Ground Tire Rubber Modified by Ethylene-Vinyl Acetate Copolymer: Processing, Physico-Mechanical Properties, Volatile Organic Compounds Emission and Recycling Possibility
Abstract
1. Introduction
2. Experimental
2.1. Materials
- -
- Ground tire rubber (GTR)—obtained from passenger and truck tires, with particle sizes up to 0.4 mm, was received from Grupa Recykl S.A. (Śrem, Poland). The basic components of GTR are: natural rubber (NR), styrene-butadiene rubber (SBR), butadiene rubber (BR), additives (curing system, activators, plasticizers, etc.), carbon black, silica, and ash. Thermogravimetric analysis of GTR was presented in the work [29].
- -
- Vestenamer®8012—semi-crystalline thermoplastic elastomer known as trans-polyoctenamer rubber (TOR) imported from the Evonik company (Essen, Germany). This additive acts as a plasticizer during rubber processing or reclaiming. TOR also reacts in the vulcanization process due to the presence of unsaturated bonds in the structure. It is characterized by a high compatibility with other materials.
- -
- Sipchem EVA 2518—ethylene-vinyl acetate copolymer containing 18.2% vinyl acetate, manufactured by Sipchem (Khobar, Kingdom of Saudi Arabia).
- -
- Escorene Ultra EVA FL00218—ethylene-vinyl acetate copolymer containing 18.0% vinyl acetate, obtained from Exxon Mobil Chemical (Machelen, Belgium).
2.2. Sample Preparation
2.2.1. Reclaiming of GTR Modified by Thermoplastics
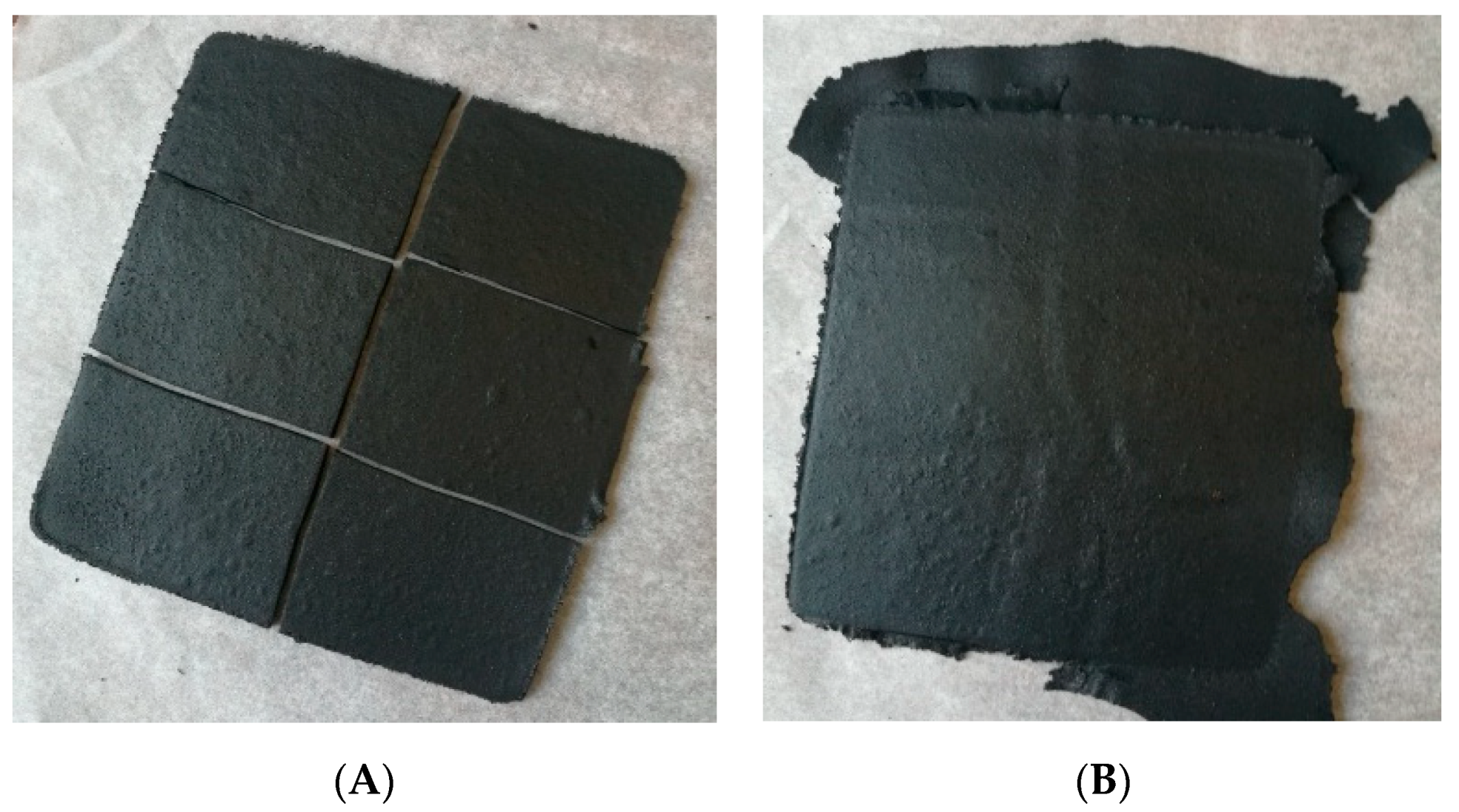
2.2.2. Formulation of Modified Reclaimed GTR by Compression Molding
2.3. Measurements
3. Results and Discussion
3.1. Temperature and Energy Consumption Measurements
3.2. MFI and Mooney Viscosity Measurements
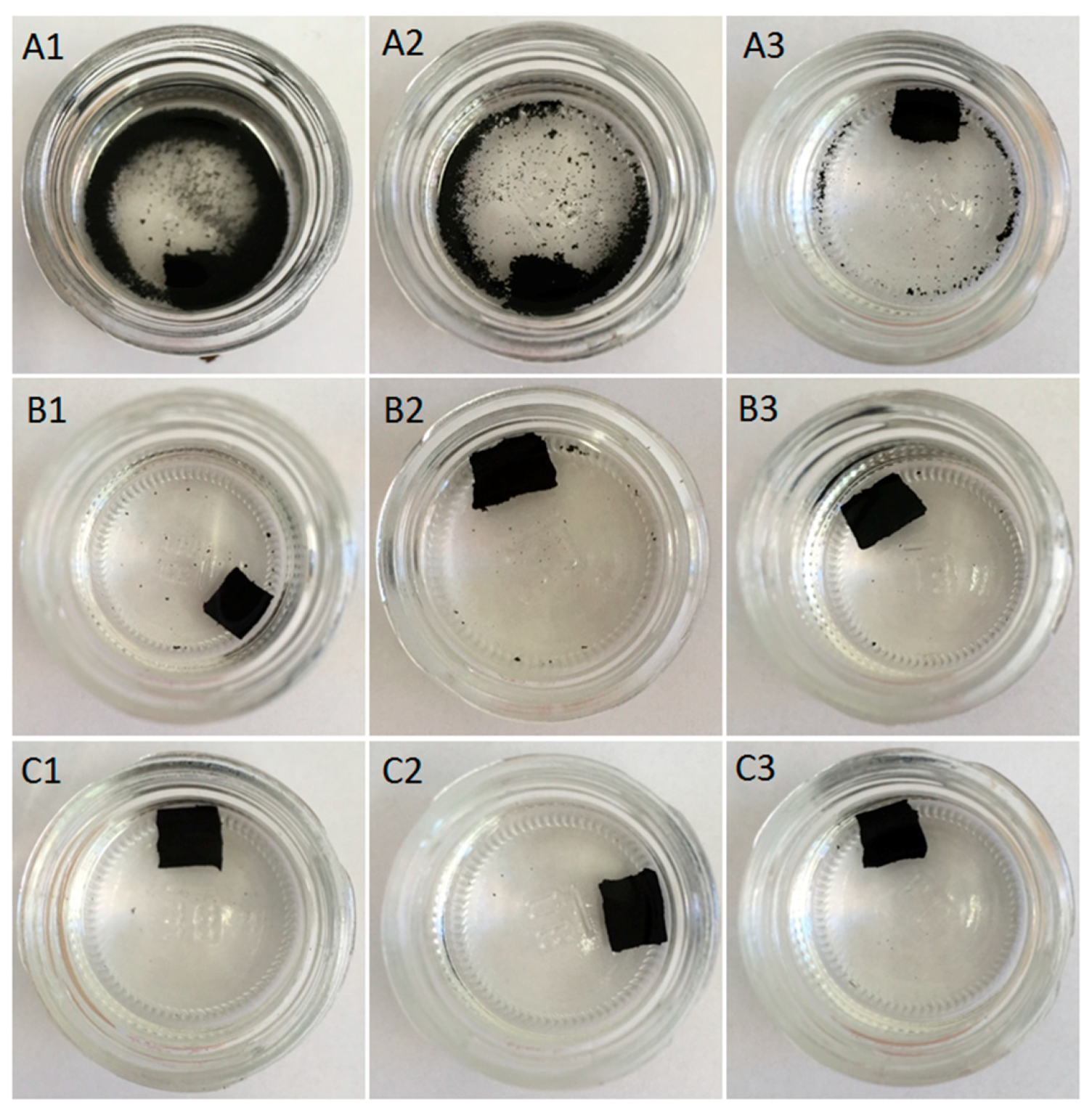
