The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
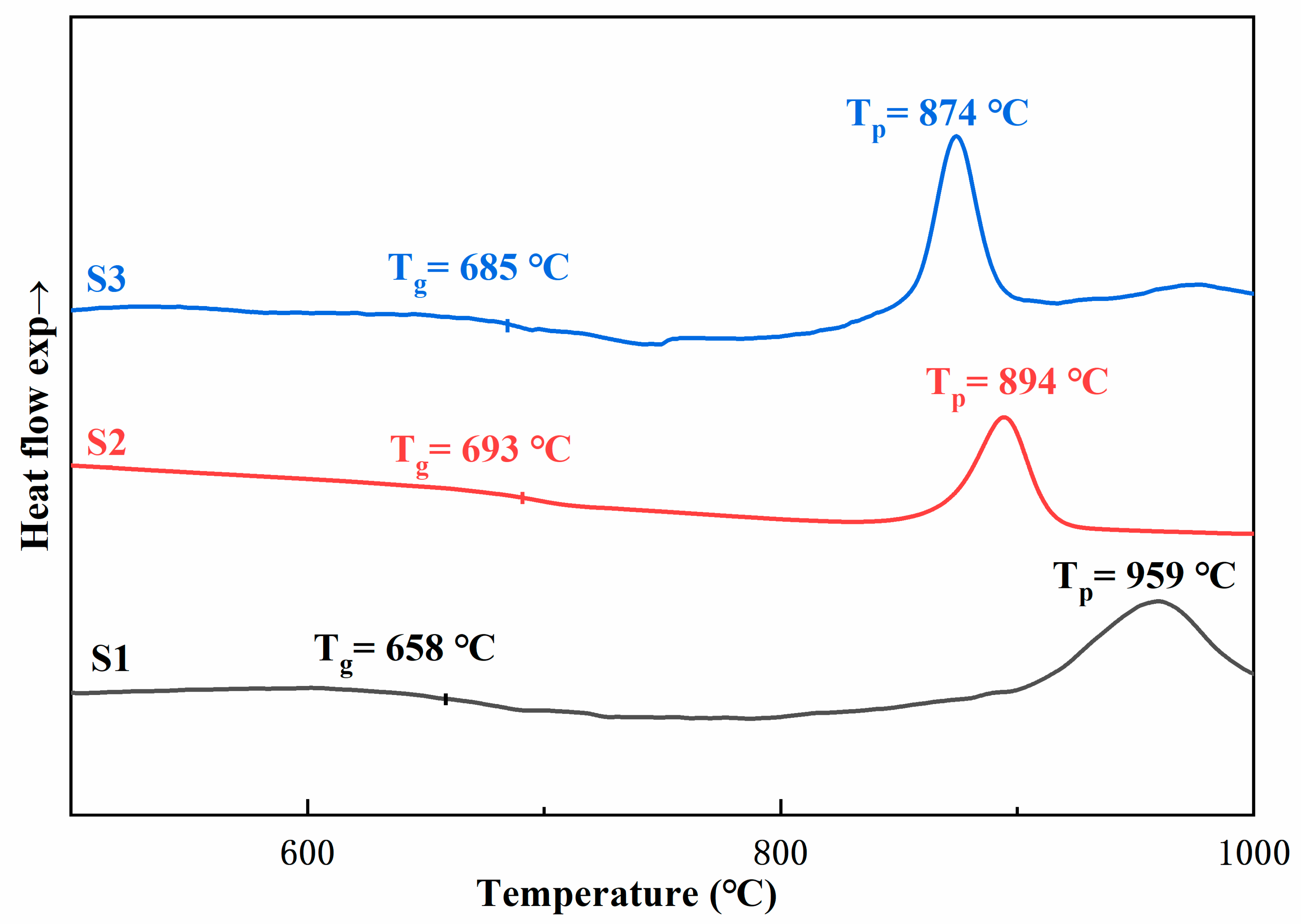
3.1. Thermal Analysis
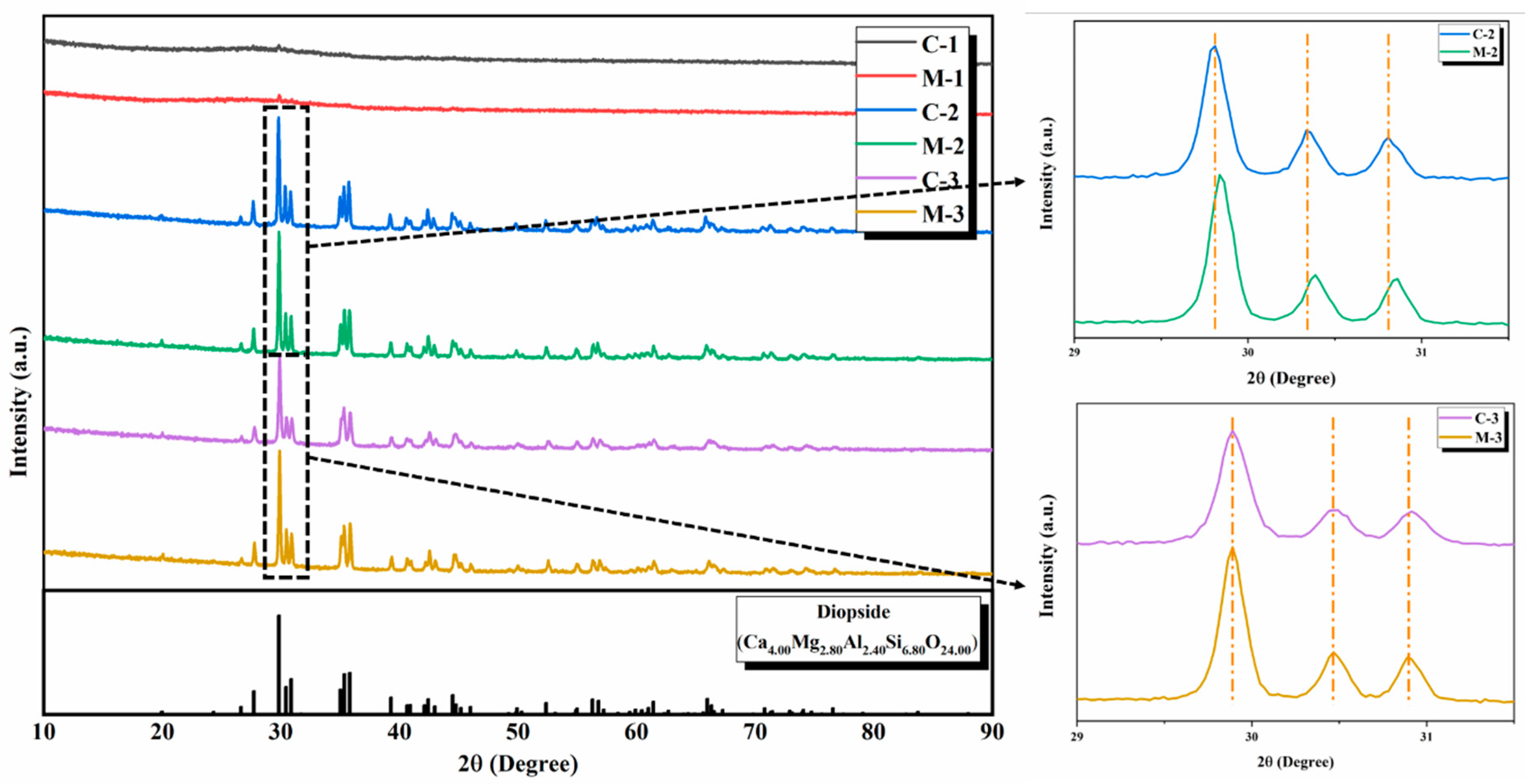
3.2. X-ray Diffraction Analysis
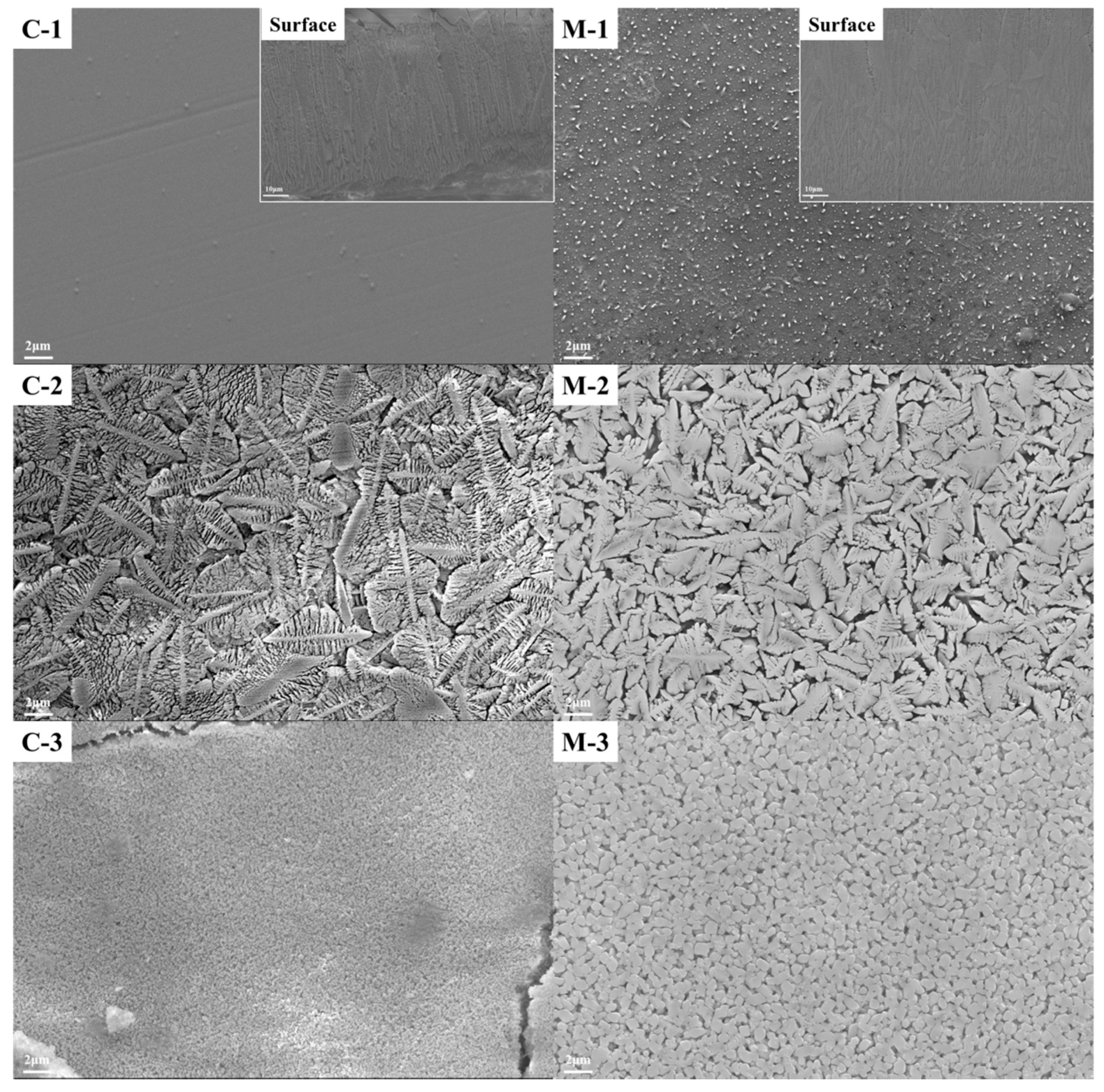
3.3. Scanning Electron Microscopy
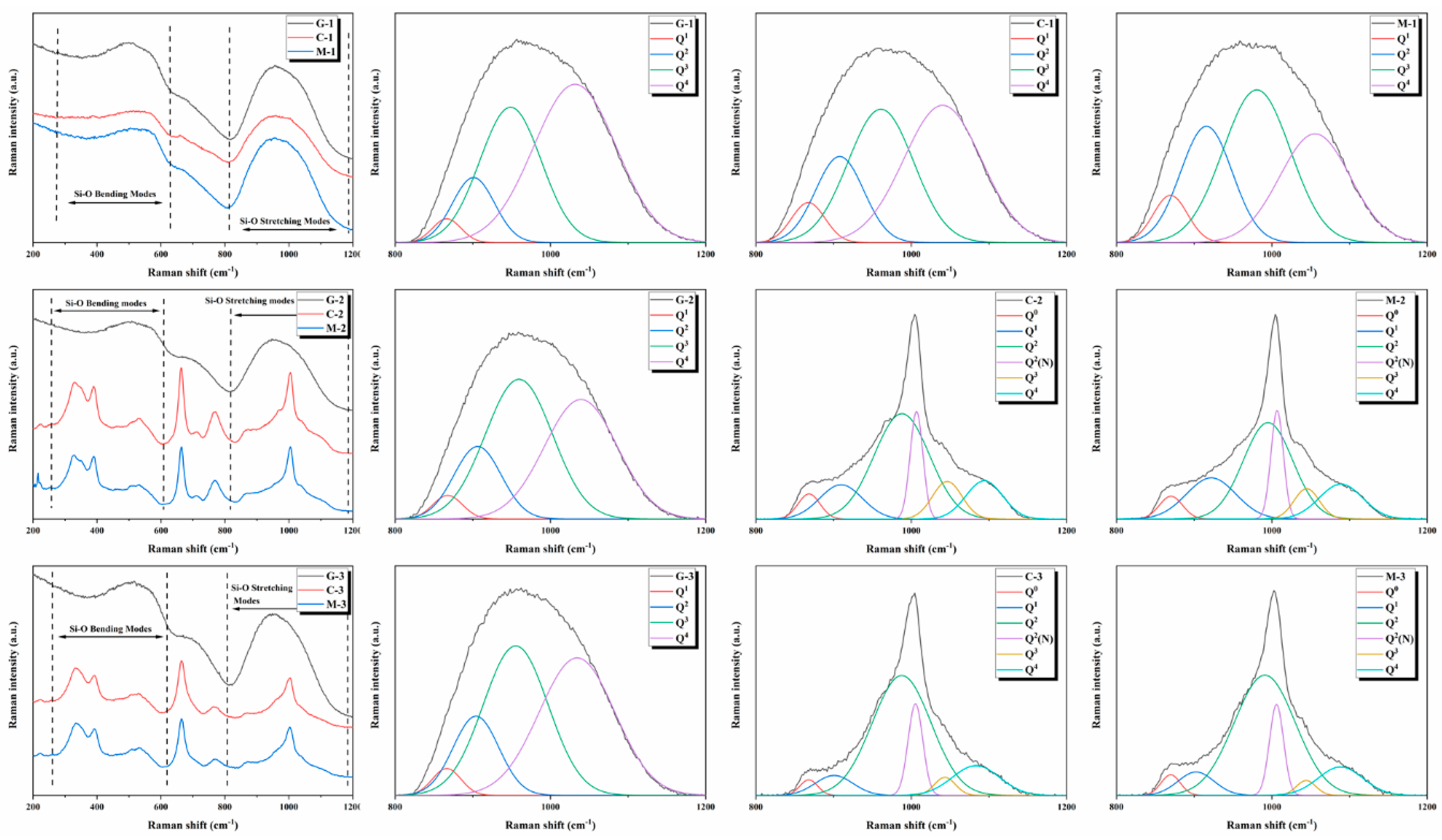
3.4. Raman Spectroscopy
3.5. Vickers Hardness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.; Krikun, E.V. Microwave Sintering: Fundamentals and Modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Raveendran, A.; Sebastian, M.T.; Raman, S. Applications of Microwave Materials: A Review. J. Electron. Mater. 2019, 48, 2601–2634. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Siligardi, C.; Leonelli, C.; So, J.; Kim, H. Evolution of Macropores in a Glass-Ceramic Under Microwave and Conventional Sintering. J. Porous Mater. 2002, 9, 299–305. [Google Scholar] [CrossRef]
- Bykov, Y.V.; Egorov, S.V.; Eremeev, A.G.; Rybakov, K.I.; Semenov, V.E.; Sorokin, A.A.; Gusev, S.A. Evidence for microwave enhanced mass transport in the annealing of nanoporous alumina membranes. J. Mater. Sci. 2001, 36, 131–136. [Google Scholar] [CrossRef]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Folz, D.C.; West, J.K. Processing materials with microwave energy. Mater. Sci. Eng. A 2000, 287, 153–158. [Google Scholar] [CrossRef]
- Barnsley, B.P. Microwave processing of materials. Met. Mater. Bury St Edmunds 1989, 5, 633–636. [Google Scholar]
- Das, S.; Basu, D.; Datta, S.; Mukhopadhyay, A.K. Crystallization of Glass Coating by Microwave Heating. Trans. Indian Ceram. Soc. 2008, 67, 139–146. [Google Scholar] [CrossRef]
