Compd. 2003, 356–357, 486–489. [Google Scholar] [CrossRef]
- Lee, B.-M.; Jang, J.-W.; Shim, J.-H.; Cho, Y.W.; Lee, B.-J. Thermodynamic assessment of the NaH ↔ Na3AlH6 ↔ NaAlH4 hydride system. J. Alloys Compd. 2006, 424, 370–375. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Han, L.; Li, S.; Ge, H.; Wang, Q. Hydriding-dehydriding kinetics and the microstructure of La-and Sm-doped NaAlH4 prepared via direct synthesis method. Int. J. Hydrogen Energy 2011, 36, 10861–10869. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1315. [Google Scholar] [CrossRef]
- Palumbo, M.; Torres, F.J.; Ares, J.R.; Pisani, C.; Fernandez, J.F.; Baricco, M. Thermodynamic and ab initio investigation of the Al–H–Mg system. Comput. Coupling Phase Diagr. Thermochem. 2007, 31, 457–467. [Google Scholar] [CrossRef]
- Cuevas, F. Advanced Materials and Technologies; Cuevas, F., Burzo, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 252–260. [Google Scholar]
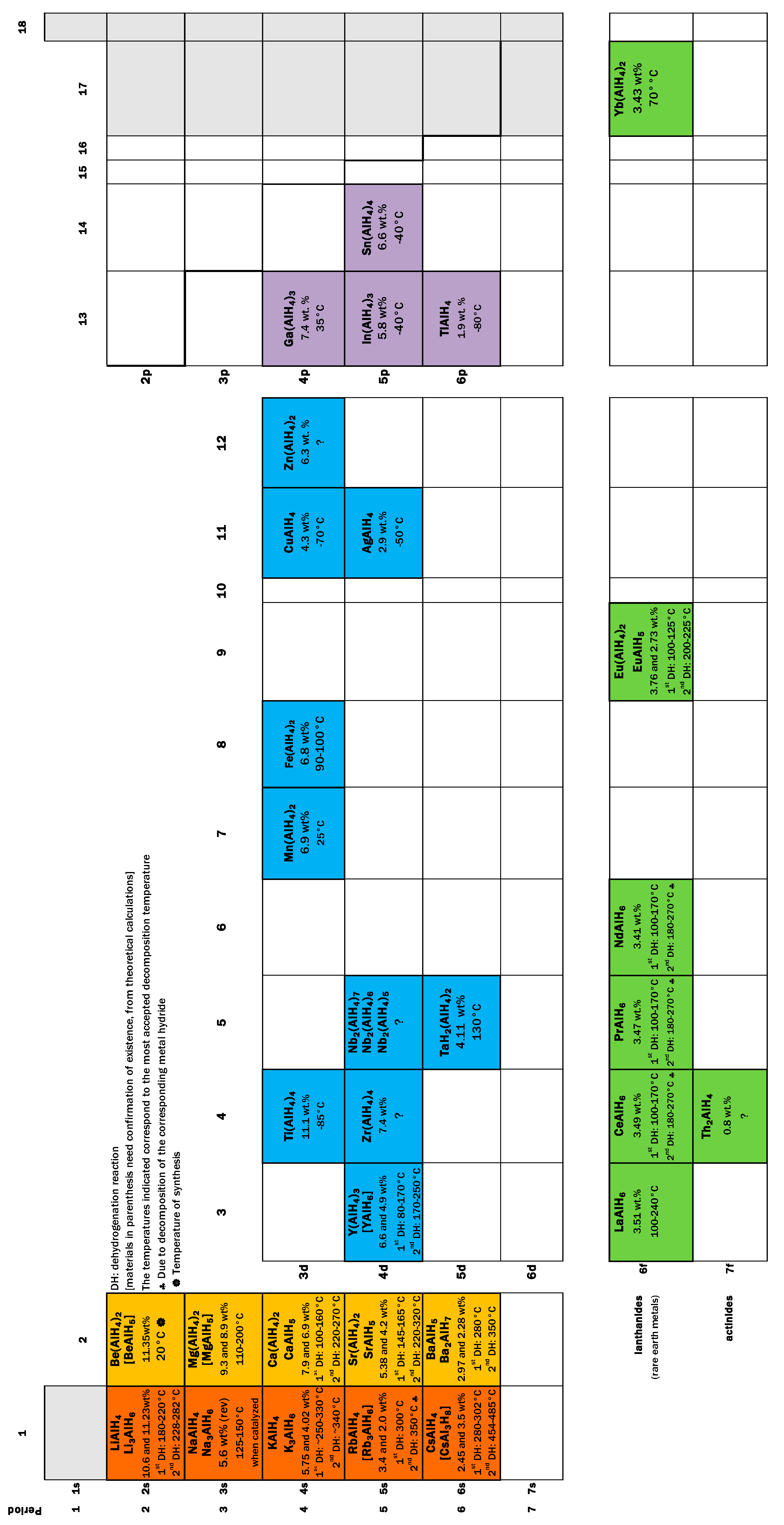


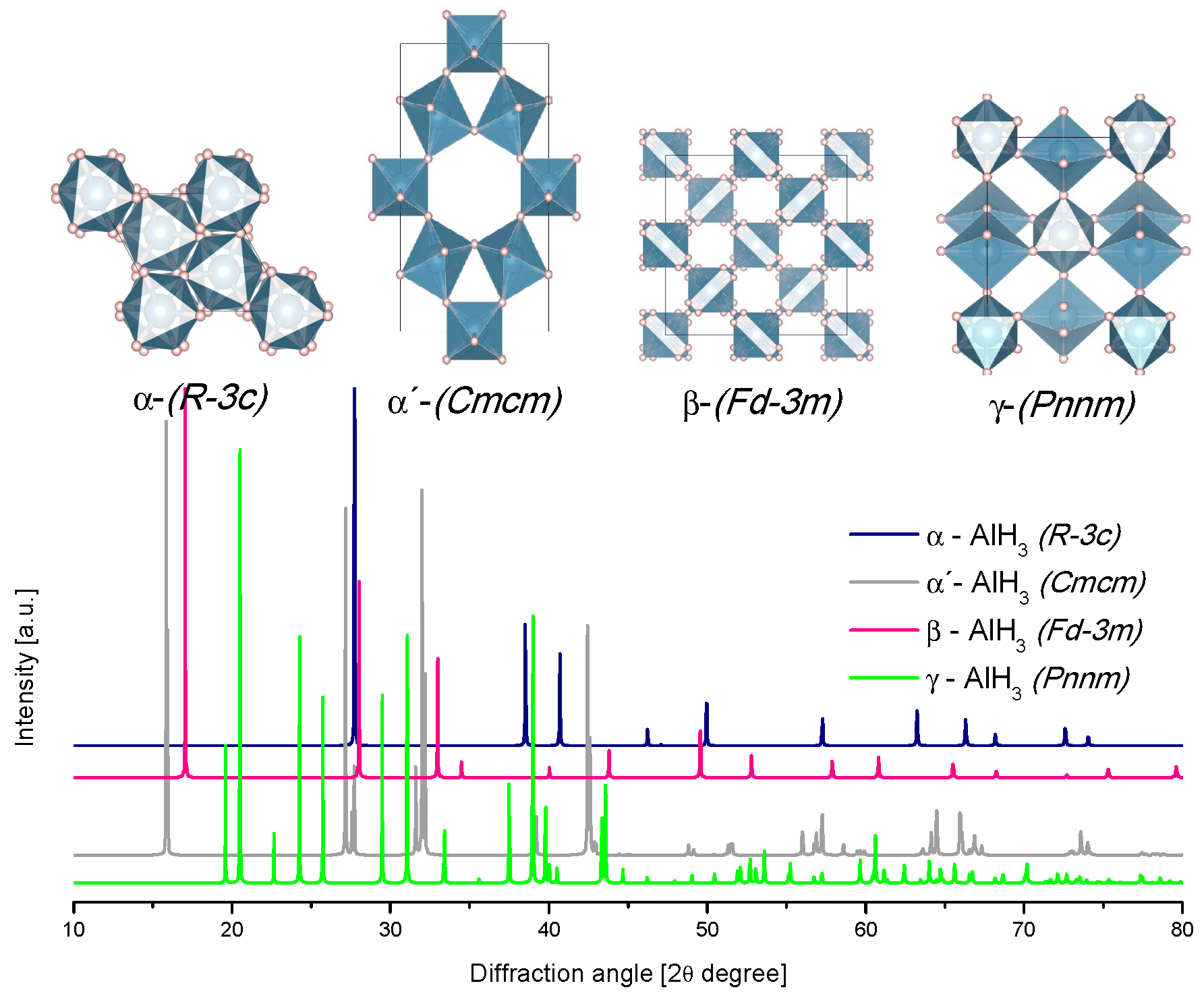
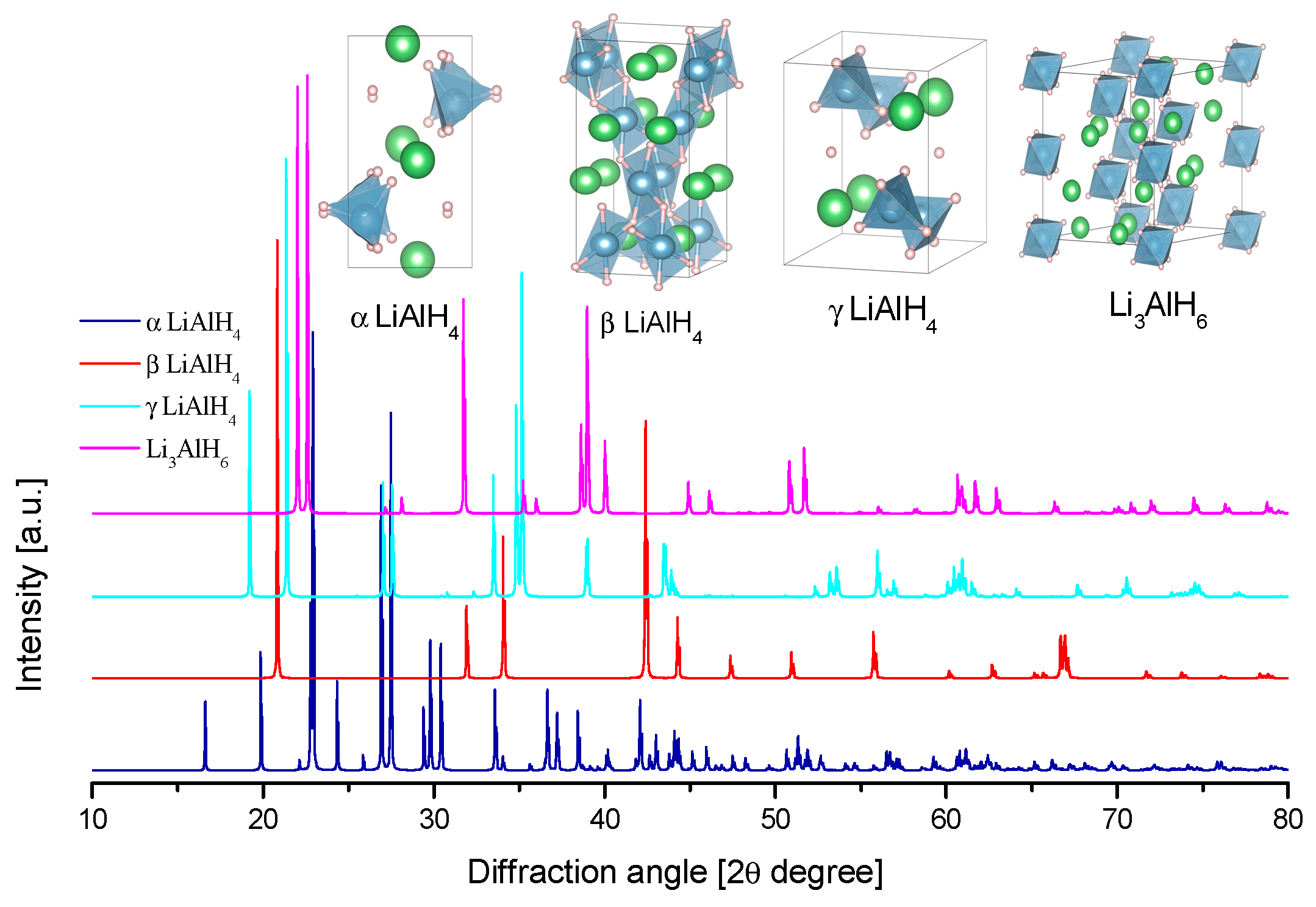
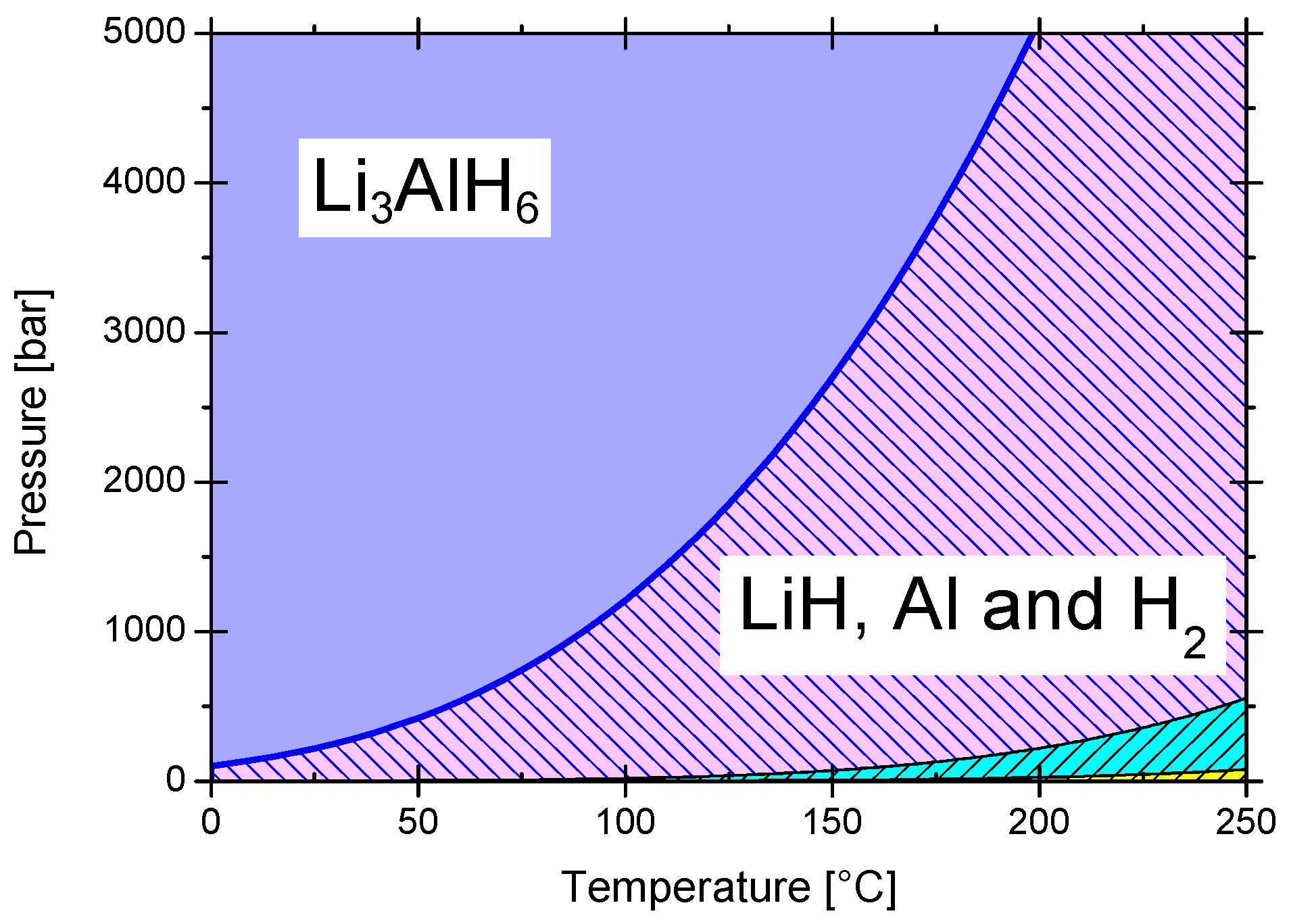
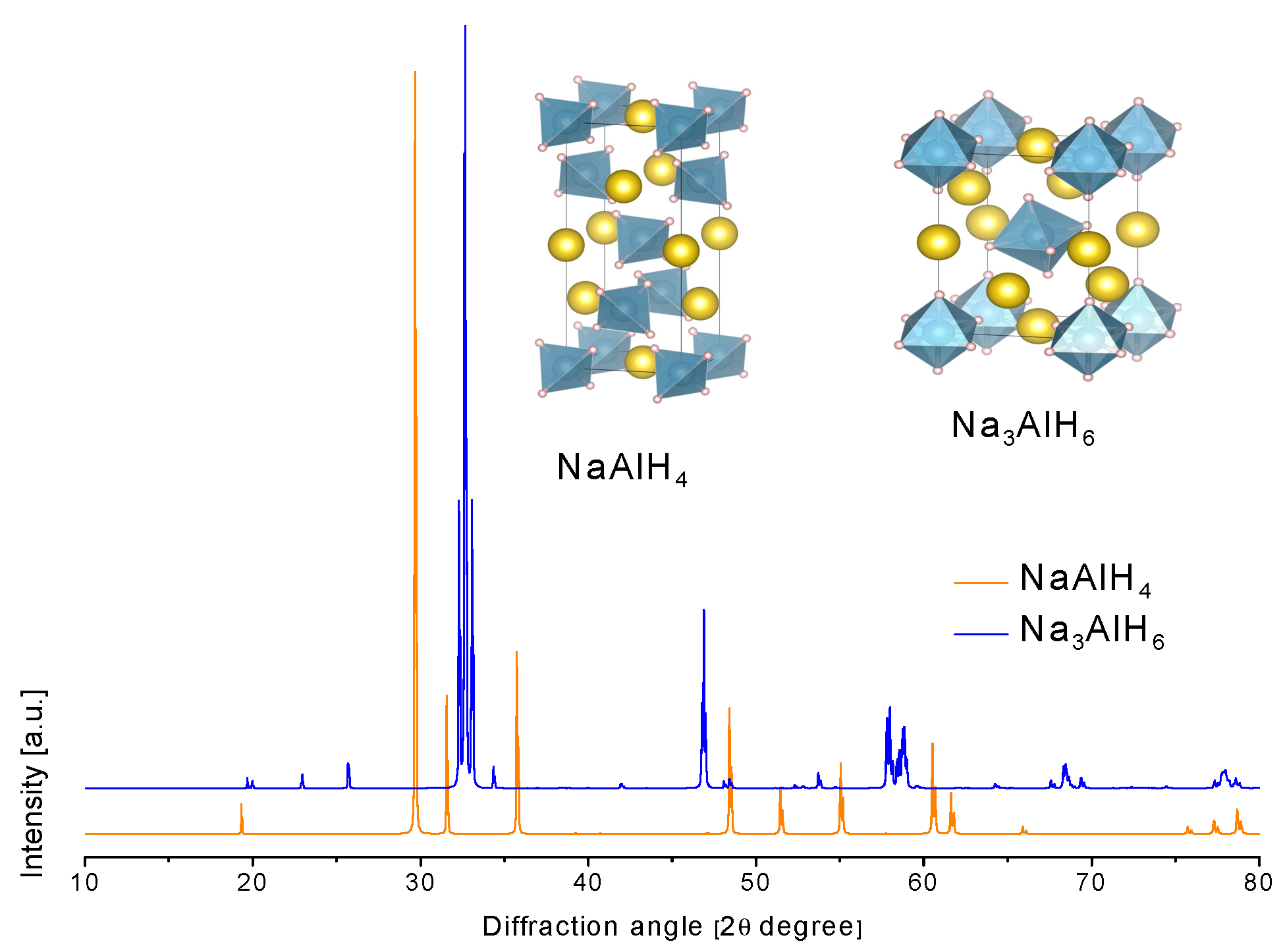
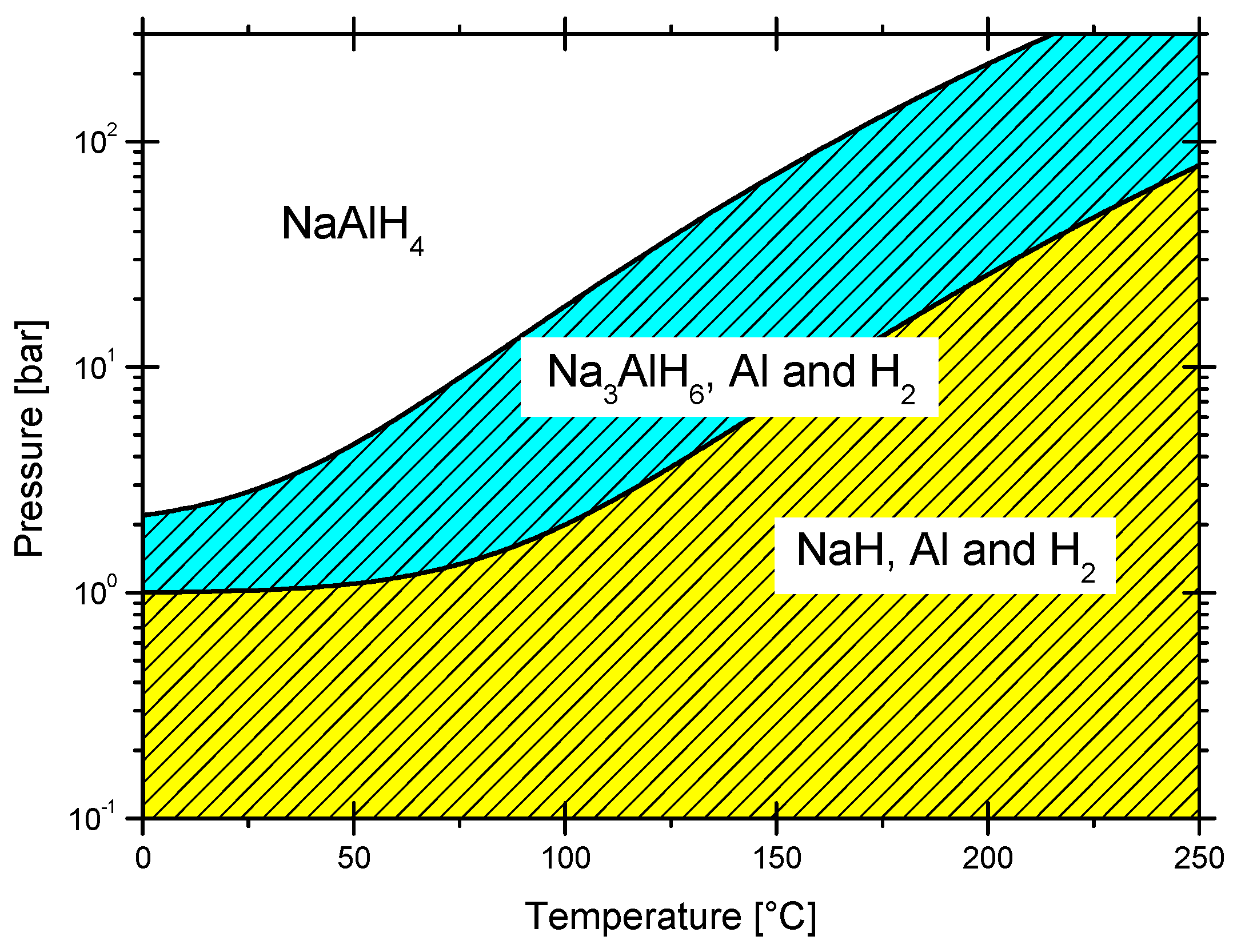
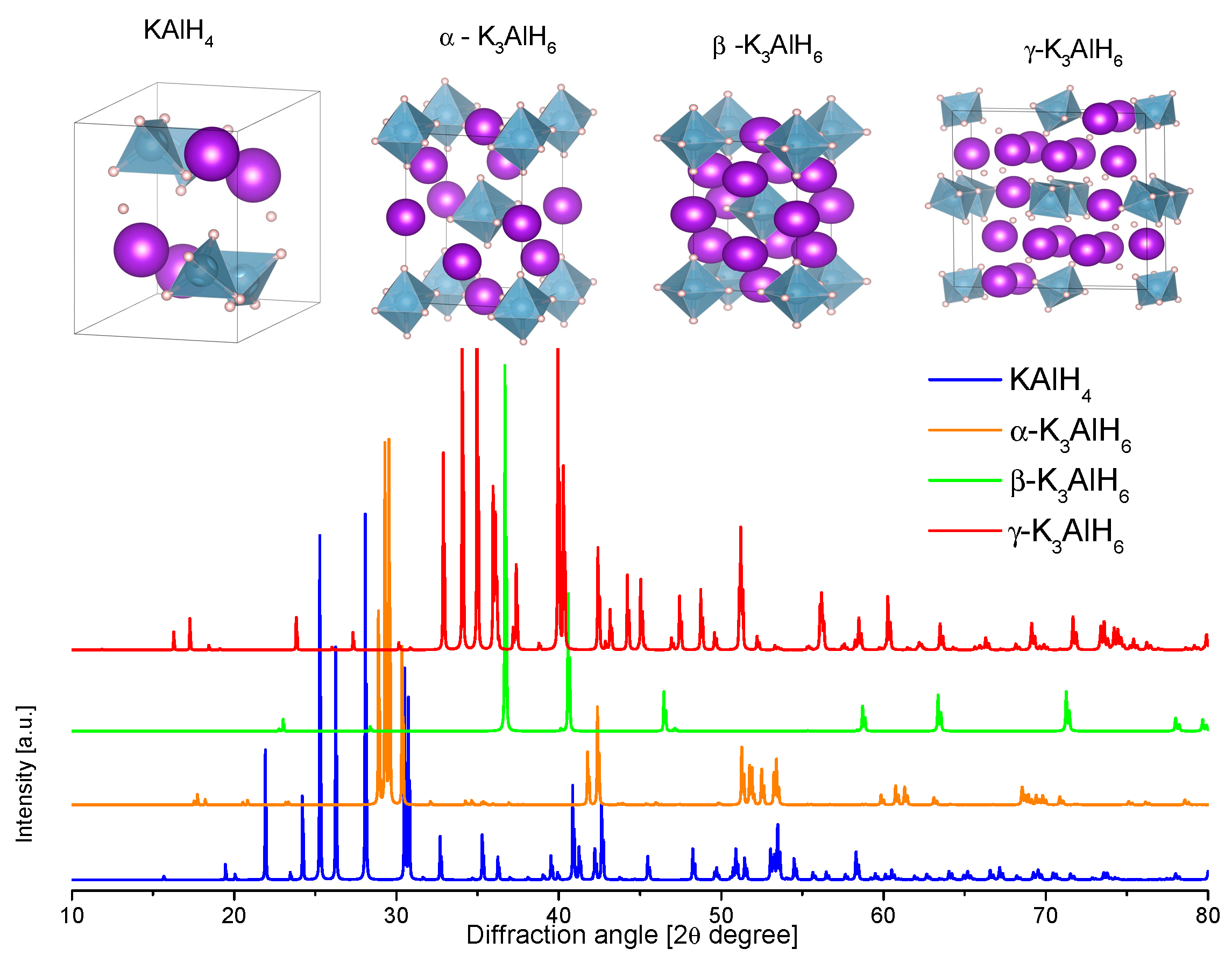
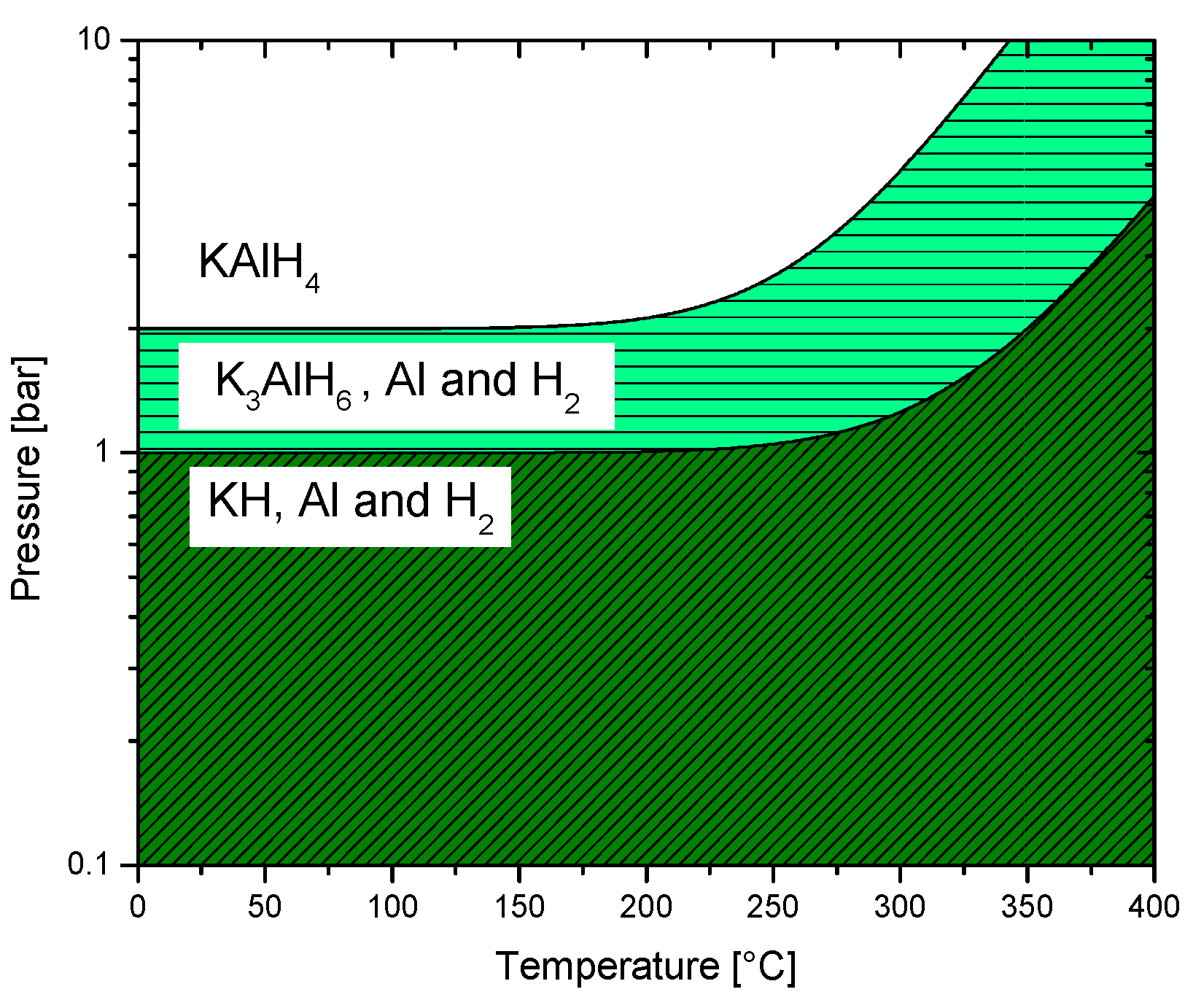

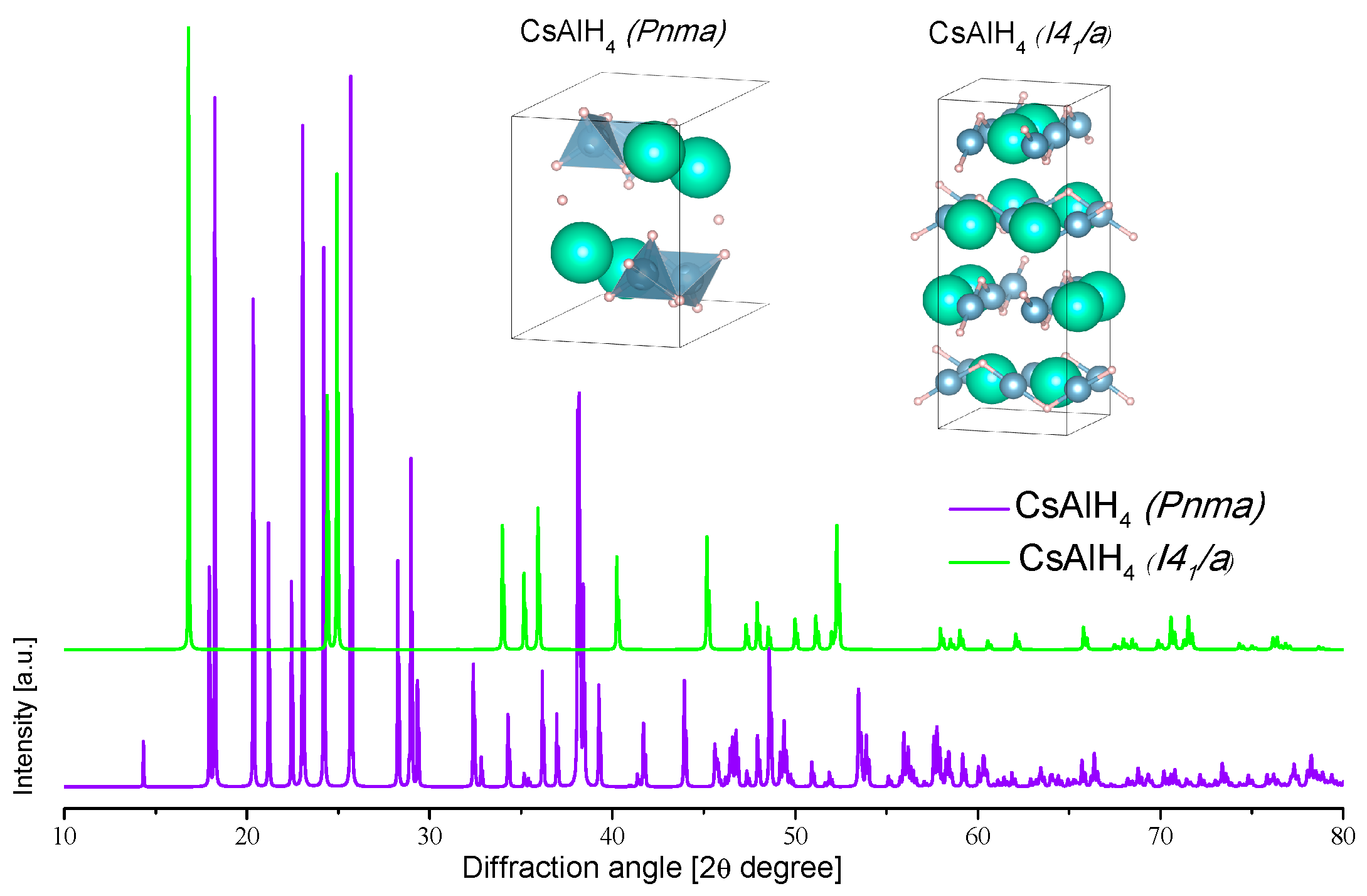
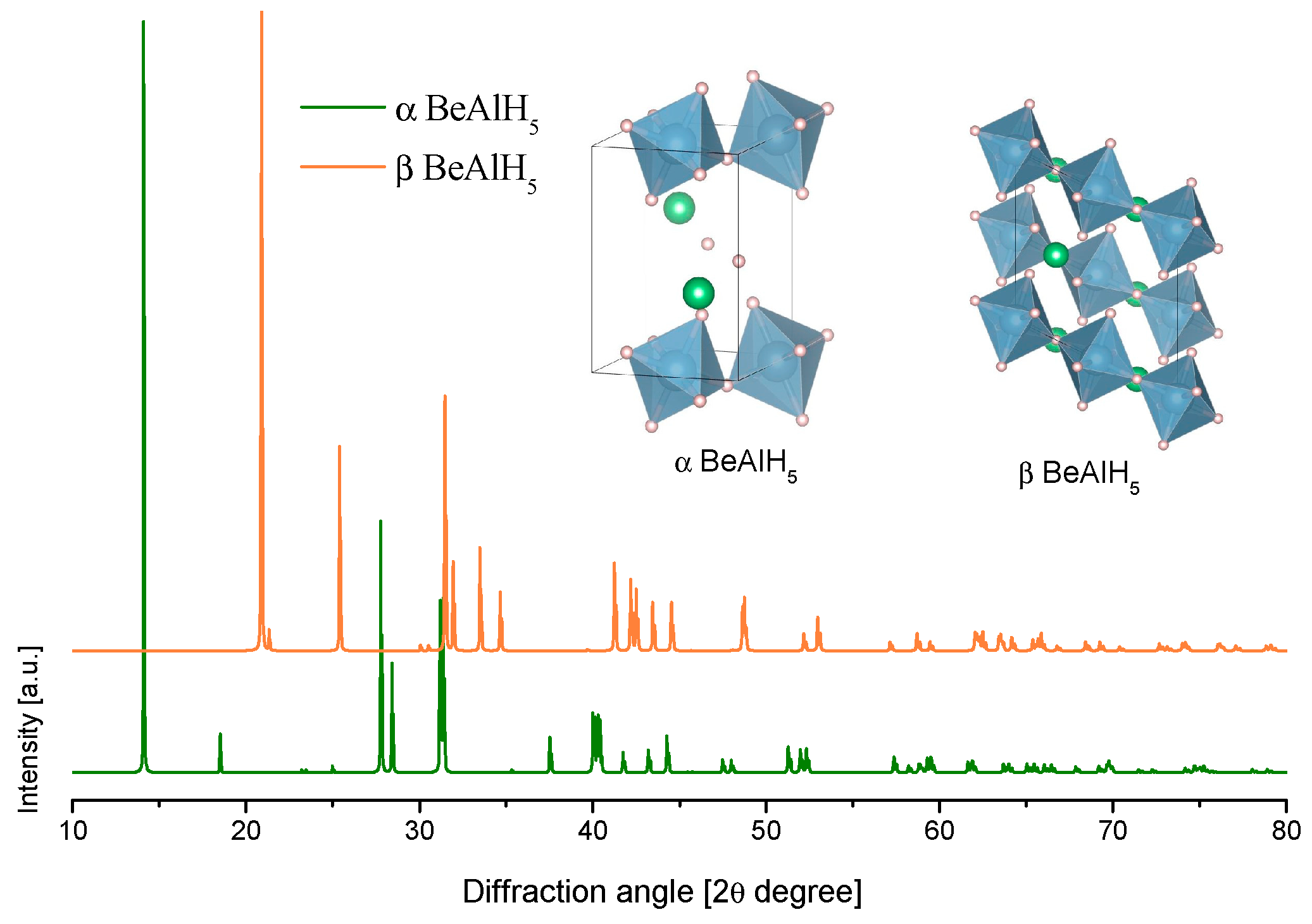

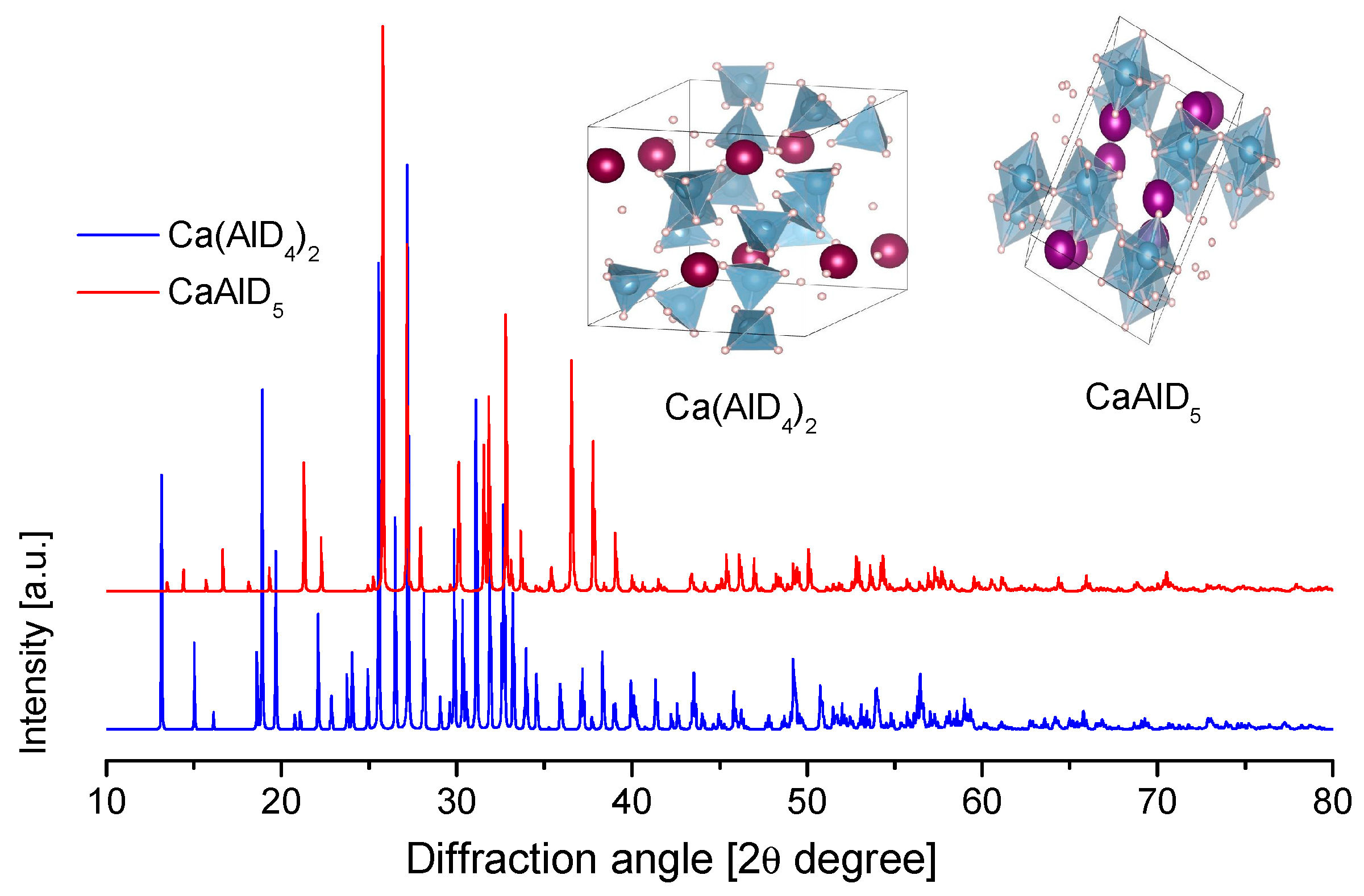
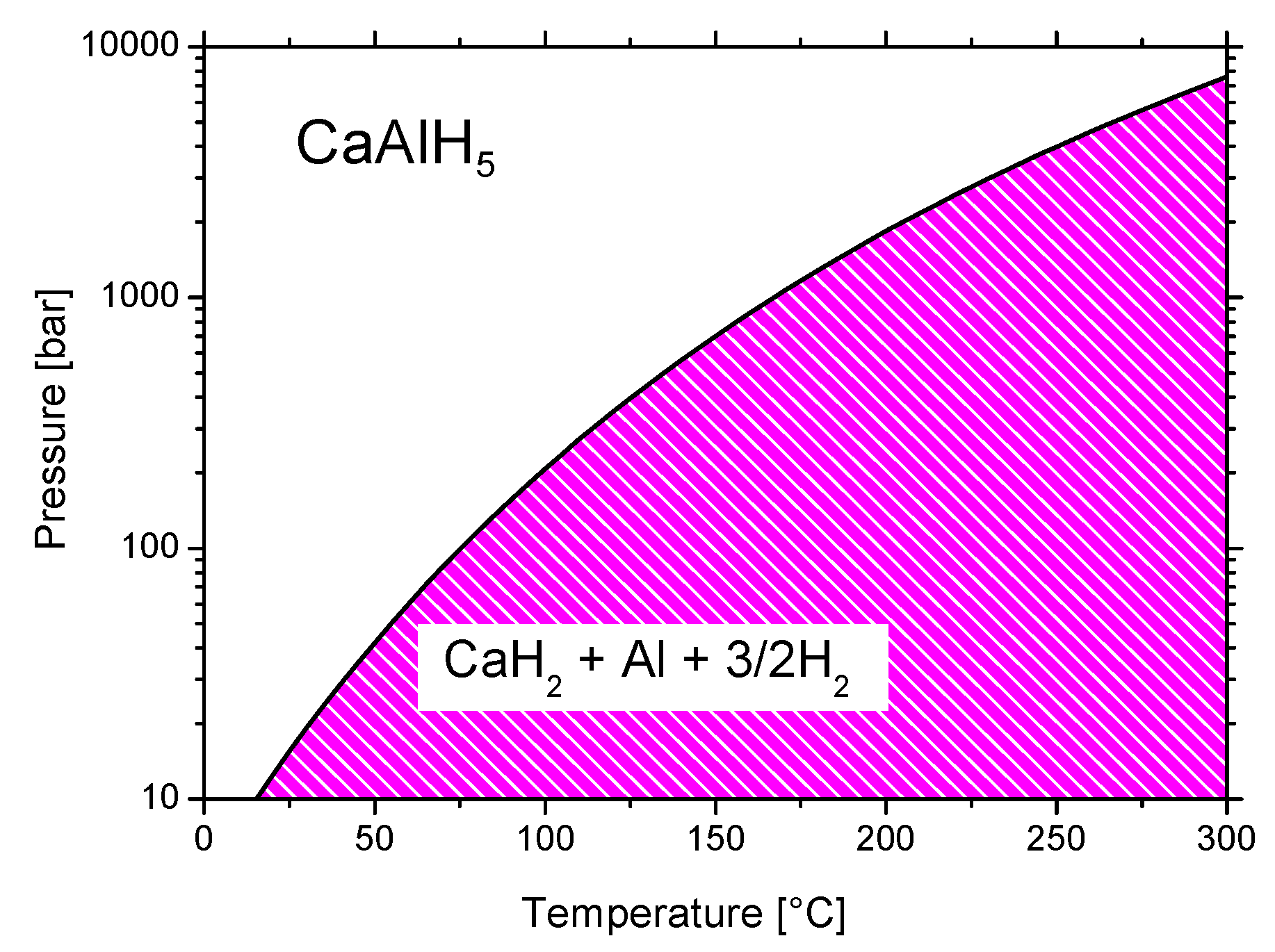
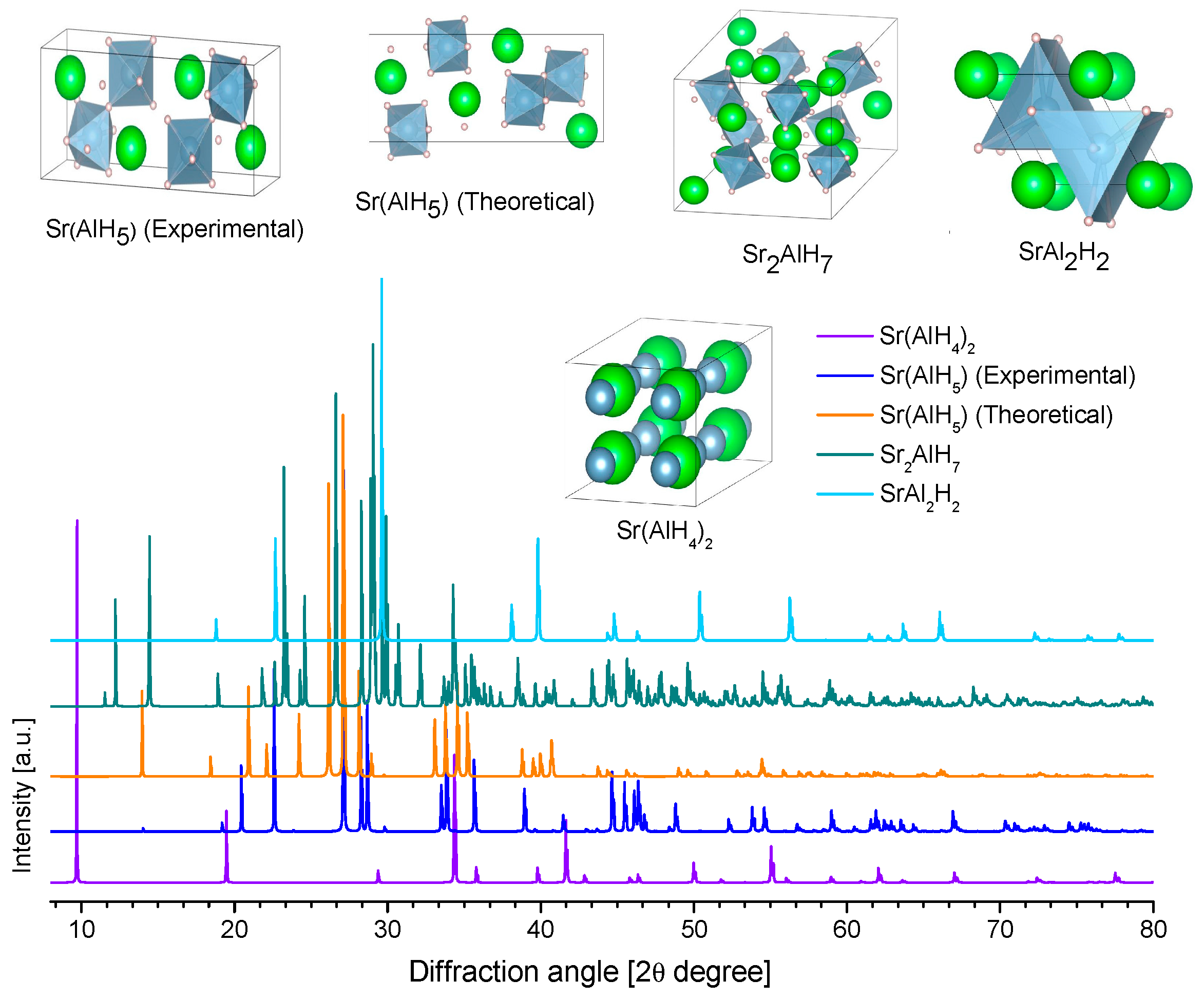
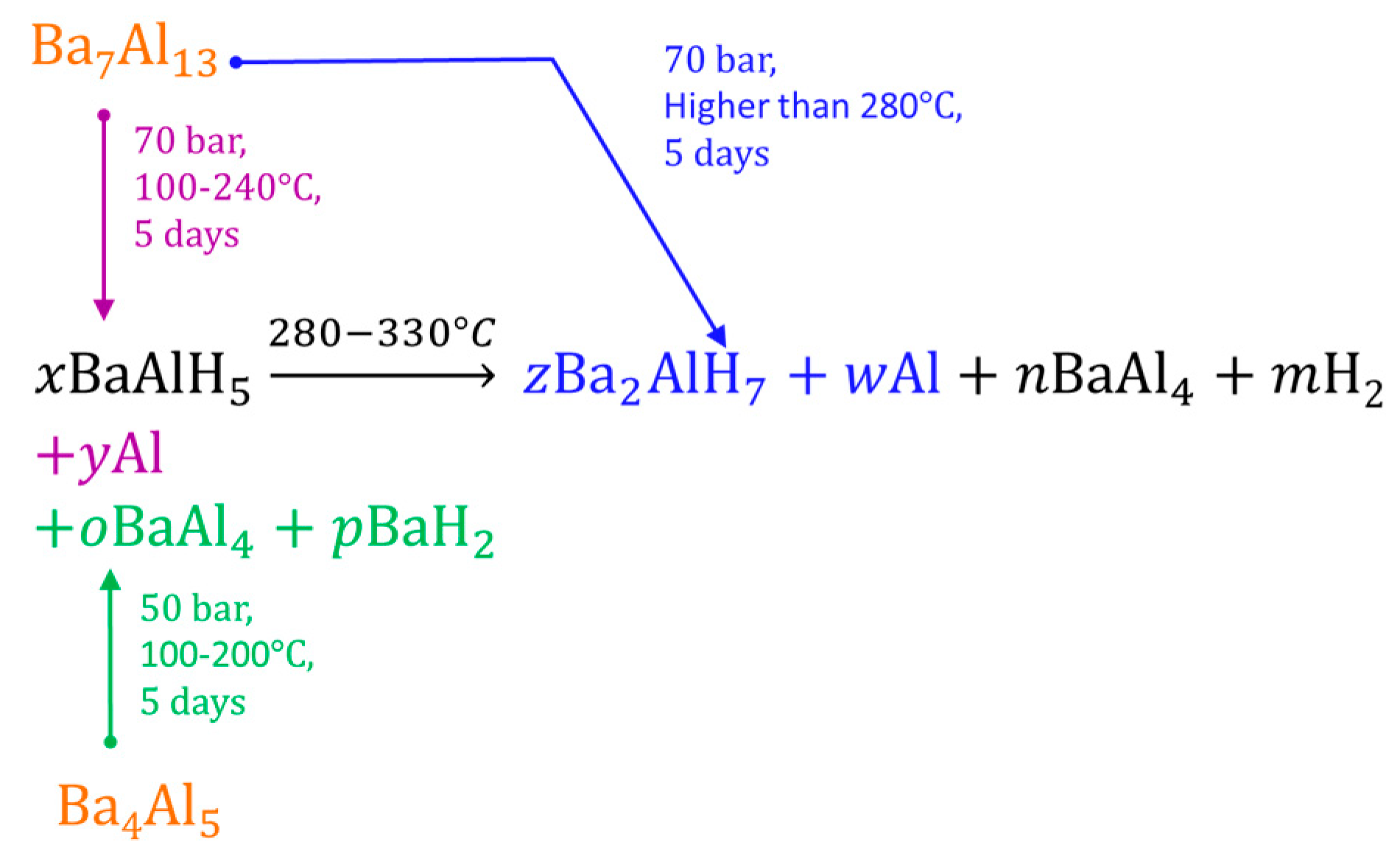
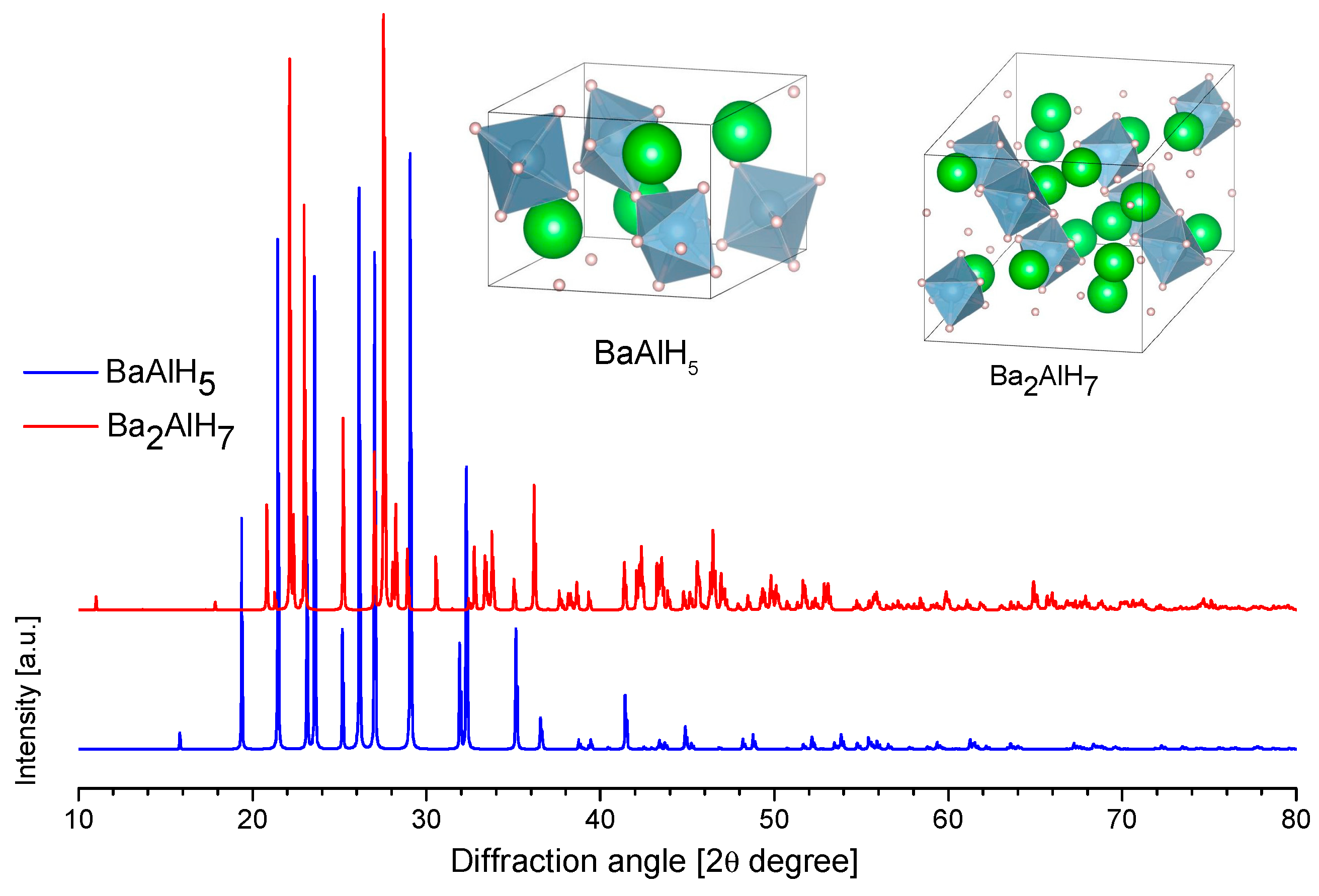


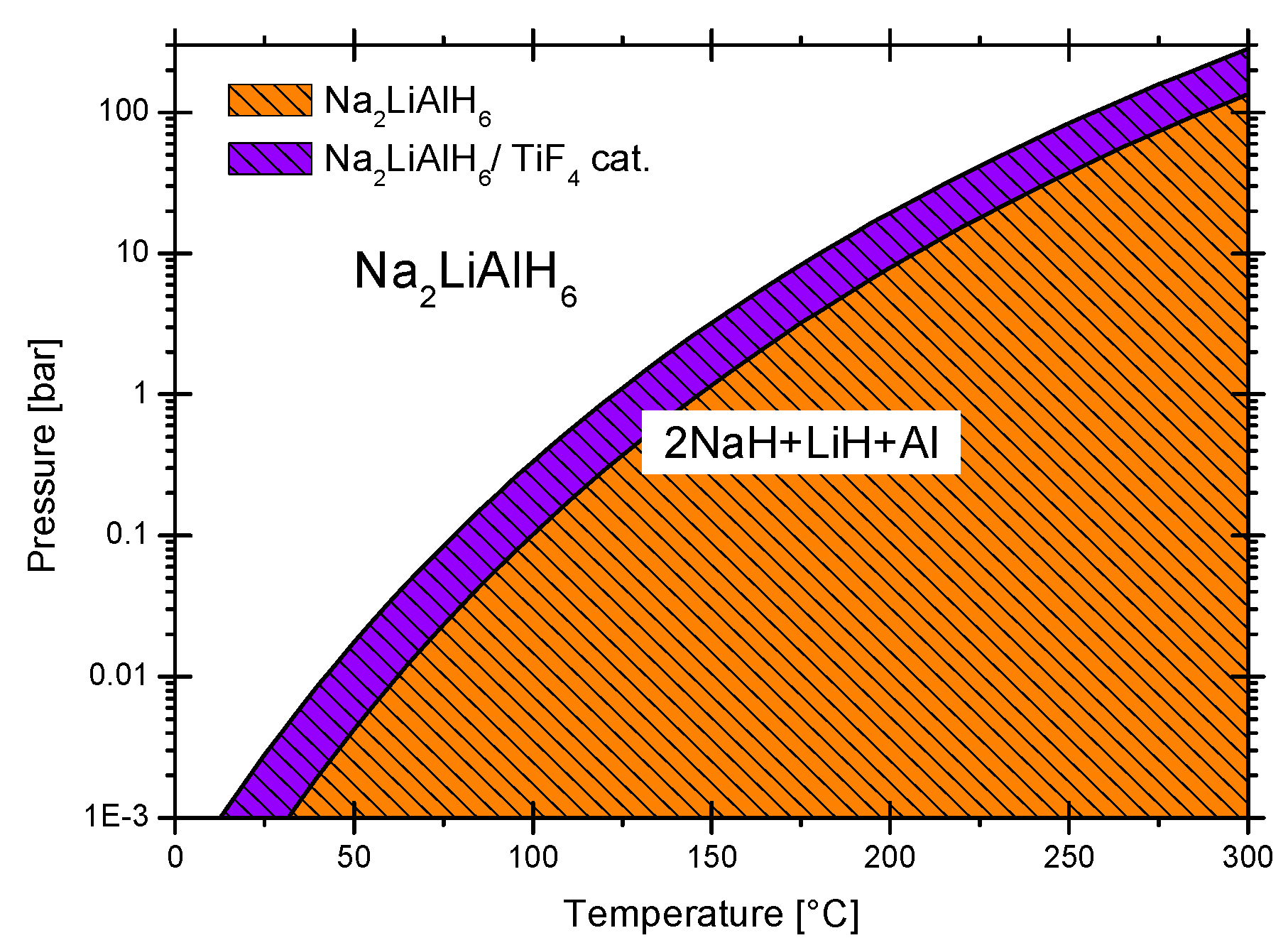
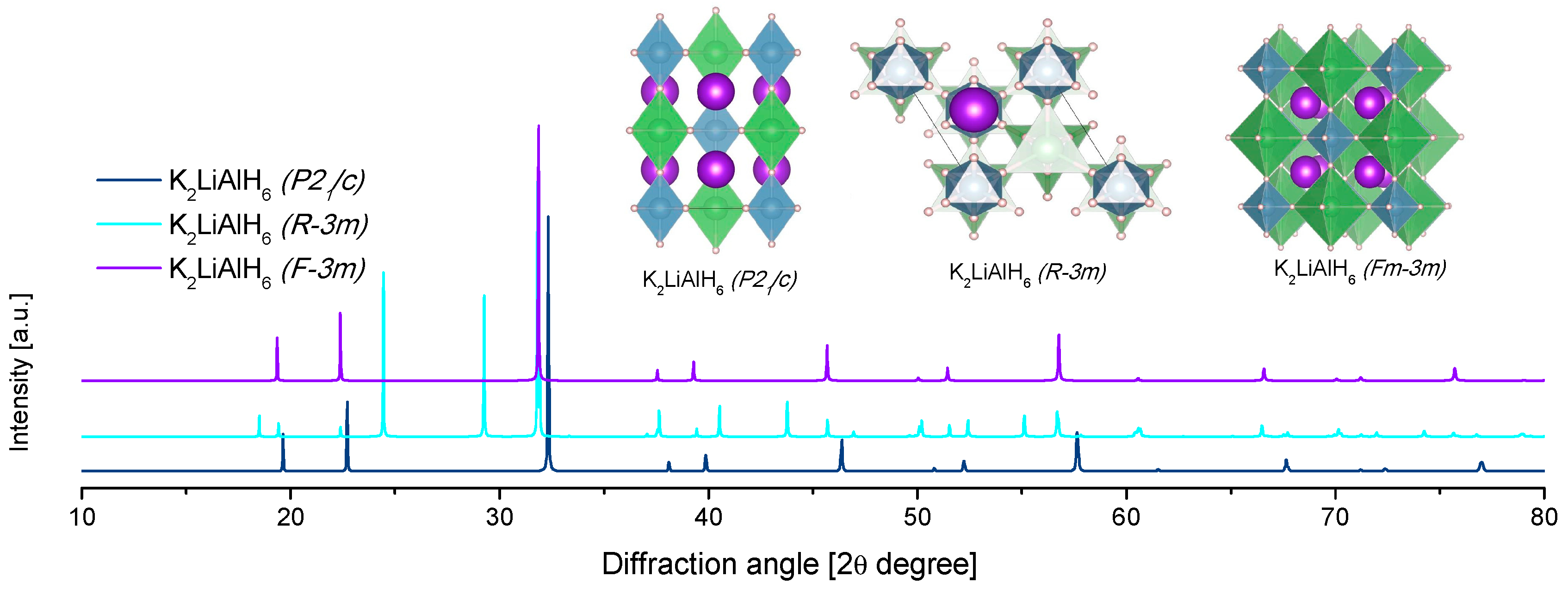
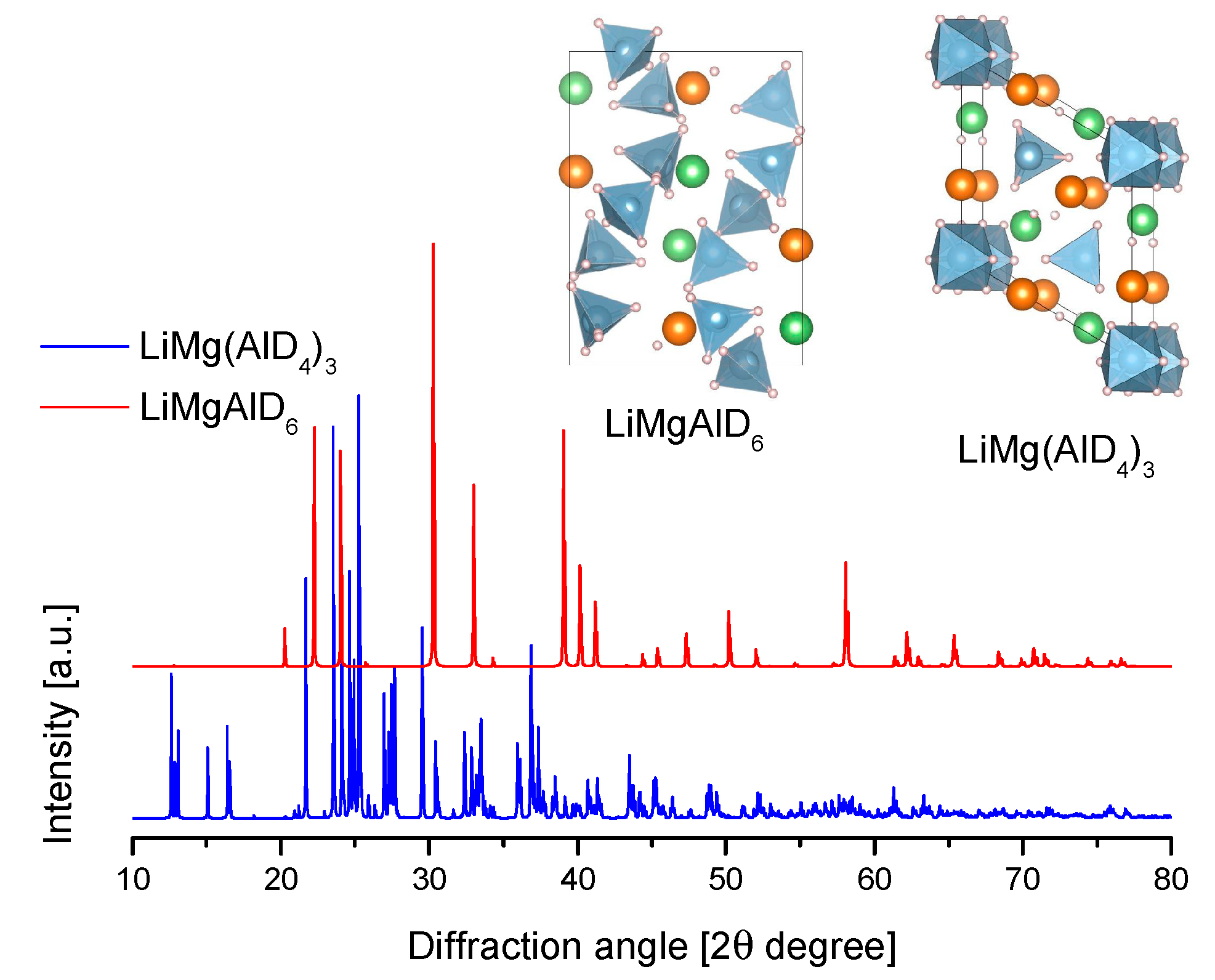
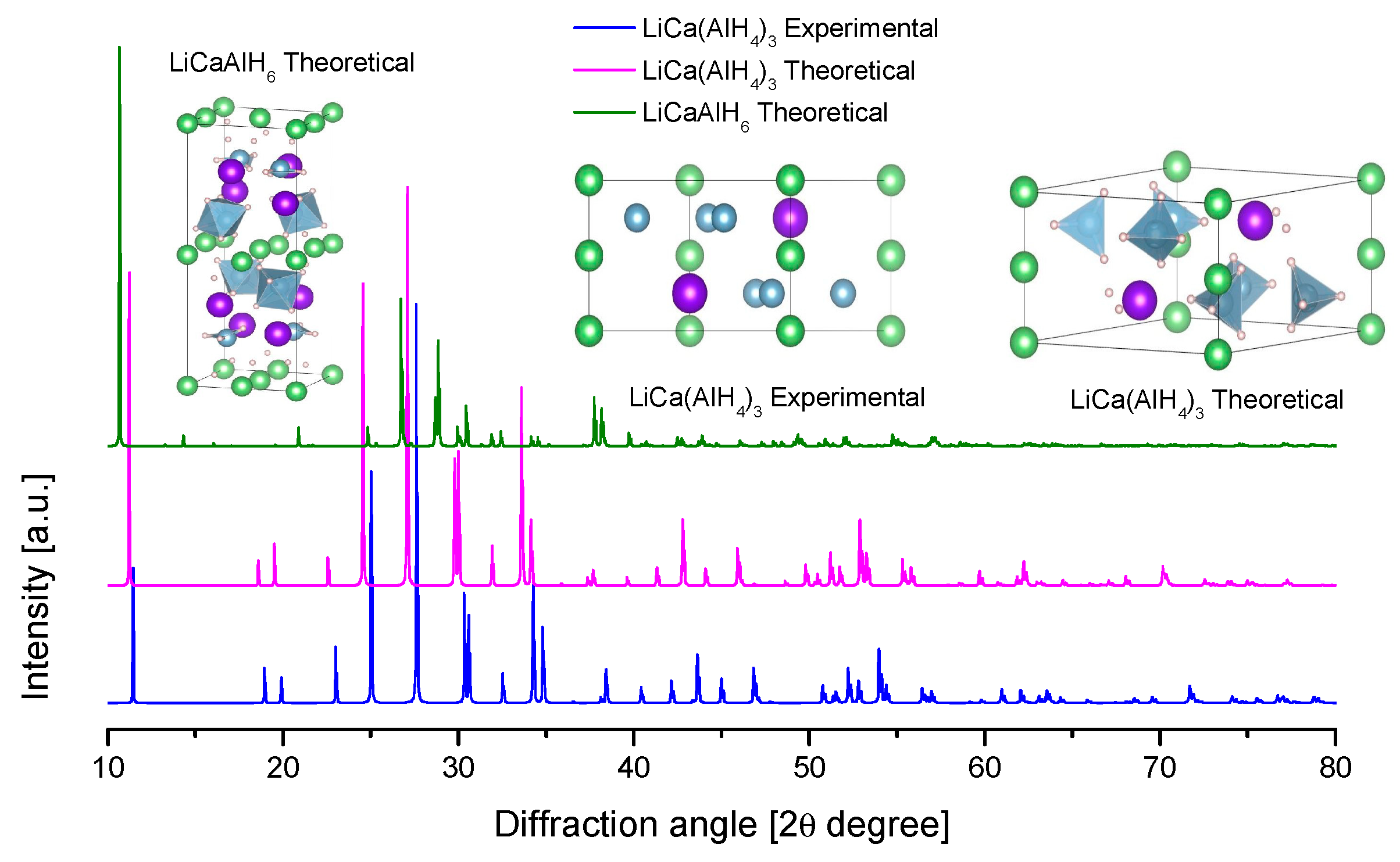
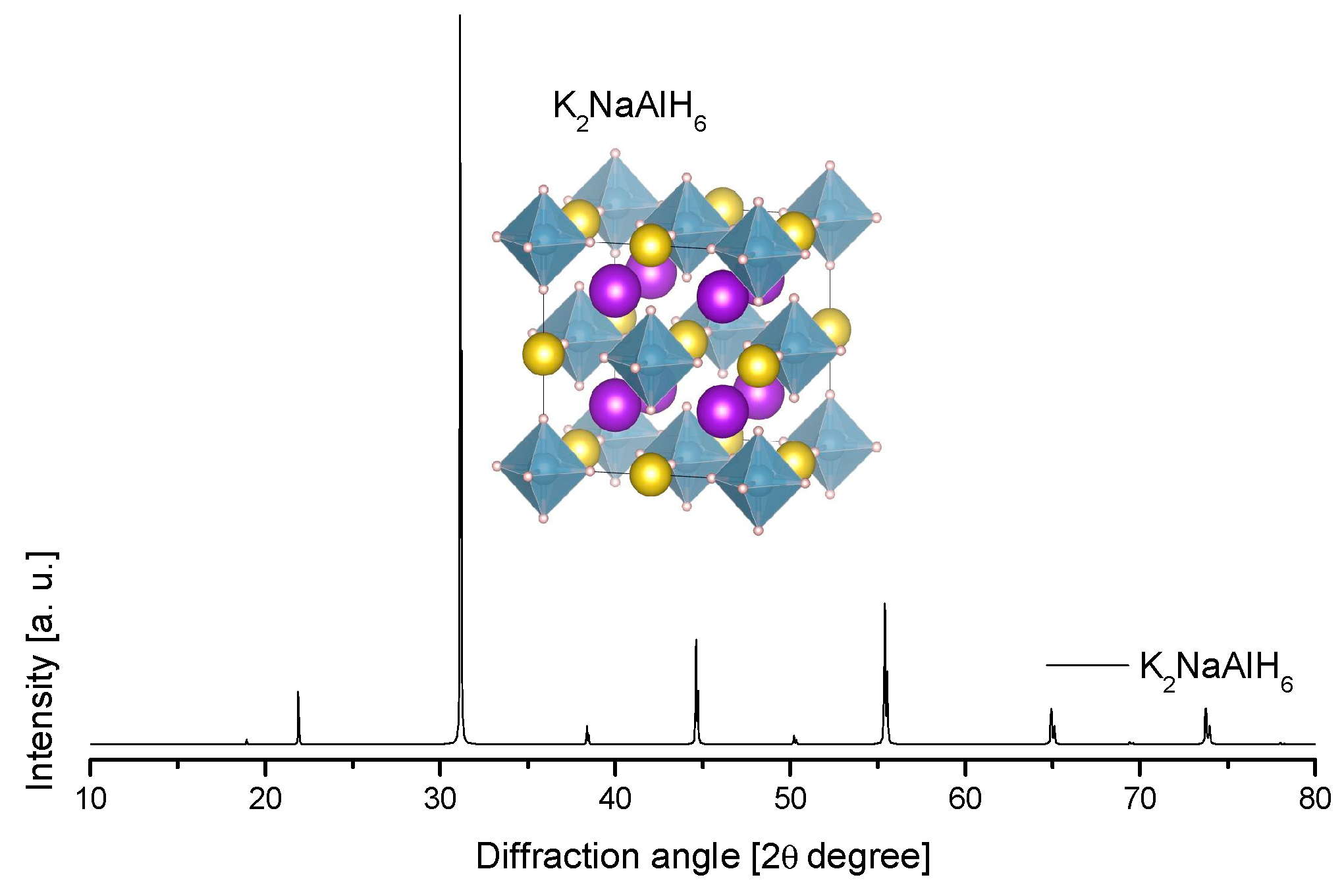
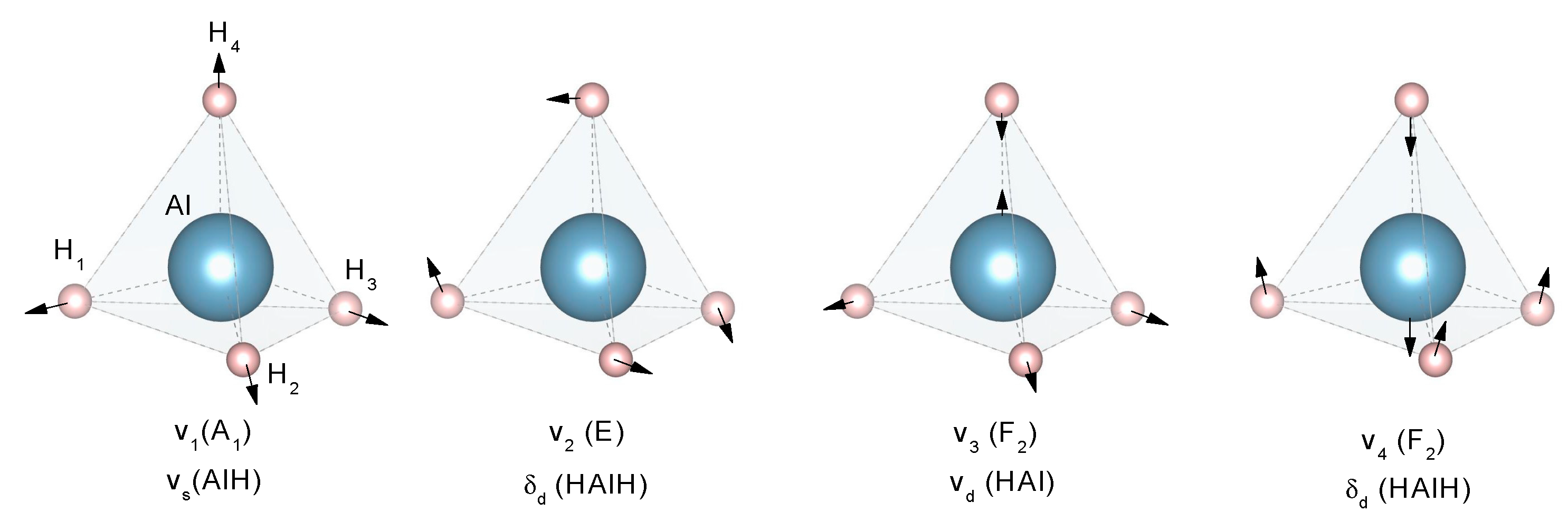
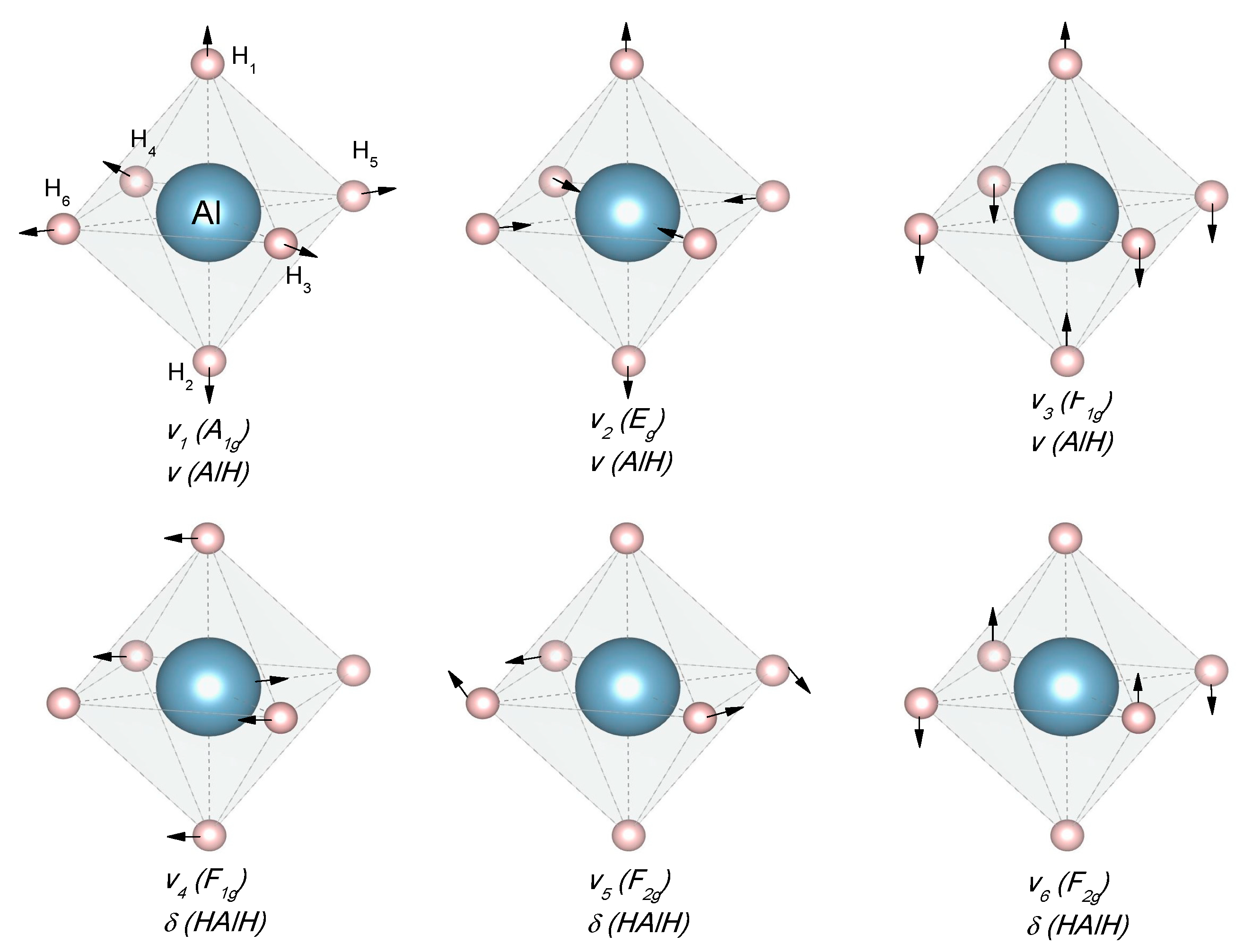
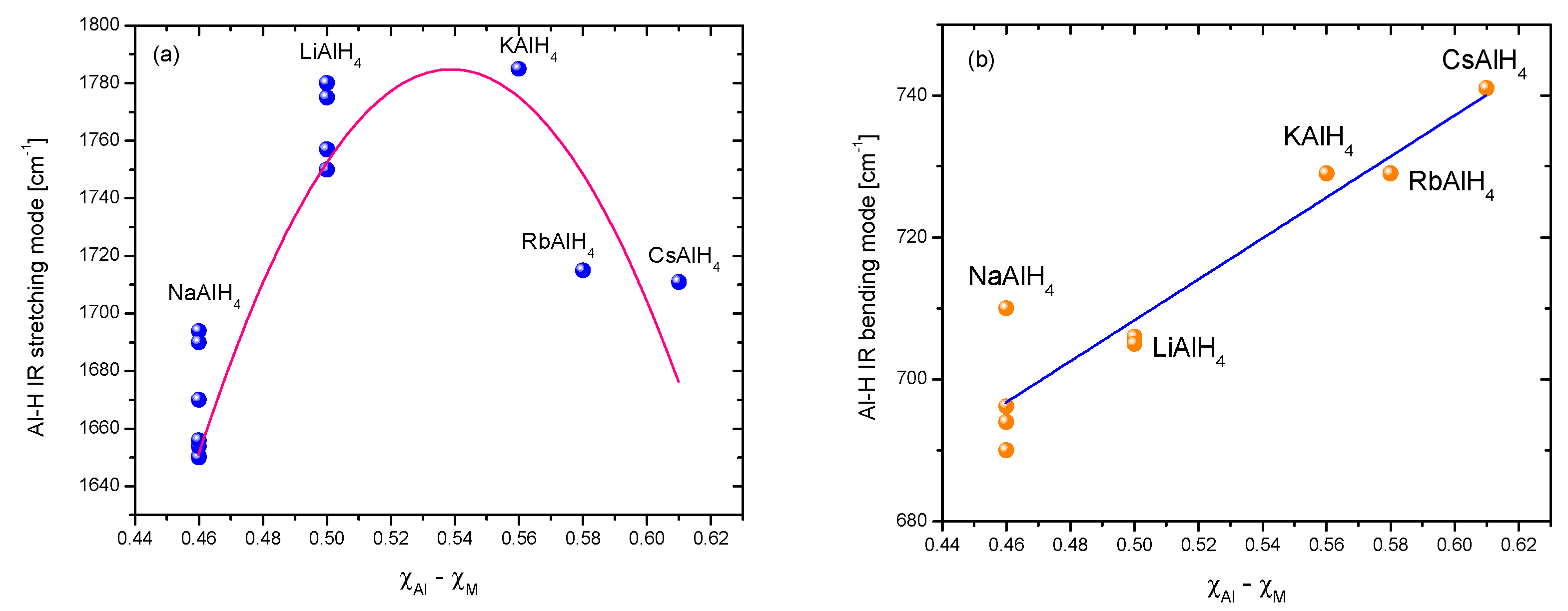
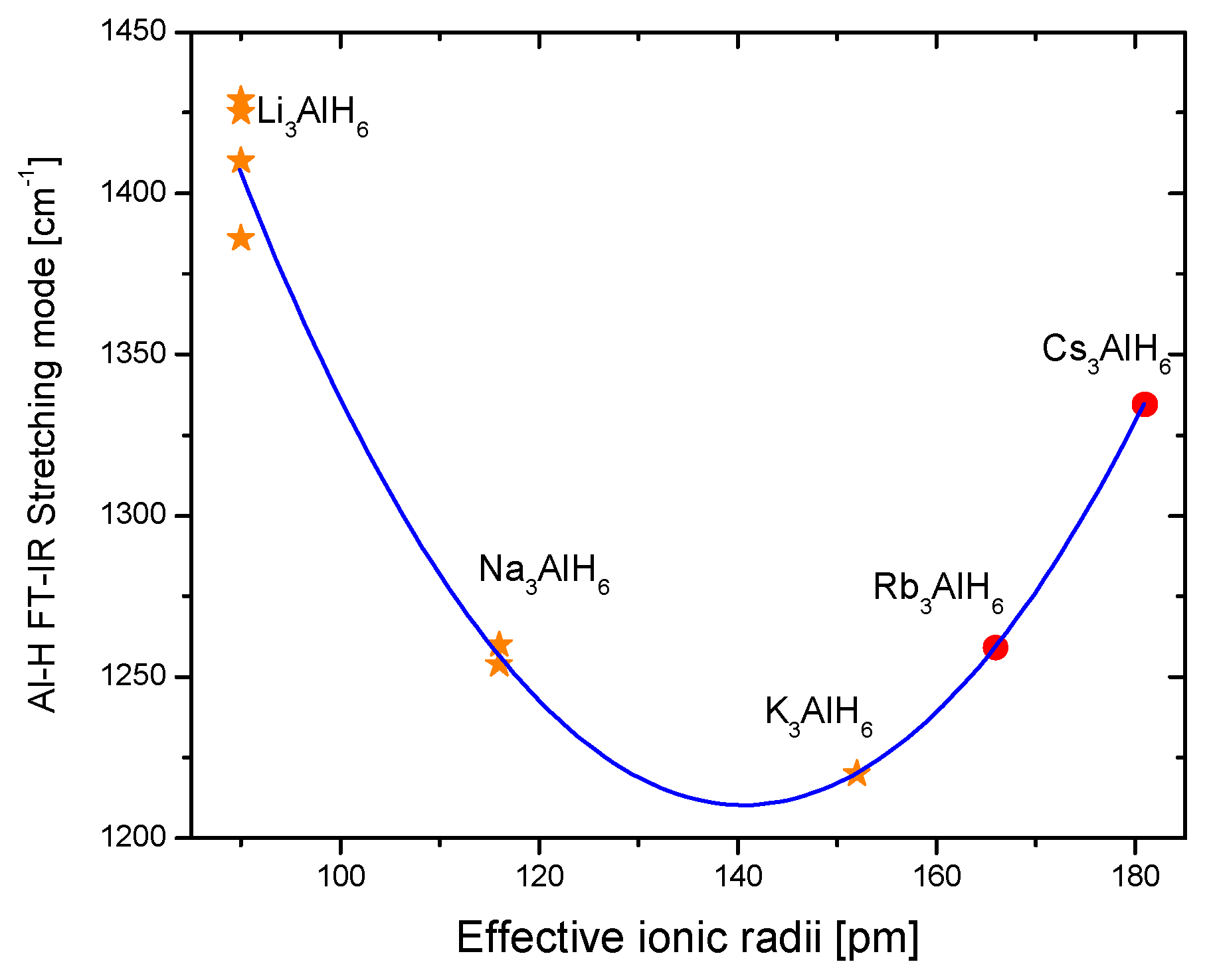

| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| α-AlD3 | (R-3c) No. 167 [66] a = 4.227; b = 4.227; c = 11.244 α = β = 90; γ = 120 | Al: 0, 0, 0 D: 0.63, 0, ¼ |
| α’-AlD3 | (Cmcm) No. 63 [47] a = 6.470(3); b = 11.117(5); c = 6.562(2) α = β = 90; γ = 90 | Al1: 0, ½, 0 Al2: ¼, ¼, 0 D1: 0, 0.197(2), 0.451(4) D2: 0.312(2), 0.1000(14), 0.047(3) D3: 0, 0.465(3), ¼ D4: 0.298(4), 0.277(2), ¼ |
| β-AlD3 | (Fd-3m) No. 227 [67] a = 9.0037(1); b = 9.0037(1); c = 9.0037(1) α = β = γ = 90 | Al1: ½, 0, 0 D1: 0.4301(1); 0.125; 0.125 |
