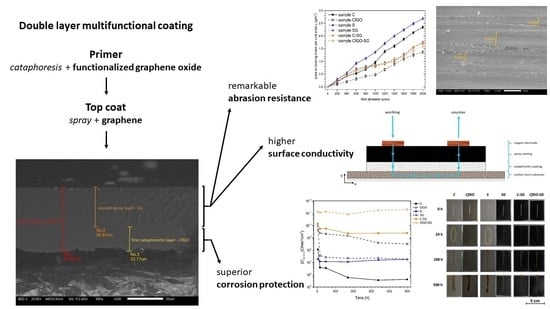
Graphene-Based Reinforcing Filler for Double-Layer Acrylic Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalized Graphene Oxide Filler Preparation
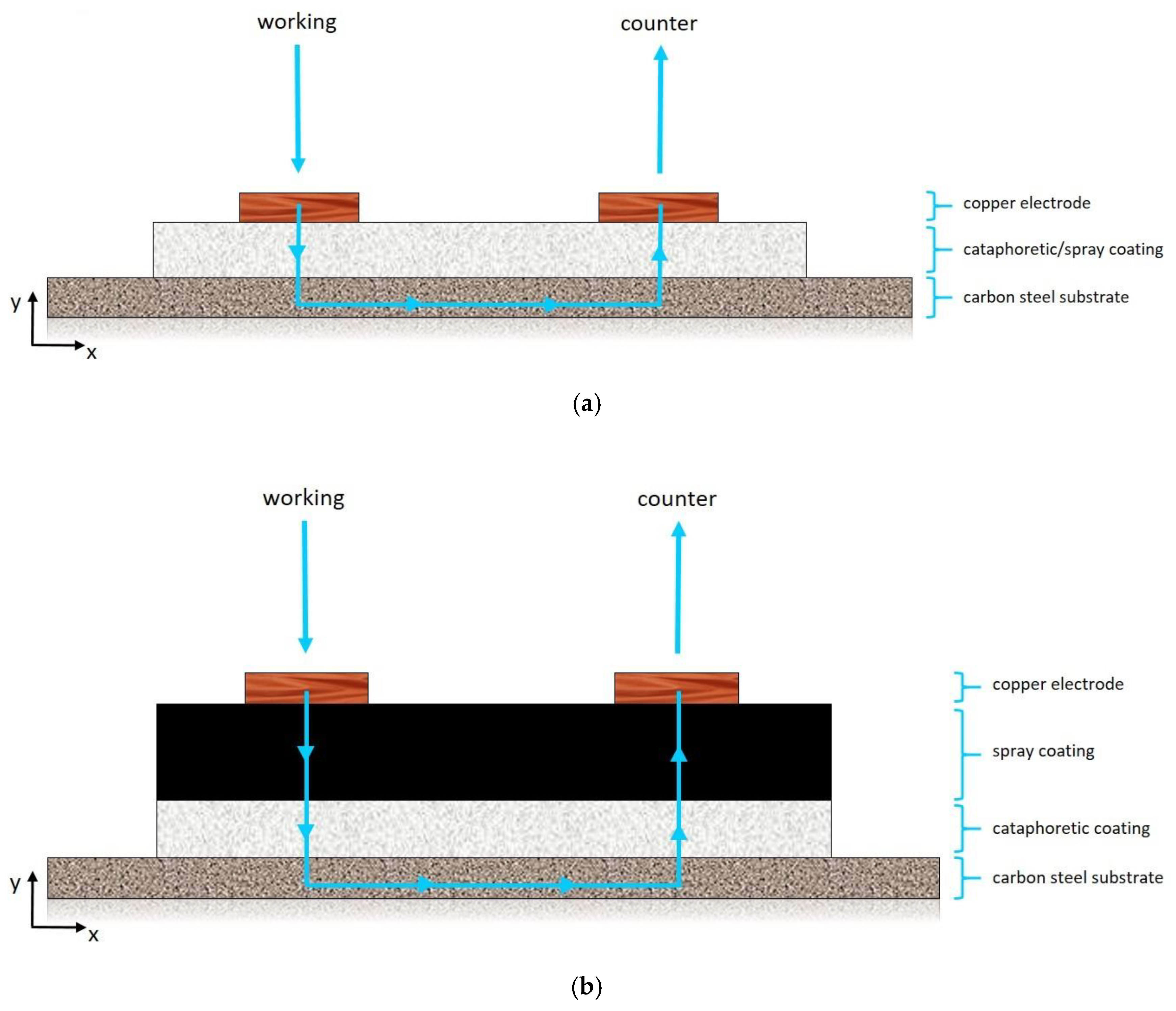
2.3. Coatings Deposition
2.4. Characterization
3. Results
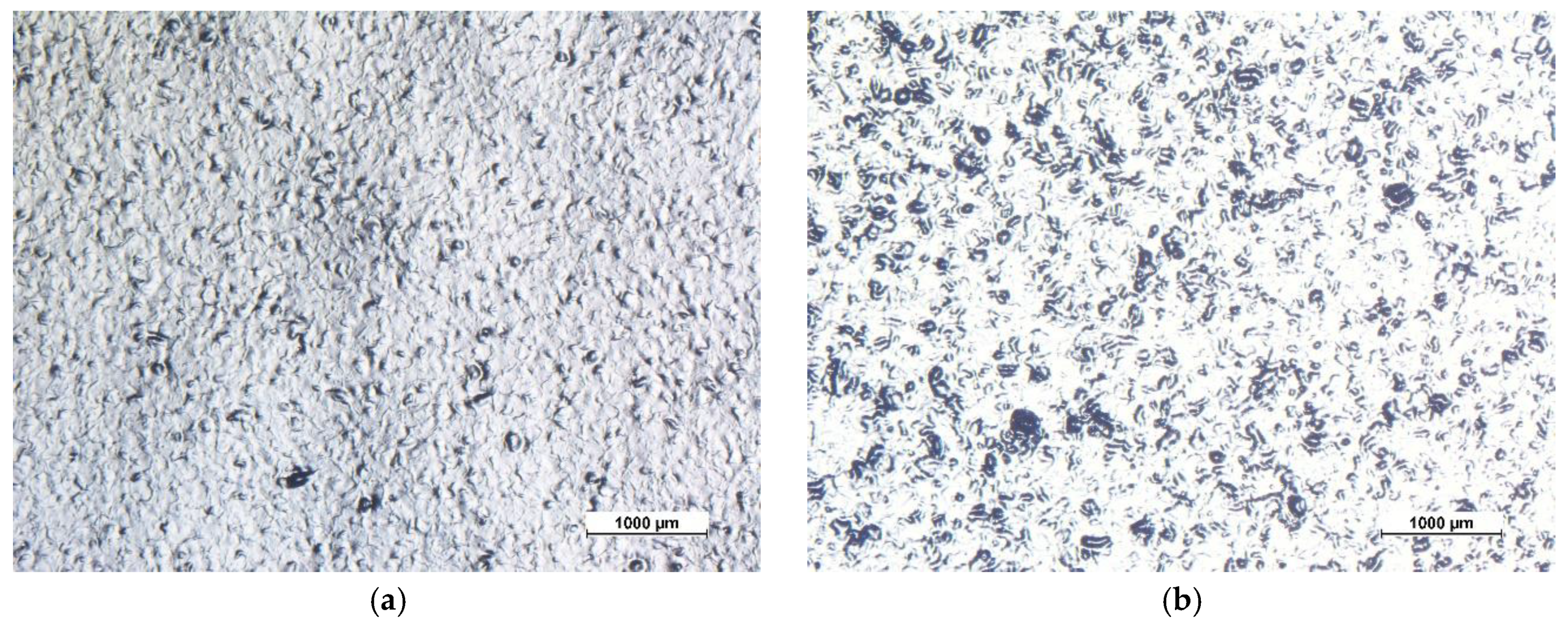
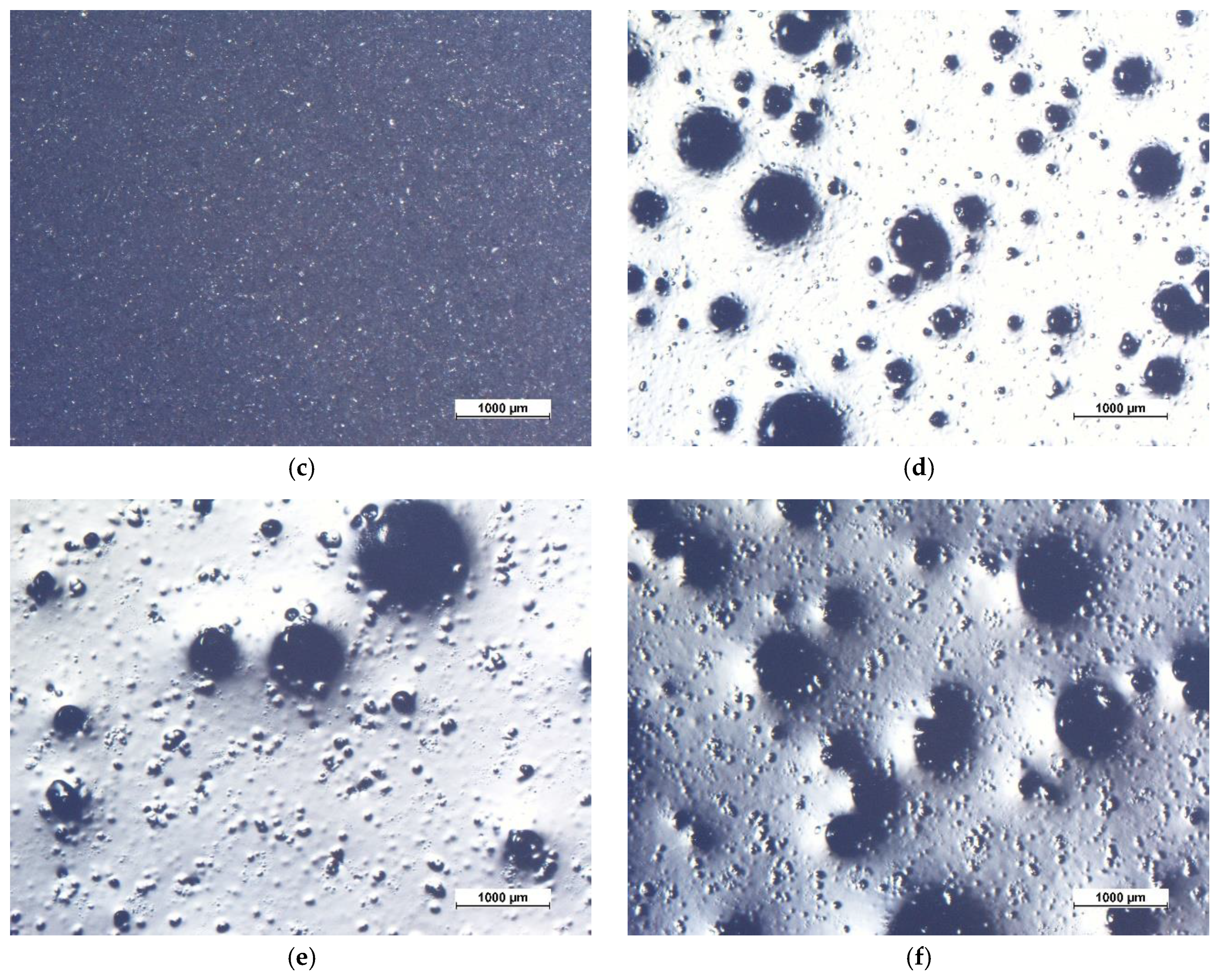
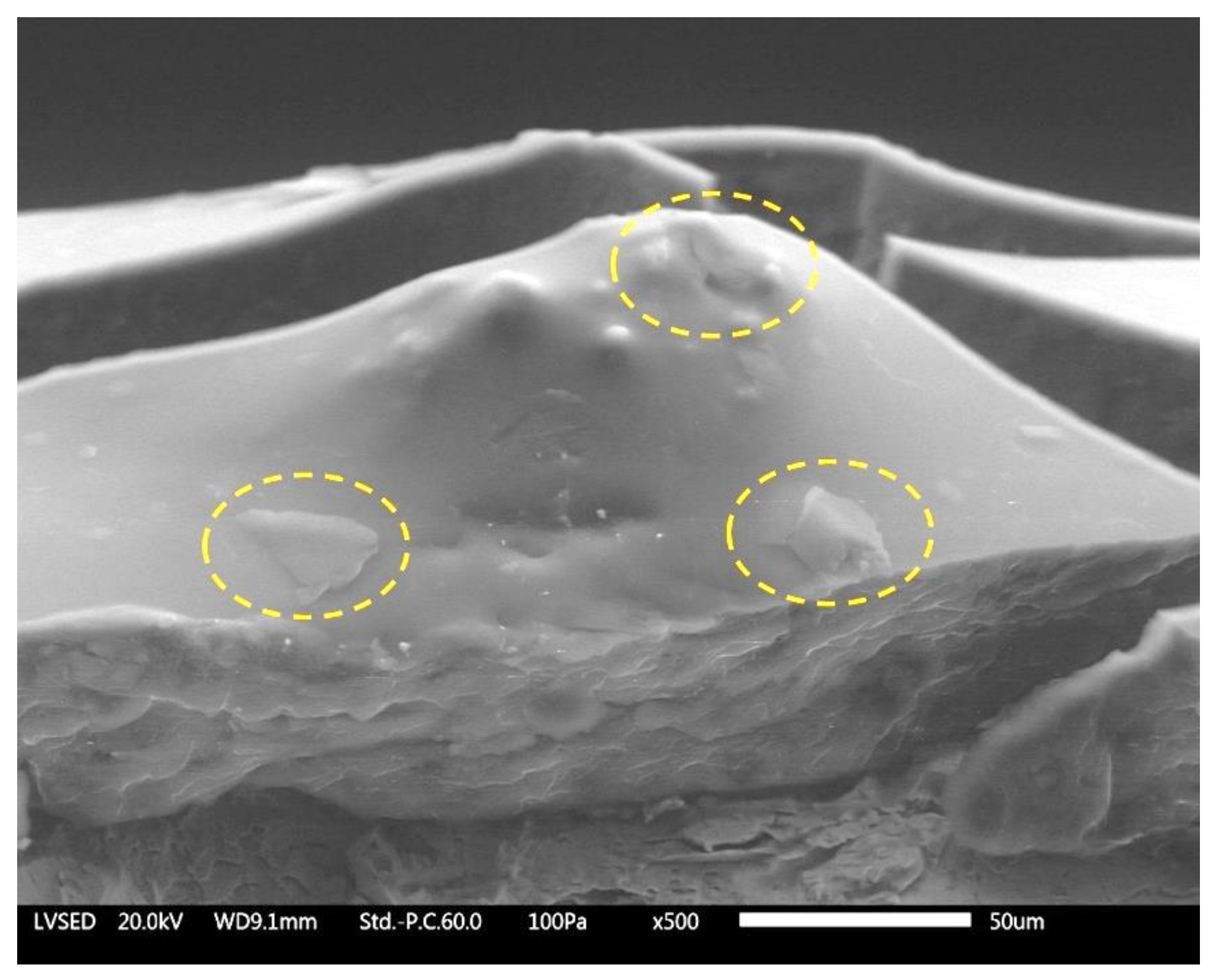
3.1. Coatings Morphology
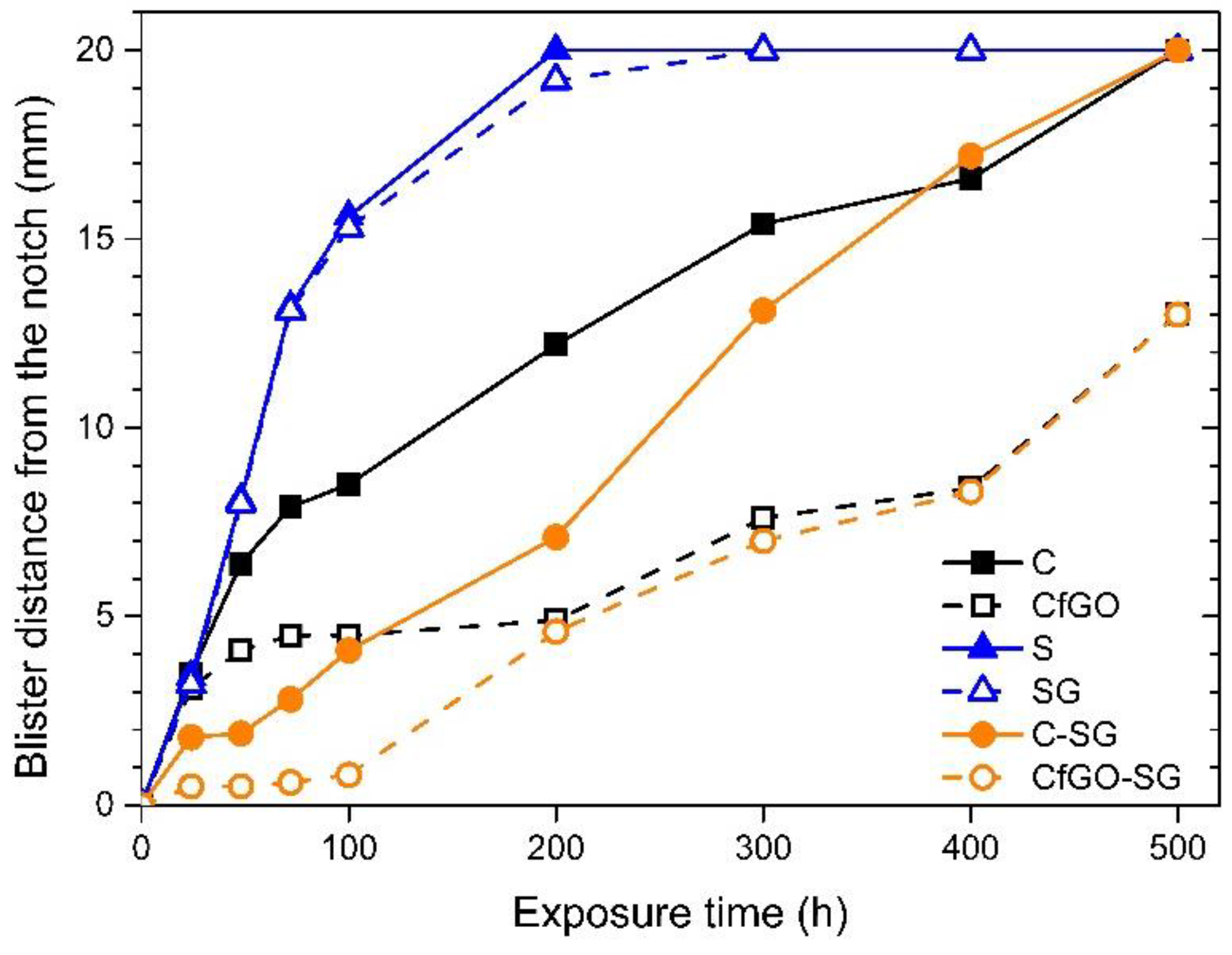
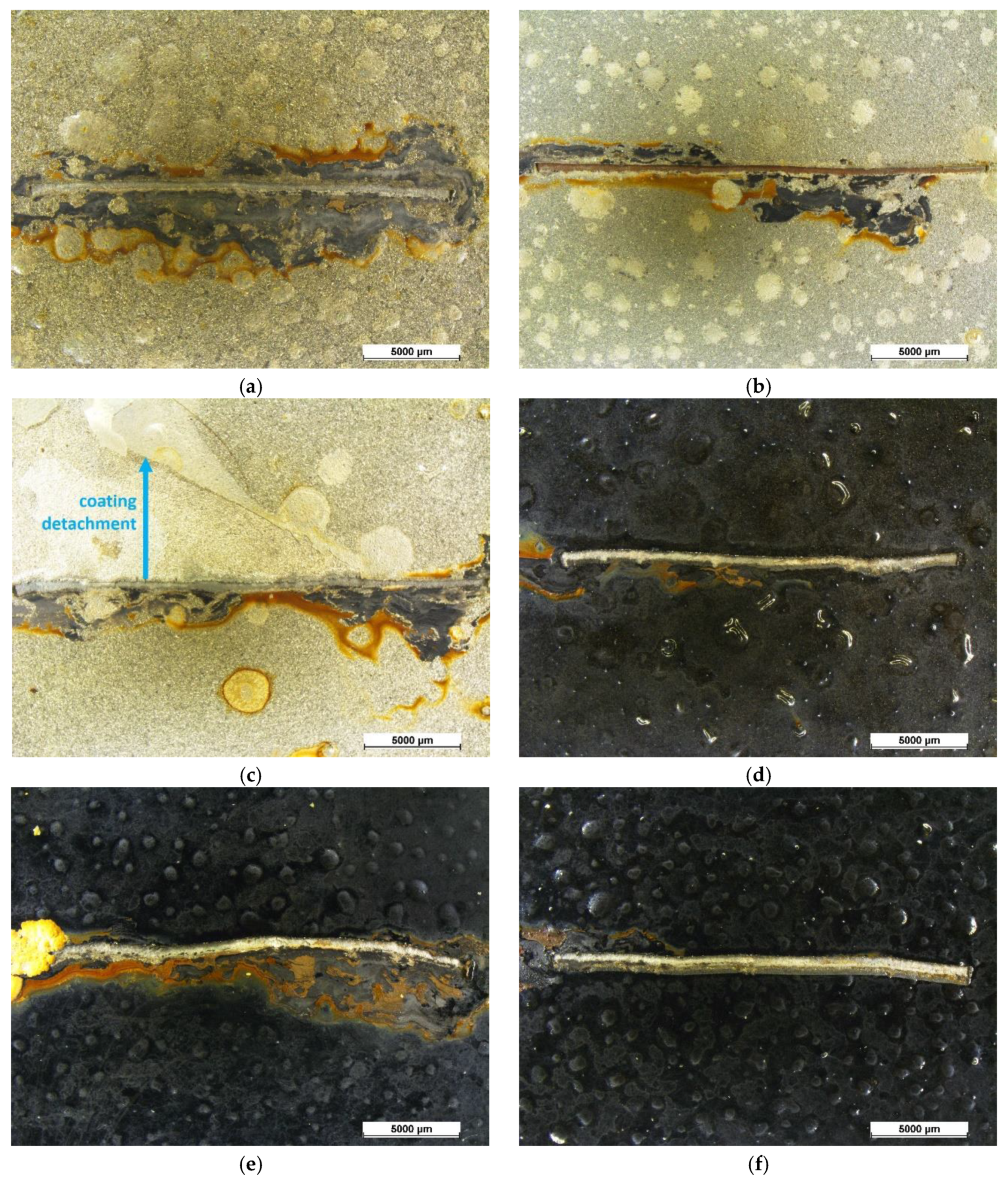
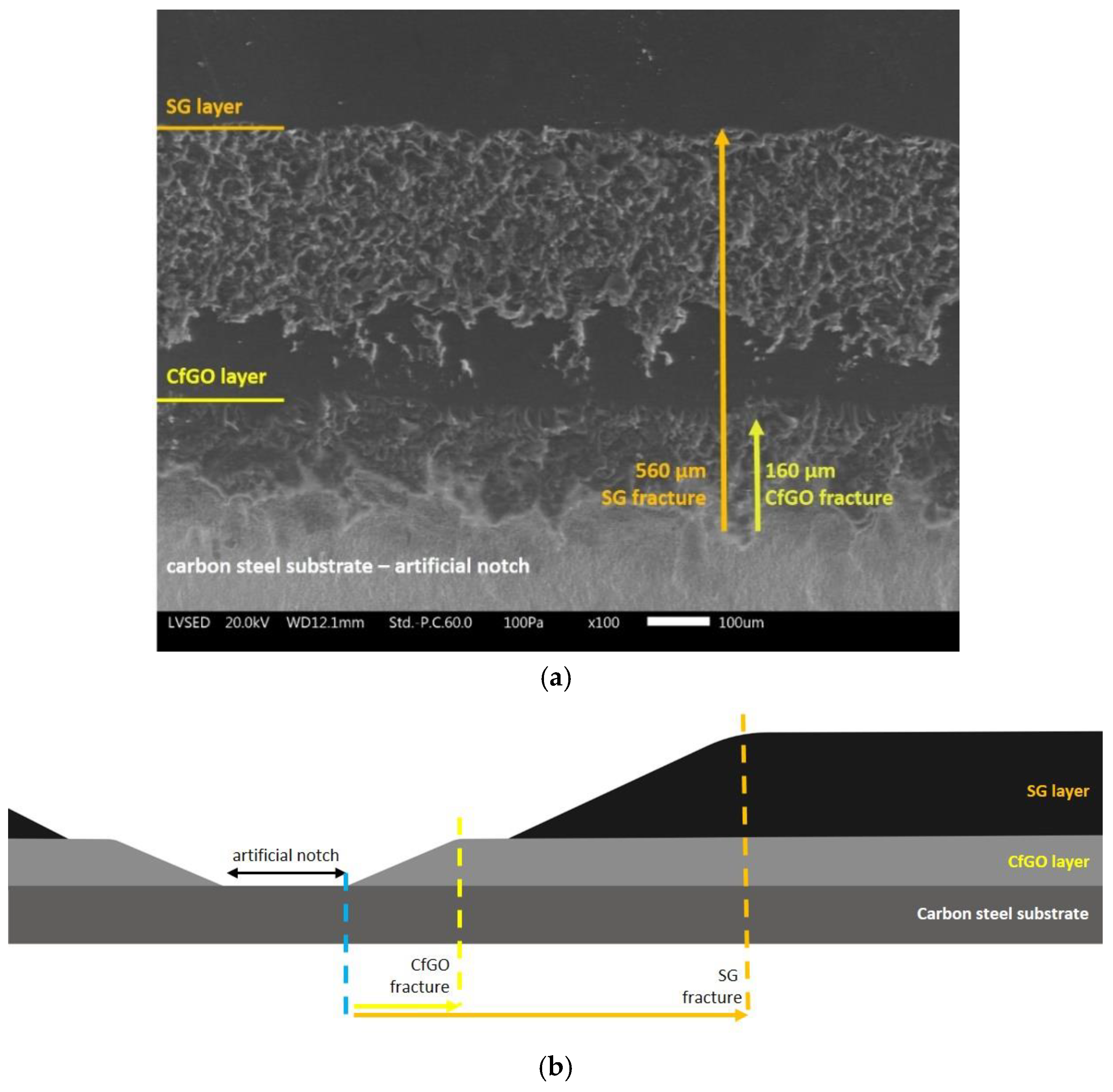
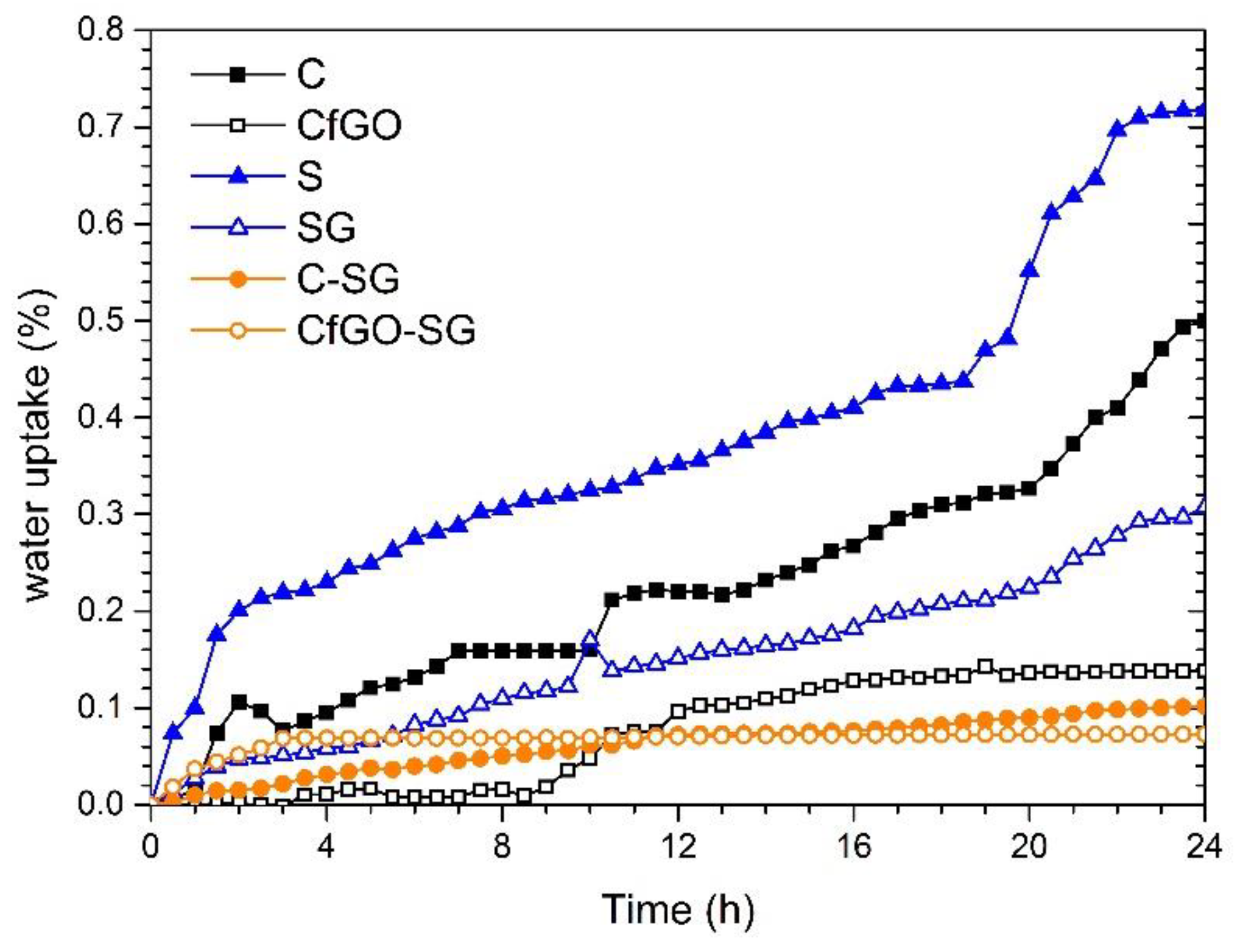
3.2. Exposure in Aggressive Environment
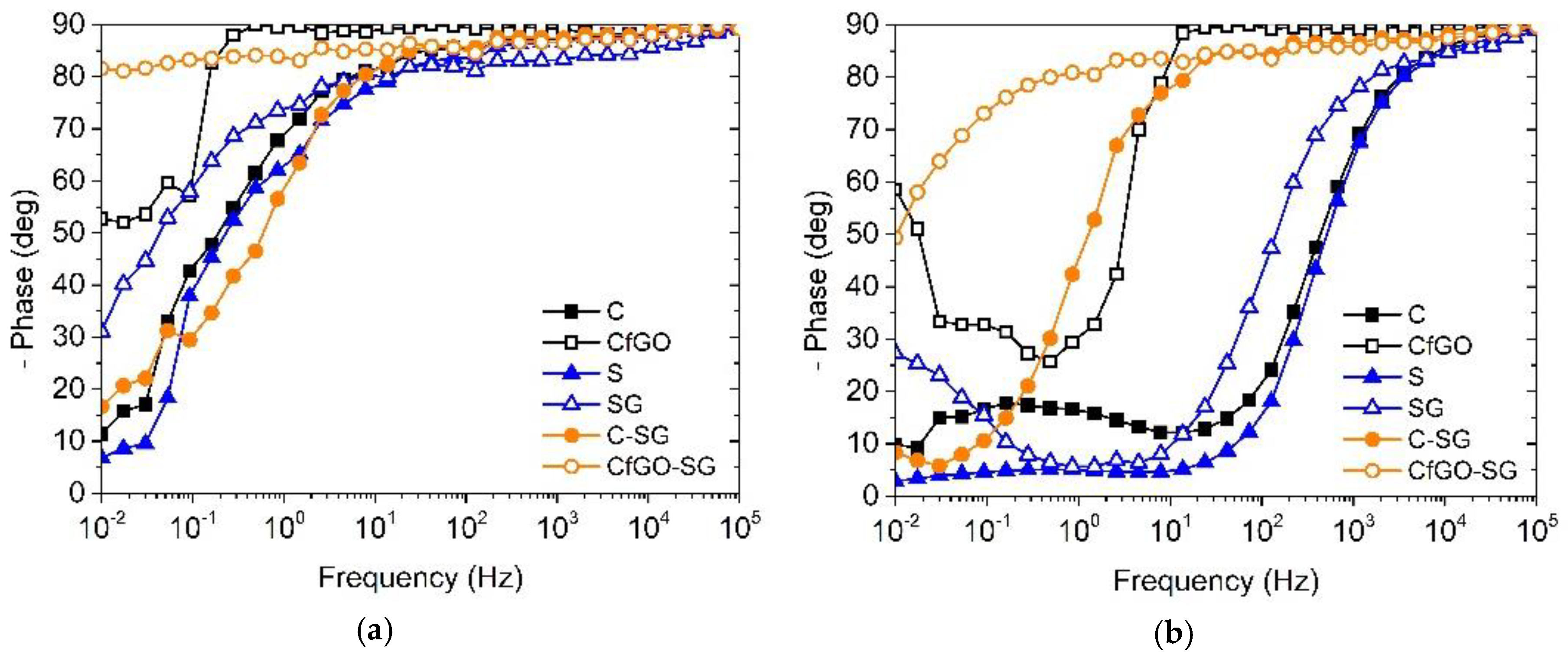
3.3. Electrochemical Impedance Spectroscopy Measurements
3.4. Coatings Conductivity Analysis
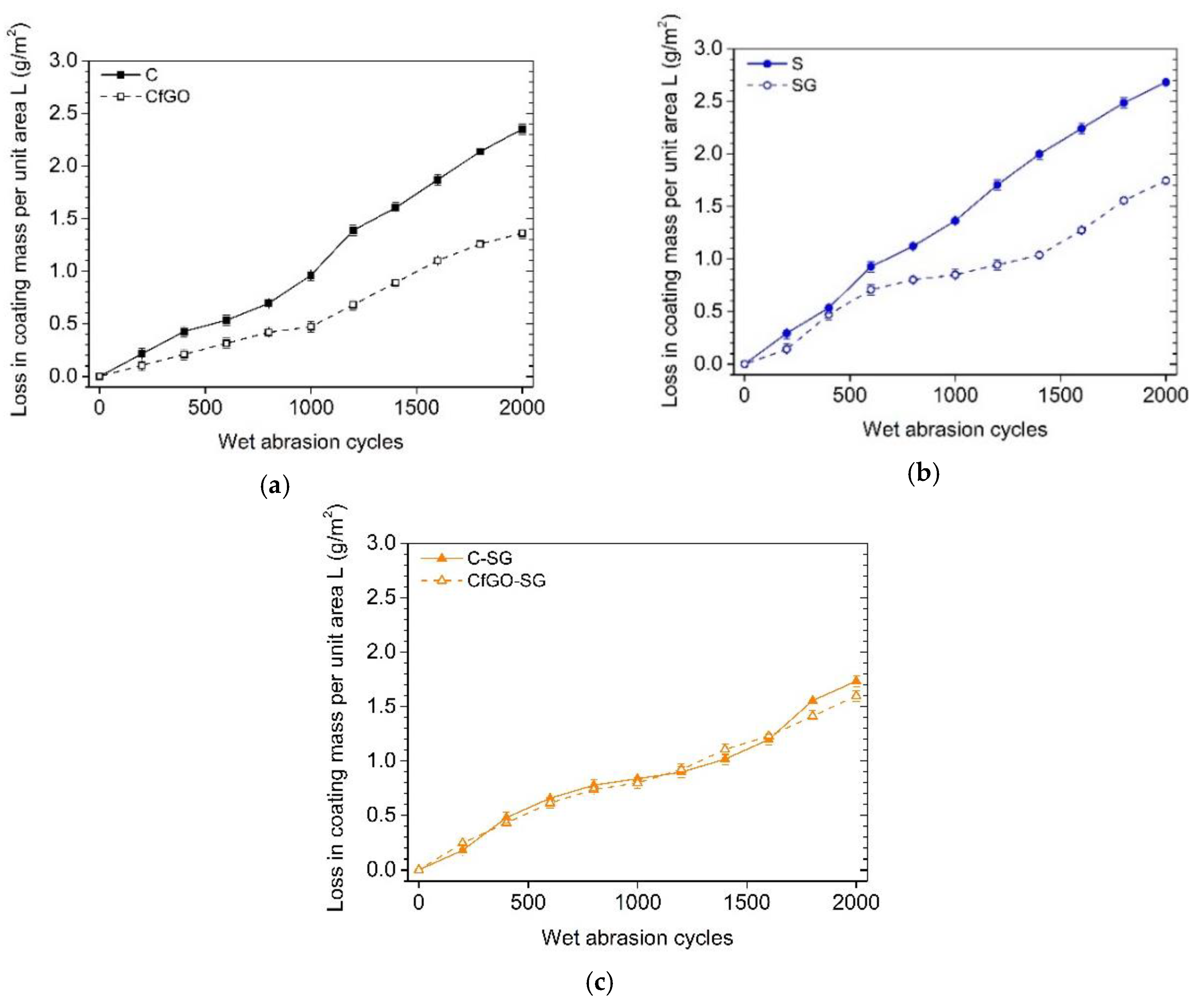
3.5. Scrub Abrasion Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Dubonos, S.V.; Grigorieva, I.V. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Shahil, K.M.F.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Wirth-Lima, A.J.; Silva, M.G.; Sombra, A.S.B. Comparisons of electrical and optical properties between graphene and silicene—A review. Chin. Phys. B 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Bøggild, P.; Mackenzie, D.; Whelan, P.; Petersen, D.; Buron, J.; Zurutuza, A.; Gallop, J.; Hao, L.; Jepsen, P. Mapping the electrical properties of large-area graphene. 2D Mater. 2017, 4, 042003. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Novoselov, K.; Hong, B. Engineering electrical properties of graphene: Chemical approaches. 2D Mater. 2015, 2, 042001. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Singh, S.; Rathi, K.; Pal, K. Synthesis, characterization of graphene oxide wrapped silicon carbide for excellent mechanical and damping performance for aerospace application. J. Alloys Compd. 2018, 740, 436–445. [Google Scholar] [CrossRef]
- Bkakri, R.; Sayari, A.; Shalaan, E.; Wageh, S.; Al-Ghamdi, A.A.; Bouazizi, A. Effects of the graphene doping level on the optical and electrical properties of ITO/P3HT:Graphene/Au organic solar cells. Superlattices Microstruct. 2014, 76, 461–471. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. Fabricating graphene supercapacitors: Highlighting the impact of surfactants and moieties. Chem. Commun. 2012, 48, 1425–1427. [Google Scholar] [CrossRef]
