Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
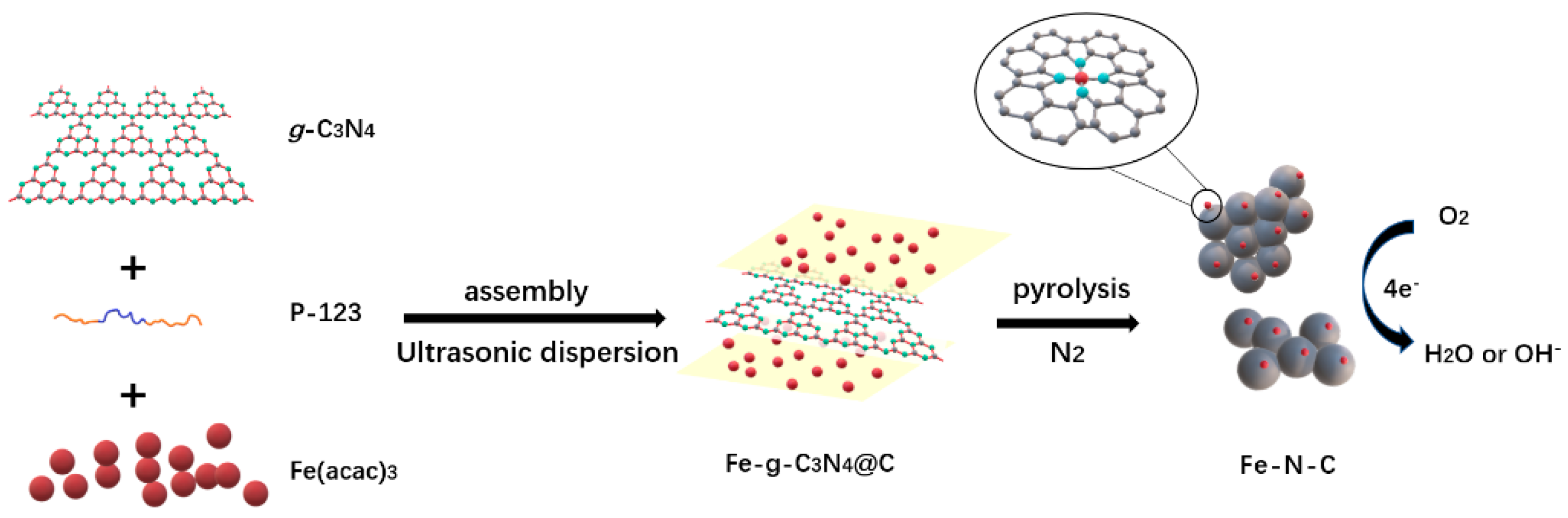
2.2. Preparation of g-C3N4
2.3. Preparation of FeX-N-C-Y Electrocatalysts
2.4. Materials Characterization
2.5. Preparation of the Working Electrodes
2.6. Electrocatalytic Measurements
3. Results
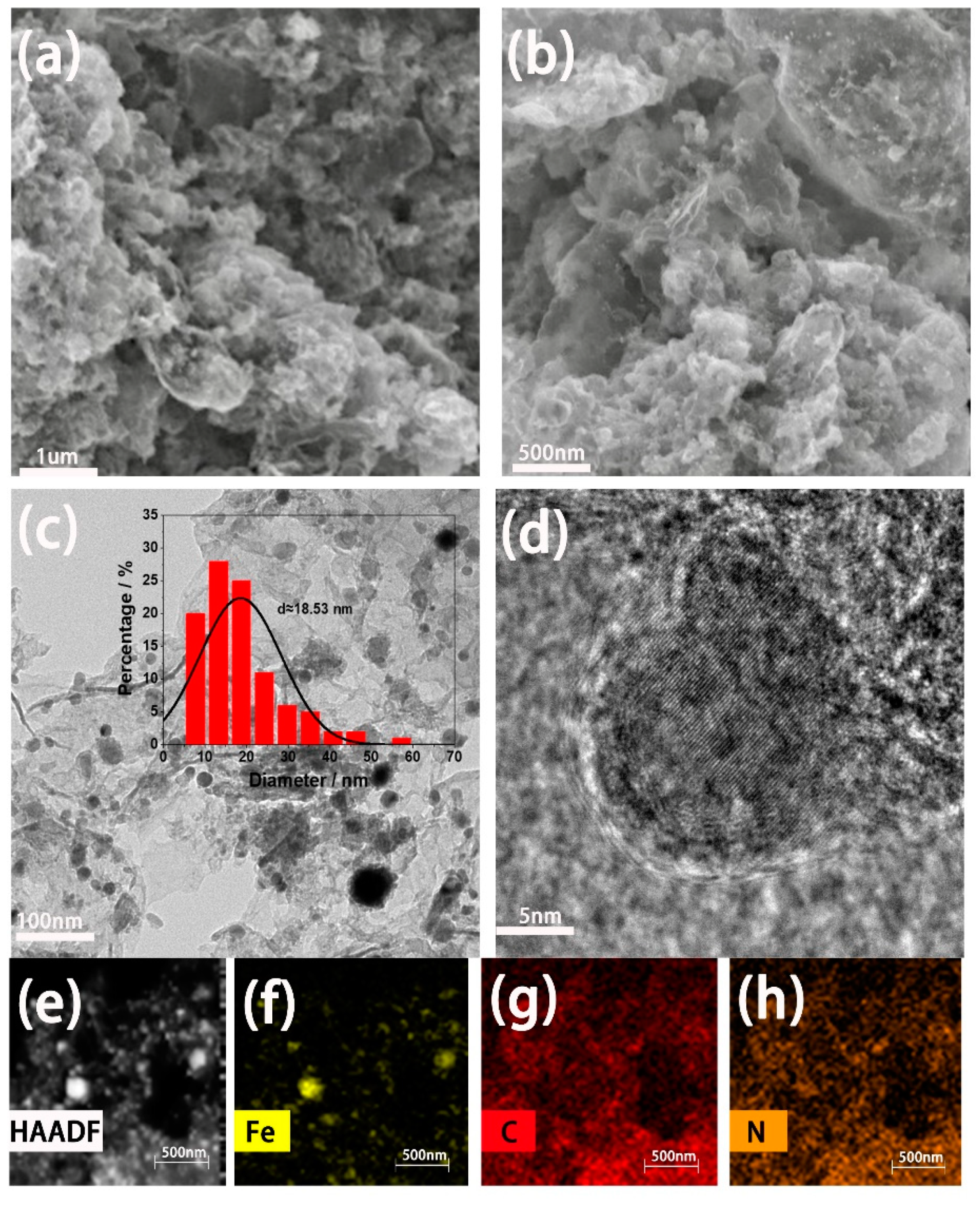
3.1. Preparation and Physical Characterization of Fe-N-C
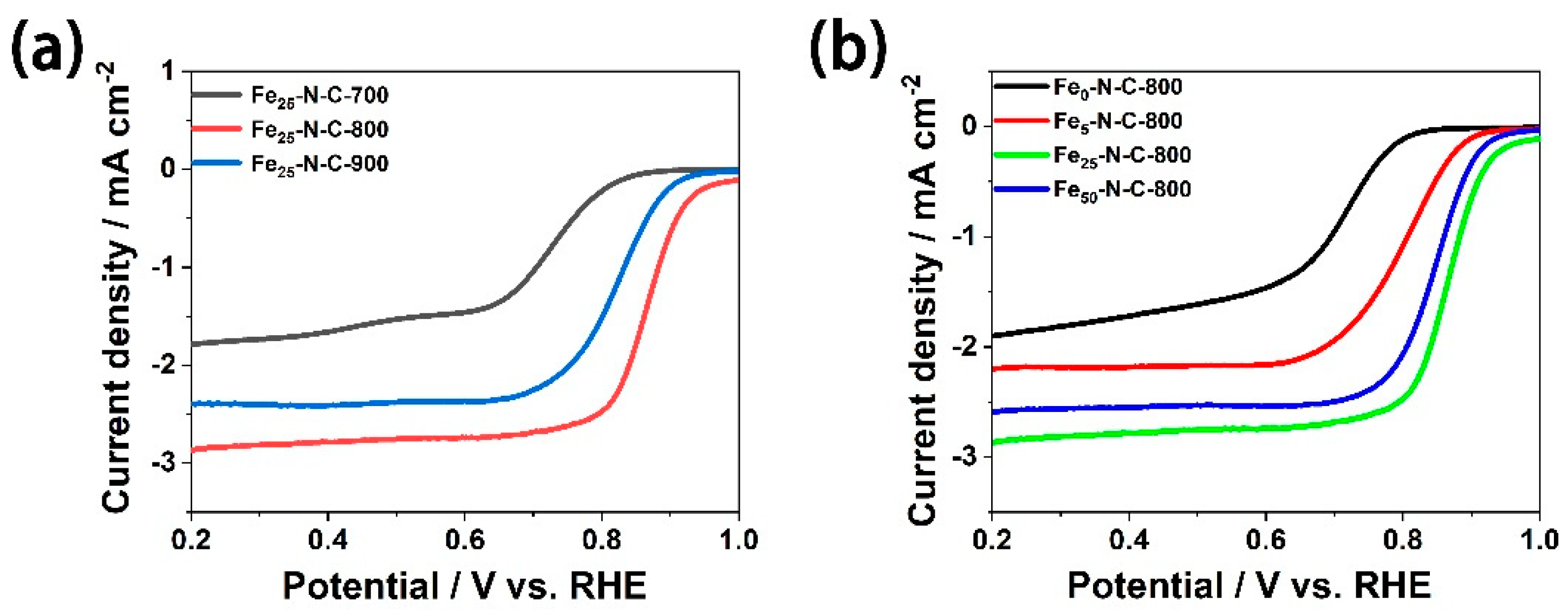
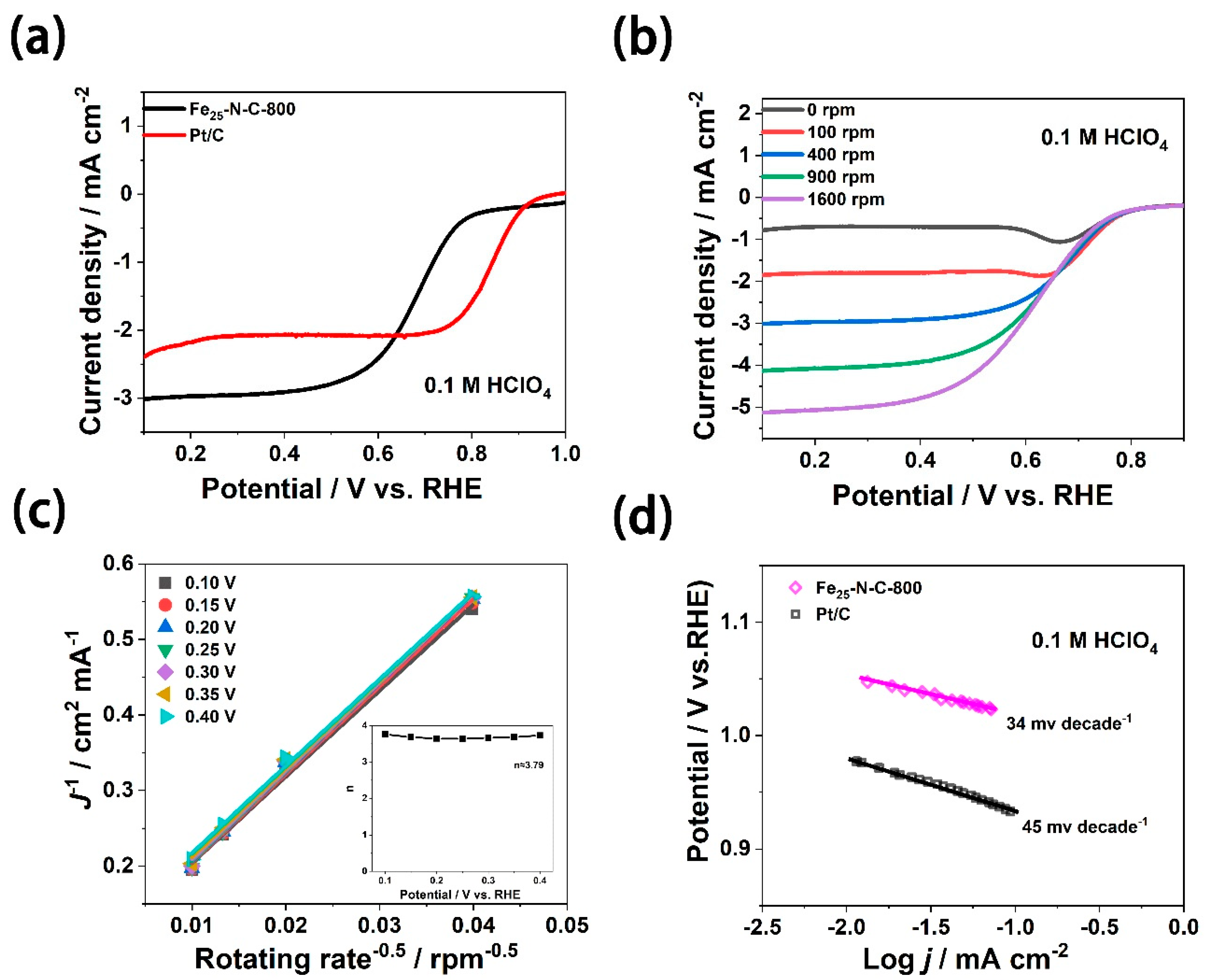
3.2. ORR Catalytic Activity Evaluation
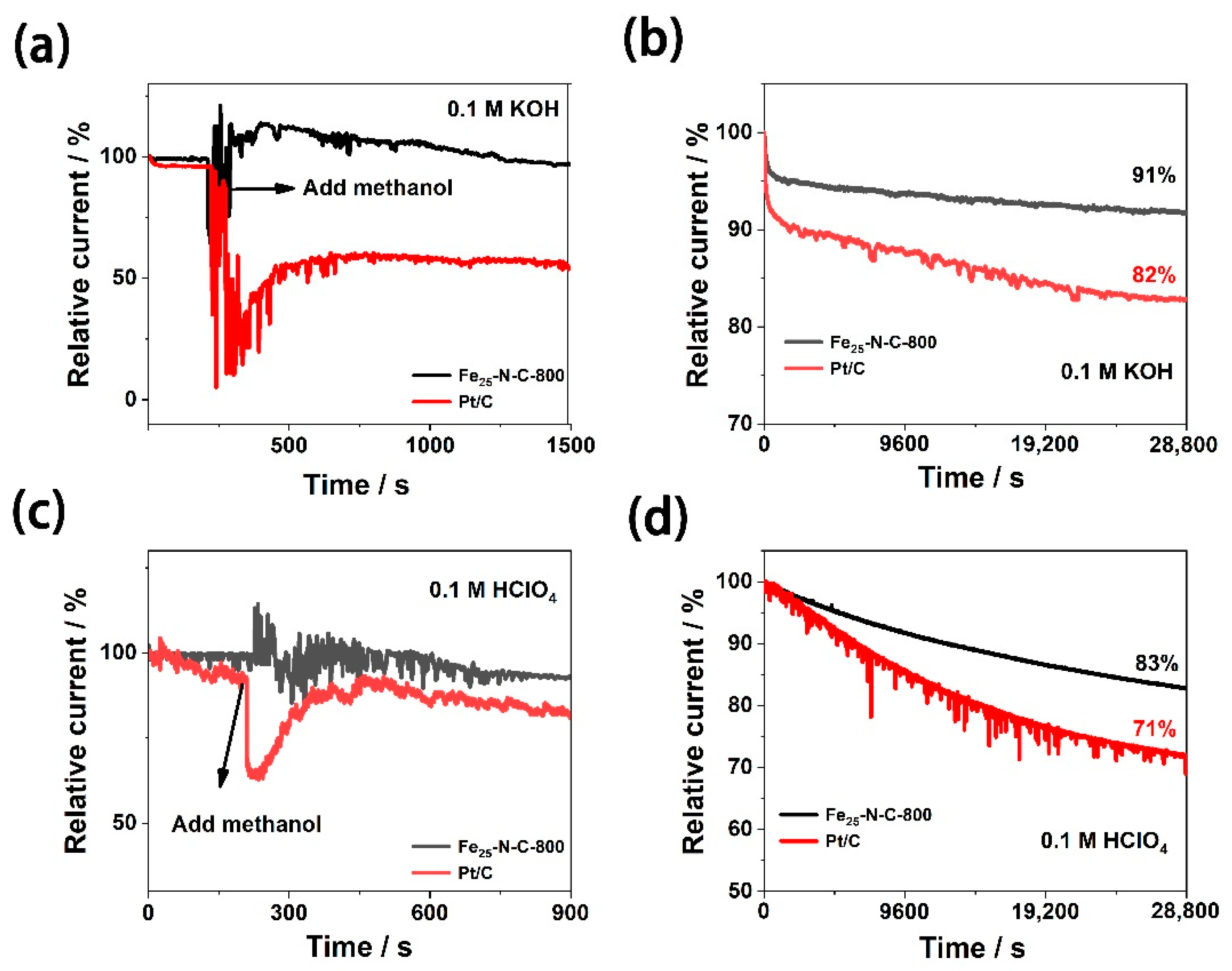
3.3. ORR Durability Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.G.; Lee, J.S.; Liu, M.L. Recent progress in non-precious catalysts for mental-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, H.; Yang, D.; Gao, Y.; Li, H. Using nitrogen-rich polymeric network and iron (II) acetate as precursors to synthesize highly efficient electrocatalyst for oxygen reduction reaction in alkaline media. J. Power Sources 2016, 307, 152–159. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Cui, X.; Yang, S.; Yan, X.; Leng, J.; Shuang, S.; Ajayan, P.M.; Zhang, Z. Pyridinic-nitrogen-dominated graphene aerogels with Fe-N-C coordination for highly efficient oxygen reduction reaction. Adv. Funct. Mater. 2016, 26, 5708–5717. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Accounts Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Bezerra, C.W.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe-N/C and Co-N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-Metal-Free Fe-N/C Catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nat. Cell Biol. 