| γ-AlD3 | (Pnnm) No. 58 [68] a = 7.3360(3); b = 5.3672(2); c = 5.7562(1) α = β = γ = 90 | Al1: 0, 0, 0 Al2: 0.4174(5), 0.7127(6), 0 D1: 0.2044(9), 0.8269(11), 0 D2: 0.3668(10), 0.3931(13), 0 D3: 0, ½, ½ D4: 0.4174(6), 0.7038(8), 0.3009(6) |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| α-LiAlD4 | (P21/c) No. 14 [78] a = 4.8254(1); b = 7.8040(1); c = 7.8968(1) α = 90; β = 112.268(1); γ = 90 | Al: 0.1428(2), 0.2013(1), 0.9311(1) Li: 0.5601(12), 0.4657(6), 0.8236(6) D1: 0.1902(10), 0.0933(8), 0.7710(6) D2: 0.3526(10), 0.3726(7), 0.9769(6) D3: 0.2384(11), 0.0840(7), 0.1141(7) D4: 0.8024(14), 0.2644(7), 0.8689(8) |
| β-LiAlH4 | (I 41/a) No. 88 [76] a = 4.6611; b = 4.6611; c = 10.5219 α = β = γ = 90 | Li: 0, ¼, 0.625 Al: 0, ¼, 0.125 H: 0.2527, 0.4237, 0.5413 |
| γ-LiAlH4 | (Pnma) No. 62 [76] a = 6.4667; b = 5.3478; c = 6.5931 α = β = γ = 90 | Li: 0.2428, ¼, 0.2467 Al: 0.513, ¼, 0.8221 H1: 0.3067, ¼, 0.9617 H2: 0.7162, ¼, 0.9631 H3:0.4889, 0.9833, 0.2943 |
| Li3AlH6 | (R-3) No. 148 [81] a = 8.0389(2); b = 8.0389(2); c = 9.4755(5) α = β = 90, γ = 120 | Al1: 0, 0, 0 Al2: 0, 0, ½, Li: 0.966(2), 0.236(3), 0.3007(17) D1: 0.8325(11), 0.8030(7), 0.1008(6) D2: 0.1593(10), 0.1799(8), 0.3884(6) |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| NaAlH4 | (I 41/a) No. 88 [118] a = b = 5.020(2); c = 11.330(3) α = β = γ = 90 | Al: 0, 0, 0 Na: 0, 0, ½ H: 0.228(1) 0.117(2) 0.838(9) |
| Na3AlH6 | (P 21/c) No. 14 [121] a = 5.4145(3); b = 5.5402(3); c = 7.7620(4) α = 90; β = 89.871(4), γ = 90 | Al: 0, 0, 0 Na1: 0, 0, ½ Na2: −0.00129(5), 0.46129(4), 0.25008(4) H1: 0.0918, 0.0352, 0.2207 H2: 0.222, 0.3283, 0.5454 H3:0.1649, 0.2689, 0.95 |
| Na3AlD6 | (P 21/c) No. 14 [122] a = 5.390(2); b = 5.514(2); c = 7.725(3) α = 90; β = 89.86(3), γ = 90 | Na1: 0, 0, ½ Na2: −0.006(5), 0.461(4), 0.252(5) Al: 0, 0, 0 D1:0.091(3), 0.041(3), 0.215(3) D2: 0.234(3), 0.328(3), 0.544(3) D3: 0.165(3), 0.266(3), 0.944(3) |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| KAlD4 | (Pnma) No. 62 [173] a = 8.8514(14); b = 5.8119(8); c = 7.3457(11) α = β = γ = 90 | K: 0.1839(12), ¼, 0.1522(17) Al: 0.5578(11), ¼, 0.8209(13) D1: 0.4018(10), ¼, 0.9156(9) D2: 0.7050(9), ¼, 0.9630(12) D3: 0.4209(6), 0.9741(8), 0.3098(7) |
| α-K3AlH6 | (P 21/c) No. 14 [175] a = 6.1771; b = 5.8881; c = 8.6431 α = 90; β = 89.3, γ = 90 | K1: 0, 0, ½ K2: −0.0058, 0.4828, 0.2544 Al: 0, 0, 0 H1: 0.0617, 0.0089, 0.2042 H2: 0.2799, 0.3136, 0.5349 H3: 0.1786, 0.2281, 0.9652 |
| β-K3AlH6 | (I 4/mmm) No. 139 [175] a = b = 4.4441; c = 7.8098 α = β = γ = 90 | K1: 0, 0, ½ K2: 0, ½, ¼ Al1: 0, 0, 0 H1: 0, 0, 0.2128 H2: 0.3429, 0, 0 |