- Kim, R.; Yu, J.; Jin, H. Graphene analogue in (111)-oriented BaBiO3 bilayer heterostructures for topological electronics. Sci. Rep. 2018, 8, 555–562. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites—Processing, properties and applications: A review. Compos. Part B Eng. 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Iskandar, L.; Deb, S. Green synthetic routes to alginate-graphene oxide composite hydrogels with enhanced physical properties for bioengineering applications. Eur. Polym. J. 2018, 103, 198–206. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, M.K.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Cai, D.; Yusoh, K.; Song, M. The mechanical properties and morphology of a graphite oxide nanoplatelet/polyurethane composite. Nanotechnology 2009, 20, 085712. [Google Scholar] [CrossRef]
- Ansari, S.; Giannelis, E.P. Functionalized graphene sheetpoly(inylidene fluoride) conductive nanocomposites. J. Polym. Sci. Pt. B Polym. Phys. 2009, 47, 888–897. [Google Scholar] [CrossRef]
- Yang, Y.G.; Chen, C.M.; Wen, Y.F.; Yang, Q.H.; Wang, M.Z. Oxidized graphene and graphene based polymer composites. New Carbon Mater. 2008, 23, 193–200. [Google Scholar] [CrossRef]
- Chen, F.; Ying, J.; Wang, Y.; Du, S.; Liu, Z.; Huang, Q. Effects of graphene content on the microstructure and properties of copper matrix composites. Carbon 2016, 96, 836–842. [Google Scholar] [CrossRef]
- Tapasztó, O.; Tapasztó, L.; Lemmel, H.; Puchy, V.; Dusza, J.; Balázsi, C.; Balázsi, K. High orientation degree of graphene nanoplatelets in silicon nitride composites prepared by spark plasma sintering. Ceram. Int. 2016, 42, 1002–1006. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Lv, Y.; Yan, J.; Yun, J.; Zhao, W.; Kou, W.L.; Zhai, C. Synthesis and characterization of ZnO NWAs/graphene composites for enhanced optical and field emission performances. Compos. B Eng. 2016, 99, 366–372. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites—A review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.; Lin, X.; Shen, X.; Jia, J.; Sharif, F.; Kim, J.K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A Appl. Sci. Manuf. 