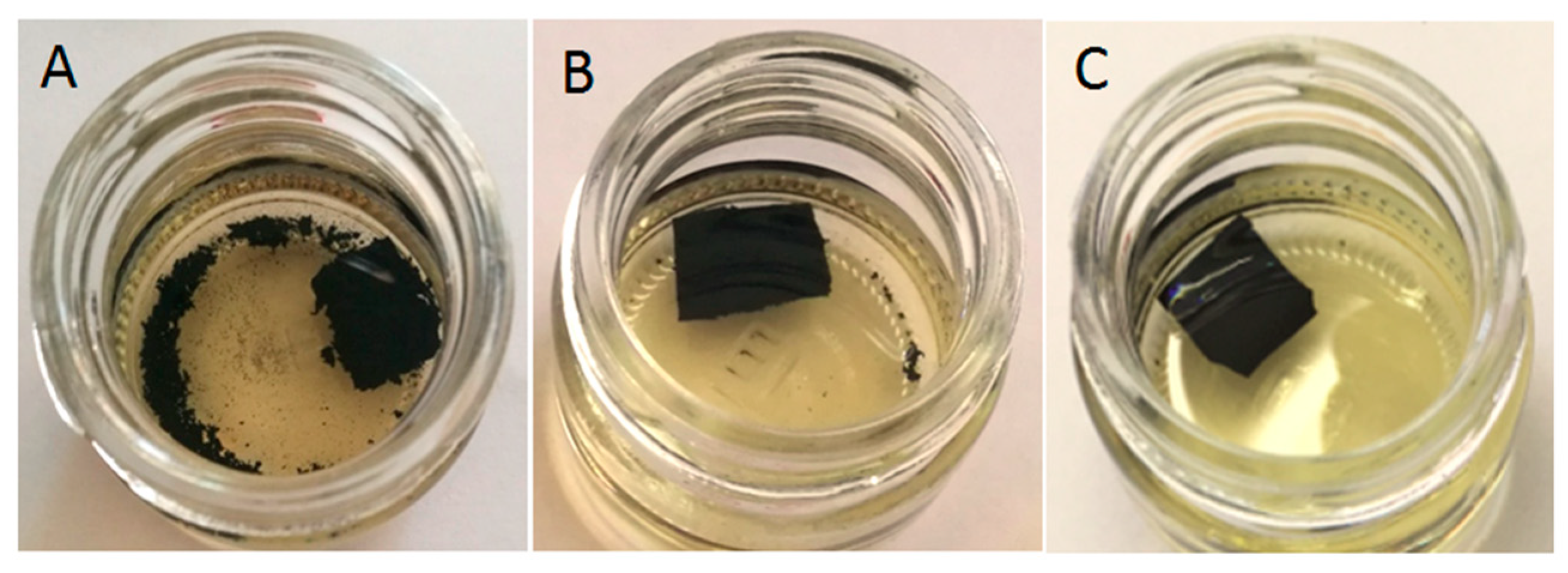
3.3. Swelling Behavior
3.4. Physico-Mechanical Properties
| Sample Composition | Sample Preparation | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Sh A) | References |
|---|---|---|---|---|---|
| GTR/EVA 100/10 | extrusion at 60 °C, compression molding at 140–180 °C | 2.7–3.4 | 125–164 | 63–65 | This study |
| GTR/recycled PE/TOR 90/10/9 | extrusion at 150–180 °C injection molding at 180–190 °C | ~2.1 | ~70 | ~78 | [28] |
| EVA/GTR 90/10 | batch mixer at 140 °C, compression molding at 123 °C | ~9* | ~550 * | ~34 * | [39,40] |
| EVA/GTR 60/40 | batch mixer at 140 °C, compression molding at 123 °C | ~6* | ~310 * | ~81 * | [41] |
| LDPE/GTR/EVA 35/45/20 | extrusion at 165–175 °C, injection molding at 165–190 °C | ~5* | ~185 * | - | [42] |
| recycled LDPE/GTR/EVA 30/40/30 | extrusion at 165–175 °C, injection molding at 165–190 °C | ~7.8* | ~180 * | - | [43] |
| Reclaimed GTR/EVA 70/30 and 50/50 | batch mixer at 120 °C, compression molding at 130 °C | 2.0–5.4 | 300–999 | 72–80 | [44] |
3.5. Evaluation of Modified Reclaimed GTR Recycling Possibility
3.6. Determination of Volatile Organic Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products—A review. SMT 2019, 22, e00125. [Google Scholar] [CrossRef]
- Simon, D.A.; Pirityi, D.; Tamás-Bényei, P.; Bárán, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 48351. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Markovski, S.; Rodwell, G.; Rahman, M.T.; Kurmus, H.; Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: A review. Resour. Conserv. Recycl. 2020, 155, 104679. [Google Scholar] [CrossRef]
