Wetting Kinetics and Microstructure Analysis of BNi2 Filler Metal over Selective Laser Melted Ti-6Al-4V Substrate
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Filler Metal Characterization
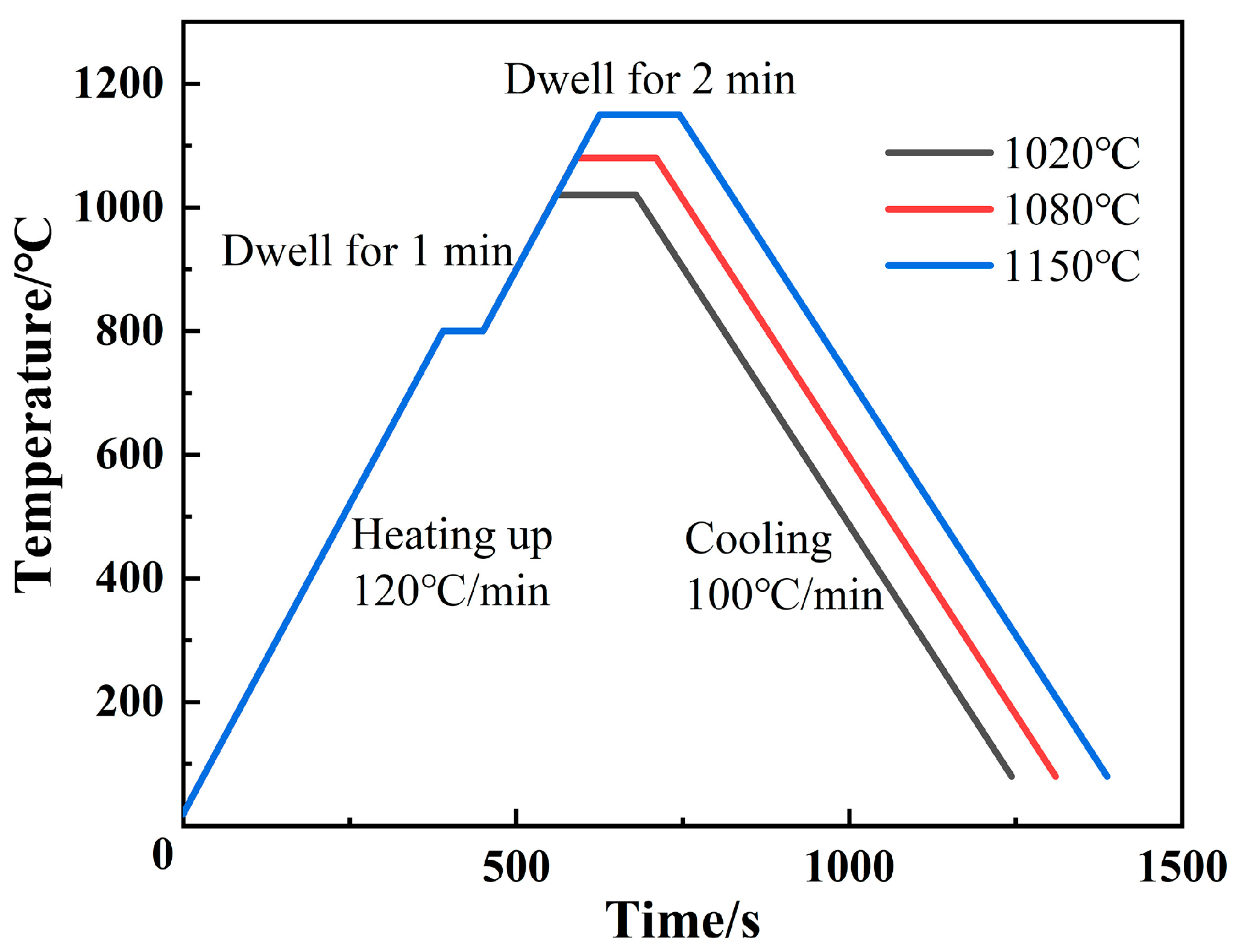
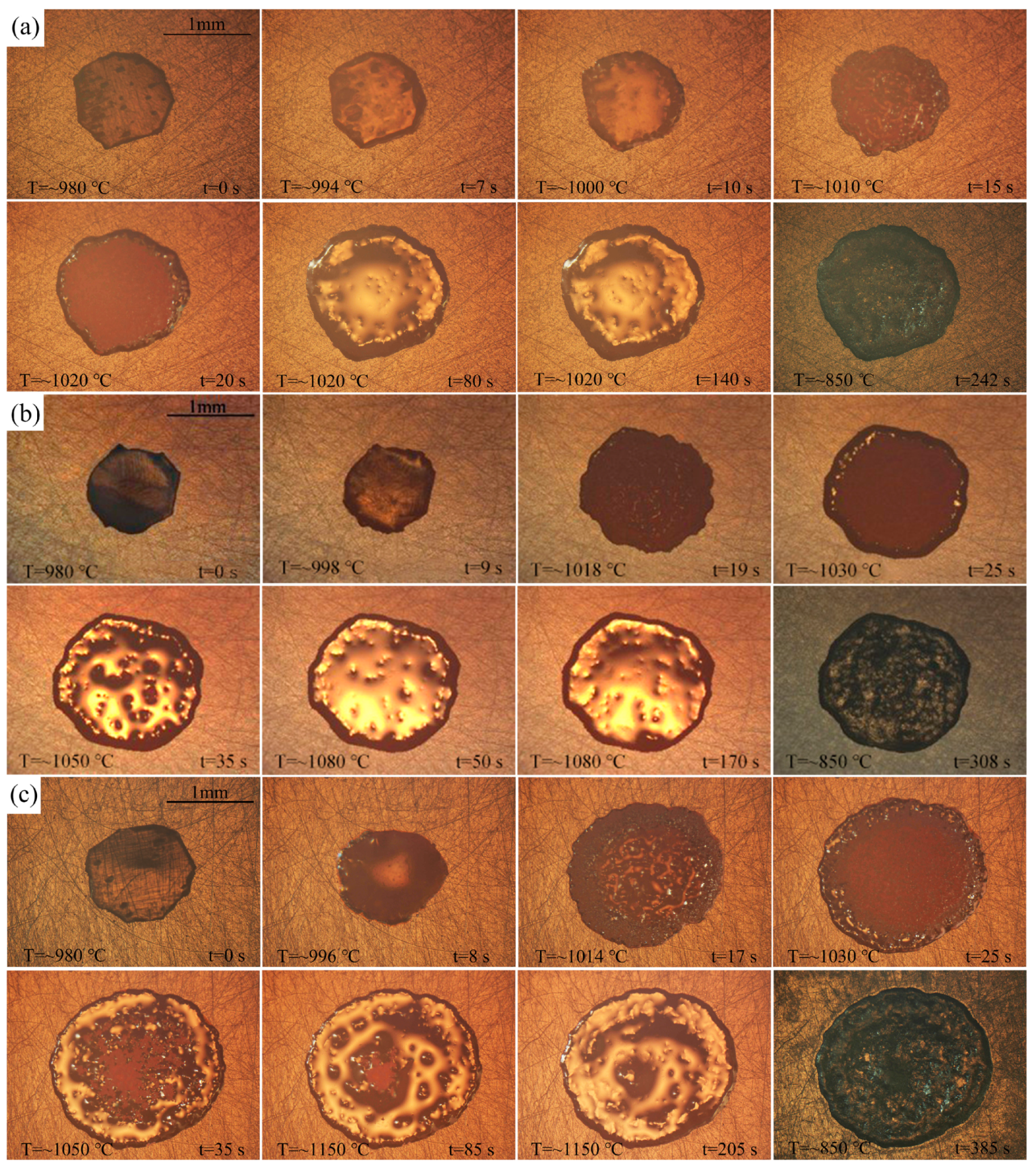
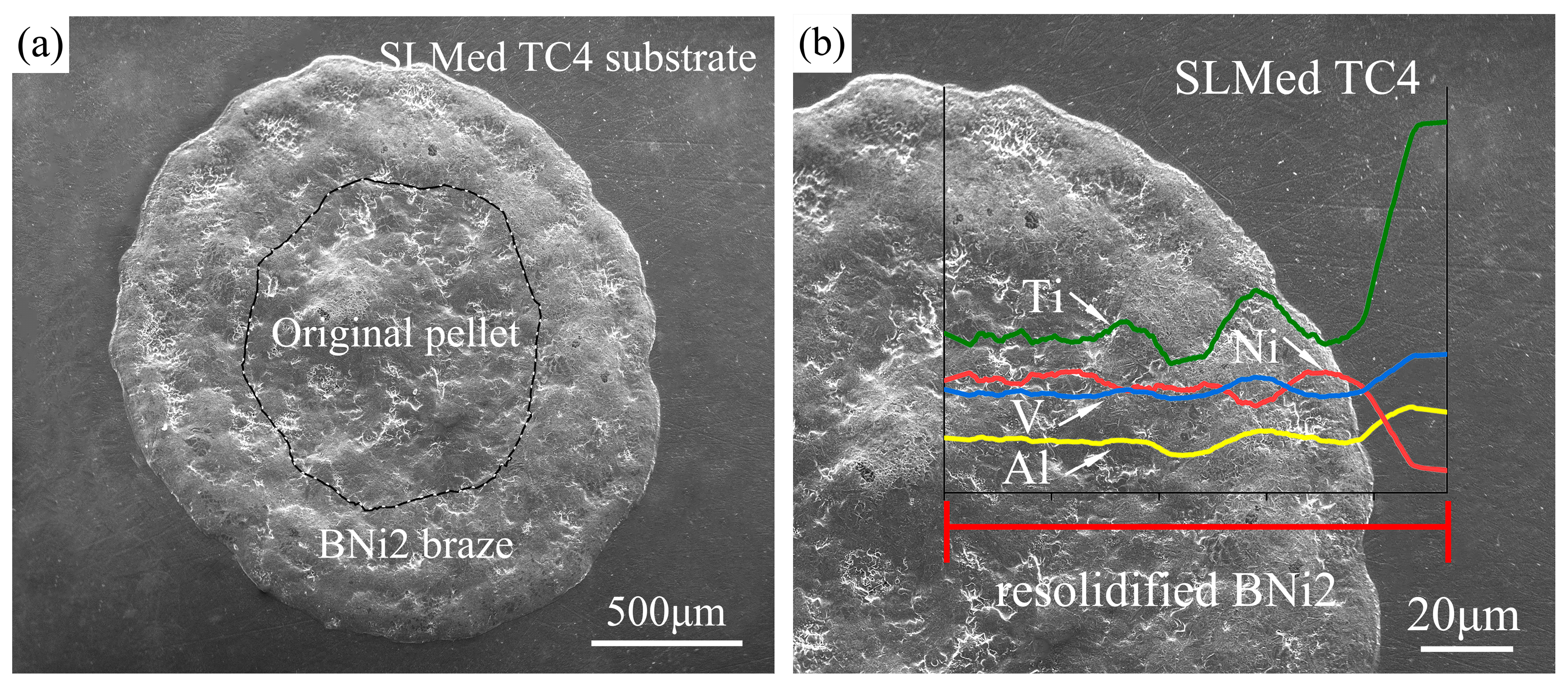
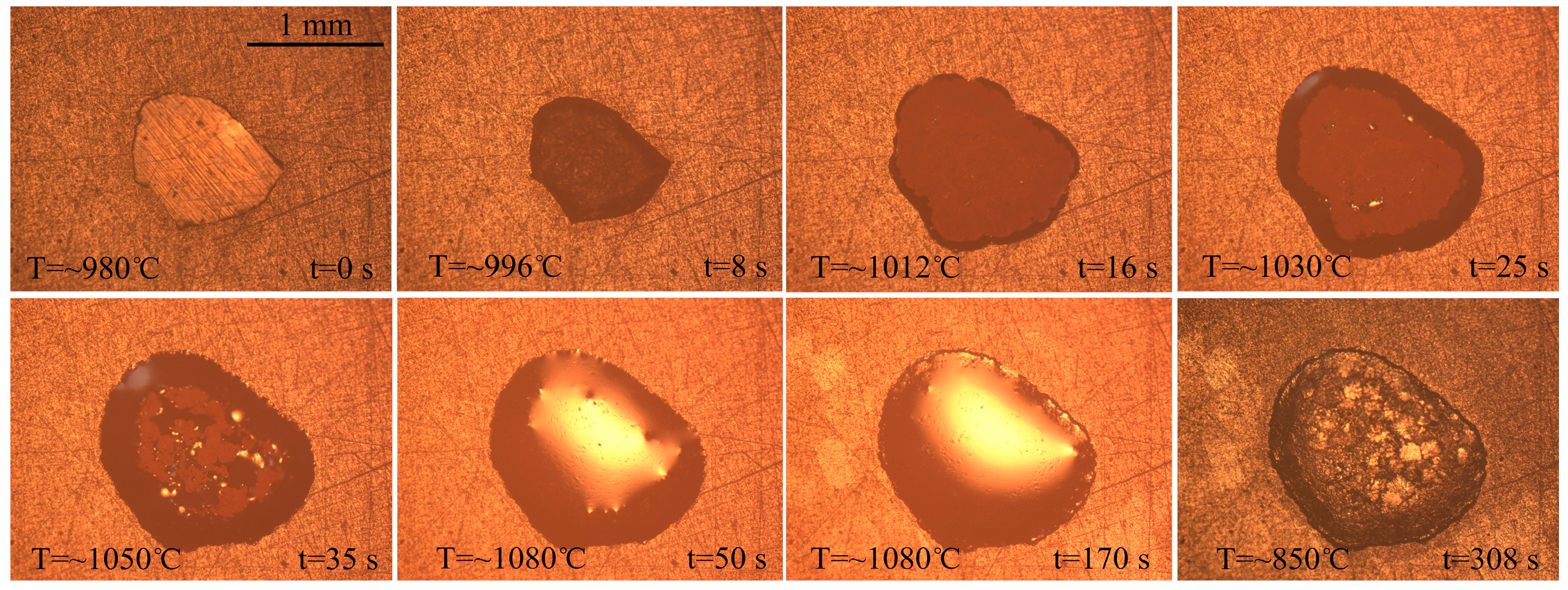
3.2. Spreading Phenomena and Data
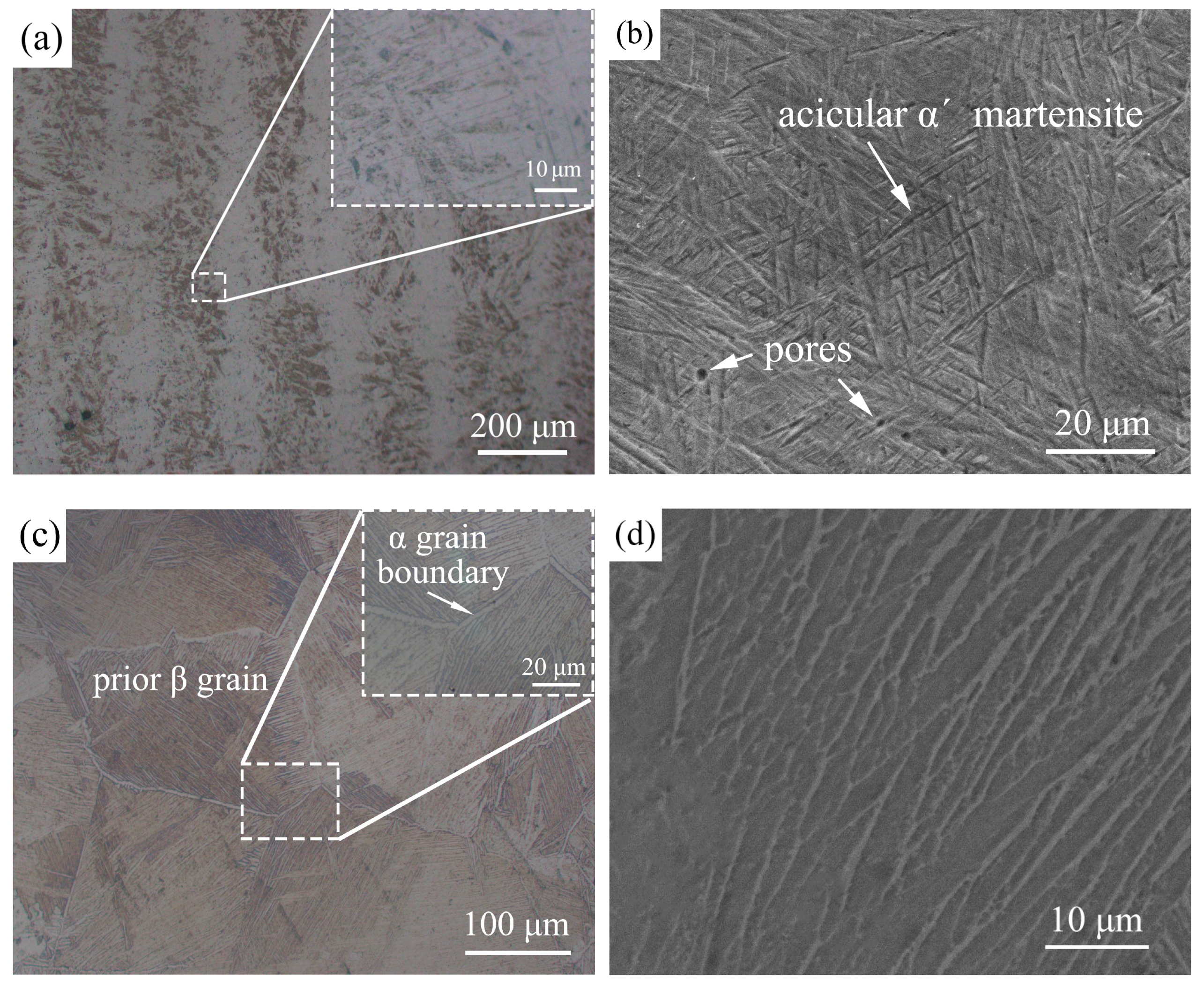
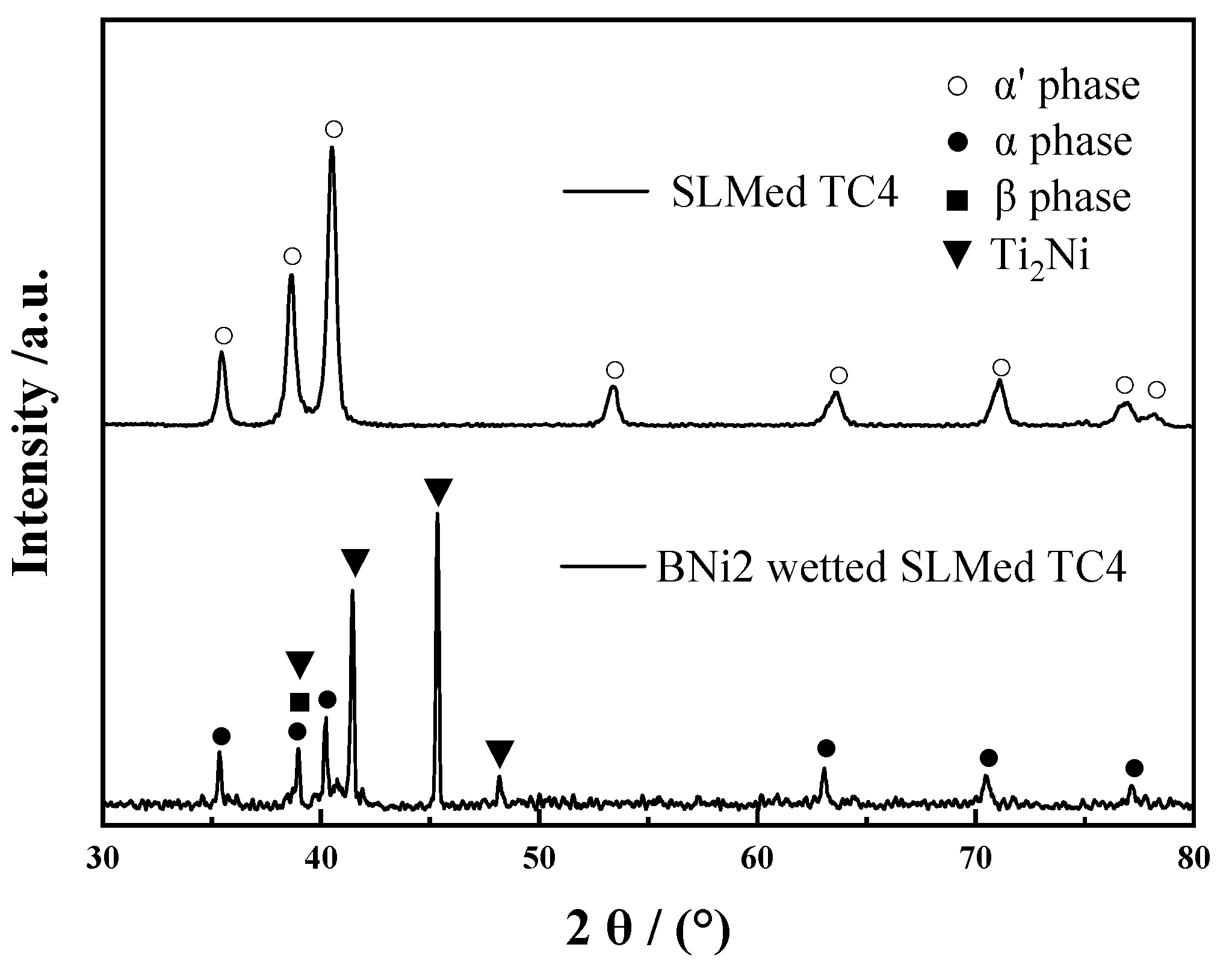
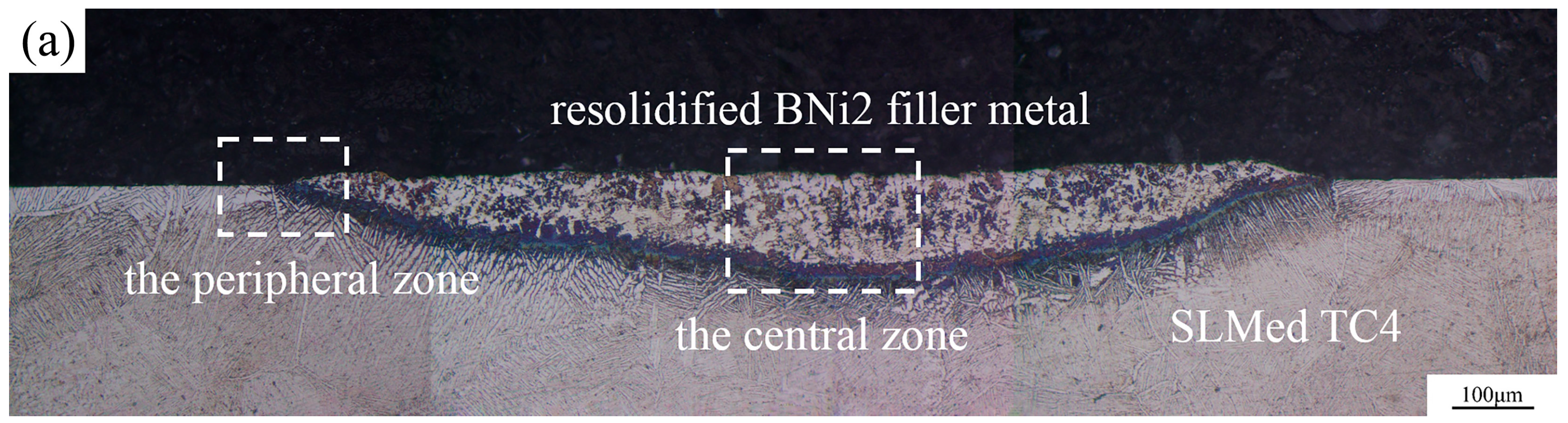
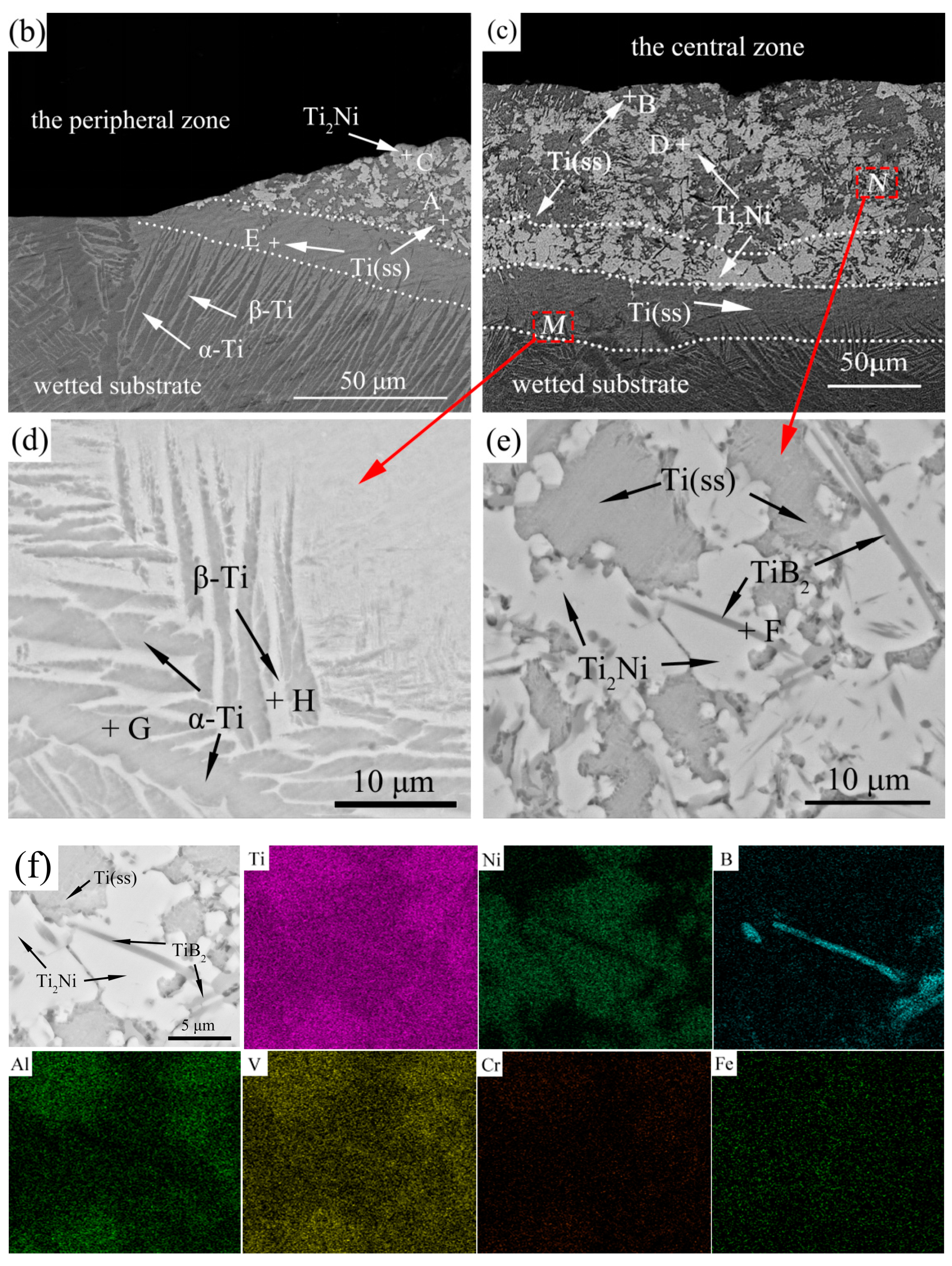
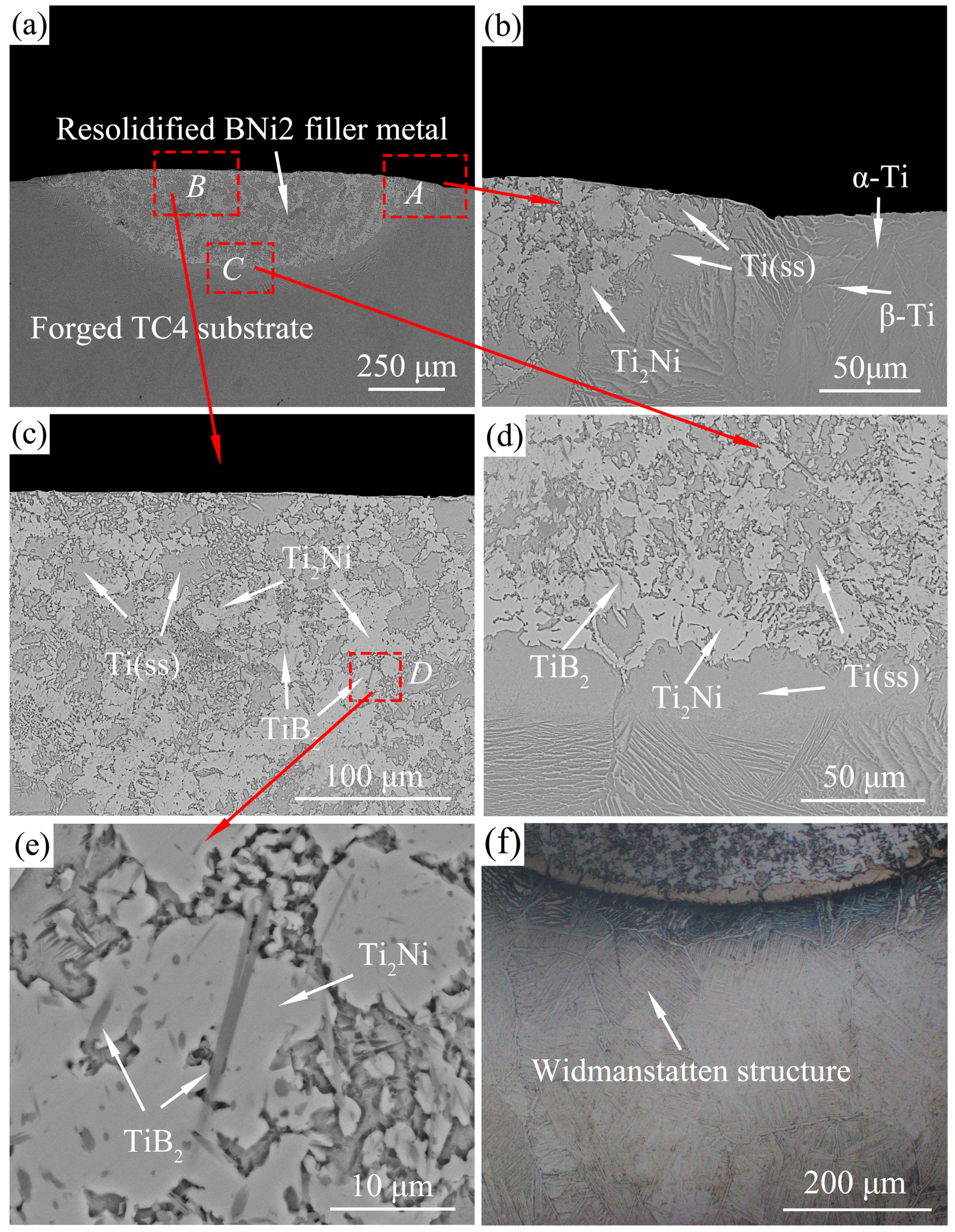
3.3. Microstructure Analysis
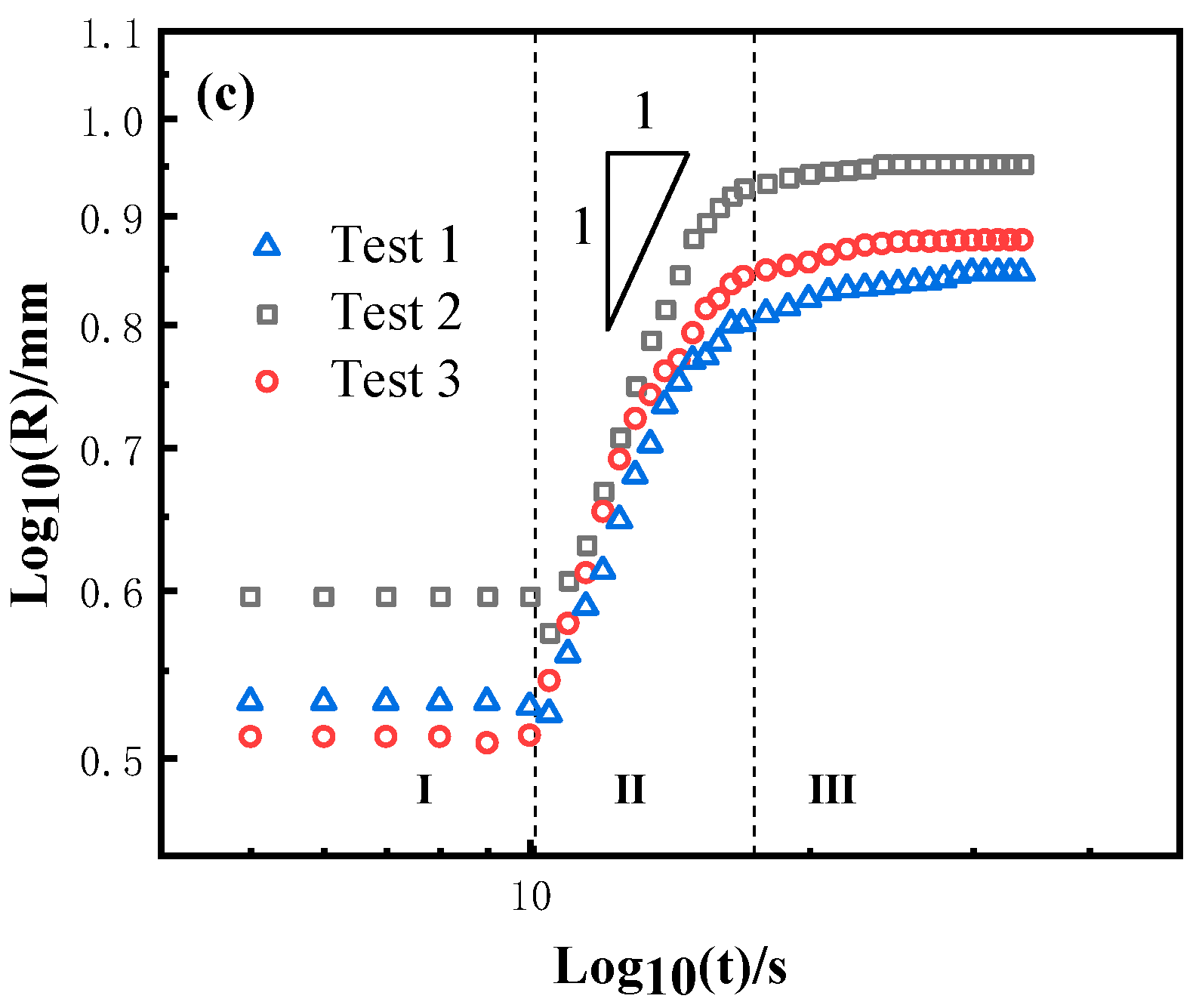
3.4. Wetting Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, D.; Krasowska, M.; Priest, C.; Popescu, M.N.; Ralston, J. Dynamics of Capillary-Driven Flow in Open Microchannels. J. Phys. Chem. C 2011, 115, 18761–18769. [Google Scholar] [CrossRef]
- Yin, L.; Murray, B.; Singler, T. Dissolutive wetting in the Bi–Sn system. Acta Mater. 2006, 54, 3561–3574. [Google Scholar] [CrossRef]
- Villanueva, W.; Boettinger, W.; McFadden, G.B.; Warren, J. A diffuse-interface model of reactive wetting with intermetallic formation. Acta Mater. 2012, 60, 3799–3814. [Google Scholar] [CrossRef]
- Hanzl, P.; Zetek, M.; Bakša, T.; Kroupa, T. The Influence of Processing Parameters on the Mechanical Properties of SLM Parts. Procedia Eng. 2015, 100, 1405–1413. [Google Scholar] [CrossRef]
