Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. DSC Measurement Methodology
2.3.1. Preliminary Steps
- The DSC was turned on approximately an hour before the measurement to allow the furnace to reach thermal equilibrium.
- Purge gas flow has been set to 20 mL min−1, and protective gas always 10 mL min−1 higher to avoid the entrance of sample vapors in the inner parts of the DSC. Nitrogen (N2) has been used.
- The DSC has been checked regularly, at least once per month, and a calibration using Indium (In) prior every series of measurements was performed.
- Gloves and tweezers have been used for sample handling to avoid any contamination of the sample and the furnace.
2.3.2. Sample Preparation
2.3.3. Temperature Program
- Tup = Tm + 30 K
- Tlow = Tm − 20 K
2.3.4. Measurement
2.3.5. Data analysis
2.4. TGA Measurement Methodology
2.4.1. Preliminary Steps
2.4.2. Blank Measurement
2.4.3. Sample Preparation
- The material of the crucible has to be compatible with the substances measured. Alumina crucibles have been used for all measurements.
- The sample should be representative of the bulk material, so it was stirred or mixed properly before extraction of the sample.
- A syringe or a disposable pipette was used when handling liquid samples. Solid samples were ground with a mortar, generating a powder as fine as possible. A micro spatula was then used to pour the sample into the crucible. The bottom of the crucibles should be fully covered. If grounding the PCM was not possible, samples with the same shape and size have been prepared to ensure repeatability.
- The amount of material poured on each crucible depends on the nature of the PCM: 5 to 10 mg for organic PCM and 10 to 30 mg for inorganic PCM, accounting for the general difference in density of these material categories, thus ensuring the coverage of the crucible bottom.
- The sample mass has been measured using a scale with a maximum tolerance of ± 0.001 mg. This mass has also been checked by the balance of the TGA itself.
- Caution was required to not alter the samples thermally before measurement, in order to avoid untrue results.
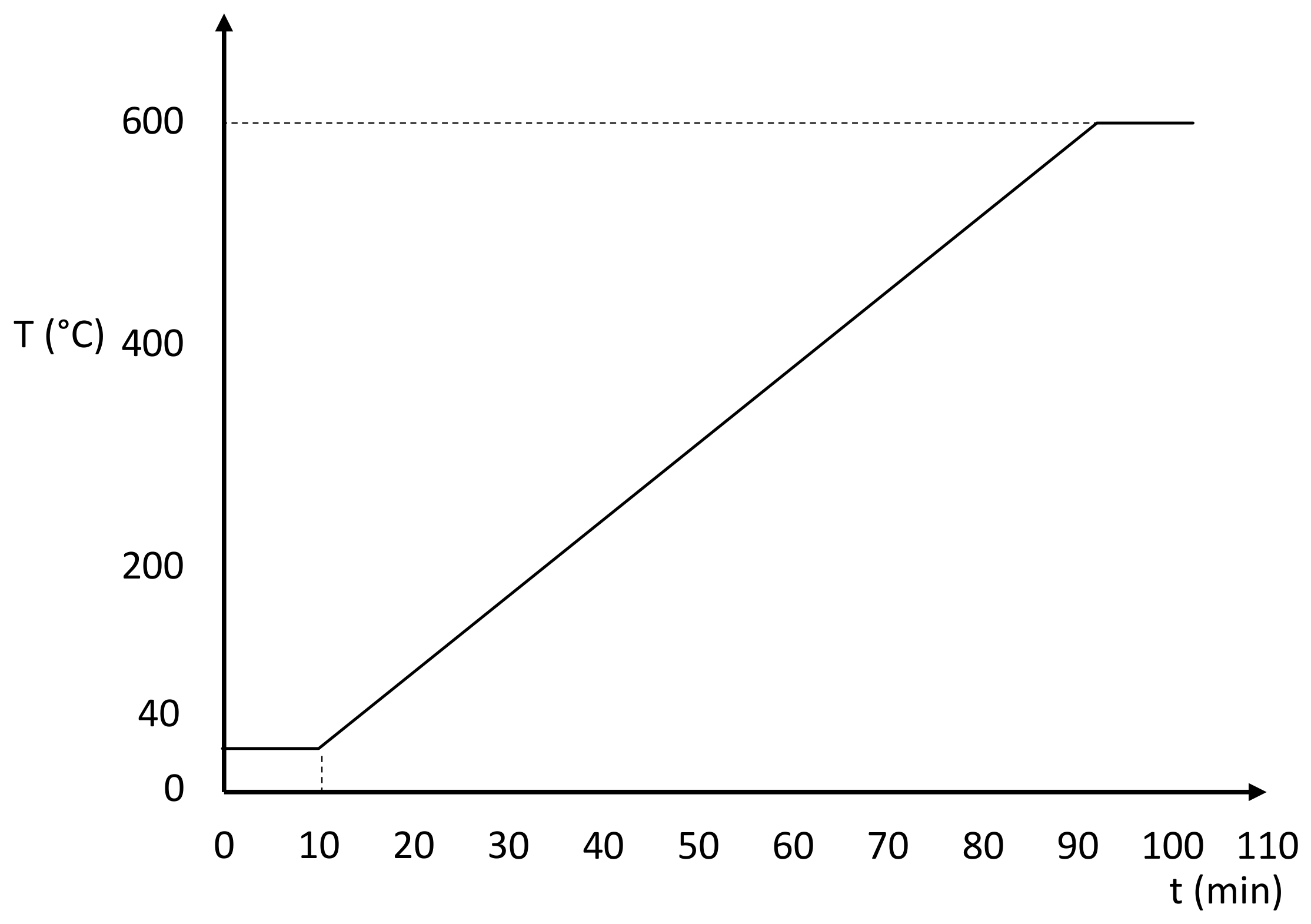
2.4.4. Temperature Program
- An isothermal step of 10 min at 40 °C, to ensure thermal stability of the sample before the heating step.