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Song, L.; Hu, Y. UV-Curable Functionalized Graphene Oxide/Polyurethane Acrylate Nanocomposite Coatings with Enhanced Thermal Stability and Mechanical Properties. Ind. Eng. Chem. Res. 2012, 51, 14629–14636. [Google Scholar] [CrossRef]
- Shi, Y.; Qian, X.; Zhou, K.; Tang, Q.; Jiang, S.; Wang, B.; Wang, B.; Yu, B.; Hu, Y.; Yuen, R.K.K. CuO/Graphene Nanohybrids: Preparation and Enhancement on Thermal Stability and Smoke Suppression of Polypropylene. Ind. Eng. Chem. Res. 2013, 2, 13654–13660. [Google Scholar] [CrossRef]
- Zhuo, D.; Wang, R.; Wu, L.; Guo, Y.; Ma, L.; Weng, Z.; Qi, J. Flame Retardancy Effects of Graphene Nanoplatelet/Carbon Nanotube Hybrid Membranes on Carbon Fiber Reinforced Epoxy Composites. J. Nanomater. 2013, 2013, 820901. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Lu, H.; Hu, Y. Synergistic Effect of Graphene on Antidripping and Fire Resistance of Intumescent Flame Retardant Poly(butylene succinate) Composites. Ind. Eng. Chem. Res. 2011, 50, 5376–5383. [Google Scholar] [CrossRef]
- Gong, L.; Kinloch, I.A.; Young, R.J.; Riaz, I.; Jalil, R.; Novoselov, K.S. Interfacial stress transfer in a graphene monolayer nanocomposite. Adv. Mater. 2010, 22, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Anandan, S.; Narasinga Rao, T.; Sathish, M.; Rangappa, D.; Honma, I.; Miyauchi, M. Superhydrophilic Graphene-Loaded TiO2 Thin Film for Self-Cleaning Applications. ACS Appl. Mater. Interfaces 2012, 5, 207–212. [Google Scholar] [CrossRef]
- Jin, J.; Wang, X.; Song, M. Graphene-Based Nanostructured Hybrid Materials for Conductive and Superhydrophobic Functional Coatings. J. Nanosci. Nanotechnol. 2011, 11, 7715–7722. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Mohamadzadeh Moghadam, M.H. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Chang, K.C.; Hsu, M.H.; Lu, H.I.; Lai, M.C.; Liu, P.J.; Hsu, C.H.; Ji, W.F.; Chuang, T.L.; Wei, Y.; Yeh, J.M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Mohamadzadeh Moghadam, M.H. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Ray, S.C. Application and Uses of Graphene, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kamal, M.R.; Uribe-Calderon, J. Nanoparticles and Polymer Nanocomposites. In Graphite, Graphene, and Their Polymer Nanocomposites, 1st ed.; CRC Press: London, UK, 2013; pp. 353–392. [Google Scholar]