- Amato, K.; Gaytan, S.; Murr, L.; Martinez, E.; Shindo, P.; Hernandez, J.; Collins, S.; Medina, F. Microstructures and mechanical behavior of Inconel 718 fabricated by selective laser melting. Acta Mater. 2012, 60, 2229–2239. [Google Scholar] [CrossRef]
- Prashanth, K.; Debalina, B.; Wang, Z.; Gostin, P.; Gebert, A.; Calin, M.; Kühn, U.; Kamaraj, M.; Scudino, S.; Eckert, J. Tribological and corrosion properties of Al–12Si produced by selective laser melting. J. Mater. Res. 2014, 29, 2044–2054. [Google Scholar] [CrossRef]
- Edwards, P.; Ramulu, M. Fatigue performance evaluation of selective laser melted Ti–6Al–4V. Mater. Sci. Eng. A 2014, 598, 327–337. [Google Scholar] [CrossRef]
- Cain, V.; Thijs, L.; Van Humbeeck, J.; Van Hooreweder, B.; Knutsen, R. Crack propagation and fracture toughness of Ti6Al4V alloy produced by selective laser melting. Addit. Manuf. 2015, 5, 68–76. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Faria, S.; Carvalho, O.; Pinto, E.; Alves, N.; Silva, F.; Miranda, G. Predictive models for physical and mechanical properties of Ti6Al4V produced by Selective Laser Melting. Mater. Sci. Eng. A 2016, 663, 181–192. [Google Scholar] [CrossRef]