- A dynamic step from 40 °C to 600 °C at a constant heating rate of 10 K min−1.
- If a DSC/TGA has been used and the DSC curve is needed, an isothermal step of 10 min at 600 °C to correct the heat flow curve drift if necessary.
2.4.5. Measurement
2.4.6. Data Analysis
3. Results and Discussion
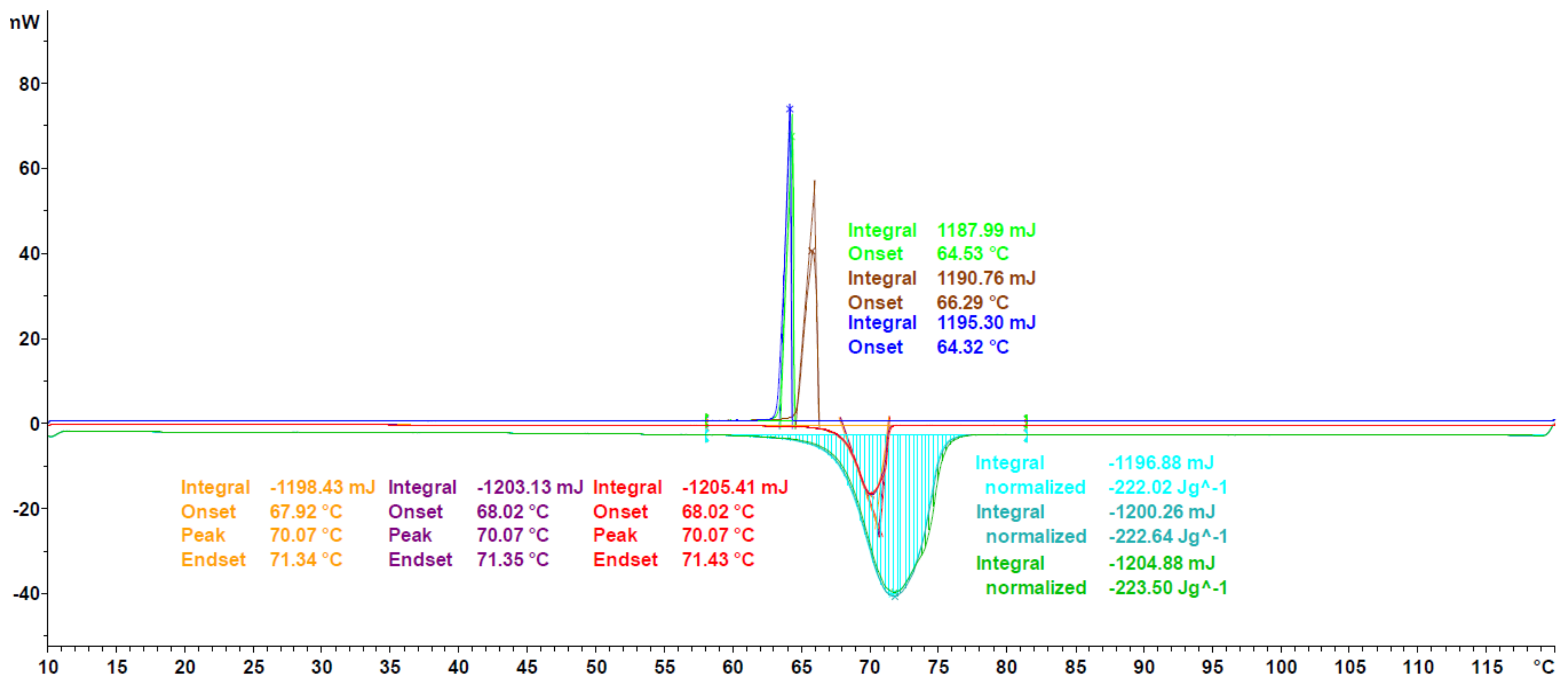
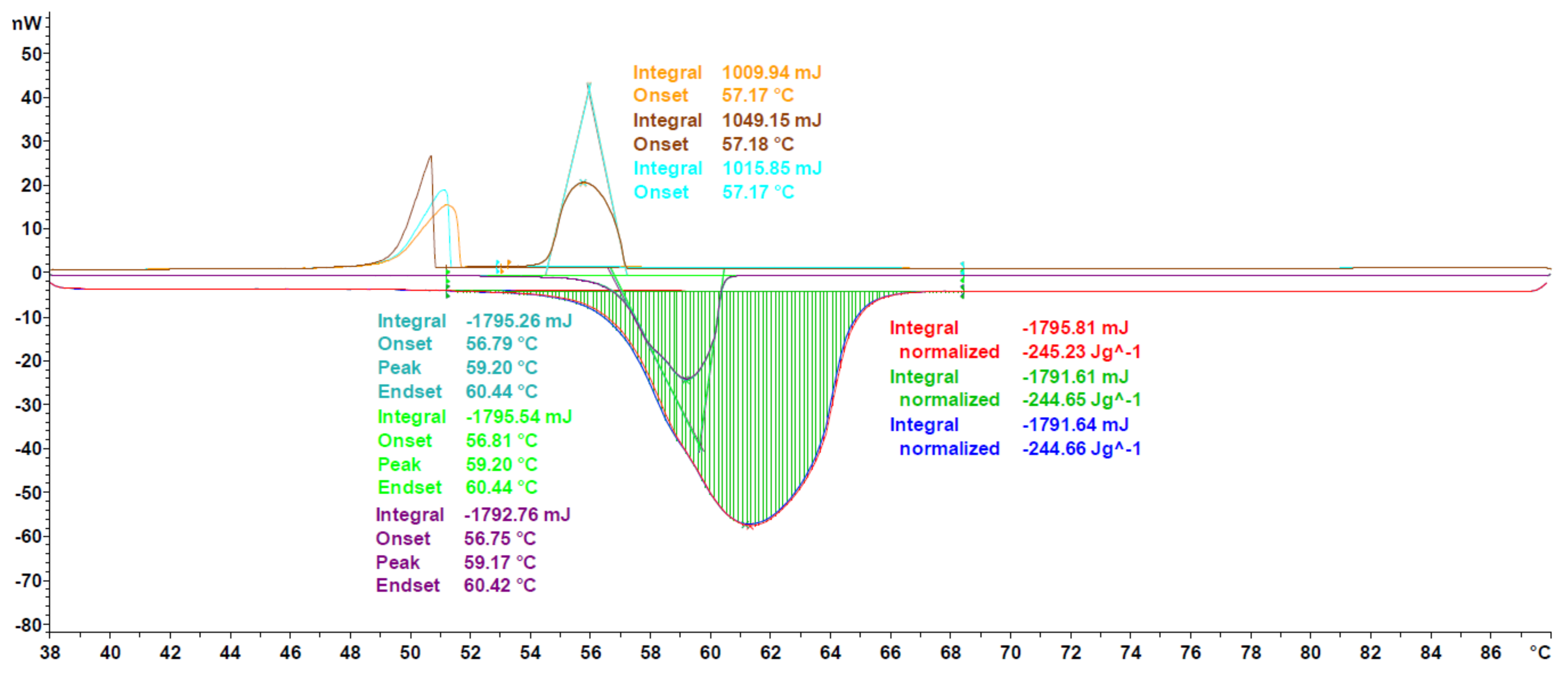
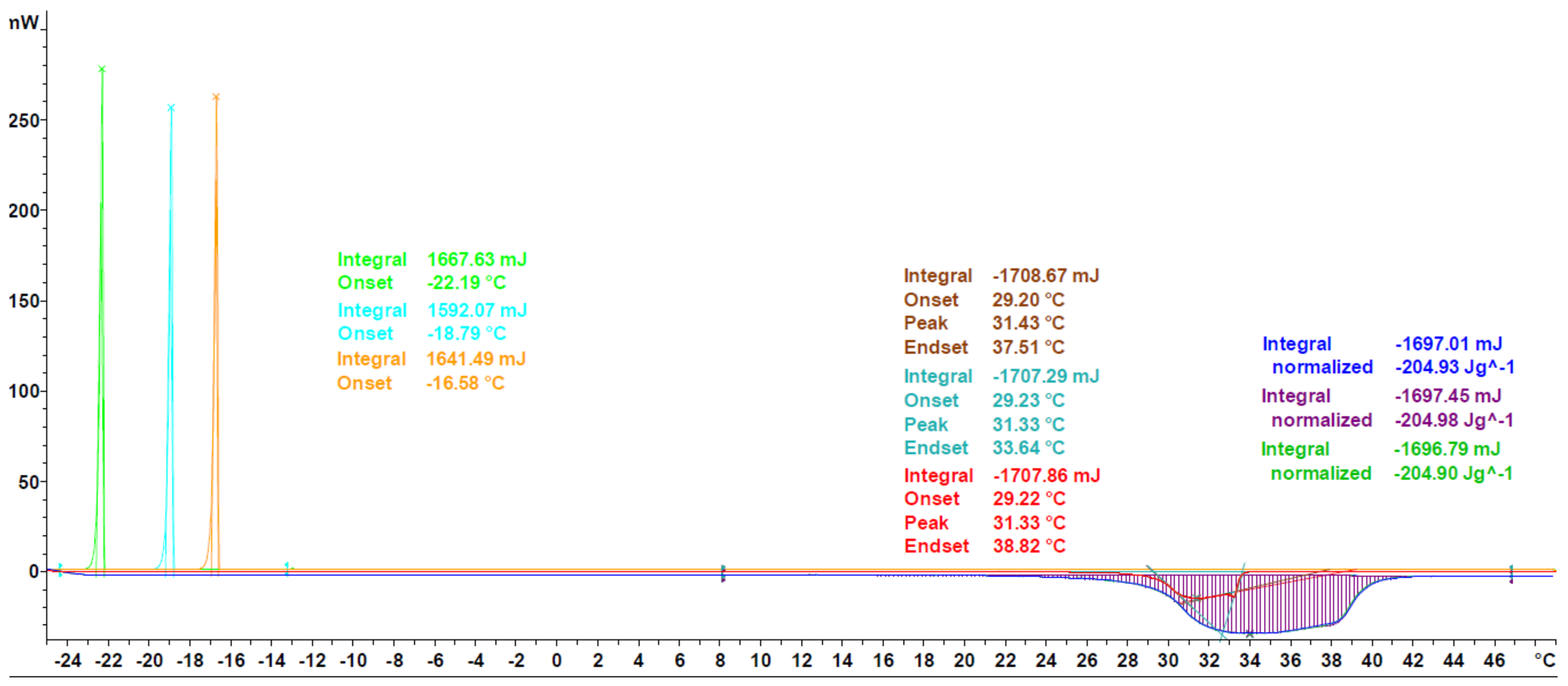
3.1. DSC Measurements
3.1.1. Validation
3.1.2. Extension to Different PCM Classes
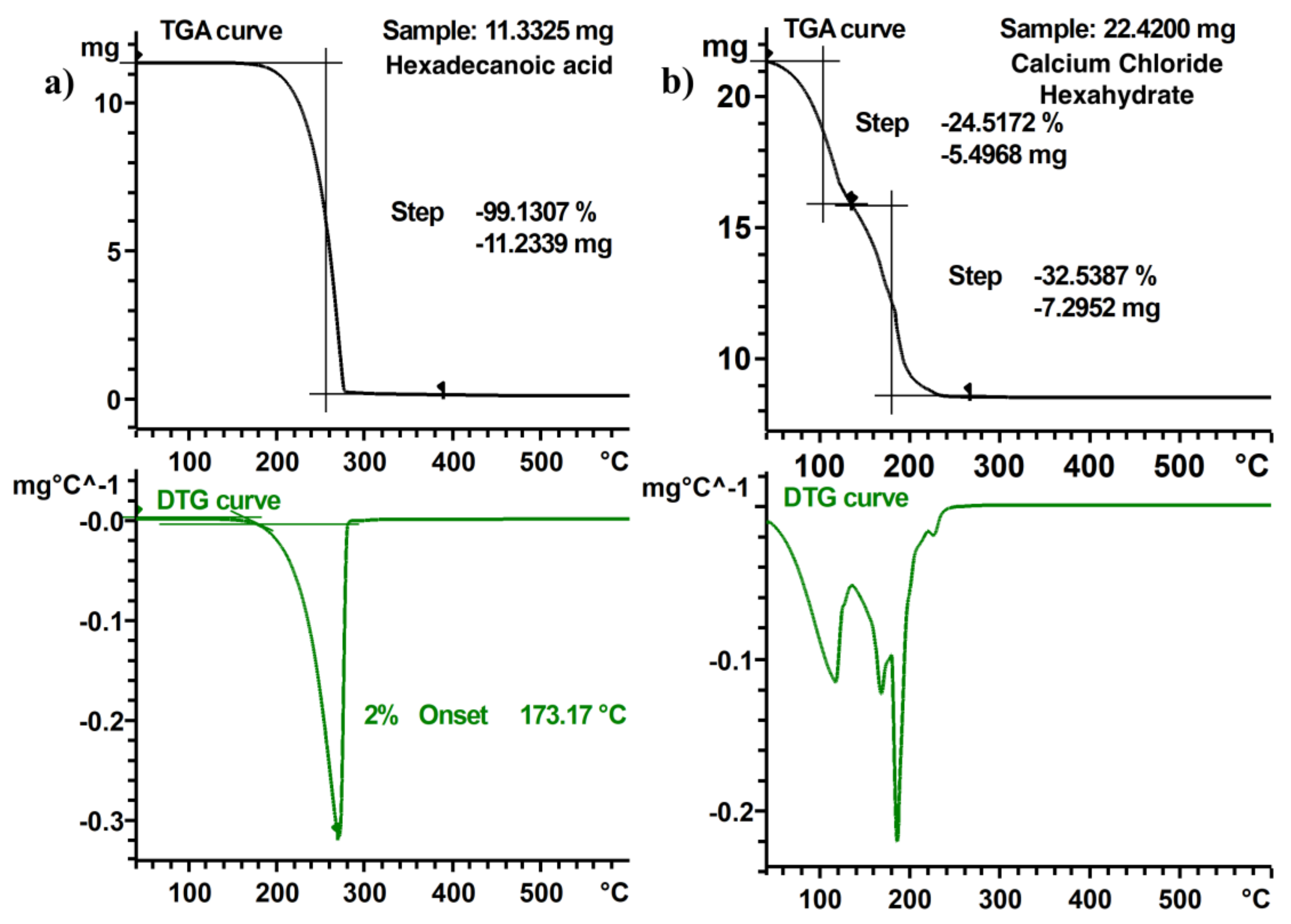
3.2. TGA Measurements
3.3. Database Measurements
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosspietsch, D.; Saenger, M.; Girod, B. Matching decentralized energy production and local consumption: A review of renewable energy systems with conversion and storage technologies. Wires Energy Environ. 2019, 8, e336. [Google Scholar] [CrossRef]
- Isaac, M.; van Vuuren, D.P. Modeling global residential sector energy demand for heating and air conditioning in the context of climate change. Energy Policy 2009, 37, 507–521. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Palacios, A.; de Gracia, A.; Cabeza, L.F.; Julià, E.; Fernández, A.I.; Barreneche, C. New formulation and characterization of enhanced bulk-organic phase change materials. Energy Build. 2018, 167, 38–48. [Google Scholar] [CrossRef]
- Eanest Jebasingh, B.; Valan Arasu, A. A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Mater. 2020, 24, 52–74. [Google Scholar] [CrossRef]
- Martín, M.; Villalba, A.; Inés Fernández, A.; Barreneche, C. Development of new nano-enhanced phase change materials (NEPCM) to improve energy efficiency in buildings: Lab-scale characterization. Energy Build. 2019, 192, 75–83. [Google Scholar] [CrossRef]
- Oh, K.; Kwon, S.; Xu, W.; Wang, X.; Toivakka, M. Effect of micro- and nanofibrillated cellulose on the phase stability of sodium sulfate decahydrate based phase change material. Cellulose 2020, 27, 5003–5016. [Google Scholar] [CrossRef]