- Gerrard, J.; Kandlikar, M. Is European end-of-life vehicle legislation living up to expectations? Assessing the impact of the ELV Directive on ‘green’ innovation and vehicle recovery. J. Clean. Prod. 2007, 15, 17–27. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Ding, Y.N.; Jalali, S. Properties and durability of concrete containing polymeric wastes (type rubber and polyethylene terephthalate bottles): An overview. Constr. Build. Mater. 2012, 30, 714–724. [Google Scholar] [CrossRef]
- Li, X.; Ling, T.C.; Hung Mo, K. Functions and impacts of plastic/rubber wastes as eco-friendly aggregate in concrete—A review. Constr. Build. Mater. 2020, 240, 117869. [Google Scholar] [CrossRef]
- Liu, L.; Cai, G.; Zhang, J.; Liu, X.; Liu, K. Evaluation of engineering properties and environmental effect of recycled waste tire-sand/soil in geotechnical engineering: A compressive review. Renew. Sustain. Energy Rev. 2020, 126, 109831. [Google Scholar] [CrossRef]
- Bisht, K.; Ramana, P.V. Waste to resource conversion of crumb rubber for production of sulphuric acid resistant concrete. Constr. Build. Mater. 2019, 194, 276–286. [Google Scholar] [CrossRef]
- Feraldi, R.; Cashman, S.; Huff, M.; Raahauge, L. Comparative LCA of treatment options for US scrap tires: Material recycling and tire-derived fuel combustion. Int. J. Life Cycle Assess. 2013, 18, 613–625. [Google Scholar] [CrossRef]
- Park, J.-M.; An, J.-Y.; Bang, D.; Kim, B.-S.; Oh, M.-H. Characteristics studies of waste tire rubber powders using the different grinding methods. J. Korean Inst. Resour. Recycl. 2014, 23, 44–50. [Google Scholar]
- ETRMA—End of Life Tyres Management-Europe-2017 Status. 19 November 2019. Available online: https://www.etrma.org/wp-content/uploads/2019/11/ELT-Management-Figures-2017-vf.xlsx.pdf (accessed on 14 September 2020).