| γ-K3AlH6 | (Pnnm) No. 58 [175] a = 10.8885; b = 10.2576; c = 2.5538 α = β = γ = 90 | K1: 0.2347, 0.03444, 0 K2: 0.55047, ¾, 0 K3: 0.691, 0.2178, 0 Al1: ½, ½, 0 Al2: 0, ½, 0 H1: 0.9388, 0.0715, 0 H2: 0.5928, 0.3931, 0 H3: 0.3085, 0.3814, 0 H4: 0.0632, 0.3708, 0 H5: 0.4194, 0.0352, 0 H6: 0.8387, 0.3512, 0 |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| α-RbAlD4 | (Pnma) No. 62 [176] a = 9.2862(6); b = 5.9392(3); c = 7.5784(6) α = β = γ = 90 | Rb: 0.1813(4), ¼, 0.1574(7) Al: 0.5639(6), ¼, 0.8121(7) D1: 0.4045(7), ¼, 0.9073(7) D2: 0.6884(7), ¼, 0.9615(8) D3: 0.4204(4), 0.9691(6), 0.3080(6) |
| α-RbAlD4 | (Cmc21) No. 36 [176] a = 3.9933; b = 14.6472; c = 6.4933 α = β = γ = 90 | Rb: ½, 0.6206, 0.2833 Al: ½, 0.1154, 0.7607 D1: ½, 0.7996, 0.0670 D2: ½, 0.1717, 0.9990 D3: ½, 0.5992, 0.7814 D4: ½, 0.9888, 0.1074 |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| CsAlD4 (o) | (Pnma) No. 62 [185] a = 9.8847(5); b = 6.15949(29); c = 7.9182(4) α = β = γ = 90 | Al: 0.55462(33), ¼, 0.80887(30) D1: 0.5755(4), 0.04042(31), 0.69536(32) D2: 0.6641(6), ¼, 0.9620(5) D3: 0.4017(5), ¼, 0.8868(6) Cs: 0.1847(4), ¼, 0.1652(8) |
| CsAlD4 (t) | (I 41/a) No. 88 [185] a = b = 5.67231(9); c = 14.2823(5) α = β = γ = 90 | Al: 0, ¾, 0.875 Cs: ½, ¾, 0.125 D1: 0.19658(31), 0.7115(9), 0.95567(12) D2:0.25993(26), 0.7644(19), 0.92159(17) |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| α-BeAlH5 | (P21) No. 4 [192] a = 4.790; b = 4.324; c = 6.227 α = γ = 90; β = 89.408 | Be: 0.002, 0.230, 0.623 Al: 0.243, 0.990, 1.000 H1: 0.247, 0.162, 0.749 H2: 0.001, 0.740, 0.902 H3: 0.501, 0.740, 0.914 H4: 0.240, 0.821, 0.251 H5: 0.890, 0.965, 0.515 |
| β-BeAlH5 | (C2/c) No. 15 [192] a = 5.959; b = 7.008; c = 6.241 α = γ = 90; β = 116.205 | Be: 0, 0.333, 0.250 Al: 0,0,0 H1: 0, 0.904, 0.250 H2: 0.902, 0.777, 0.881 H3: 0.688, 0.044; 0.913 |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| Mg(AlH4)2 | (P −3 m 1) No. 164 [202] a = b = 5.1949(2); c = 5.8537(2) α = 90; β = 90; γ = 120 | Mg: 0, 0, 0 Al: 0.3333, 0.6667, 0.7057(5) H1: 0.3333, 0.6667, 0.439(2) H2: 0.1589(14), −0.1589(14), 0.804(2) |
| MgAlH5 | (P 21 21 21) No. 19 [206] a = 4.55; b = 4.26; c = 13.024 α = 90; β = 90; γ = 90 | Mg: −0.2504, −0.2466, −0.3204 Al: 0.2486, 0.2528, −0.4083 H1: −0.4756, −0.0559, 0.4069 H2: −0.03, 0.0912, 0.3051 H3: 0.4719, −0.0516, −0.4063 H4: 0.0284, 0.0975, −0.3045 H5: −0.0024, 0.0916, −0.4994 |
| α-MgAlH5 | (P 21 /c) No. 14 [207] a = 4.7499; b = 8.8127; c = 6.6281 α = 90; β = 90; γ = 109.75 | Mg: 0.527, 0.985, 0.253 Al: 0.092, 0.245, 0.395 H1: 0.400, 0.121, 0.444 H2: 0.349, 0.390, 0.495 H3: 0.121, 0.592, 0.201 H4: 0.197, 0.862, 0.142 H5: 0.130, 0.305, 0.156 |