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Bagotzky, V.; Tarasevich, M.; Radyushkina, K.; Levina, O.; Andrusyova, S. Electrocatalysis of the oxygen reduction process on metal chelates in acid electrolyte. J. Power Sources 1978, 2, 233–240. [Google Scholar] [CrossRef]
- Yeager, E. Dioxygen electrocatalysis: Mechanisms in relation to catalyst structure. J. Mol. Catal. 1986, 38, 5–25. [Google Scholar] [CrossRef]
- Wu, G.; Johnston, C.M.; Mack, N.H.; Artyushkova, K.; Ferrandon, M.; Nelson, M.; Lezama-Pacheco, J.S.; Conradson, S.D.; More, K.L.; Myers, D.J.; et al. Synthesis-structure-performance correlation for polyaniline-Me-C non-precious metal cathode catalysts for oxygen reduction in fuel cells. J. Mater. Chem. 2011, 21, 11392–11405. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, B.; Liu, X.; Wang, D.-W.; Su, D.S. Unravelling the Structure of Electrocatalytically Active Fe-N Complexes in Carbon for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herranz, J.; Larouche, N.; Arruda, T.M.; Lefèvre, M.; Jaouen, F.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Mukerjee, S.; et al. Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 2012, 14, 11673–11688. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, S.; Li, X.; Zhang, M. Template free fabrication of porous g-c3n4/graphene hybrid with enhanced photocatalytic capability under visible light. Mater. Technol. 2014, 29, 172–178. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhu, Y. Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir 2013, 29, 10566–10572. [Google Scholar] [CrossRef]