- Fazli, A.; Rodrique, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Picado-Santosa, L.G.; Capitão, S.D.; Neves, J.M.C. Crumb rubber asphalt mixtures: A literature review. Constr. Build. Mater. 2020, 247, 118577. [Google Scholar] [CrossRef]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Youssf, O.; Mills, J.E. A comprehensive review on the mechanical properties of waste tire rubber concrete. Constr. Build. Mater. 2020, 237, 117651. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling – recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Formela, K.; Cysewska, M.; Haponiuk, J. Thermomechanical reclaiming of ground tire rubber via extrusion at low temperature: Efficiency and limits. J. Vinyl Addit. Technol. 2014, 22, 213–221. [Google Scholar] [CrossRef]
- Zedler, Ł.; Klein, M.; Saeb, M.R.; Colom, X.; Cañavate, J.; Formela, K. Synergistic effects of bitumen plasticization and microwave treatment on short-term devulcanization of ground tire rubber. Polymers 2018, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Aït Hocine, N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym. Degrad. Stab. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Xu, J.Y.; Shen, M.; Wang, X.Q.; Chen, C.H.; Xin, Z.X. The effect of reclaim softener on properties of reclaimed rubber. J. Macromol. Sci. B 2014, 53, 1182–1192. [Google Scholar] [CrossRef]
- Cheng, X.; Song, P.; Zhao, X.; Peng, Z.; Wang, S. Liquefaction of ground tire rubber at low temperature. Waste Manag. 2018, 71, 301–310. [Google Scholar] [CrossRef]
- Hassan, A.; Formela, K.; Wang, S. Reclaimed rubber in-situ grafted with soybean oil as a novel green reactive plasticizer in SBR/silica compounds. ACS Sustain. Chem. Eng. 2019, 7, 14991–15001. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.T.; Xu, Z.; Chen, X.; Zhang, M. Process for devulcanization of rubber. U.S. Patent 2009/0082475A1, 26 March 2009. [Google Scholar]
- Formela, K.; Cysewska, M.; Korol, J. Effect of compounding conditions on static and dynamic mechanical properties of high density polyethylene/ground tire rubber blends. Int. Polym. Proc. 2014, 29, 272–279. [Google Scholar] [CrossRef]
- Barbosa, R.; Ambrósio, J.D. Devulcanization of natural rubber compounds by extrusion using thermoplastics and characterization of revulcanized compounds. J. Polym. Res. 2019, 26, 160. [Google Scholar] [CrossRef]
- Nunes, A.T.; dos Santos, R.E.; Pereira, J.S.; Barbosa, R.; Ambrósio, J.D. Characterization of waste tire rubber devulcanized in twin-screw extruder with thermoplastics. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 143–157. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Chen, Y.-K.; Rodrigue, D. Production of thermoplastic elastomers based on recycled PE and ground tire rubber: Morphology, mechanical properties and effect of compatibilizer addition. Int. Polym. Proc. 2018, 33, 525–534. [Google Scholar] [CrossRef]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Vahabi, H.; Saeb, M.R.; Colom, X.; Cañavate, J.; Wang, S.; Formela, K. Preliminary investigation on auto-thermal extrusion of ground tire rubber. Materials 2019, 12, 2090. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B. An investigation of selected monoaromatic hydrocarbons released from the surface of polystyrene lids used in coffee-to-go cups. Microchem. J. 2017, 133, 496–505. [Google Scholar] [CrossRef]
- Marć, M. Emissions of selected monoaromatic hydrocarbons as a factor affecting the removal of single-use polymer barbecue and kitchen utensils from everyday use. Sci. Total Environ. 2020, 720, 137485. [Google Scholar] [CrossRef]
- Galeja, M.; Wypiór, K.; Wachowicz, J.; Kędzierski, P.; Hejna, A.; Marć, M.; Klewicz, K.; Gabor, J.; Okła, H.; Swinarew, A.S. POM/EVA blends with future utility in fused deposition modeling. Materials 2020, 13, 2912. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Colomines, G.; Peuvrel-Disdier, E.; Deterre, R. Thermo-mechanical recycling of rubber: Relationship between material properties and specific mechanical energy. J. Mater. Process. Technol. 2018, 252, 454–468. [Google Scholar] [CrossRef]
- Garcia, P.S.; de Sousa, F.D.B.; de Lima, J.A.; Cruz, S.A.; Scuracchio, C.H. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Mangili, I.; Lasagni, M.; Anzano, M.; Collina, E.; Tatangelo, V.; Franzetti, A.; Caracino, P.; Isayev, A.I. Mechanical and rheological properties of natural rubber compounds containing devulcanized ground tire rubber from several methods. Polym. Degrad. Stab. 2015, 121, 369–377. [Google Scholar] [CrossRef]
- Sripornsawat, B.; Saiwari, S.; Pichaiyut, S.; Nakason, C. Influence of ground tire rubber devulcanization conditions on properties of its thermoplastic vulcanizate blends with copolyester. Eur. Polym. J. 2016, 85, 279–297. [Google Scholar] [CrossRef]
- Song, P.; Wan, C.; Xie, Y.; Formela, K.; Wang, S. Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Manag. 2018, 78, 238–248. [Google Scholar] [CrossRef]
- Anis Sakinah, Z.A.; Ratnam, C.T.; Luqman Chuah, A.; Yaw, T.C.S. Performance of irradiated and crosslinked ethylene vinyl acetate/waste tire dust blend. J. Elastomers Plast. 2011, 43, 239–256. [Google Scholar] [CrossRef]
- Ratnam, C.T.; Ramarad, S.; Siddiqui, M.K.; Abidin, A.S.Z.; Chuah, L.T.G. Irradiation cross-linking of ethylene vinyl acetate/waste tire dust:Effect of multifunctional acrylates. J. Compos. Mater. 2016, 29, 464–478. [Google Scholar] [CrossRef]
- Ratnam, C.T.; Ramarad, S.; Khalid, M.; Noraini, N. Effect of pre-irradiation of waste tire dust on the properties of ethylene vinyl acetate/waste tire dust blend (EVA/WTD) blends. J. Compos. Biodegrad. Polym. 2013, 1, 16–22. [Google Scholar] [CrossRef]
- Mészáros, L.; Tábi, T.; Kovács, J.G.; Bárány, T. The effect of EVA content on the processing parameters and the mechanical properties of LDPE/ground tire rubber blends. Polym. Sci. Eng. 2008, 48, 868–874. [Google Scholar] [CrossRef]