- Manias, E. Polymer Nanocomposite Technology, Fundamentals of Barrier. J. Nat. Mater. 2007, 6, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.D.; Ren, P.G.; Chen, J.; Zhang, W.Q.; Ji, X.; Li, Z.M. High barrier graphene oxide nanosheet/poly(vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409–410, 156–163. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon 2007, 45, 1446–1452. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R. Effect of functionalized graphene oxide concentration on the corrosion resistance properties provided by cataphoretic acrylic coatings. Mat. Chem. Phys. 2020, 239, 121984–121996. [Google Scholar] [CrossRef]
- Yousefi, N.; Lin, X.; Zheng, Q.; Shen, X.; Pothnis, J.; Jia, J.; Zussman, E.; Kim, J.K. Simultaneous in situ reduction, self-alignment and covalent bonding in graphene oxide/epoxy composites. Carbon 2013, 59, 406–417. [Google Scholar] [CrossRef]
- Lin, X.; Shen, X.; Zheng, Q.; Yousefi, N.; Ye, L.; Mai, Y.W.; Kim, J.K. Fabrication of highly-aligned, conductive, and strong graphene papers using ultralarge graphene oxide sheets. ACS Nano 2012, 6, 10708–10719. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Xu, J.Z.; Xu, L.; Zhong, G.; Hsiao, B.S.; Li, Z.M. Improved barrier properties of poly(lactic acid) with randomly dispersed graphene oxide nanosheets. J. Membr. Sci. 2014, 464, 110–118. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N.; Huang, Y.L.; Tien, H.W.; Wang, J.Y.; Ma, C.C.M.; Li, S.M.; Wang, Y.S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Zhang, P.; Song, L.; Yang, H.; Hu, Y. Covalent functionalization of graphene with organosilane and its use as a reinforcement in epoxy composites. Compos. Sci. Technol. 2012, 72, 737–743. [Google Scholar] [CrossRef]
- Jing, Q.; Liu, W.; Pan, Y.; Silberschmidt, V.V.; Li, L.; Dong, Z.L. Chemical functionalization of graphene oxide for improving mechanical and thermal properties of polyurethane composites. Mater. Des. 2015, 85, 808–814. [Google Scholar] [CrossRef]