- Kumar, N.; Ness, R.V.O.N.; Chavez, R., Jr.; Banerjee, D.; Muley, A.; Stoia, M. Experimental Analysis of Salt Hydrate Latent Heat Thermal Energy Storage System With Porous Aluminum Fabric and Salt Hydrate as Phase Change Material With Enhanced Stability and Supercooling. J. Energy Resour. Technol. 2020, 143. [Google Scholar] [CrossRef]
- Ravotti, R.; Lardon, N.; Stamatiou, A.; Worlitschek, J.; Fischer, L.; Fellmann, O. Analysis of Bio-Based Fatty Esters PCM’s Thermal Properties and Investigation of Trends in Relation to Chemical Structures. Appl. Sci. 2019, 9, 225. [Google Scholar] [CrossRef]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.J.; Stamatiou, A.; Worlitschek, J. Investigation of Lactones as Innovative Bio-sourced PCM for Latent Heat Storage. Molecules 2019, 24, 1300. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. A Review on Polymeric-Based Phase Change Material for Thermo-Regulating Fabric Application. Polym. Rev. 2020, 60, 389–419. [Google Scholar] [CrossRef]
- Barreneche, C.; Navarro, M.E.; Cabeza, L.F.; Fernández, A.I. New database to select phase change materials: Chemical nature, properties, and applications. J. Energy Storage 2015, 3, 18–24. [Google Scholar] [CrossRef]
- Barreneche, C.; Navarro, H.; Serrano, S.; Cabeza, L.F.; Fernández, A.I. New Database on Phase Change Materials for Thermal Energy Storage in Buildings to Help PCM Selection. Energy Procedia 2014, 57, 2408–2415. [Google Scholar] [CrossRef]
- Lager, D. Evaluation of Thermophysical Properties for Thermal Energy Storage Materials-Determining Factors, Prospects and Limitations; Technische Universität: Wien, Austria, 2018. [Google Scholar]
- Barreneche, C.; Pisello, A.L.; Fernández, A.I.; Cabeza, L.F. Experimental Methods for the Characterization of Materials for Latent Thermal Energy Storage. In Recent Advancements in Materials and Systems for Thermal Energy Storage; Frazzica, A., Cabeza, L.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 89–101. ISBN 978-3-319-96639-7. [Google Scholar]
