Preparation of Mg(OH)2/Calcined Fly Ash Nanocomposite for Removal of Heavy Metals from Aqueous Acidic Solutions
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
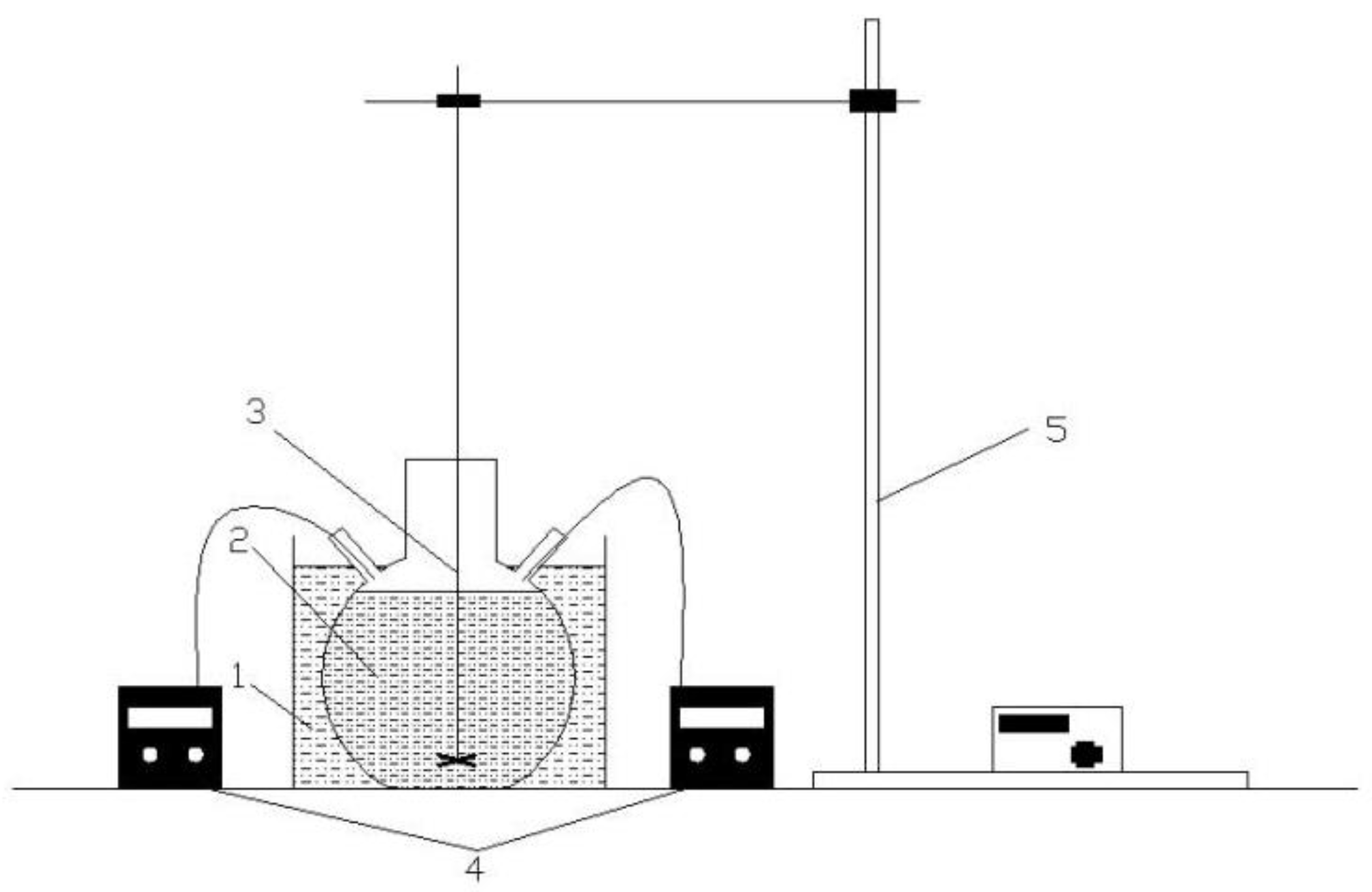
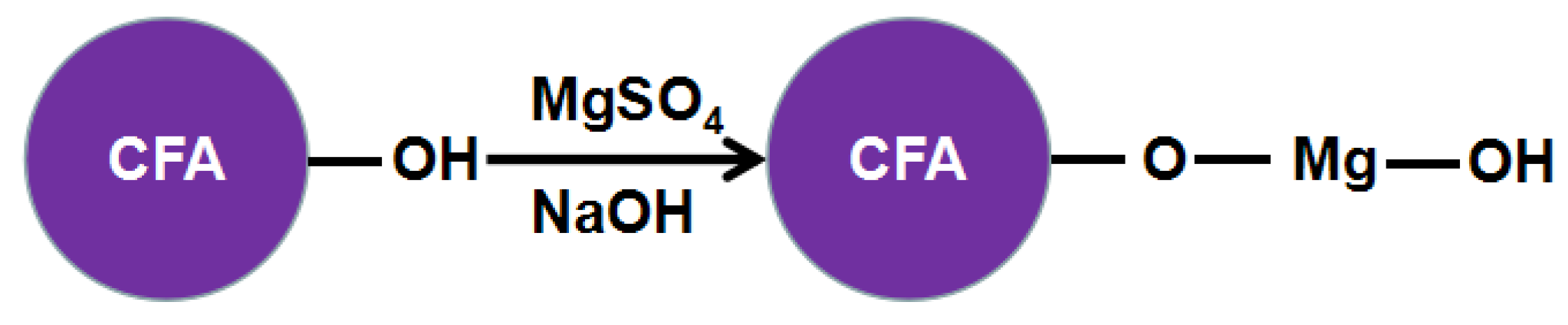
2.2. Preparation of CFAMH
2.3. Characterization
2.4. Adsorption Studies
3. Results and Discussion
3.1. SEM Observation
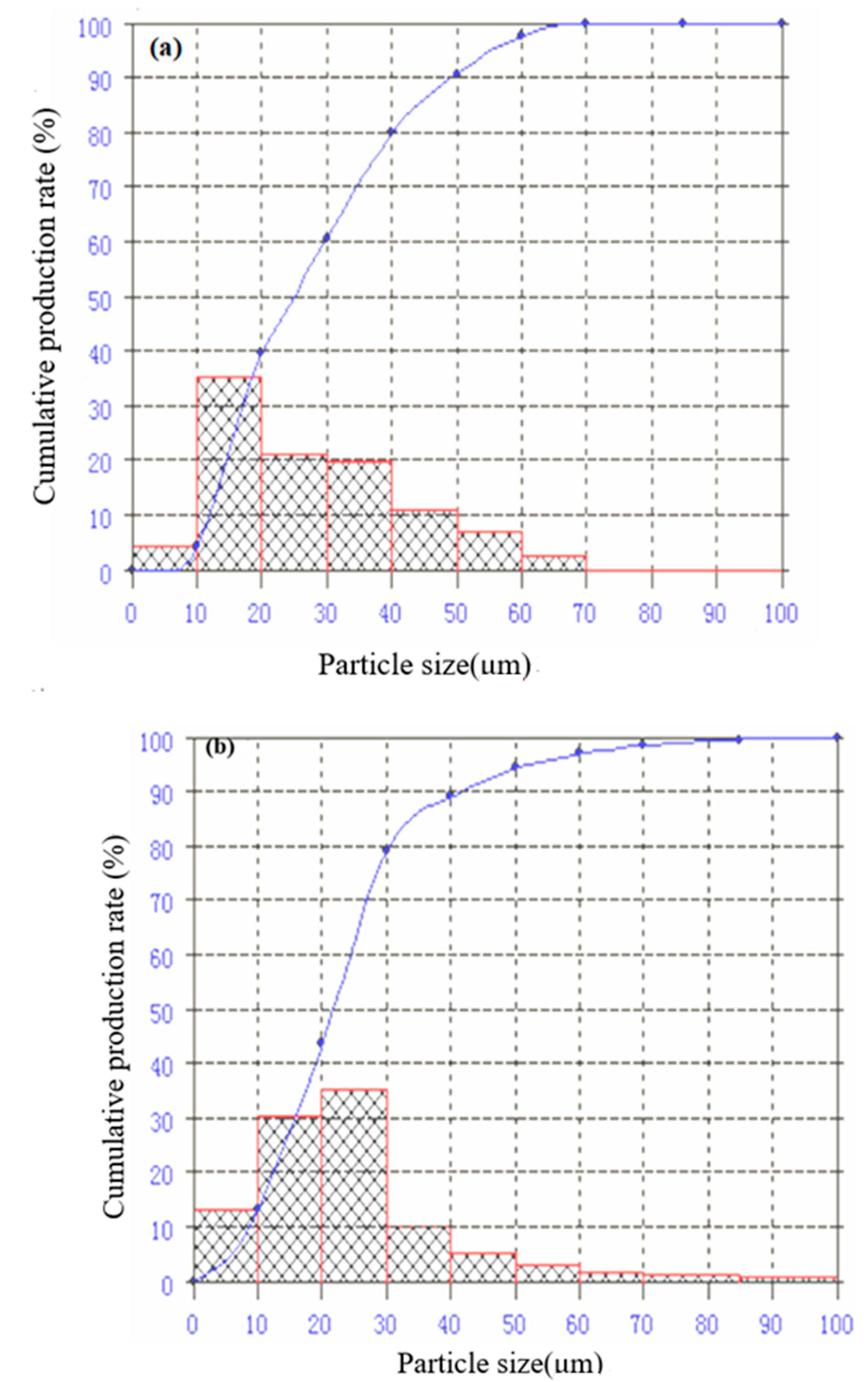
3.2. Particle Size Distribution
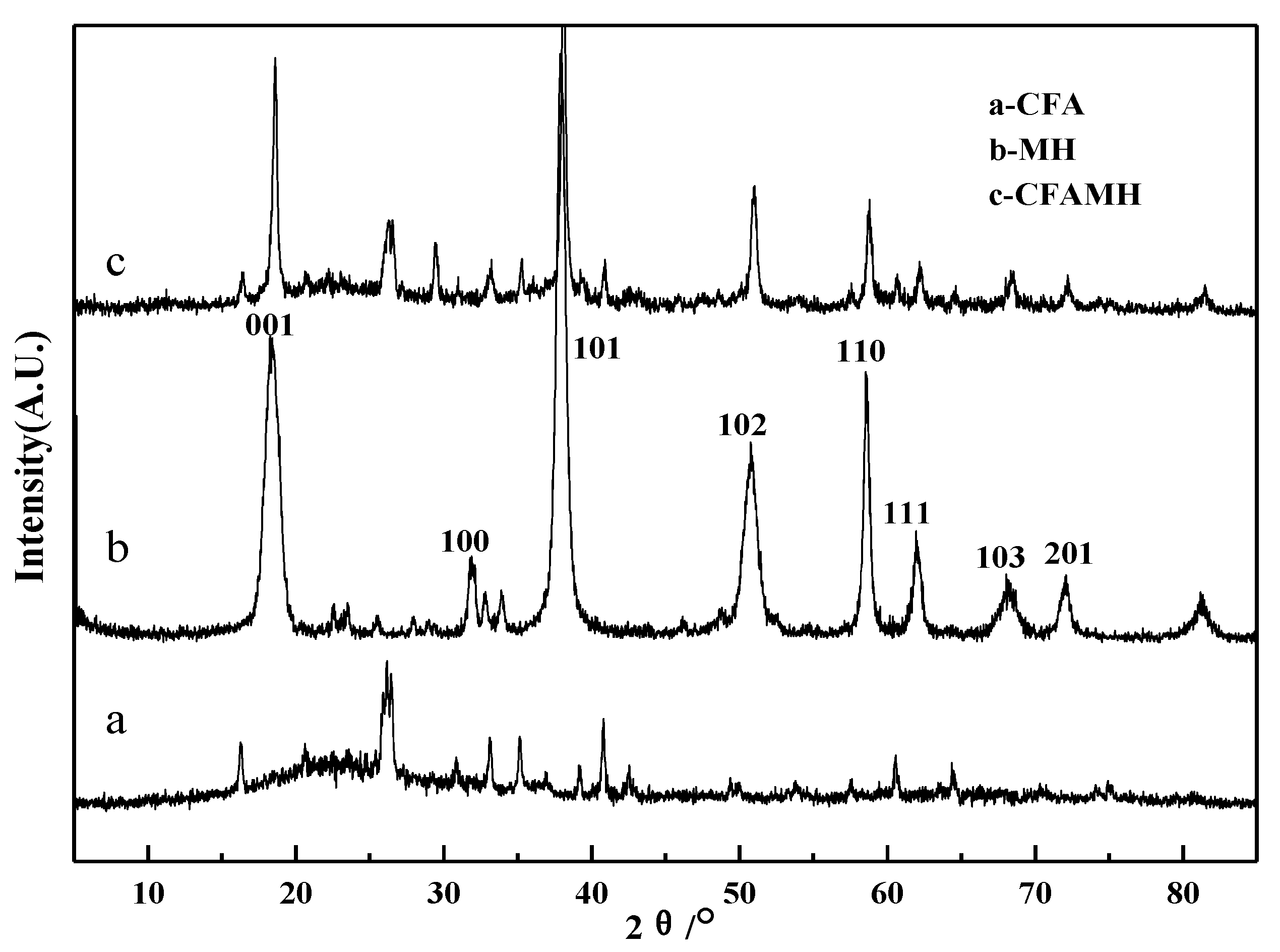
3.3. XRD Analysis
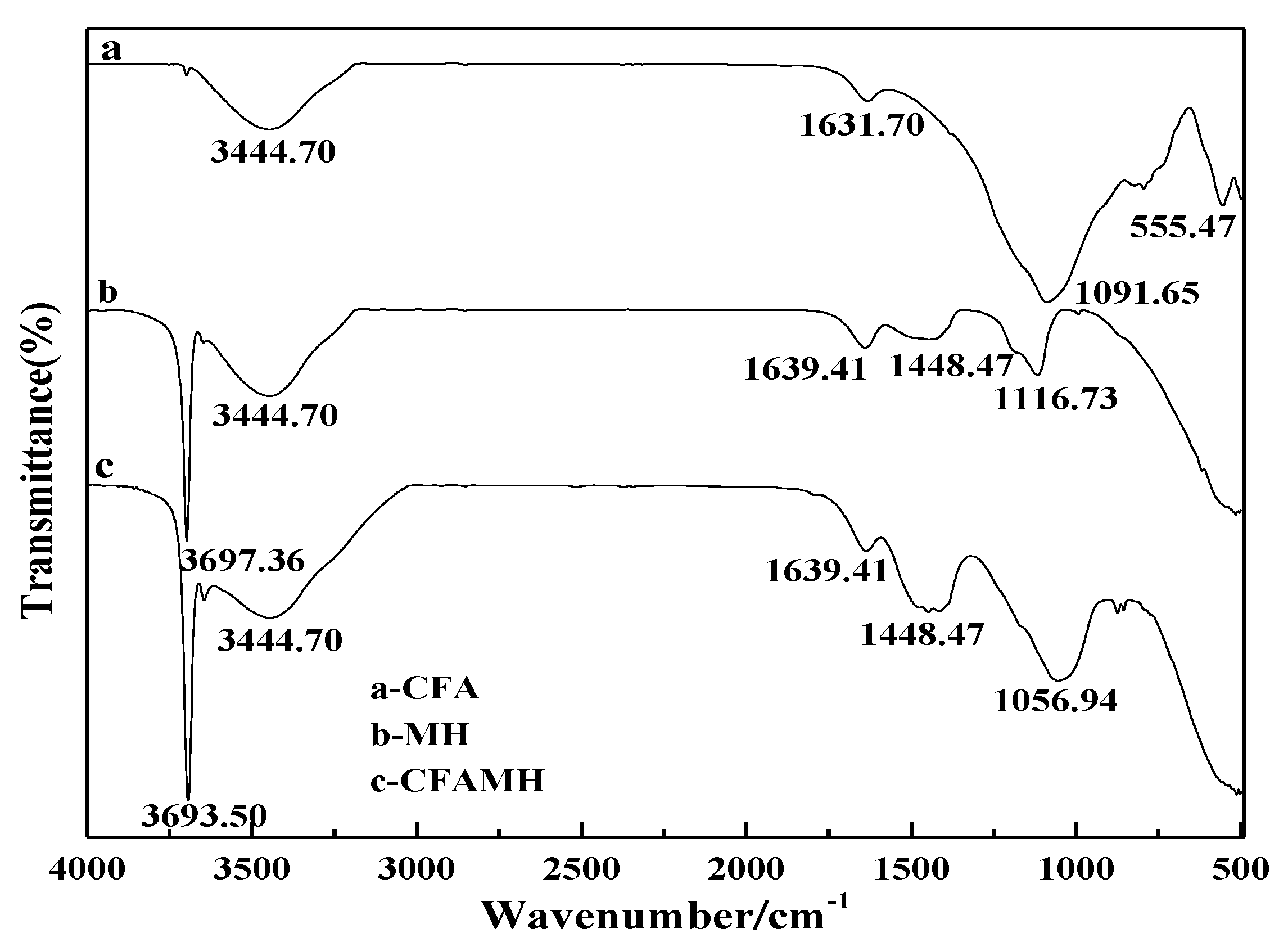
3.4. Fourier Translation Infrared Spectroscopy (FTIR) Analysis
3.5. The Preparation Mechanism of Core-Shell CFAMH
3.6. Adsorption Tests
3.6.1. Comparison of CFA, MH and CFAMH for Adsorption of Heavy Metal Ions
3.6.2. Adsorption Test of CFAMH for Cu2+, Zn2+ and Ni2+
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.H.; Pang, H.W.; Liu, Y.; Wang, X.X.; Yu, S.J.; Fu, D.; Chen, J.R.; Wang, X.K. Environmental remediation of heavy metal ions by novel-nanomaterials:A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, S.; Huang, S.Y.; Zhang, R.; Yin, L.; Hu, Y.Z.; Wen, T.; Zhuang, L.; Hu, B.W.; Wang, X.K. A novel multi-shelled Fe3O4@MnOx hollow microspheres for immobilizing U(VI) and Eu(III). Chem. Eng. J. 2019, 355, 697–709. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. In situ ion exchange synthesis of strongly coupled Ag@AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 22116–22125. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Camacho, A.; Serrano, I.; Devesa, R.; Matia, L.; Vallés, I. Removal of radionuclides in drinking water by membrane treatment using ultrafiltration, reverse osmosis and electrodialysis reversal. J. Environ. Radioact. 2013, 125, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Yao, W.; Wang, J.; Ji, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Macroscopic, spectroscopic, and theoretical investigation for the interaction of phenol and naphthol on reduced graphene oxide. Environ. Sci. Technol. 2017, 51, 3278–3286. [Google Scholar] [CrossRef]
- Kumar, P.; Varjani, S.; Suganya, S. Treatment of dye wastewater using an ultrasonic aided nanoparticle stacked activated carbon:kinetic and isotherm modelling. Bioresour. Technol. 2018, 250, 716–722. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) present in aqueous solutions by aluminium electrocoagulation. J. Hazard. Mater. 2008, 152, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mcphedran, K.; Sun, N.; Chelmeayala, P.; Gamal, E. Investigation of the impact of organic solvent type and solution pH on the extraction efficiency of naphthenic acids from oil sands process-affected water. Chemosphere 2016, 146, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Sudilovskiy, P.S.; Kagramanov, G.G.; Kolesnikov, V.A. Use of RO and NF for treatment of copper containing wastewaters in combination with flotation. Desalination 2008, 221, 192–201. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Huang, X.B.; Tang, Z.W.; Huang, Q.F.; Niu, F.L.; Wang, X.K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution:a review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.X.; Zhao, G.X.; Chen, C.L.; Chai, Z.F.; Alsaedi, A.; Hayat, T.; Wang, X.K. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Ai, Y.; Liu, Y.H.; Li, J.X.; Ji, Y.; Wang, X. Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides:batch experimental and theoretical calculation study. Environ. Sci. Technol. 2016, 50, 3658–3667. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions:a review. Chem. Eng. J. 2018, 333, 343–360. [Google Scholar] [CrossRef]