- Rossi, S.; Calovi, M. Addition of graphene oxide plates in cataphoretic deposited organic coatings. Prog. Org. Coat. 2018, 424, 40–47. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Vaezi, M.R.; Bagherzadeh, M.R. Excellent corrosion protection performance of epoxy composite coatings filled with aminosilane functionalized graphene oxide. Surf. Coat. Technol. 2017, 317, 1–9. [Google Scholar] [CrossRef]
- Mo, M.; Zhao, W.; Chen, Z.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. Excellent tribological and anti-corrosion performance of polyurethane composite coatings reinforced with functionalized graphene and graphene oxide nanosheets. RSC Adv. 2015, 5, 56486–56497. [Google Scholar] [CrossRef]
- Calovi, M.; Callone, E.; Ceccato, R.; Deflorian, F.; Rossi, S.; Dirè, S. Effect of the organic functional group on the grafting ability of trialkoxysilanes onto graphene oxide: A combined NMR, XRD and ESR study. Materials 2019, 12, 3828. [Google Scholar] [CrossRef] [PubMed]
- Calovi, M.; Dirè, S.; Ceccato, R.; Deflorian, F.; Rossi, S. Corrosion protection properties of functionalized graphene—Acrylate coatings produced by cataphoretic deposition. Prog. Org. Coat. 2019, 136, 105261. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R.; Guo, X.; Frankel, G.S. Effects of Graphene-Based Fillers on Cathodic Delamination and Abrasion Resistance of Cataphoretic Organic Coatings. Coatings 2020, 10, 602. [Google Scholar] [CrossRef]
- Ramdé, T.; Ecco, L.G.; Rossi, S. Visual appearance durability as function of natural and accelerated ageing of electrophoretic styrene-acrylic coatings: Influence of yellow pigment concentration. Prog. Org. Coat. 2017, 103, 23–32. [Google Scholar] [CrossRef]
- Almeida, E.; Alves, I.; Brites, C.; Fedrizzi, L. Cataphoretic and autophoretic automotive primers: A comparative study. Prog. Org. Coat. 2003, 46, 8–20. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Prosseda, S. Improvement of corrosion protection system for aluminium body bus used in public transportation. Mater. Des. 2006, 27, 758–769. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Rodriguez, F.J.; Rossi, S.; Deflorian, F. Corrosion study of industrial painting cycles for garden furniture. Prog. Org. Coat. 2003, 46, 62–73. [Google Scholar] [CrossRef]