- Vastola, G.; Zhang, G.; Pei, Q.-X.; Zhang, Y. Modeling the Microstructure Evolution During Additive Manufacturing of Ti6Al4V: A Comparison Between Electron Beam Melting and Selective Laser Melting. JOM 2016, 68, 1370–1375. [Google Scholar] [CrossRef]
- Do, D.K.; Li, P. The effect of laser energy input on the microstructure, physical and mechanical properties of Ti-6Al-4V alloys by selective laser melting. Virtual Phys. Prototyp. 2016, 11, 41–47. [Google Scholar] [CrossRef]
- Yu, H.; Li, F.; Yang, J.; Shao, J.; Wang, Z.; Zeng, X. Investigation on laser welding of selective laser melted Ti-6Al-4V parts: Weldability, microstructure and mechanical properties. Mater. Sci. Eng. A 2018, 712, 20–27. [Google Scholar] [CrossRef]
- Xia, C.; Zhao, M.; Sun, W.; Li, H.; Liu, P. Microstructure and Properties of 3D Printed Inconel 718 Joint Brazed with BNi-2 Amorphous Filler Metal. Mater. Res. 2018, 22. [Google Scholar] [CrossRef]
- Prashanth, K.; Scudino, S.; Klauss, H.; Surreddi, K.; Löber, L.; Wang, Z.; Chaubey, A.; Kühn, U.; Eckert, J. Microstructure and mechanical properties of Al–12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Kaban, I.; Damodaram, R.; Maity, T.; Wang, P.; Eckert, J. Friction welding of selective laser melted Ti6Al4V parts. Mater. Sci. Eng. A 2017, 704, 66–71. [Google Scholar] [CrossRef]
- Nahmany, M.; Rosenthal, I.; Benishti, I.; Frage, N.; Stern, A. Electron beam welding of AlSi10Mg workpieces produced by selected laser melting additive manufacturing technology. Addit. Manuf. 2015, 8, 63–70. [Google Scholar] [CrossRef]