- Feng, J.; Gao, M.M.; Zhang, Z.Q.; Liu, S.N.; Zhao, X.Y.; Ren, Y.M.; Lv, Y.Z.; Fan, Z.J. Fabrication of mesoporous magnesium oxide nanosheets using magnesium powder and their excellent adsorption of Ni(II). J. Colloid Interface Sci. 2018, 510, 69–76. [Google Scholar] [CrossRef]
- Das, P.S.; Bakuli, S.; Samanata, A.; Mandal, A.K.; Ghosh, J.; Dey, A.; Mukhopadhyay, A.K. Very high Cu(II) adsorption efficacy of designed nano-platelet Mg(OH)2 assembly. Mater. Res. Express 2017, 4, 025025. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment:a critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Skwarek, E.; Matysek-Nawrocka, M.; Janusz, W.; Zarko, V.I.; Gun’ko, V.M. Adsorption of heavy metal ions at the Al2O3-SiO2/NaClO4 electrolyte interface. Physicochem. Probl. Miner. Process. 2008, 42, 153–164. [Google Scholar]
- Babel, S.; Kurniawan, T. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Wang, S.B.; Li, L.; Zhu, Z.H. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, B139, 254–259. [Google Scholar] [CrossRef]
- He, X.P.; Yao, B.; Xia, Y.; Huang, H.; Gan, Y.P.; Zhang, W. Coal fly ash derived zeolite for highly efficient removal of Ni2+ in waste water. Powder Technol. 2020, 367, 40–46. [Google Scholar] [CrossRef]