- Yang, M.; Feng, J.; Huang, Q. Influence of mechanical milling on photocatalytic activity of g-C3N4 prepared by heating melamine. J. Wuhan Univ. Technol. Sci. Ed. 2010, 25, 914–918. [Google Scholar] [CrossRef]
- Guan, Y.B.; Zhang, S.L.; Lou, X.W. Realization of walnut-shaped particles with macro-/mesoporous open channels through pore architecture manipulation and their use in electrocatalytic oxygen reduction. Angew. Chem. Int. Ed. Engl. 2018, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, K.; Cheong, W.-C.; Zheng, L.; Zhang, C.; Liu, S.; Cao, X.; Wu, K.; Pan, Y.; Zhuang, Z.; et al. Synergistically interactive pyridinic-N-MoP sites: Identified active centers for enhanced hydrogen evolution in alkaline solution. Angew. Chem. Int. Ed. 2019, 59, 8982–8990. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, C.; Robertson, A.W.; Gao, Y.; Ding, J.; Zhang, Y.; Ma, T.; Sun, Z. N-Doping of graphene oxide at low temperature for the oxygen reduction reaction. Chem. Commun. 2017, 53, 873–876. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Jiang, Y.; Zhou, F.; Wang, G.; Wang, R. Tungsten carbide encapsulated in nitrogen-doped carbon with iron/cobalt carbides electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2016, 389, 157–164. [Google Scholar] [CrossRef]
- Zhang, J.; He, D.; Su, H.; Chen, X.; Pan, M.; Mud, S. Porous polyaniline-derived FeNxC/C catalysts with high activity and stability towards oxygen reduction reaction using ferric chloride both as an oxidant and iron source. J. Mater. Chem. A 2014, 2, 1242–1246. [Google Scholar] [CrossRef]