| β-MgAlH5 | (Cc) No. 9 [207] a = 7.8033; b = 5.7251; c = 6.7393 α = 90; β = 90; γ = 115.39 | Mg: 0.542, 0.025, 0.257 Al: 0.000, 0.000, 0.000 H1: 0.008, 0.924, 0.256 H2: 0.201, 0.289, 0.034 H3: 0.771, 0.969, 0.882 H4: 0.027, 0.299, 0.979 H5: 0.246, 0.031, 0.130 |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| Ca(AlD4)2 | (Pbca) No. 61 [221] a = 13.4491(27); b = 9.5334(19); c = 9.0203(20) α = β = γ=90 | Ca: 0.8958(1), 0.4662(2), 0.2818(3) Al1: 0.4389(3), 0.7757(5), −0.0011(8) Al2: 0.8460(3), 0.1060(4), 0.1839(5) D1: 0.3710(9), 0.6842(11), 0.1087(12) D2: 0.5280(8), 0.8546(12), 0.0825(14) D3: 0.4877(9), 0.6706(12), −0.1183(13) D4: 0.3647(8), 0.8817(11), −0.0835(13) D5: 0.8264(10), 0.0829(11), 0.0086(8) D6: 0.8094(8), 0.2610(8), 0.2337(14) D7: 0.9590(5), 0.0702(12), 0.2407(16) D8: 0.7762(9), −0.0075(10), 0.2636(16) |
| CaAlD5 | (P 21/c) No. 14 [221] a = 9.8000(19); b = 6.9081(13); c = 12.4503(23) α = 90; β = 137.936(4); γ = 90 | Ca1: 0.7845(16), 0.2166(19), 0.7382(13) Ca2: 0.3275(14), 0.2676(16), 0.1816(11) Al1: 0.8017(15), 0.3097(16), 0.4907(12) Al2: 0.2071(14), 0.2175(14), 0.8706(11) D1: 0.0058(17), 0.3009(19), 0.5190(14) D2: 0.6406(16), 0.4242(18), 0.3076(12) D3: 0.6070(14), 0.2725(17), 0.4696(13) D4: 0.7010(18), 0.3865(14), 0.8592(15) D5: 0.9589(14), 0.1915(15), 0.6767(10) D6: 0.1259(17), 0.0329(14), 0.9070(13) D7: 0.1154(19), 0.3773(14), 0.9139(15) D8: 0.2848(16), 0.0634(15), 0.8156(14) D9: 0.2612(19), 0.4064(13), 0.8154(13) D10: 0.4470(13), 0.1884(16), 0.0707(12) |
| Compound | Space Group, Cell Dimensions [Å] and Angles [°] | Atomic Coordinates |
|---|---|---|
| Sr(AlH4)2 | Pmmn (No. 59) [44] a = 9.1165(18); b = 5.2164(11); c = 4.3346(8) α = β = γ = 90 | Sr: 0.1958(3), ¼, ¾ Al1: 0.9665(11), ¼, ¼ Al2: 0.37309(11), ¾, ¼ |
| SrAlD5 (experimental) | Pbcm (No. 57) [229] a = 4.6226(10); b = 12.6213(30); c = 5.0321(10) α = β = γ = 90 | Sr: 0.2532(7), 0.8925(3), ¼ Al: 0.3296(11), 0.1597(3), ¼ D1: 0.4366(13), ¼, 0 D2: 0.3461(13), 0.5790(5), ¼ D3: 0.0311(13), 0.7146(3), ¼ D4: 0.1914(7), 0.0718(3), 0.4986(9) |
| SrAlH5 (theoretical) | P 212121 (No. 19) [192] a = 12.679; b = 5.200; c = 4.508 α = β = γ =90 | Sr: 0.908, 0.104, 0.036 Al: 0.165, 0.117, 0.071 H1: 0.763, 0.859, 0.278 H2: 0.078, 0.337, 0.918 H3: 0.093, 0.860, 0.945 H4: 0.079, 0.114, 0.374 H5: 0.254, 0.116, 0.768 |
| Sr2AlD7 | I2 (No. 5) [230] a = 12.552(1); b = 9.7826(8); c = 7.9816(7) α = γ = 90; β = 100.286(4) | Sr1: 0.0935(3), 0.3289(4), 0.3195(6) Sr1´: 0.9065(3), 0.6711(4), 0.3195(6) Sr2: 0.8609(4), 0.0684(4), 0.0882(6) Sr2´: 0.1391(4), 0.9316(4), 0.4118(6) Al1: 0.671(1), 0.847(1), 0.232(2) Al1´: 0.329(1), 0.153(1), 0.268(2) D1: 0.7494(7), 0.8594(7), 0.077(1) D1´: 0.2506(7), 0.1406(7), 0.423(1) D2: 0.6014(7), 0.7106(7), 0.117(1) D2´: 0.3986(7), 0.2894(7), 0.383(1) D3: 0.7658(6), 0.7378(8), 0.341(1) D3´: 0.2342(6), 0.2622(8), 0.159(1) D4: 0.5885(6), 0.8298(8), 0.379(1) D4´: 0.4115(6), 0.1702(8), 0.121(1) D5: 0.7395(6), 0.9919(7), 0.3291(9) D5´: 0.2605(6), 0.0081(7), 0.1709(9) D6: 0.5748(6), 0.9558(7), 0.1157(8) D6´: 0.4252(6), 0.0442(7), 0.3843(8) D7: 0.4375(6), 0.6037(7), 0.3189(9) D7´: 0.5625(6), 0.3963(7), 0.1811(9) |