- Visa, M. Synthesis of characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Zhao, X.W.; Hu, B.; Ye, J.J.; Jia, Q. Preparation, characterization and application of Graphene-zinc oxide composites(G-ZnO) for the adsorption of Cu(II), Pb(II) and Cr(III). J. Chem. Eng. Data 2013, 58, 2395–2401. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Chen, L.; Yan, Z.; Zhang, L. Mg(OH)2 supported nanoscale zero valent iron enhancing the removal of Pb(II) from aqueous solution. ACS Appl. Mater. Interfaces 2015, 7, 7961–7969. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Wang, J.; Bai, L.Q.; Yang, R.Q.; Wang, H.F. Preparation and Characterization of Fly Ash Coated with Zinc Oxide Nanocomposites. Materials 2019, 12, 3550. [Google Scholar] [CrossRef]
- Kameda, T.; Takeuchi, H.; Yoshioka, T. Preparation of organic acid anion-modifified magnesium hydroxides by coprecipitation: A novel material for the uptake of heavy metal ions from aqueous solutions. J. Phys. Chem. Solids 2019, 70, 1104–1108. [Google Scholar] [CrossRef]
- Wang, C.L.; Yang, R.Q.; Wang, H.F. Synthesis of ZIF-8/Fly Ash Composite for Adsorption of Cu2+, Zn2+ and Ni2+ from Aqueous Solutions. Materials 2020, 13, 214. [Google Scholar] [CrossRef]
- Bayat, B. Comparative study of adsorption properties of Turkish fly ashes I. The case of nickel(II), copper(II) and zinc(II). J. Hazard. Mater. 2002, 92, 251–273. [Google Scholar] [CrossRef]