- ASTM B117. Operating Salt Spray (Fog) Apparatus; ASTM: West Conshohocken, PA, USA, 2011; pp. 1–12. [Google Scholar] [CrossRef]
- ISO 4628. Evaluation of Degradation of Coatings; ISO: Geneva, Switzerland, 2012; pp. 1–11. [Google Scholar]
- ASTM D 3359-17. Standard Test Methods for Rating Adhesion by Tape Test; ASTM: West Conshohocken, PA, USA, 2017; pp. 1–10. [Google Scholar]
- ASTM D 257-07. Standard Test Methods for DC Resistance or Conductance of Insulating Materials; ASTM: West Conshohocken, PA, USA, 2007; pp. 1–18. [Google Scholar]
- BS EN ISO 11998:2006. Paints and Varnishes—Determination of Wet-Scrub Resistance and Cleanability of Coatings; BSI British Standards: London, UK, 2006; pp. 1–11. [Google Scholar]
- Bonora, P.L.; Deflorian, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S. An EIS study of ion diffusion through organic coatings. Electrochim. Acta 2006, 51, 1736–1744. [Google Scholar] [CrossRef]
- Rossi, S.; Deflorian, F.; Fontanari, L.; Cambruzzi, A.; Bonora, P.L. Electrochemical measurements to evaluate the damage due to abrasion on organic protective system. Prog. Org. Coat. 2005, 52, 288–297. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.R.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Vosgien Lacombre, C.; Bouvet, G.; Trinh, D.; Mallarino, S.; Touzain, S. Water uptake in free films and coatings using the Brasher and Kingsbury equation: A possible explanation of the different values obtained by electrochemical Impedance spectroscopy and gravimetry. Electrochim. Acta 2017, 231, 162–170. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Lopez, A.B.; De la Cal, J.C.; Asua, J.M. From fractal polymer dispersions to mechanically resistant waterborne superhydrophobic coatings. Polymer 2017, 124, 12–19. [Google Scholar] [CrossRef]
- Kaew-on, N.; Katemake, P.; Prasongsuk, S. Primer formulations with antibacterial properties for murals. Prog. Org. Coat. 2020, 138, 105395. [Google Scholar] [CrossRef]
- Kok, K.; Young, T.M. Evaluation of insect residue resistant coatings—Correlation of a screening method with a conventional assessment technique. Prog. Org. Coat. 2014, 77, 1382–1390. [Google Scholar] [CrossRef]