| SrAl2D2 | P-3m1 (No. 164) [233] a = b = 4.5253(1); c = 4.7214(2) α = γ = 90; β = 120 | Sr: 0,0,0 Al: 0.3333, 0.6667, 0.4589(7) D: 0.3333, 0.6667, 0.0976(4) |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| BaAlH5 | Pna21 (No. 33) [207] a = 9.1568; b = 7.0718; c = 5.1039 α = β = γ = 90 | Ba: 0.686, 0.156, 0.256 Al: 0.041, 0.846, 0.229 H1: 0.008, 0.946, 0.905 H2: 0.584, 0.844, 0.025 H3: 0.578, 0.786, 0.504 H4: 0.357, 0.695, 0.233 H5: 0.708; 0.545, 0.214 |
| Ba2AlD7 | I2/a (No. 15) [235] a = 13.197(3); b = 10.237(2); c = 8.509(2) α = γ = 90; β = 101.290(9) | Ba1: 0.3459, 0.5848, 0.3249 Ba2: 0.1084, 0.3247, 0.0852, Al1: 0.927, 0.096, 0.235 D1: 0.004(1), 0.116(1), 0.077(2) D2: 0.846(1), 0.974(1), 0.135(2) D3: 0.023(1), 0.999(2), 0.325(2) D4: 0.844(1), 0.104(2), 0.387(2) D5: 0.983(1), 0.249(2), 0.324(2) D6: 0.832(1), 0.207(1), 0.115(2) D7: 0.693(1), 0.864(1), 0.322(2) |
| Material | Hydrogen Content | Hydrogen Release * | Decomposition Temperature [°C] | Crystal Structure (R-3m, No.166) [Å] | |
|---|---|---|---|---|---|
| [wt.%] | Experimental | DFT ** | |||
| LaAlH6 | 3.51 | 0.98 | Beginning 100, ending 240 | a = 6.4732 c = 6.2765 | a = 6.5272(4) c = 6.3212(7) H: 0.2149, 0.7851, 0.4904 |
| CeAlH6 | 3.49 | 0.80 | First step: Beginning 100, ending 170 Second step: Beginning 180, ending 270 | a = 6.4711 c = 6.2527 | a = 6.4637(4) c = 6.2609(7) H: 0.2147, 0.7853, 0.4910 |
| PrAlH6 | 3.47 | 0.78 | a = 6.4217 c = 6.2028 | a = 6.4106(7) c = 6.2118(11) H: 0.2139, 0.7861, 0.4894 | |
| NdAlH6 | 3.41 | 0.78 | a = 6.3796 c = 6.1616 | a = 6.3846(7) c = 6.1741(10) H: 0.2132, 0.7868, 0.4883 | |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| Eu(AlH4)2 | Pmmn (No. 59) a: 9.1003(13); b: 5.1912(8); c: 4.2741(5) | Eu: 0.1966(3), 0.25, 0.75 Al: 0.9625(12), 0.25, 0.25 Al: 0.3821(5), 0.75, 0.25 |
| EuAlH5 | Pnma (No. 62) a: 12.481(3); b: 5.0103(12); c: 4.5887(11) | Eu: 0.6517(3), 0.25, 0.2016(12) Al: 0.4105(14), 0.25, 0.586(4) |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| Na2LiAlD6 (experimental) | Fm-3m (No. 225) [284] a: 7.38484 (5) | Na: 0.25, 0.25, 0.25 Li: 0.5, 0.5, 0.5 Al: 0, 0, 0 D: 0.238(4), 0, 0 |
| Na2LiAlH6 (calculated) | P 21/c (No. 14) [282] a = 5.165; b = 5.251; c = 7.339 α = 90, β = 90.03, γ = 90 | Li: 0, 0, 0.5 Na: 0.99, 0.47, 0.25 Al: 0, 0, 0 H: 0.07, 0.02, 0.23 H: 0.23, 0.3, 0.53 H: 0.2, 0.27, 0.96 |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| K2LiAlH6 | R-3m (No. 166) [286] a: 5.62068(8) c: 27.3986(6) | Li: 0, 0, 0.4036(8) Al: 0, 0, 0 Al: 0, 0, ½ K: 0, 0, 0.1270(1) K: 0, 0, 0.2853 (1) H: 0.096(7), −0.096(7), 0.466(3) H: 0.205(5), −0.205(5), 0.638(2) |