- Tian, G.-L.; Zhang, Q.; Zhang, B.; Jin, Y.-G.; Huang, J.; Su, D.S.; Wei, F. Toward full exposure of “Active Sites”: Nanocarbon electrocatalyst with surface enriched nitrogen for superior oxygen reduction and evolution reactivity. Adv. Funct. Mater. 2014, 24, 5956–5961. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2019, 12, 250–260. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Song, J.; Shi, Q.; Su, D.; Engelhard, M.H.; Li, X.; Xiao, D.; Li, D.; Estevez, L.; et al. Self-assembled Fe-N-doped carbon nanotube aerogels with single-atom catalyst feature as high-efficiency oxygen reduction electrocatalysts. Small 2017, 13, 1603407. [Google Scholar] [CrossRef] [PubMed]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen reduction fuel cell catalyst derived from carbendazim: Synthesis, structure, and reactivity. Adv. Energy Mater. 2014, 4, 919–926. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Waghmare, U.V.; Hegde, M.S. Origin of activation of lattice oxygen and synergistic interaction in bimetal-ionic Ce0.89Fe0.1Pd0.01O2−δ Catalyst. Chem. Mater. 2009, 21, 4880–4891. [Google Scholar] [CrossRef]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. One-step fabrication of magnetic γ-Fe2O3/polyrhodanine nanoparticles using in situ chemical oxidation polymerization and their antibacterial properties. Catal. Commun. 2010, 46, 6735–6737. [Google Scholar] [CrossRef]
- Maldonado, S.; Stevenson, K.J. Direct preparation of carbon nanofiber electrodes via pyrolysis of iron (II) phthalocyanine: Electrocatalytic aspects for oxygen reduction. J. Phys. Chem. B 2004, 108, 11375–11383. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Li, J.Z.; Zhang, H.G.; Samarakoon, W.; Shan, W.T.; Karakalos, S.; Chen, M.J.; Gu, D.M.; More, K.L.; Wang, G.F.; Feng, Z.X.; et al. Thermally driven structure and performance evolution of atomically dispersed fen4 sites for oxygen reduction. Angew. Chem. Int. Ed. 2019, 58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Xu, X.-X.; Hu, B.-C.; Liang, H.-W.; Lin, Y.; Chen, L.-F.; Yu, S. Iron carbide nanoparticles encapsulated in mesoporous Fe-N-doped carbon nanofibers for efficient electrocatalysis. Angew. Chem. 2015, 127, 8297–8301. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal–nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed]
- Varnell, J.A.; Tse, E.C.M.; Schulz, C.E.; Fister, T.T.; Haasch, R.T.; Timoshenko, J.; Frenkel, A.I.; Gewirth, A.A. Identification of carbon-encapsulated iron nanoparticles as active species in non-precious metal oxygen reduction catalysts. Nat. Commun. 2016, 7, 12582. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.-T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: Linking surface science to coordination chemistry. J. Am. Chem. Soc. 2013, 135, 15443–15449. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, B.; Dai, W.; Ouyang, L.; Ye, J. Carbon network framework derived iron-nitrogen co-doped carbon nanotubes for enhanced oxygen reduction reaction through metal salt-assisted polymer blowing strategy. Appl. Surf. Sci. 2019, 463, 767–774. [Google Scholar] [CrossRef]