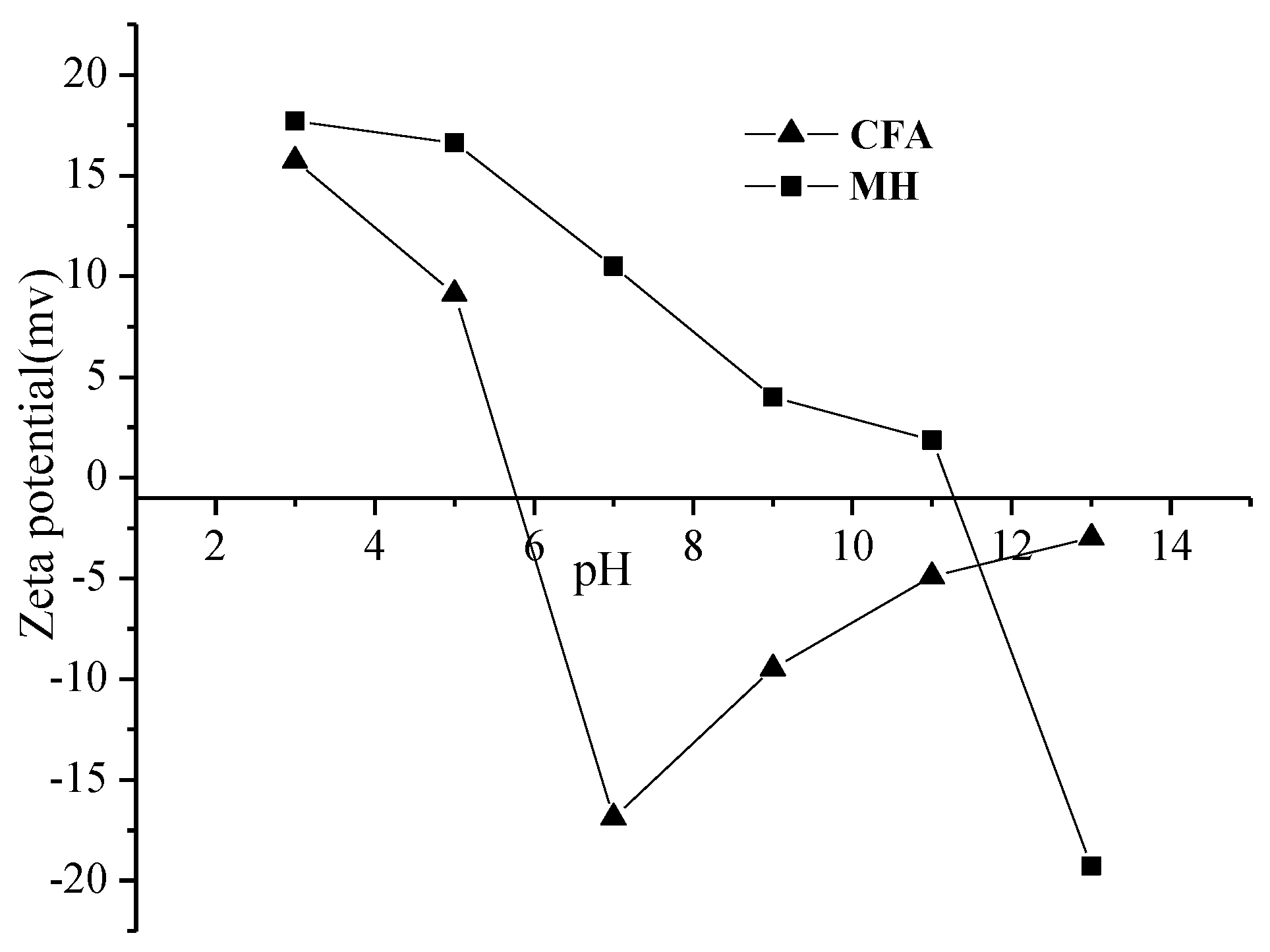

| Samples | SBET (m2/g) | Vtotal (cm3/g) | Vmeso (cm3/g) | Vmac (cm3/g) |
|---|---|---|---|---|
| FA | 3.7 | 0.0115 | 0.0113 | 0.0002 |
| CFA | 2.5 | 0.0093 | 0.0091 | 0.0002 |
| CFAMH | 31.0 | 0.0329 | 0.0325 | 0.0004 |
| Crystal Face | 2θ (°) | h (mm) | β (rad) | D (nm) |
|---|---|---|---|---|
| (101) | 37.88 | 601.13 | 0.00672 | 20.99 |
| (001) | 18.32 | 314.34 | 0.01012 | 13.4 |
| (110) | 58.54 | 278.00 | 0.00375 | 40.83 |
| Samples (Adsorbent Dosage) (g/L) | Heavy Metals | C0 (mg/L) | InitialpH | Time (min) | Equilibrium pH | Filtration Time (min) | Ct (mg/L) | Removal Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| CFA (2 g/L) | Cu2+ | 35 | 2 | 120 | 3.20 | 10 | 34.61 | 1.11 |
| Zn2+ | 35 | 2 | 120 | 3.13 | 10 | 34.78 | 0.63 | |
| Ni2+ | 35 | 2 | 120 | 3.15 | 10 | 34.86 | 0.4 | |
| CFAMH (2 g/L) | Cu2+ | 35 | 2 | 120 | 6.66 | 10 | 3.03 | 91.34 |
| Zn2+ | 35 | 2 | 120 | 6.8 | 10 | 3.7 | 89.43 | |
| Ni2+ | 35 | 2 | 120 | 7.01 | 10 | 4.1 | 88.29 | |
| Cu2+/ Zn2+/ Ni2+ | 11.67/11.67/ 11.67 | 2 | 120 | 7.08 | 10 | 0.92/ 8.21/ 11.40 | 92.12/ 29.65/ 2.31 | |
| MH (0.6 g/L) | Cu2+ | 35 | 2 | 120 | 4.51 | 10 | 28.3 | 19.14 |