- Tillmann, W.; Henning, T.; Wojarski, L. Vacuum brazing of 316L stainless steel based on additively manufactured and conventional material grades. IOP Conf. Series: Mater. Sci. Eng. 2018, 373, 012023. [Google Scholar] [CrossRef]
- Akselsen, O.M. Advances in brazing of ceramics. J. Mater. Sci. 1992, 27, 1989–2000. [Google Scholar] [CrossRef]
- Chai, K.; Lin, T.; He, P.; Sun, J. Wetting and spreading of Sn on molten ZnCl2 treated amorphous Zr55Cu30Al10Ni5 surface. J. Alloy. Compd. 2016, 678, 389–395. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Yan, M.; Hu, X.; Li, Y. Kinetics of wetting and spreading of AgCu filler metal over Ti–6Al–4V substrates. J. Mater. Sci. 2016, 51, 10960–10969. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Long, W.; Hu, X.; Cao, J.; Yan, M. Wetting kinetics and spreading phenomena of the precursor film and bulk liquid in the AgCuTi/TC4 system. J. Alloy. Compd. 2019, 802, 345–354. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Sekulic, D.P.; He, P. Reactive wetting of AgCuTi filler metal on the TiAl-based alloy substrate. Appl. Surf. Sci. 2012, 259, 343–348. [Google Scholar] [CrossRef]
- Murray, D.; Corbin, S. Determining the kinetics of transient liquid phase bonding (TLPB) of inconel 625/BNi-2 couples using differential scanning calorimetry. J. Mater. Process. Technol. 2017, 248, 92–102. [Google Scholar] [CrossRef]