- Choi, C.H.; Lim, H.-K.; Chung, M.W.; Chon, G.; Sahraie, N.R.; Altin, A.; Sougrati, M.-T.; Stievano, L.; Oh, H.S.; Park, E.S.; et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 2018, 11, 3176–3182. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W.-H.; Wang, H.-H.; Chen, C.; Zhou, Z.-Y.; Sun, S. Pyrolyzed Fe-N-C Composite as an Efficient Non-precious Metal Catalyst for Oxygen Reduction Reaction in Acidic Medium. ACS Catal. 2014, 4, 3928–3936. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe-N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Byon, H.R.; Suntivich, J.; Crumlin, E.J.; Shao-Horn, Y. Fe-N-modified multi-walled carbon nanotubes for oxygen reduction reaction in acid. Phys. Chem. Chem. Phys. 2011, 13, 21437–21445. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, B.; Lin, C.; Ye, J.; Ouyang, L. Porous carbon supported Fe-N-C composite as an efficient electrocatalyst for oxygen reduction reaction in alkaline and acidic media. Appl. Surf. Sci. 2017, 411, 487–493. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal-organic framework-derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater. 2014, 4, 1220–1225. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, X.; Mao, L.; Liu, F. Fe-N Co-doped porous carbon derived from ionic liquids as an efficient electrocatalyst for the oxygen reduction reaction. Ind. Eng. Chem. Res. 2018, 57, 15638–15646. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem. 2012, 125, 389–393. [Google Scholar] [CrossRef]


| Samples | Weight (g) | Constant Volume (mL) | Fe (wt.%) |
|---|---|---|---|
| Fe5-N-C-800 | 0.0227 | 10 | 1.18 |
| Fe25-N-C-800 | 0.0248 | 10 | 5.35 |
| Fe50-N-C-800 | 0.0311 | 10 | 12.65 |
| Samples | C% | N% | Fe% |
|---|---|---|---|
| Fe25-N-C-700 | 94.36 | 5.22 | 0.42 |
| Fe25-N-C-800 | 94.78 | 4.59 | 0.63 |