- Féat, A.; Federle, W.; Kamperman, M.; Murray, M.; Van der Gucht, J.; Taylor, P. Slippery paints: Eco-friendly coatings that cause ants to slip. Prog. Org. Coat. 2019, 135, 331–344. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Reggiani Silva, C.; Muller Guerrini, L. Effect of itaconic acid on the wet scrub resistance of highly pigmented paints for architectural coatings. J. Coat. Technol. Res. 2011, 8, 439–447. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, P.; Li, J.; Xu, H.; Wang, Q.; Zhang, X.; Zheng, L.; Lu, Y.; Dai, N.; Song, W. Sol-gel derived near-UV and visible antireflection coatings from hybridized hollow silica nanospheres. J. Sol Gel Sci. Technol. 2014, 71, 267–275. [Google Scholar] [CrossRef]
- Khanjani, J.; Hanifpour, A.; Pazokifard, S.; Zohuriaan-Mehr, M.J. Waterborne acrylic-styrene/PDMS coatings formulated by different particle sizes of PDMS emulsions for outdoor applications. Prog. Org. Coat. 2020, 141, 105267. [Google Scholar] [CrossRef]







| Sample | Number of Layers | Deposition Technique | Filler Addition |
|---|---|---|---|
| C | 1 | Cataphoresis | none |
| CfGO | 1 | Cataphoresis | fGO—0.2 wt % |
| S | 1 | Spray | none |
| SG | 1 | Spray | G—1 wt % |
| C-SG | 2 | Cataphoresis | none |
| Spray | G—1 wt % | ||
| CfGO-SG | 2 | Cataphoresis | fGO—0.2 wt % |
| Spray | G—1 wt % |
| Sample | First Layer [µm] | Second Layer [µm] | Total Coating [µm] |
|---|---|---|---|
| C | 29.0 ± 0.8 | / | 29.0 ± 0.8 |
| CfGO | 28.6 ± 1.2 | / | 28.6 ± 1.2 |
| S | 55.0 ± 5.9 | / | 55.0 ± 5.9 |
| SG | 55.6 ± 5.2 | / | 55.6 ± 5.2 |
| C-SG | 30.6 ± 1.2 | ≈60 | 92.4 ± 2.8 |
| CfGO-SG | 30.9 ± 1.2 | ≈60 | 93.0 ± 3.2 |
| Time [h] | Pore Resistance Rpore [Ω*cm2] | |||||
|---|---|---|---|---|---|---|
| C | CfGO | S | SG | C-SG | CfGO-SG | |
| 3 | 6.85 × 105 | 1.53 × 108 | 2.36 × 106 | 1.11 × 1010 | 7.15 × 109 | 2.59 × 1011 |
| 15 | 2.81 × 105 | 1.72 × 108 | 2.95 × 106 | 8.43 × 106 | 5.96 × 109 | 2.02 × 1011 |
| 48 | 3.45 × 105 | 4.21 × 108 | 3.26 × 106 | 1.19 × 107 | 2.14 × 109 | 2.34 × 1011 |
| 170 | 5.29 × 104 | 4.38 × 108 | 3.43 × 106 | 1.14 × 107 | 1.57 × 109 | 2.54 × 1011 |
| 340 | 2.25 × 104 | 1.50 × 108 | 7.50 × 106 | 1.38 × 107 | 1.28 × 109 | 2.54 × 1011 |
| 500 | 2.45 × 104 | 1.36 × 108 | 6.84 × 106 | 1.04 × 107 | 1.87 × 109 | 3.74 × 1011 |