- Binner, J.; Vaidhyanathan, B.; Wang, J.; Price, D.; Reading, M. Evidence for Non-Thermal Microwave Effects Using Single and Multimode Hybrid Conventional/Microwave Systems. J. Microw. Power Electromagn. Energy 2007, 42, 47–63. [Google Scholar] [CrossRef]
- Clark, D.E.; Sutton, W.H. Microwave processing of materials. Mater. Res. Soc. 2003, 18, 347–351. [Google Scholar] [CrossRef]
- Wong, B. Understanding Non-Thermal Microwave Effects in Materials Processing—A Classical Non-Quantum Approach; Wiley: Hoboken, NJ, USA, 2014; pp. 329–338. [Google Scholar]
- Kokubo, T. Bioactive glass-ceramicss: Properties and applications. Biomaterials 1991, 12, 155. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.; Boccaccini, A.R. Glass-ceramics: Their production from wastes—A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahmoud, M.M.; Seifert, H.J.; Mahmood, M.M. Crystallization of two rare-earth aluminosilicate glass-ceramics using conventional and microwave heat-treatments. J. Alloys Compd. 2019, 797, 45–57. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Folz, D.C.; Suchicital, C.T.A.; Clark, D.E. Crystallization of Lithium Disilicate Glass Using Microwave Processing. J. Am. Ceram. Soc. 2011, 95, 579–585. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Thumm, M. Crystallization of lithium disilicate glass using high frequency microwave processing. J. Eur. Ceram. Soc. 2015, 35, 2915–2922. [Google Scholar] [CrossRef]
- Das, S.; Mukhopadhyay, A.K.; Datta, S.; Das, G.C.; Basu, D. Hard glass-coating bu microwave processing. J. Eur. Ceram. Soc. 2008, 28, 729–738. [Google Scholar] [CrossRef]