- DIN 53765. Testing of Plastics and Elastomeres; Thermal Analysis; DSC-Method; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1994. [Google Scholar]
- ISO 11357–3. Plastics-Differential Scanning Calorimetry (DSC)-Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization; International Organization for Standardization (ISO): Geneva, Switzerland, 2011. [Google Scholar]
- ASTM E793. Standard Test Method for Enthalpies of Fusion and Crystallization by Differential Scanning Calorimetry; ASTM International (ASTM): West Conshohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- ASTM D4318. Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning; ASTM International (ASTM): West Conshohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- Haillot, D.; Bauer, T.; Kröner, U.; Tamme, R. Thermal analysis of phase change materials in the temperature range 120–150 °C. Acta 2011, 513, 49–59. [Google Scholar] [CrossRef]
- Harish, S.; Orejon, D.; Takata, Y.; Kohno, M. Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets. Appl. Eng. 2015, 80, 205–211. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Tang, F.; Cao, L.; Fang, G. Preparation and thermal properties of stearic acid/titanium dioxide composites as shape-stabilized phase change materials for building thermal energy storage. Energy Build. 2014, 80, 352–357. [Google Scholar] [CrossRef]
- Tong, B.; Tan, Z.C.; Shi, Q.; Li, Y.S.; Yue, D.T.; Wang, S.X. Thermodynamic investigation of several natural polyols (I): Heat capacities and thermodynamic properties of xylitol. Acta 2007, 457, 20–26. [Google Scholar] [CrossRef]
- Barreneche, C.; Solé, A.; Miró, L.; Martorell, I.; Fernández, A.I.; Cabeza, L.F. Study on differential scanning calorimetry analysis with two operation modes and organic and inorganic phase change material (PCM). Acta 2013, 553, 23–26. [Google Scholar] [CrossRef]
- Castellón, C.; Günther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC—A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 32, 1258–1265. [Google Scholar] [CrossRef]