- Tolkacheva, A.S.; Shkerin, S.N.; Korzun, I.V.; Plaksin, S.V.; Khrustov, V.R.; Ordinartsev, D.P. Phase transition in mayenite Ca12Al14O33. Russ. J. Inorg. Chem. 2012, 57, 1014–1018. [Google Scholar] [CrossRef]
- Arafin, M.; Medraj, M.; Turner, D.; Bocher, P. Transient liquid phase bonding of Inconel 718 and Inconel 625 with BNi-2: Modeling and experimental investigations. Mater. Sci. Eng. A 2007, 447, 125–133. [Google Scholar] [CrossRef]
- Kozlova, O.; Voytovych, R.; Protsenko, P.; Eustathopoulos, N. Non-reactive versus dissolutive wetting of Ag–Cu alloys on Cu substrates. J. Mater. Sci. 2009, 45, 2099–2105. [Google Scholar] [CrossRef]
- Landry, K.; Kalogeropoulou, S.; Eustathopoulos, N. Wettability of carbon by aluminum and aluminum alloys. Mater. Sci. Eng. A 1998, 254, 99–111. [Google Scholar] [CrossRef]
- Li, Z.; Xu, R.; Zhang, Z.; Kucukkoc, I. The influence of scan length on fabricating thin-walled components in selective laser melting. Int. J. Mach. Tools Manuf. 2018, 126, 1–12. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti–6Al–4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Viswanathan, G.; Denkenberger, R.; Furrer, D.; Fraser, H.L. The role of crystallographic and geometrical relationships between α and β phases in an α/β titanium alloy. Acta Mater. 2003, 51, 4679–4691. [Google Scholar] [CrossRef]