- Tian, J.; Mukhopadhyay, A.; Datta, S.; Basu, D. Evaluation of microwave processed glass–ceramic coating on nimonic superalloy substrate. Ceram. Int. 2010, 36, 1125–1130. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Link, G.; Miksch, S.; Thumm, M. High Frequency Microwave Processing of Lithium Disilicate Glass-Ceramic; Wiley: Hoboken, NJ, USA, 2012; pp. 115–122. [Google Scholar]
- Li, H.-X.; Li, B.; Deng, L.-B.; Xu, P.-F.; Du, Y.-S.; Ouyang, S.-L. Microwave-assisted processing of graded structural tailing glass-ceramics. J. Eur. Ceram. Soc. 2018, 38, 2632–2638. [Google Scholar] [CrossRef]
- Li, H.-X.; Li, H.-X.; Deng, L.-B.; Xu, P.-F.; Du, Y.-S.; Ouyang, S.-L.; Liu, Z.-X. Evidence for non-thermal microwave effect in processing of tailing-based glass-ceramics. J. Eur. Ceram. Soc. 2019, 39, 1389–1396. [Google Scholar] [CrossRef]
- Baert, K.; Meulebroeck, W.; Wouters, H.; Cosyns, P.; Nys, K.; Thienpont, H.; Terryn, H. Using Raman spectroscopy as a tool for the detection of iron in glass. J. Raman Spectrosc. 2011, 42, 1789–1795. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.; Li, M.; He, F. Raman spectra of soda–lime–silicate glass doped with rare earth. Phys. B Condens. Matter 2011, 406, 3865–3869. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.-S.; Li, M.; He, F. Structure and properties of soda lime silicate glass doped with rare earth. Phys. B Condens. Matter 2011, 406, 187–191. [Google Scholar] [CrossRef]