- RAL Institute. Quality & Testing Specifications for Phase Change Materials; RAL Deutsches Institut für Gütesicherung und Kennzeichnung e.V.: Bonn, Germany, 2018. [Google Scholar]
- Gschwander, S.; Lazaro, A.; Cabeza, L.F.; Günther, E.; Fois, M.; Chui, J. Development of a Test-Standard for PCM and TCM Characterization Part 1: Characterization of Phase Change Materials; International Energy Agency (IEA): Paris, France, 2011. [Google Scholar]
- Lazaro, A.; Peñalosa, C.; Solé, A.; Diarce, G.; Haussmann, T.; Fois, M.; Zalba, B.; Gshwander, S.; Cabeza, L.F. Intercomparative tests on phase change materials characterisation with differential scanning calorimeter. Appl. Energy 2013, 109, 415–420. [Google Scholar] [CrossRef]
- ASTM C1872. Standard Test Method for Thermogravimetric Analysis of Hydraulic Cement. Astm Stand. 2018. [Google Scholar] [CrossRef]
- ISO 11358–1. Plastics–Thermogravimetry (TG) of Polymers–Part 1: General Principles; International Organization for Standardization (ISO): Geneva, Switzerland, 2014. [Google Scholar]
- ASTM E1131. Standard Test Method for Compositional Analysis by Thermogravimetry; ASTM International (ASTM): West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- ASTM E2253. Standard Test Method for Temperature and Enthalpy Measurement Validation of Differential Scanning Calorimeters; ASTM International (ASTM): West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- DIN 65583-04. Aerospace–Fibre Reinforced Materials – Determination of Glass Transition of Fibre Composites under Dynamic Load; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1999. [Google Scholar] [CrossRef]
- Gschwander, S.; Haussmann, T.; Hagelstein, G.; Solé, A.; Cabeza, L.F.; Diarce, G.; Hohenauer, W.; Lager, D.; Ristic, A.; Rathgeber, C.; et al. Standardization of PCM Characterization via DSC. In Proceedings of the 13th International Conference on Energy Storage, Greenstock, Trondheim, Norway, 19–21 May 2015. [Google Scholar]