| Zn2+ | 35 | 2 | 120 | 4.87 | 10 | 29.7 | 15.14 | |
| Ni2+ | 35 | 2 | 120 | 4.91 | 10 | 31.4 | 10.29 | |
| Cu2+/ Zn2+/ Ni2+ | 11.67/11.67/ 11.67 | 2 | 120 | 4.96 | 10 | 8.64/ 10.61/ 11.24 | 25.96/ 8.6/ 3.68 |
| Initial Concentration of Cu2+ (mg/L) | Adsorbent Concentration (g/L) | Initial pH Value | Time (min) | Equilibrium Concentration (mg/L) | Equilibrium pH Value |
|---|---|---|---|---|---|
| 50 | 1 | 4 | 15 | 30.14 | / |
| 50 | 1 | 4 | 120 | 20.88 | / |
| 50 | 1 | 4 | 180 | 15.86 | / |
| 50 | 1 | 4 | 600 | 5.24 | / |
| 50 | 1 | 4 | 1080 | 0.87 | / |
| 50 | 1 | 4 | 1440 | 0.42 | 6.42 |
| 50 | 2 | 4 | 120 | 0.44 | 7.68 |
| 50 | 4 | 2 | 240 | 0.36 | 8.56 |
| 35 | 1 | 4 | 15 | 13.33 | / |
| 35 | 1 | 4 | 60 | 8.07 | / |
| 35 | 1 | 4 | 120 | 4.25 | / |
| 35 | 1 | 4 | 180 | 2.34 | / |
| 35 | 1 | 4 | 1080 | 0.38 | 6.31 |
| 35 | 3 | 2 | 120 | 0.11 | 7.94 |
| 20 | 1 | 4 | 20 | 0.36 | 6.67 |
| 20 | 2 | 2 | 20 | 0.42 | 6.56 |
| Initial Concentration of Zn2+(mg/L) | Adsorbent Concentration (g/L) | Initial pH Value | Time (min) | Equilibrium Concentration (mg/L) | Equilibrium pH Value |
|---|---|---|---|---|---|
| 50 | 6 | 2 | 240 | 4.3 | 7.84 |
| 35 | 3 | 2 | 60 | 3.2 | 7.62 |
| 20 | 2 | 2 | 20 | 2.4 | 7.13 |