- Wu, D.; Tian, Y.; Wen, X.; Zuo, W.; Liu, H.; Lee, D.-J. Studies on the use of microwave for enhanced properties of glass-ceramics produced from sewage sludge pyrolysis residues (SSPR). J. Taiwan Inst. Chem. Eng. 2015, 48, 81–86. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Chen, D. Crystallization evolution, microstructure and properties of sewage sludge-based glass–ceramics prepared by microwave heating. J. Hazard. Mater. 2011, 196, 370–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ouyang, S.; Zhang, X.; Zhao, Z.; Jia, X.; Du, Y.; Deng, L.; Li, B. Transformation of unstable heavy metals in solid waste into stable state by the preparation of glass-ceramics. Mater. Chem. Phys. 2020, 252, 123061. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhang, Y.; Chen, Y.; Zhao, Z.; Wen, M.; Li, B.; Shi, Y.; Zhang, M.; Liu, S. Preparation of Glass-ceramics Using Chromium-containing Stainless Steel Slag: Crystal Structure and Solidification of Heavy Metal Chromium. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Wiesendanger, R. Characterization of Solid Materials and Heterogeneous Catalysts; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Yadav, A.K.; Singh, P. ChemInform Abstract: A Review of the Structures of Oxide Glasses by Raman Spectroscopy. RSC Adv. 2015, 46, 67583–67609. [Google Scholar] [CrossRef]
- Dickinson, J.E.; Scarfe, C.M. Raman spectroscopic study of glasses on the join diopside-albite. Geochim. Cosmochim. Acta 1990, 54, 1037–1043. [Google Scholar] [CrossRef]
- Furukawa, T. Raman spectroscopic investigation of the structure of silicate glasses. III. Raman intensities and structural units in sodium silicate glasses. J. Chem. Phys. 1981, 75, 3226. [Google Scholar] [CrossRef]