- Ravotti, R.; Fellmann, O.; Lardon, N.; Fischer, L.; Stamatiou, A.; Worlitschek, J. Synthesis and Investigation of Thermal Properties of Highly Pure Carboxylic Fatty Esters to Be Used as PCM. Appl. Sci. 2018, 8, 1069. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 31–Lipids and Biological Membranes. In Organic Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1001–1032. ISBN 978-0-12-812838-1. [Google Scholar]
- Acree, W.E.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2018. [Google Scholar]
- Domalski, E.S.; Hearing, E.D. Condensed Phase Heat Capacity Data. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2018. [Google Scholar]
- Debenedetti, P.G. Metastable Liquids; Princeton University Press: Princeton, NJ, USA, 1996; Volume 1, ISBN 9780691085951. [Google Scholar]
- Ventolà, L.; Ramírez, M.; Calvet, T.; Solans, X.; Cuevas-Diarte, M.A.; Negrier, P.; Mondieig, D.; van Miltenburg, J.C.; Oonk, H.A.J. Polymorphism of N-Alkanols: 1-Heptadecanol, 1-Octadecanol, 1-Nonadecanol, and 1-Eicosanol. Chem. Mater. 2002, 14, 508–517. [Google Scholar] [CrossRef]
- Carlsson, B. Phase change behaviour of some latent heat storage media based on calcium chloride hexahydrate. Sol. Energy 2009, 83, 485–500. [Google Scholar] [CrossRef]
- Schmit, H.; Rudaleviciene, D.; Rathgeber, C.; Hiebler, S. Calorimetric Investigation of Two Factors Influencing the Maximum Storage Capacity of Calcium Chloride Hexahydrate. Iop Conf. Ser. Mater. Sci. Eng. 2019, 660, 12076. [Google Scholar] [CrossRef]
- Wan, X.; Su, L.; Guo, B. Design and preparation of novel shapeable PEG/SiO2/AA shape-stabilized phase change materials based on double-locked network with enhanced heat storage capacity for thermal energy regulation and storage. Powder Technol. 2019, 353, 98–109. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, L.; Shan, F.; Fang, G. Preparation and characteristics of microencapsulated stearic acid as composite thermal energy storage material in buildings. Energy Build. 2013, 62, 469–474. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Fang, G. Synthesis and properties of microencapsulated stearic acid/silica composites with graphene oxide for improving thermal conductivity as novel solar thermal storage materials. Sol. Energy Mater. Sol. Cells 2019, 189, 197–205. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Song, G.; Tang, G.; Shi, X. Organic-inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized pickering emulsion polymerization. Sol. Energy Mater. Sol. Cells 2018, 175, 102–110. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Liang, H.; Yan, X. Preparation and characterization of PMMA/TiO2 hybrid shell microencapsulated PCMs for thermal energy storage. Energy 2019, 167, 1031–1039. [Google Scholar] [CrossRef]
- Arpagaus, C.; Berthold, M.; Eschmann, M. Bericht « WP-Feldmessung » (Auswertung verlängert bis Dez. 2019). Jahresbericht 2017 Energ. Schweiz 2019, 2018. [Google Scholar]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical Characterization of MgCl2·6H2O, Xylitol and Erythritol as Phase Change Materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials (Basel) 2017, 10, 444. [Google Scholar] [CrossRef]
- Gombás, Á.; Szabó-Révész, P.; Regdon, G.; Erős, I. Study of thermal behaviour of sugar alcohols. J. Anal. Calorim. 2003, 73, 615–621. [Google Scholar] [CrossRef]
- Solé, A.; Neumann, H.; Niedermaier, S.; Cabeza, L.F.; Palomo, E. Thermal stability test of sugar alcohols as phase change materials for medium temperature energy storage application. Energy Procedia 2014, 48, 436–439. [Google Scholar] [CrossRef]
- Fischer, L.J. N7 Phasenwechselmaterialien (PCM) für Latent-Wärmespeicher BT–VDI-Wärmeatlas: Fachlicher Träger VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen; Stephan, P., Kabelac, S., Kind, M., Mewes, D., Schaber, K., Wetzel, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1989–2008. ISBN 978-3-662-52989-8. [Google Scholar]




| PCM | CAS Number | Purity | Manufacturer | PCM Class |
|---|---|---|---|---|
| 1-Octadecanol | 112-92-5 | 99% | Sigma-Aldrich | Alcohol |