| K2LiAlH6 | Fm-3m (225) [267] a = 7.9383 | K: ¼, ¼, ¼ Li: ½, ½, ½ Al: 0, 0, 0 H: 0.216, 0, 0 |
| K2LiAlH6 (calculated) | P 21/n (No. 14) [282] a = 5.528 b = 5.536 c = 7.832 α = 90, β = 90.03, γ = 90 | K: 0, ½, ¼ Li: 0, 0, ½ Al: 0, 0, 0 H: 0, 0, 0.23 H: 0.27, 0.27, ½ H: 0.23, 0.23, 0 |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| LiMg(AlD4)3 | P 21/c (No. 14) [288] a = 8.37113(16) b = 8.73910(17) c = 14.3012(3) α = γ = 90, β = 124.8308(8) | Mg: 0.6305(6), 0.5292(4), 0.8833(3) Li: 0.127(3), 0.4720(19), 0.3822(14) Al1: 0.7615(5), 0.6282(4), 0.1512(3) Al2: 0.4745(5), 0.8809(4), 0.8581(3) A13: 0.9593(5), 0.2510(4), 0.4986(3) D1: 0.6057(14), 0.5722(12), 0.1782(9) D2: 0.6523(14), 0.5907(11), 0.0190(6) D3: 0.7843(17), 0.8088(9), 0.1721(10) D4: 0.9475(12), 0.5201(10), 0.2158(9) D5: 0.4888(15), 0.7127(10), 0.8153(9) D6: 0.6918(11), 0.9294(11), 0.9554(8) D7: 0.3783(15), 0.9895(12), 0.7474(8) D8: 0.3312(15), 0.8752(13), 0.8981(10) D9: 0.9500(15), 0.3124(13), 0.3908(8) D10: 0.7599(14), 0.1597(12), 0.4549(10) D11: 1.1293(13), 0.1222(10), 0.5635(8) D12: 0.9941(14), 0.3727(11), 0.5902(7) |
| LiMgAlD6 | P321 (No. 150) [290] a = b = 7.985550(2) c = 4.378942(7) α = β = 90, γ = 120 | Mg: 1, 0.3570(13), 0 Li: 0, 0.686(6), ½ Al1: 0, 0, 0 Al2: 1/3, 2/3, 0.492(10) D1: 0.540(3), 0.763(2), 0.278(3) D2: 0.119(3), 0.576(2), 0.734(3) D3: 0.904(2), 0.117(2), 0.228(3) |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| LiCa(AlH4)3 (experimental) | P63/m (No. 176) [292] a = b = 8.91978(12); c = 5.8887(7) α = γ = 90, β = 120 | Li: 0, 0, 0 Ca: 2/3, 1/3, ¼ Al: 0.2805(3), 0.9027(4), ¼ |
| LiCa(AlH4)3 (theoretical) | P63/m (No. 176) [293] a = b = 9.093 c = 5.996 α = γ = 90, β = 120 | Li: 0, 0, 0 Ca: 2/3, 1/3, ¼ Al: 0.3, 0.9, ¼ H1: 0.544, 0.501, ¼ H2: 0.807, 0.815, ¼ H3: 0.535, 0.754, 0.029 |
| LiCaAlH6 (theoretical) | P–4 (No. 81) [294] a = b = 6.6652 c = 16.5607 α = γ = β= 90 | Li1: 0, 0, 0 Li2: 0, 0, ½ Li3: ½, ½, 0 Li4: ½, ½, ½ Li5: 0, ½, 0.4843 Li6: 0, ½, 0.0085 Ca1: 0.3119, 0.2730, 0.1937 Ca2: 0.2380, 0.1803, 0.6978 Al1: 0.2812, 0.2452, 0.3777 Al2: 0.2812, 0.2398, 0.8264 H1: 0.4729, 0.2445, 0.3245 H2: 0.2947, 0.0386, 0.4346 H3: 0.1523, 0.0958, 0.2964 H4: 0.1623, 0.4222, 0.3053 H5: 0.2861, 0.4340, 0.4450 H6: 0.2449, 0.0032, 0.5840 H7: 0.0609, 0.3014, 0.8263 H8: 0.2532, 0.4729, 0.9283 H9: 0.3881, 0.3663, 0.7959 H10: 0.2891, 0.0307, 0.8161 H11: 0.2130, 0.1039, 0.9599 H12: 0.2158, 0.4740, 0.0859 |
| Compound | Space Group, Cell Dimensions [Å] | Atomic Coordinates |
|---|---|---|