| Fe25-N-C-900 | 96.51 | 2.48 | 1.01 |
| Samples | Alkaline Electrolyte | Acidic Electrolyte | Reference | ||
|---|---|---|---|---|---|
| Electron Transfer Number | Relative Current (%)/Test Time | Electron Transfer Number | Relative Current (%)/Test Time | ||
| Fe25-N-C-800 | 3.80–3.93 | 91%/28,800 s | 3.74–3.86 | 83%/28,800 s | this work |
| Fe-N-CNP-CNF | 3.91 | 86.8%/20,000 s | - | - | [51] |
| Fe-N-MWCNTs | - | - | 3.3 | 60%/86,400 s | [52] |
| Fe-N-CNFs | 3.93 | 83.3%/20,000 s | - | - | [42] |
| Fe-N-C | 3.8–3.9 | 90%/25,500 s | - | 73%/25,500 s | [53] |
| N-doped Fe/Fe3C@C/RGO | 3.08–3.52 | 91%/6000 s | - | - | [54] |
| Fe-N/C800 | 3.78–3.89 | 75.64%/7000 s | - | - | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Zhu, B.; Wang, X.; Zhang, L.; Song, D.; Guo, J.; Tao, H. Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media. Materials 2020, 13, 4551. https://doi.org/10.3390/ma13204551
Jin L, Zhu B, Wang X, Zhang L, Song D, Guo J, Tao H. Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media. Materials. 2020; 13(20):4551. https://doi.org/10.3390/ma13204551
Chicago/Turabian StyleJin, Linglei, Baikang Zhu, Xuesong Wang, Le Zhang, Debin Song, Jian Guo, and Hengcong Tao. 2020. "Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media" Materials 13, no. 20: 4551. https://doi.org/10.3390/ma13204551
APA StyleJin, L., Zhu, B., Wang, X., Zhang, L., Song, D., Guo, J., & Tao, H. (2020). Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Efficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media. Materials, 13(20), 4551. https://doi.org/10.3390/ma13204551