- Mészáros, L.; Fejős, M.; Bárány, T. Mechanical properties of recycled LDPE/EVA/ground tyre rubber blends: Effects of EVA content and postirradiation. J. Appl. Polym. Sci. 2012, 125, 512–519. [Google Scholar] [CrossRef]
- Ramarad, S.; Ratnam, C.T.; Khalid, M.; Chuah, A.L. Improving the properties of reclaimed waste tire rubber by blending with poly(ethylene-co-vinyl acetate) and electron beam irradiation. J. Appl. Polym. Sci. 2014, 132, 41649. [Google Scholar] [CrossRef]
- Kamarulzaman, N.H.; Le-Minh, N.; Stuetz, R.M. Identification of VOCs from natural rubber by different headspace techniques coupled using GC-MS. Talanta 2019, 191, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Morand, J.L. Chain scission in the oxidation of polyisoprene. Rubber Chem. Technol. 1977, 50, 373–396. [Google Scholar] [CrossRef]
- Ginsberg, G.; Toal, B.; Simcox, N.; Bracker, A.; Golembiewski, B.; Kurland, T.; Hedman, C. Human health risk assessment of synthetic turf fields based upon investigation of five fields in Connecticut. J. Toxicol. Environ. Health Part. A 2011, 74, 1150–1174. [Google Scholar] [CrossRef] [PubMed]
- Gągol, M.; Boczkaj, G.; Haponiuk, J.; Formela, K. Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym. Degrad. Stab. 2015, 119, 113–120. [Google Scholar] [CrossRef]
- Sato, S.; Honda, Y.; Kuwahara, M.; Watanabe, T. Degradation of vulcanized and nonvulcanized polyisoprene rubbers by lipid peroxidation catalyzed by oxidative enzymes and transition metals. Biomacromolecules 2003, 4, 321–329. [Google Scholar] [CrossRef]

| Properties | Additive | ||
|---|---|---|---|
| Vestenamer®8012 * | Sipchem EVA 2518 * | Escorene Ultra EVA FL00218 * | |
| Abbreviation | TOR | EVA1 | EVA2 |
| Density at 25 °C, g/cm3 | 0.910 | 0.935 | 0.940 |
| MFI190 °C, 2.16 kg, g/10 min | 13.8 | 2.5 | 1.7 |
| Vicat softening temperature, °C | - | 64 | 62 |
| Melting temperature, °C | 54 | 87 | 87 |
| Sample Composition * | Compression Molding Temperature (°C) | Sample Coding |
|---|---|---|
| GTR + 10 phr Vestenamer®8012 | 140 | GTR+TOR-140 °C |
| 160 | GTR+TOR-160 °C | |
| 180 | GTR+TOR-180 °C | |
| GTR + 10 phr Sipchem EVA 2518 | 140 | GTR+EVA1-140 °C |
| 160 | GTR+EVA1-160 °C | |
| 180 | GTR+EVA1-180 °C | |
| GTR + 10 phr Escorene Ultra EVA FL00218 | 140 | GTR+EVA2-140 °C |
| 160 | GTR+EVA2-160 °C | |
| 180 | GTR+EVA2-180 °C |
| Item | Specification * |
|---|---|
| Measuring range | −30 to 650 °C |
| Accuracy | ± 2 °C/±2% |
| Infrared resolution | 320 × 240 |
| Thermal sensitivity | 60 mK |
| Geometric resolution | 2.3 mrad |
| SuperResolution | 640 × 480 pixels/1.3 mrad |
| IR image refresh rate | 9 Hz |
| Spectral range | 7.5–14 μm |
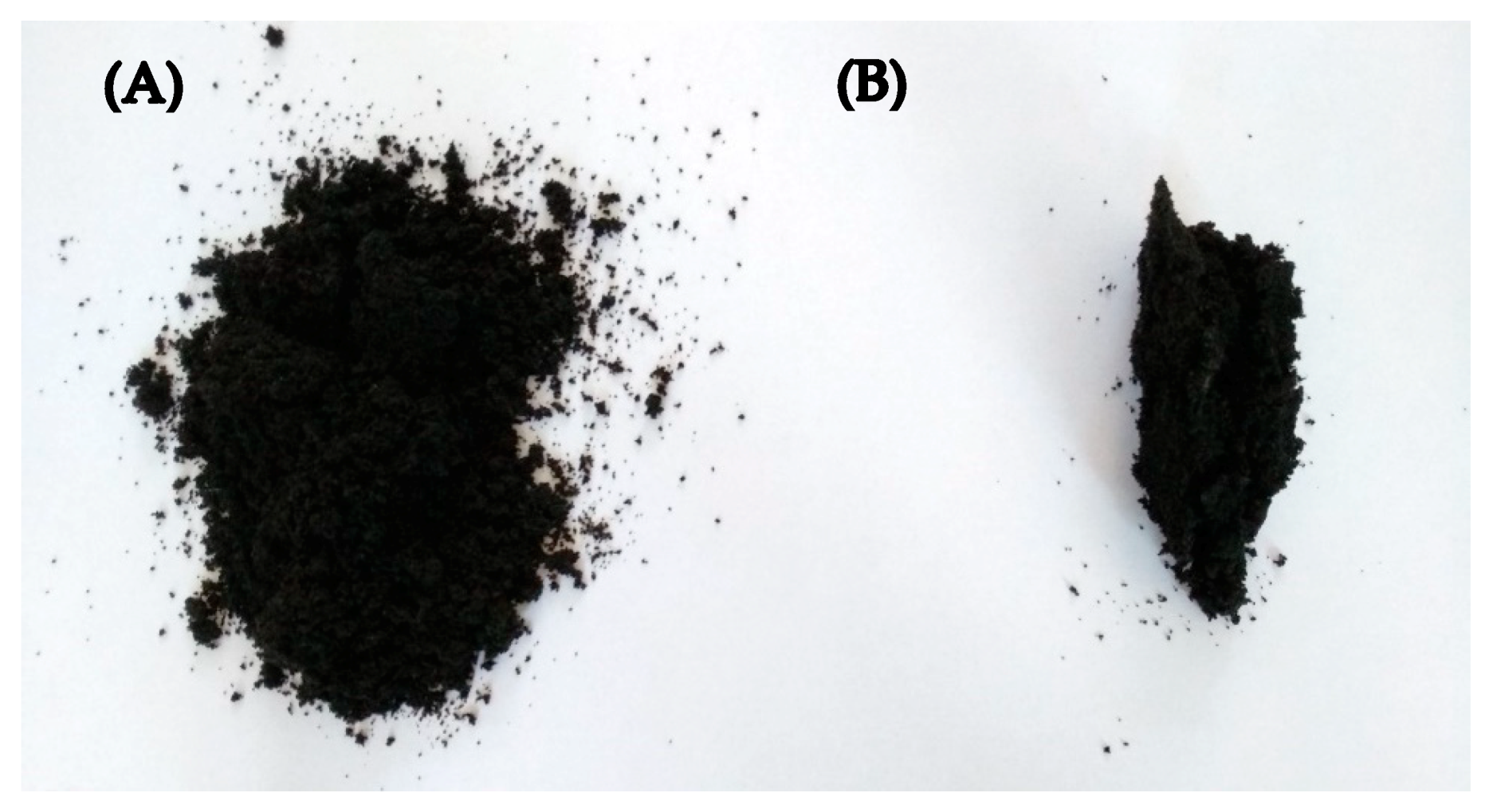
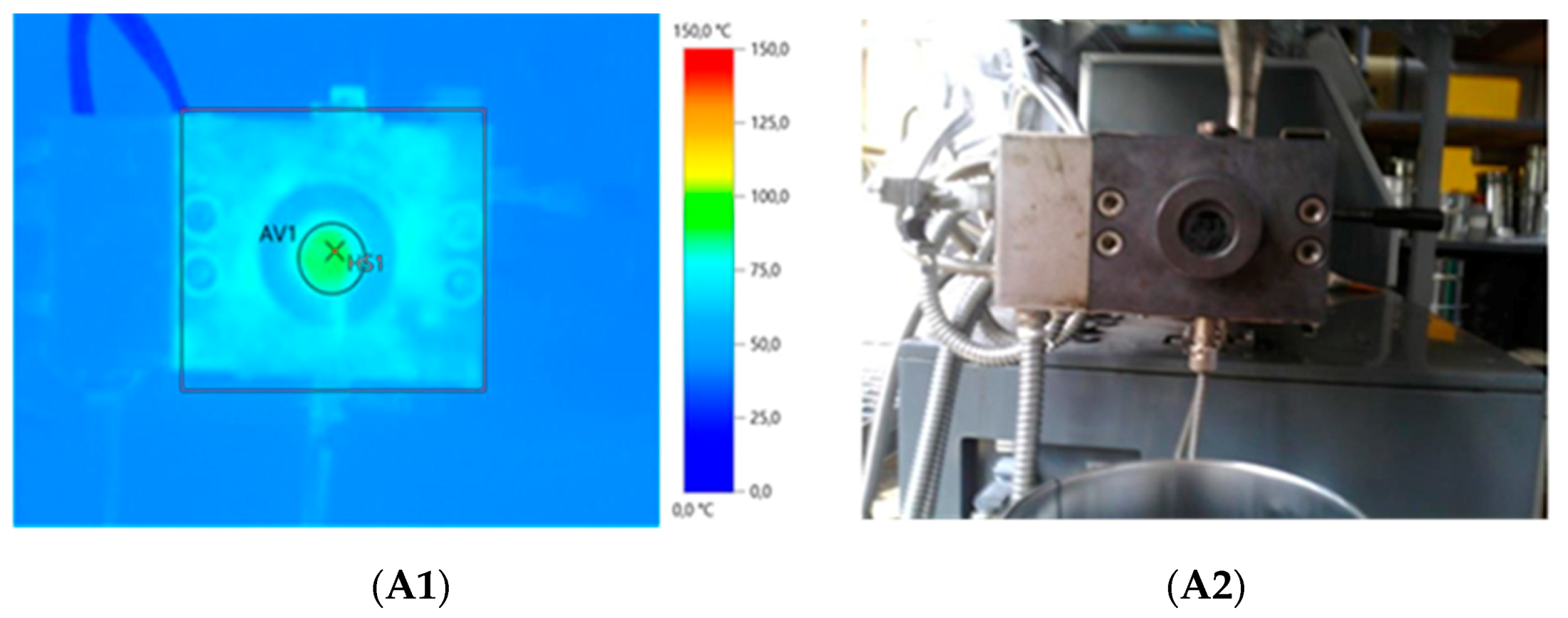
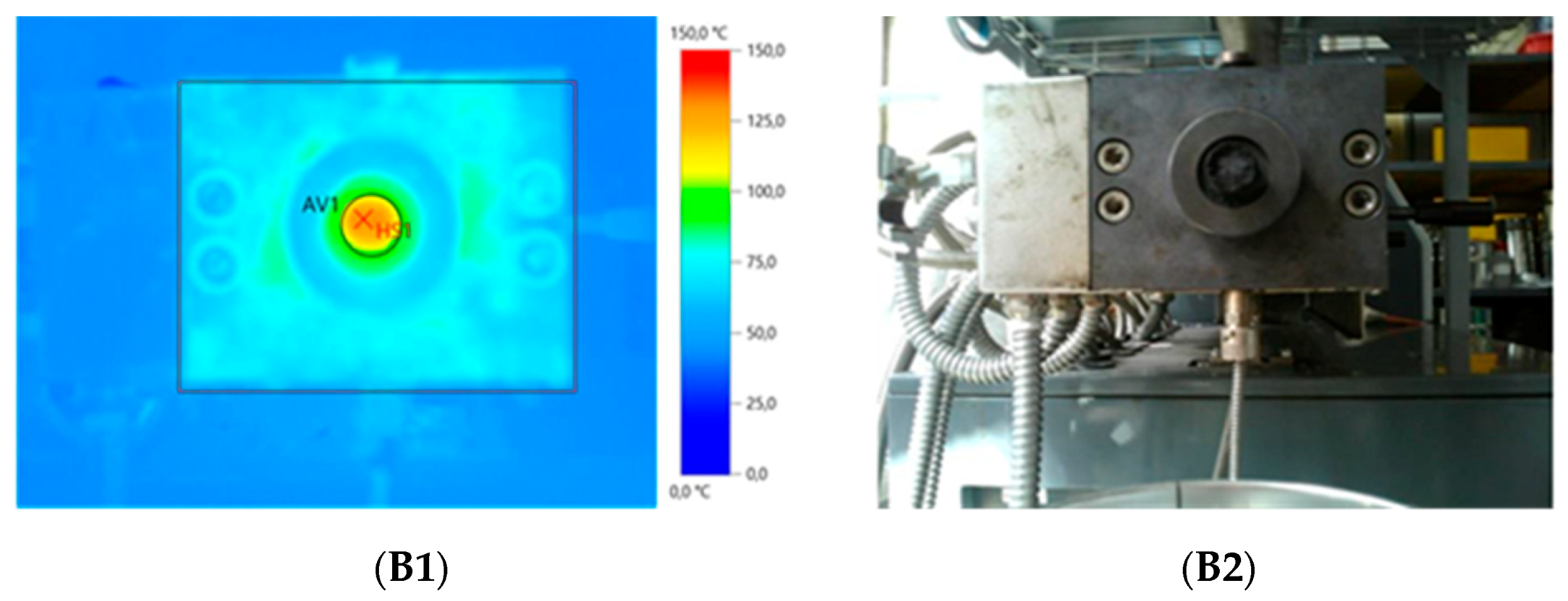