- Gangireddy, S. Effect of Initial Microstructure on High-Temperature Dynamic Deformation of Ti-6Al-4V Alloy. Met. Mater. Trans. A 2018, 49, 4581–4594. [Google Scholar] [CrossRef]
- Ya, B.; Zhou, B.; Yang, H.; Huang, B.; Jia, F.; Zhang, X. Microstructure and mechanical properties of in situ casting TiC/Ti6Al4V composites through adding multi-walled carbon nanotubes. J. Alloy. Compd. 2015, 637, 456–460. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Yadroitsava, I.; Kazantseva, N.; Du Plessis, A.; Yadroitsev, I. Deformation Behavior and Microstructure of Ti6Al4V Manufactured by SLM. Phys. Procedia 2016, 83, 778–788. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Li, L.; Bai, P.-K.; Jin, Y.; Wu, L.-Y.; Li, J.; Guan, R.-G.; Qu, H.-Q. The Heat Treatment Influence on the Microstructure and Hardness of TC4 Titanium Alloy Manufactured via Selective Laser Melting. Materials 2018, 11, 1318. [Google Scholar] [CrossRef]
- Tanner, L.H. The spreading of silicone oil drops on horizontal surfaces. J. Phys. D Appl. Phys. 1979, 12, 1473–1484. [Google Scholar] [CrossRef]
- Eustathopoulos, N. Dynamics of wetting in reactive metal/ceramic systems. Acta Mater. 1998, 46, 2319–2327. [Google Scholar] [CrossRef]