- Rezvani, M.; Eftekhari-Yekta, B.; Solati-Hashjin, M.; Marghussian, V.K. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2-Al2O3-CaO-MgO(R2O) glass-ceramics. Ceram. Int. 2005, 31, 75–80. [Google Scholar] [CrossRef]
- Shelby, J.E. Introduction to Glass Science and Technology; Royal Society of Chemistry: London, UK, 2007; Volume II, p. 1030. [Google Scholar]
- Umesaki, N.; Takahashi, M.; Tatsumisago, M.; Minami, T. Raman spectroscopic study of alkali silicate glasses and melts. J. Non-Cryst. Solids 1996, 205, 225–230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


| Sample | Chemical Composition (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | MgO | SiO2 | CaO | Na2CO3 | Na2B4O7 | Al2O3 | Fe2O3 | Cr2O3 |
| S1 | 14.17 | 52.54 | 20.24 | 3.74 | 1.36 | 5.60 | 2.36 | 0.00 |
| S2 | 14.36 | 52.35 | 20.03 | 3.63 | 1.33 | 5.74 | 2.35 | 0.20 |
| S3 | 14.62 | 52.11 | 19.82 | 3.52 | 1.32 | 5.92 | 2.29 | 0.40 |
| Sample No. | Nucleation | Crystallization | Heat Treatment |
|---|---|---|---|
| C-1 | 720 °C, 3 h | 880 °C, 3 h | Conventional heating |
| C-2 | 720 °C, 3 h | 880 °C, 3 h | Conventional heating |
| C-3 | 720 °C, 3 h | 880 °C, 3 h | Conventional heating |
| M-1 | 720 °C, 30 min | 880 °C, 20 min | Microwave heating |
| M-2 | 720 °C, 30 min | 880 °C, 20 min | Microwave heating |
| M-3 | 720 °C, 30 min | 880 °C, 20 min | Microwave heating |
| Item | G-1 | C-1 | M-1 | G-2 | C-2 | M-2 | G-3 | C-3 | M-3 |
|---|---|---|---|---|---|---|---|---|---|
| ν1 | 865.09 | 866.00 | 867.98 | 866.26 | 907.56 | 919.78 | 864.94 | 898.67 | 900.45 |
| ν2 | 899.03 | 905.82 | 913.72 | 904.25 | 985.36 | 992.57 | 902.19 | 985.11 | 988.42 |
| ν3 | 946.28 | 958.43 | 978.10 | 957.09 | 1043.84 | 1042.41 | 952.90 | 1040.82 | 1041.72 |