| Sample Composition | Physical form of Extruded Material | IR Camera Record | Energy Consumption (kWh/kg) | Mooney Viscosity ML(1+4) 125 °C (MU) | |||
|---|---|---|---|---|---|---|---|
| TAverage (°C) | TMaximal (°C) | SME | Total * | SME in Total Energy (%) | |||
| GTR | Powder with developed surface | 63.0 | 69.0 | 0.142 | 0.420 | 33.9 | - ** |
| GTR+TOR | Solid profile | 107.4 | 136.1 | 0.489 | 0.567 | 86.3 | 124.6 ± 0.6 |
| GTR+EVA1 | Solid profile | 110.4 | 126.9 | 0.457 | 0.545 | 83.8 | 150.6 ± 1.7 |
| GTR+EVA2 | Solid profile | 102.9 | 120.3 | 0.423 | 0.524 | 80.7 | 142.3 ± 0.3 |
| Sample Coding | Swelling Degree (%) | Cross-Link Density (mol/cm310−4) | Sol Fraction (%) | Gel Fraction (%) |
|---|---|---|---|---|
| GTR-140 °C | 166 ± 2 | 1.64 ± 0.06 | 9.6 ± 0.1 | 90.4 |
| GTR-160 °C | 163 ± 1 | 1.65 ± 0.01 | 9.8 ± 0.1 | 90.2 |
| GTR-180 °C | 169 ± 4 | 1.55 ± 0.07 | 10.5 ± 0.3 | 89.5 |
| GTR+EVA1-140 °C | 178 ± 3 | 1.36 ± 0.02 | 11.0 ± 0.8 | 89.0 |
| GTR+EVA1-160 °C | 180 ± 1 | 1.34 ± 0.01 | 10.9 ± 0.2 | 89.1 |
| GTR+EVA1-180 °C | 184 ± 1 | 1.29 ± 0.04 | 10.6 ± 0.3 | 89.4 |
| GTR+EVA2-140 °C | 172 ± 3 | 1.53 ± 0.06 | 9.4 ± 0.2 | 90.6 |
| GTR+EVA2-160 °C | 174 ± 1 | 1.49 ± 0.02 | 9.6 ± 0.2 | 90.4 |
| GTR+EVA2-180 °C | 178 ± 1 | 1.40 ± 0.01 | 10.1 ± 0.1 | 89.9 |
| Sample Coding | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Sh A) | Density (g/cm3) |
|---|---|---|---|---|
| GTR-140 °C | 2.4 ± 0.2 | 77 ± 7 | 59 ± 1 | 1.170 ± 0.002 |
| GTR-160 °C | 2.4 ± 0.2 | 71 ± 6 | 55 ± 1 | 1.163 ± 0.006 |
| GTR-180 °C | 2.6 ± 0.1 | 79 ± 4 | 57 ± 1 | 1.149 ± 0.007 |
| GTR+TOR-140 °C | 1.7 ± 0.2 | 49 ± 11 | 63 ± 1 | 1.090 ± 0.007 |
| GTR+TOR-160 °C | 2.1 ± 0.2 | 73 ± 11 | 64 ± 1 | 1.121 ± 0.001 |
| GTR+TOR-180 °C | 2.4 ± 0.2 | 92 ± 14 | 65 ± 1 | 1.125 ± 0.003 |
| GTR+EVA1-140 °C | 2.7 ± 0.4 | 125 ± 13 | 64 ± 1 | 1.130 ± 0.003 |
| GTR+EVA1-160 °C | 3.1 ± 0.1 | 138 ± 10 | 64 ± 1 | 1.123 ± 0.003 |
| GTR+EVA1-180 °C | 3.4 ± 0.4 | 164 ± 14 | 64 ± 1 | 1.127 ± 0.003 |
| GTR+EVA2-140 °C | 3.4 ± 0.5 | 147 ± 16 | 65 ± 1 | 1.140 ± 0.004 |
| GTR+EVA2-160 °C | 3.2 ± 0.5 | 146 ± 11 | 63 ± 1 | 1.132 ± 0.002 |
| GTR+EVA2-180 °C | 3.2 ± 0.5 | 151 ± 21 | 63 ± 1 | 1.134 ± 0.003 |
| Sample Coding | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Sh A) |
|---|---|---|---|
| Reference | 3.2 ± 0.5 | 146 ± 11 | 63 ± 1 |
| 1st round of recycling | 2.9 ± 0.4 | 136 ± 17 | 63 ± 1 |
| 2nd round of recycling | 3.1 ± 0.4 | 143 ± 17 | 64 ± 1 |
| 3rd round of recycling | 2.7 ± 0.5 | 123 ± 25 | 63 ± 1 |
| Sample Coding | TVOCs (μg/g) |
|---|---|
| GTR-180 °C | 1.56 |
| GTR+TOR-180 °C | 0.86 |
| GTR+EVA1-180 °C | 0.76 |
| GTR+EVA2-180 °C | 0.78 |
| Retention Time (min) | Identified Compound | Chemical Structure | Molecular Weight (g/mol) | Peak Area (%) | Match Quality (%) | Source | References |
|---|---|---|---|---|---|---|---|
| 6.58 | 1-butanol |  | 74.12 | 2.68 | 90 | natural rubber present in GTR | [45] |