- Tillmann, W.; Pfeiffer, J.; Sievers, N.; Boettcher, K. Analyses of the spreading kinetics of AgCuTi melts on silicon carbide below 900 °C, using a large-chamber SEM. Colloids Surfaces A: Physicochem. Eng. Asp. 2015, 468, 167–173. [Google Scholar] [CrossRef]
- Mortensen, A.; Drevet, B.; Eustathopoulos, N. Kinetics of diffusion-limited spreading of sessile drops in reactive wetting. Scr. Mater. 1997, 36, 645–651. [Google Scholar] [CrossRef]
- Landry, K.; Eustathopoulos, N. Dynamics of wetting in reactive metal/ceramic systems: Linear spreading. Acta Mater. 1996, 44, 3923–3932. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, X.; Lei, M. Effect of temperature and substrate surface roughness on wetting behavior and interfacial structure between Sn–35Bi–1Ag solder and Cu substrate. J. Mater. Sci. Mater. Electron. 2020, 31, 4224–4236. [Google Scholar] [CrossRef]





| Process Parameters | Values |
|---|---|
| Laser energy density (VED) | 69.5 J·mm−3 |
| Laser power (P) | 275 W |
| Laser scanning speed (v) | 1100 mm·s−1 |
| Printing layer thickness (h) | 0.03 mm |
| Overlap spacing (l) | 0.12 mm |
| Sites | Main Elements (at.%) | Possible Phase(s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | Al | V | Ni | Cr | Fe | Si | B | ||
| A | 73.32 | 10.86 | 4.35 | 7.06 | 2.40 | 0.70 | 1.31 | - | Ti(ss) |
| B | 73.95 | 11.09 | 4.33 | 7.15 | 2.30 | 0.81 | 1.37 | - | |
| C | 63.25 | 5.42 | - | 27.92 | 1.24 | 1.21 | 0.95 | - | Ti2Ni |