| K2NaAlD6 | Fm-3m (No. 225) [295] a = b = c = 8.118(1) α = β = γ = 90 | K: ¼, ¼, ¼ Na: ½, ½, ½ Al: 0, 0, 0 D: 0.2167(8), 0, 0 |
| Alanate | Mode/Peak Position [cm−1] | Comments/Reference | ||
|---|---|---|---|---|
| Stretching | Bending | Librational | ||
| LiAlH4 | 1779, 1642 | 885, 811, 715 | 465 | Pure crystalline material [177] |
| 1800, 1780, 1645 | 890, 810, 700 | [306] and Refs. within | ||
| 1757, 1615 | 900, 830 | [310] and Refs. within | ||
| Li3AlH6 | 1410, 1300 | 1000, 960, 854 | [321] | |
| 1386, 1276 | 1000, 950, 850 | [310] and Refs. within | ||
| Li3AlD6 | 1020, 915 | 740, 700, 635 | [321] | |
| NaAlH4 | 1680 | 900, 811, 730, 680 | Pure crystalline material [177] | |
| 1680 | 900, 800, 735, 690 | [306] and Refs. within | ||
| Na3AlH6 | 1440, 1290 | 930, 842, 690 | [321] | |
| KAlH4 | 1715 | 811, 729 | Pure crystalline material [177] | |
| RbAlH4 | 1715 | 811, 763, 739 | Pure crystalline material [177] | |
| 1715 | 811, 769, 729 | [306] and Refs. within | ||
| CsAlH4 | 1711 | 741 | Pure crystalline material [177], Ref. [306] and Refs. within | |
| Mg(AlH4)2 | 1935 | 800, 625 | Ref. [306] and Refs. within | |
| 642, 1937 | [43] | |||
| 1620, 1700–1800 | [39] | |||
| 2013, 1905, 1850, 716, 663, 620, 360, 302, 282 | [210] | |||
| Ca(AlH4)2 | 600, 1780 | [40] | ||
| 1788 | 816, 653 | 482 | [306] and Refs. within | |
| Alanate | Assignation/Peaks Position [cm−1] | Comments/Reference | ||||
|---|---|---|---|---|---|---|
| Combination | Stretching | Bending | Librational | Translational | ||
| LiAlH4 | 1837, 1762, 1722 | 950, 882, 830, 780, 690 | 510, 438, 322 | 220, 165, 151, 143, 112, 95 | [306] | |
| Li3AlH6 | 2090, 1974 | 1604, 1311 | 1014, 975 | 577, 510 | [321] | |
| Li3AlD6 | 1478, 1397 | 1137, 940 | 730, 686 | 412, 360 | [321] | |
| NaAlH4 | 1762, 1681, | 848, 817, 770 | 521, 429 | 180, 125, 117 | [306] | |
| Na3AlH6 | 1556, 1465, 1152, 1070 | 990, 815, 760 | 560, 480 | [321] | ||
| KAlH4 | 1779, 1711 | 790 | [306] | |||
| Mg(AlH4)2 | 1969, 1944, 1808 | 824, 768, 736 | [306] | |||
| 2077, 1852, 1845, 812, 758, 742, 298, 232, 87 | [310] | |||||
| Alanate | Formation Enthalpy | Dehydrogenation Reaction/Dehydrogenation Enthalpy [kJ/mol] | Apparent Activation Energy [kJ/mol] | |
|---|---|---|---|---|
| LiAlH4 | −107.1 [330] −113.42 [81] ‖ −114.8 [87] ‡ −118.9 [331] −119 [71] | (15) | −10 [26] −9.79 [81] ‖ | 102 [75], 103 [332] (pure) 42.6 [73] (TiCl3-1/3AlCl3 2 mol%) 67 [74] (NbF3 1 mol%) 81.5 [333] (FeCl2 2 mol%) 87.4 [308] (TiN 2 mol%) |
| Li3AlH6 | −310.89 [81] ‖ −298.5 to −311.0 [81,87,330] | (16) | 15.72 [81] ‖ 25 [26] | 54.8 [73] (TiCl3-1/3AlCl3 2 mol%) 77 [74] (NbF3 1 mol%) |