| Time [h] | Coating Capacitance C [F/cm2] | |||||
|---|---|---|---|---|---|---|
| C | CfGO | S | SG | C-SG | CfGO-SG | |
| 0 | 1.69 × 10−10 | 1.86 × 10−10 | 1.38 × 10−10 | 2.86 × 10−10 | 5.88 × 10−11 | 5.93 × 10−11 |
| 4 | 2.57 × 10−10 | 1.95 × 10−10 | 3.78 × 10−10 | 3.70 × 10−10 | 6.74 × 10−11 | 8.03 × 10−11 |
| 8 | 3.40 × 10−10 | 1.99 × 10−10 | 5.28 × 10−10 | 4.62 × 10−10 | 7.32 × 10−11 | 8.03 × 10−11 |
| 12 | 4.43 × 10−10 | 2.83 × 10−10 | 6.46 × 10−10 | 5.55 × 10−10 | 8.08 × 10−11 | 8.08 × 10−11 |
| 16 | 5.45 × 10−10 | 3.26 × 10−10 | 8.35 × 10−10 | 6.35 × 10−10 | 8.22 × 10−11 | 8.13 × 10−11 |
| 20 | 7.07 × 10−10 | 3.38 × 10−10 | 1.55 × 10−9 | 7.65 × 10−10 | 8.71 × 10−11 | 8.15 × 10−11 |
| 24 | 1.50 × 10−9 | 3.40 × 10−10 | 3.20 × 10−9 | 1.10 × 10−9 | 9.17 × 10−11 | 8.17 × 10−11 |
| Sample | Voltage Applied V | Current Measured I | Volume Resistance R | Volume Resistivity ρ | Normalized Volume Resistivity |
|---|---|---|---|---|---|
| [V] | [A] | [Ω] | [Ω*cm] | / | |
| C | 10 | 4.40 × 10−10 | 2.27 × 1010 | 1.71 × 1012 | 1.00 |
| CfGO | 10 | 7.20 × 10−11 | 1.39 × 1011 | 9.51 × 1012 | 5.56 |
| S | 10 | 4.30 × 10−11 | 2.33 × 1011 | 2.36 × 1013 | 13.79 |
| SG | 10 | 3.70 × 10−5 | 2.70 × 105 | 2.55 × 107 | 1.50 × 10−5 |
| C-SG | 10 | 1.80 × 10−10 | 5.56 × 1010 | 1.75 × 1012 | 1.03 |
| CfGO-SG | 10 | 3.45 × 10−11 | 2.90 × 1011 | 9.02 × 1012 | 5.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R. Graphene-Based Reinforcing Filler for Double-Layer Acrylic Coatings. Materials 2020, 13, 4499. https://doi.org/10.3390/ma13204499
Calovi M, Rossi S, Deflorian F, Dirè S, Ceccato R. Graphene-Based Reinforcing Filler for Double-Layer Acrylic Coatings. Materials. 2020; 13(20):4499. https://doi.org/10.3390/ma13204499
Chicago/Turabian StyleCalovi, Massimo, Stefano Rossi, Flavio Deflorian, Sandra Dirè, and Riccardo Ceccato. 2020. "Graphene-Based Reinforcing Filler for Double-Layer Acrylic Coatings" Materials 13, no. 20: 4499. https://doi.org/10.3390/ma13204499
APA StyleCalovi, M., Rossi, S., Deflorian, F., Dirè, S., & Ceccato, R. (2020). Graphene-Based Reinforcing Filler for Double-Layer Acrylic Coatings. Materials, 13(20), 4499. https://doi.org/10.3390/ma13204499