| D | 63.01 | 5.3 | - | 28.42 | 1.29 | 1.16 | 0.38 | - | |
| E | 79.28 | 9.84 | 4.94 | 5.39 | - | 0.55 | - | - | Ti(ss) |
| F | 36.18 | 0.55 | - | 5.11 | - | - | - | 58.26 | TiB2 |
| G | 75.51 | 11.16 | 4.16 | 6.06 | 1.38 | 0.68 | 1.06 | - | α-Ti |
| H | 74.65 | 11.25 | 4.30 | 6.60 | 1.58 | 0.63 | 0.99 | - | β-Ti |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, G.; Ouyang, H.; Li, Y.; Yan, M.; Pecht, M. Wetting Kinetics and Microstructure Analysis of BNi2 Filler Metal over Selective Laser Melted Ti-6Al-4V Substrate. Materials 2020, 13, 4666. https://doi.org/10.3390/ma13204666
Liu J, Liu G, Ouyang H, Li Y, Yan M, Pecht M. Wetting Kinetics and Microstructure Analysis of BNi2 Filler Metal over Selective Laser Melted Ti-6Al-4V Substrate. Materials. 2020; 13(20):4666. https://doi.org/10.3390/ma13204666
Chicago/Turabian StyleLiu, Jiankun, Guanpeng Liu, Hua Ouyang, Yulong Li, Ming Yan, and Michael Pecht. 2020. "Wetting Kinetics and Microstructure Analysis of BNi2 Filler Metal over Selective Laser Melted Ti-6Al-4V Substrate" Materials 13, no. 20: 4666. https://doi.org/10.3390/ma13204666
APA StyleLiu, J., Liu, G., Ouyang, H., Li, Y., Yan, M., & Pecht, M. (2020). Wetting Kinetics and Microstructure Analysis of BNi2 Filler Metal over Selective Laser Melted Ti-6Al-4V Substrate. Materials, 13(20), 4666. https://doi.org/10.3390/ma13204666