| 7.92 | methyl-isobutyl ketone |  | 100.16 | 4.74 | 87 | natural rubber and anti-aging agents present in GTR | [18,45,46] |
| 8.64 | toluene | 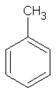 | 92.14 | 0.84 | 92 | styrene-butadiene rubber present in GTR | [47,48] |
| 8.94 | butanoic acid, ethyl-ester | 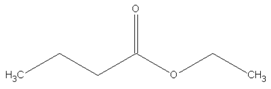 | 116.16 | 2.86 | 89 | EVA | - |
| 10.09 | 5-methyl-2-hexanone | 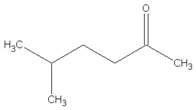 | 114.19 | 1.62 | 91 | EVA | - |
| 10.37 | 1-butanol, 3-methyl-, acetate | 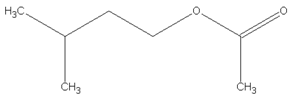 | 130.18 | 4.67 | 90 | EVA | - |
| 10.59 | xylene | 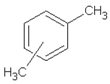 | 106.16 | 0.58 | 94 | styrene-butadiene rubber present in GTR | [47,48] |
| 11.06 | styrene | 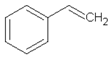 | 104.15 | 1.27 | 95 | styrene-butadiene rubber present in GTR | [47,48] |
| 11.19 | cyclohexanone | 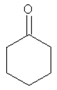 | 98.14 | 5.33 | 91 | elastomers present in GTR | [48] |
| 11.80 | cyclooctane | 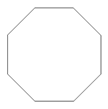 | 112.21 | 4.95 | 97 | elastomers present in GTR | - |
| 12.50 | benzaldehyde | 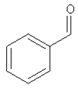 | 106.12 | 1.43 | 96 | styrene-butadiene rubber present in GTR | [48] |
| 12.70 | aniline | 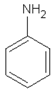 | 93.13 | 2.30 | 95 | vulcanization accelerators present in GTR | [45] |
| 13.58 | limonene |  | 136.23 | 13.25 | 94 | natural rubber present in GTR | [45,49] |
| 14.36 | acetophenone | 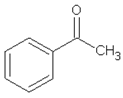 | 120.15 | 1.13 | 95 | styrene-butadiene rubber present in GTR | - |
| 17.37 | benzothiazole |  | 135.19 | 9.87 | 95 | vulcanization accelerators present in GTR | [18,45,47,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground Tire Rubber Modified by Ethylene-Vinyl Acetate Copolymer: Processing, Physico-Mechanical Properties, Volatile Organic Compounds Emission and Recycling Possibility. Materials 2020, 13, 4669. https://doi.org/10.3390/ma13204669
Zedler Ł, Burger P, Wang S, Formela K. Ground Tire Rubber Modified by Ethylene-Vinyl Acetate Copolymer: Processing, Physico-Mechanical Properties, Volatile Organic Compounds Emission and Recycling Possibility. Materials. 2020; 13(20):4669. https://doi.org/10.3390/ma13204669
Chicago/Turabian StyleZedler, Łukasz, Paulina Burger, Shifeng Wang, and Krzysztof Formela. 2020. "Ground Tire Rubber Modified by Ethylene-Vinyl Acetate Copolymer: Processing, Physico-Mechanical Properties, Volatile Organic Compounds Emission and Recycling Possibility" Materials 13, no. 20: 4669. https://doi.org/10.3390/ma13204669
APA StyleZedler, Ł., Burger, P., Wang, S., & Formela, K. (2020). Ground Tire Rubber Modified by Ethylene-Vinyl Acetate Copolymer: Processing, Physico-Mechanical Properties, Volatile Organic Compounds Emission and Recycling Possibility. Materials, 13(20), 4669. https://doi.org/10.3390/ma13204669