| NaAlH4 | −78.9 [334] −105.6 [268] −113.0 [331] −116.3 [335] ‡ | (25) | 36.7 [335] ‡ 37 [88] ѳ 36–40.9 [71] ֎  | 114.2 [336] (pure) 113.8 (NiFe2O4 3 mol%) [315], and (MnFe2O4) [317] 86.4 [336] (LaCl3 2 mol%) |
| Na3AlH6 | −238.8 [335] ‡ −172.8 [334] ‖ −260 [337] | (26) | 69.6 [335] ‡ 47 [88] ѳ 46.8–47 [71] ֎  | 162.6 [336] (pure) 86.4 [336] (LaCl3 2 mol%) |
| KAlH4 | −166.6 [331] −183.7 [161] ֎ −128 [175] ‖ | (34) | 70 [167]  ~55 [168] ‖  | 140 [164] (pure)  80 [164] (TiCl3 2% mol)  |
| K3AlH6 | −224.7 [175] ‖ | (35) | 81 [167]  | ? |
| CsAlH4 | −164.9 [330] | (39) | ? | ? |
| Mg(AlH4)2 | −79 [338] (“assessed value”) | (42) | 20.4 [43] (at 0 K, ab-initio) | 123.8 [199] (pure not milled) 123.6 [195] (with LiCl2) 123 [197] (submicron rods) 82.3 [200], 85.5 [208] (TiF4 doped) |
| Ca(AlH4)2 | −214 [192] ‖ | (45) | −7 [224]  , −7.4 [220] , −7.4 [220] | ? |
| CaAlH5 | −224 [192] ‖ | (46) | 26 [41] 32 [224]  , 31.1 [220] , 31.1 [220] | 161 [41], 153.4 [219] (pure) 57.4 [219] (TiF3 10 wt.%) |
| SrAlH5 | −248 [192] ‖ | (54) | ? | ? |
| BaAlH5 | −224 [207] ‖ | -- | ? | ? |
| LaAlH6 [261] | ? | ? | ~30 ‖,  | ? |
| MAlH6, M=Ce, Pr, Nd [261] | ? | (82) | ~28-32 ‖,  | ? |
| Eu(AlH4)2 [44] | ? | ? | −4.4 and 57 (for 2 consecutive reactions of hydrogen release,  ). ). | ? |
| Na2LiAlH6 | −84.5 [268]  ‖ ‖−55.26 [297]  (‖, 300 K) (‖, 300 K)−53.5 [267]  | (88) | 53.5 [267]  , 63.8 [274] , 63.8 [274]  56.4 [276]  TiF3 doped TiF3 doped57.3 [285] TiF4 doped | 173 [274] 143.6 [285] TiF4 doped |
| K2LiAlH6 | −100.5 [268]  ‖ ‖−102.42 [297]  (‖, 300 K) (‖, 300 K)−82 [267]  | ? | 82 [267]  | ? |
| LiMg(AlH4)3 | −192.6 [206] (‖, 0 K) | (91) | −4.16 [289]  , −5 [339] , −5 [339]  | ~66 277 |
| LiMgAlH6 | −184.8 [206] (‖, 0 K) | (92) | 8.89 [289]  , 9 [339] , 9 [339]  | ? |
| K2NaAlH6 | −107.66 [297]  (‖, 300 K) (‖, 300 K)−97 [267]  | (96) | 97 [267]  98 [295] TiF3 doped  | 124.43 [296] 88.05 TiF3 catalyzed [296] |
 Explicitly reported per mole of H2, i.e., kJ/mol H2, ? Unknown.
Explicitly reported per mole of H2, i.e., kJ/mol H2, ? Unknown.© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Alcántara, K.; Tena-Garcia, J.R.; Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. https://doi.org/10.3390/ma12172724
Suárez-Alcántara K, Tena-Garcia JR, Guerrero-Ortiz R. Alanates, a Comprehensive Review. Materials. 2019; 12(17):2724. https://doi.org/10.3390/ma12172724
Chicago/Turabian StyleSuárez-Alcántara, Karina, Juan Rogelio Tena-Garcia, and Ricardo Guerrero-Ortiz. 2019. "Alanates, a Comprehensive Review" Materials 12, no. 17: 2724. https://doi.org/10.3390/ma12172724
APA StyleSuárez-Alcántara, K., Tena-Garcia, J. R., & Guerrero-Ortiz, R. (2019). Alanates, a Comprehensive Review. Materials, 12(17), 2724. https://doi.org/10.3390/ma12172724