| ν4 | 1029.25 | 1037.86 | 1053.23 | 1036.92 | 1091.99 | 1086.92 | 1031.95 | 1081.14 | 1086.42 |
| FWHM1 | 42.50 | 50.07 | 50.88 | 44.16 | 63.91 | 74.29 | 42.49 | 56.57 | 54.14 |
| FWHM2 | 63.03 | 72.17 | 77.60 | 70.82 | 83.87 | 75.10 | 68.32 | 89.02 | 92.39 |
| FWHM3 | 92.41 | 98.80 | 101.82 | 101.34 | 45.11 | 38.49 | 97.89 | 32.07 | 29.66 |
| FWHM4 | 125.77 | 118.42 | 107.74 | 111.38 | 56.58 | 66.45 | 115.21 | 72.40 | 68.48 |
| A1 | 94,203 | 186,000 | 145,160 | 91,922 | 224,390 | 248,050 | 106,040 | 57,747 | 64,473 |
| A2 | 380,570 | 576,550 | 544,290 | 452,600 | 901,900 | 587,490 | 501,990 | 544,510 | 566,400 |
| A3 | 1,161,100 | 1,222,800 | 937,670 | 1,247,500 | 173,230 | 94,918 | 1,357,000 | 29,659 | 22,439 |
| A4 | 1,847,300 | 1,508,000 | 707,080 | 1,173,300 | 221,080 | 188,510 | 1,469,000 | 110,210 | 99,534 |
| A1% | 2.70 | 5.32 | 6.22 | 3.10 | 14.76 | 22.17 | 3.09 | 7.78 | 8.56 |
| A2% | 10.93 | 16.50 | 23.32 | 15.26 | 59.31 | 52.50 | 14.62 | 73.37 | 75.23 |
| A3% | 33.33 | 35.00 | 40.17 | 42.07 | 11.39 | 8.48 | 39.52 | 4.00 | 2.98 |
| A4% | 53.03 | 43.17 | 30.29 | 39.57 | 14.54 | 16.85 | 42.78 | 14.85 | 13.22 |
| Item | G-1 | C-1 | M-1 | G-2 | C-2 | M-2 | G-3 | C-3 | M-3 |
|---|---|---|---|---|---|---|---|---|---|
| X1% | 3.11 | 6.12 | 7.15 | 3.56 | 16.97 | 25.49 | 3.55 | 8.95 | 9.85 |
| X2% | 11.14 | 16.83 | 23.78 | 15.57 | 60.50 | 53.55 | 14.91 | 74.84 | 76.74 |
| X3% | 34.67 | 36.40 | 41.78 | 43.75 | 11.85 | 8.82 | 41.10 | 4.16 | 3.10 |
| X4% | 51.08 | 40.64 | 27.29 | 37.11 | 10.68 | 12.13 | 40.44 | 12.06 | 10.31 |
| Q1 | 1.98 | 3.90 | 4.56 | 2.28 | 10.83 | 16.27 | 2.27 | 5.72 | 6.29 |