| Initial Concentration of Ni2+ (mg/L) | Adsorbent Concentration (g/L) | Initial pH Value | Time (min) | Equilibrium Concentration (mg/L) | Equilibrium pH Value |
|---|---|---|---|---|---|
| 50 | 13 | 2 | 240 | 0.72 | 8.62 |
| 35 | 9.5 | 2 | 60 | 0.32 | 8.31 |
| 20 | 6 | 2 | 20 | 0.28 | 8.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, J.; Wang, S.; Yang, R.; Wang, H. Preparation of Mg(OH)2/Calcined Fly Ash Nanocomposite for Removal of Heavy Metals from Aqueous Acidic Solutions. Materials 2020, 13, 4621. https://doi.org/10.3390/ma13204621
Wang C, Wang J, Wang S, Yang R, Wang H. Preparation of Mg(OH)2/Calcined Fly Ash Nanocomposite for Removal of Heavy Metals from Aqueous Acidic Solutions. Materials. 2020; 13(20):4621. https://doi.org/10.3390/ma13204621
Chicago/Turabian StyleWang, Caili, Jing Wang, Shaobin Wang, Runquan Yang, and Huaifa Wang. 2020. "Preparation of Mg(OH)2/Calcined Fly Ash Nanocomposite for Removal of Heavy Metals from Aqueous Acidic Solutions" Materials 13, no. 20: 4621. https://doi.org/10.3390/ma13204621
APA StyleWang, C., Wang, J., Wang, S., Yang, R., & Wang, H. (2020). Preparation of Mg(OH)2/Calcined Fly Ash Nanocomposite for Removal of Heavy Metals from Aqueous Acidic Solutions. Materials, 13(20), 4621. https://doi.org/10.3390/ma13204621