| Q2 | 7.10 | 10.73 | 15.16 | 9.94 | 38.61 | 34.18 | 9.53 | 47.82 | 49.04 |
| Q3 | 22.10 | 23.20 | 26.63 | 27.92 | 7.56 | 5.63 | 26.26 | 2.66 | 1.98 |
| Q4 | 32.56 | 25.90 | 17.39 | 23.69 | 6.82 | 7.74 | 25.84 | 7.70 | 6.59 |
| NBO/(NBO + BO) | 0.23 | 0.31 | 0.38 | 0.30 | 0.64 | 0.67 | 0.28 | 0.63 | 0.65 |
| NBO/tetrahedron | 0.66 | 0.88 | 1.11 | 0.86 | 1.84 | 1.92 | 0.82 | 1.81 | 1.86 |
| BO/tetrahedron | 3.34 | 3.12 | 2.89 | 3.14 | 2.16 | 2.08 | 3.18 | 2.19 | 2.14 |
| Sample (No.) | C-1 | C-2 | C-3 | M-1 | M-2 | M-3 |
|---|---|---|---|---|---|---|
| Vickers hardness (HV 0.5) | 701.24 ± 18.05 | 819.79 ± 26.47 | 908.91 ± 32.44 | 739.43 ± 32.92 | 821.23 ± 28.69 | 848.04 ± 40.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, S.; Xu, W.; Zhang, Y.; Li, X.; Ouyang, S.; Zhao, G.; Liu, F.; Wu, N. The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics. Materials 2020, 13, 4555. https://doi.org/10.3390/ma13204555
Li H, Liu S, Xu W, Zhang Y, Li X, Ouyang S, Zhao G, Liu F, Wu N. The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics. Materials. 2020; 13(20):4555. https://doi.org/10.3390/ma13204555
Chicago/Turabian StyleLi, Hangren, Saiyu Liu, Wence Xu, Yuxuan Zhang, Xin Li, Shunli Ouyang, Guangkai Zhao, Fang Liu, and Nannan Wu. 2020. "The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics" Materials 13, no. 20: 4555. https://doi.org/10.3390/ma13204555
APA StyleLi, H., Liu, S., Xu, W., Zhang, Y., Li, X., Ouyang, S., Zhao, G., Liu, F., & Wu, N. (2020). The Effect of Microwave on the Crystallization Behavior of CMAS System Glass-Ceramics. Materials, 13(20), 4555. https://doi.org/10.3390/ma13204555