| 1-Tetradecanol | 112-72-1 | 97% | Sigma-Aldrich | Alcohol |
| 1-Undecanol | 112-42-5 | 99% | Sigma-Aldrich | Alcohol |
| Ethyl octadecanoate | 111-61-5 | 97% | Sigma-Aldrich | Ester |
| Methyl hexadecanoate | 112-39-0 | 99% | Sigma-Aldrich | Ester |
| Methyl octadecanoate | 112-61-8 | 96% | Sigma-Aldrich | Ester |
| (MgCl2-Mg(NO3)2)·6H2O (41 wt%/59 wt%) | 7786-30-3/13446-18-9 | 98%/99% | Sigma-Aldrich | Eutectic |
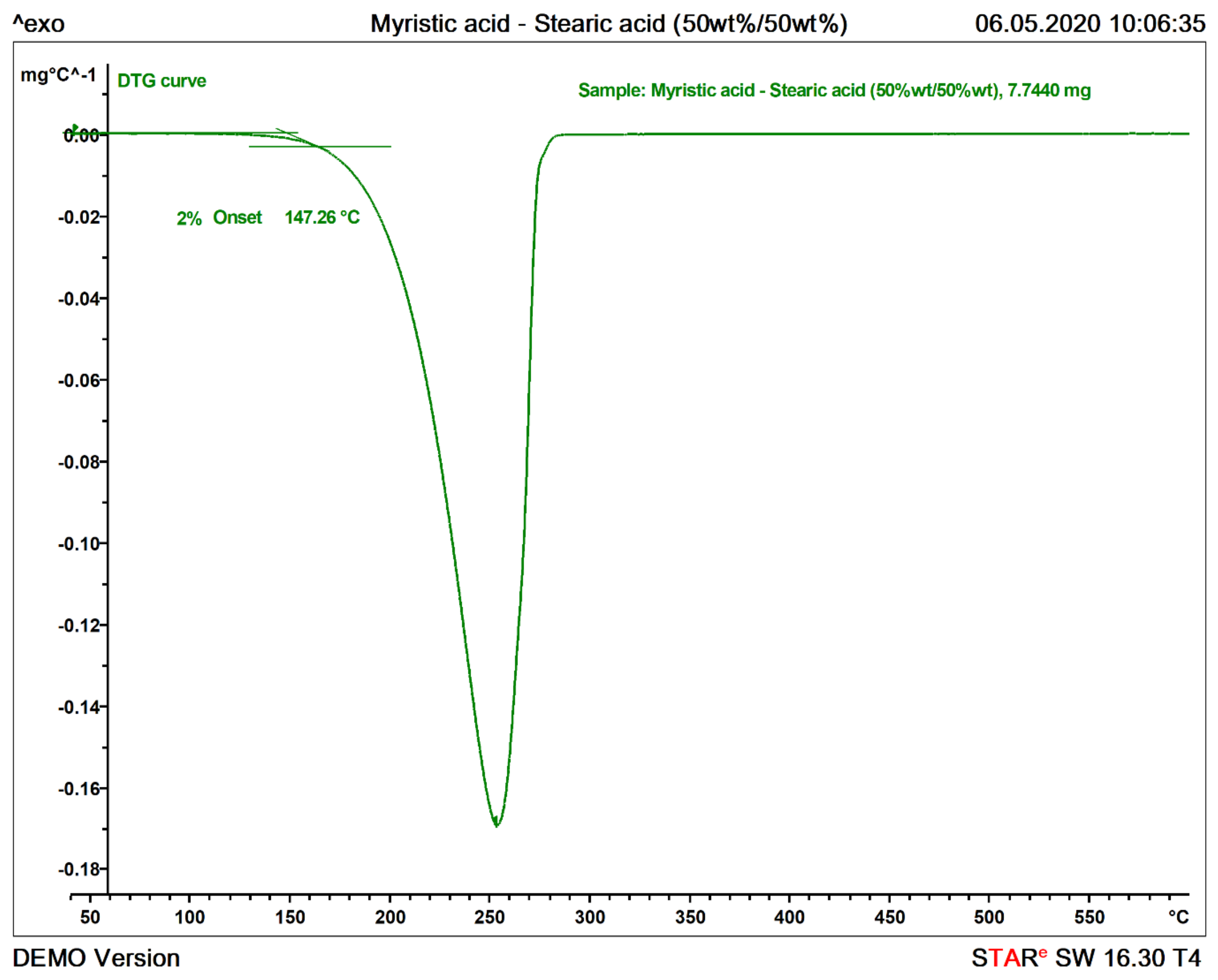
| Myristic acid-Stearic acid (50 wt%/50 wt%) | 544-63-8/57-11-4 | 99%/98% | Sigma-Aldrich/Roth | Eutectic |
| Hexadecanoic acid | 57-10-3 | 99% | Sigma-Aldrich | Fatty acid |
| Hexanoic acid | 142-62-1 | 99% | Sigma-Aldrich | Fatty acid |
| Methanoic acid | 64-18-6 | 96% | Sigma-Aldrich | Fatty acid |
| Octadecanoic acid | 57-11-4 | 95% | Sigma-Aldrich | Fatty acid |
| Propionic acid | 79-09-4 | 99.50% | Sigma-Aldrich | Fatty acid |
| Crodatherm 17 | n/a 1 | As purchased 1 | Croda Europe | Organic |
| PEG 10000 | 25322-68-3 | n/a | Sigma-Aldrich | Organic |
| Decane | 124-18-5 | 99% | Sigma-Aldrich | Paraffin |
| Dodecane | 112-40-3 | 99% | Sigma-Aldrich | Paraffin |
| Eicosane | 112-95-8 | 99% | Sigma-Aldrich | Paraffin |
| CaBr2·6H2O | 71626-99-8 | 98% | Sigma-Aldrich | Salt hydrate |
| CaCl2·6H2O | 7774-34-7 | 98% | Sigma-Aldrich | Salt hydrate |
| SAT | n/a 1 | As purchased 1 | Cowa Thermal Solutions | Salt hydrate |
| Dulcitol | 608-66-2 | 99% | Sigma-Aldrich | Sugar alcohol |
| meso-Erythritol | 149-32-6 | 99% | Sigma-Aldrich | Sugar alcohol |
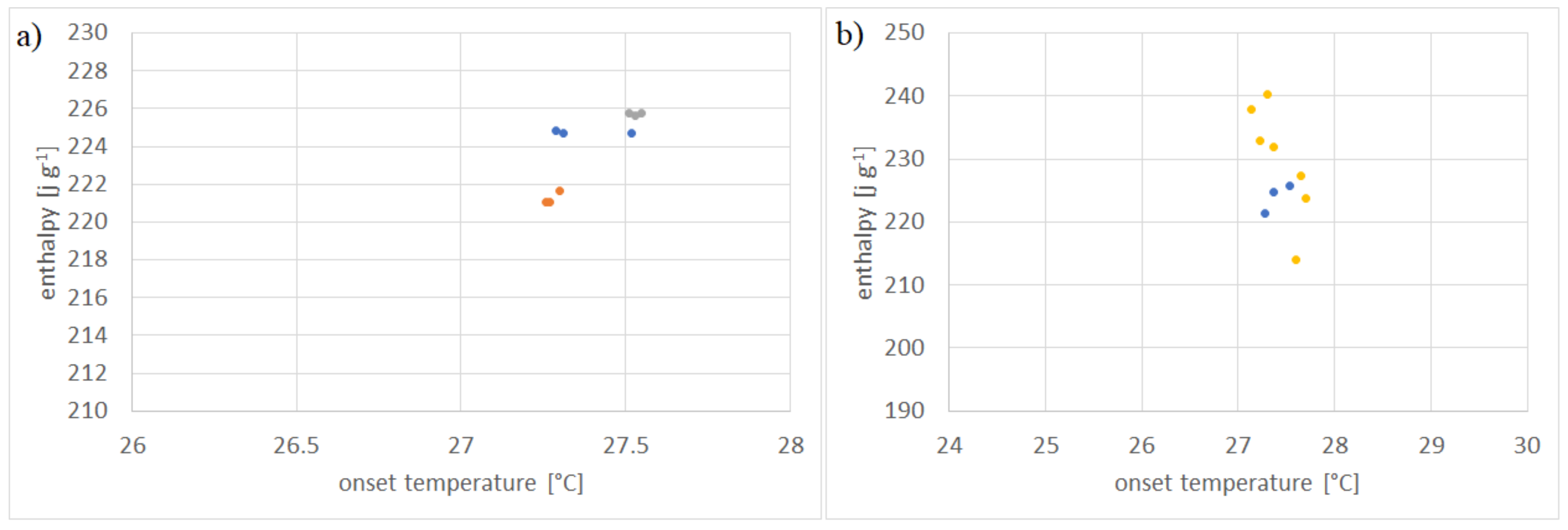
| Laboratory | Operator | Instrument 1 | Enthalpy (J/g) | CV (%) | Onset Temperature (°C) | CV (%) |
|---|---|---|---|---|---|---|
| HSLU 2 | 1 | A | 165.32 ± 0.63 | 0.38 | 15.84 ± 0.06 | 0.37 |
| HSLU 2 | 1 | B | 156.41 ± 0.72 | 0.46 | 16.48 ± 0.04 | 0.26 |
| MT 3 | 2 | C | 171.15 ± 0.61 | 0.35 | 16.08 ± 0.21 | 1.3 |
| MT 3 | 2 | D | 169.32 ± 0.71 | 0.42 | 15.77 ± 0.03 | 0.19 |
| Mean | - | - | 165.55 ± 6.56 | 3.43 | 16.05 ± 0.32 | 1.73 |
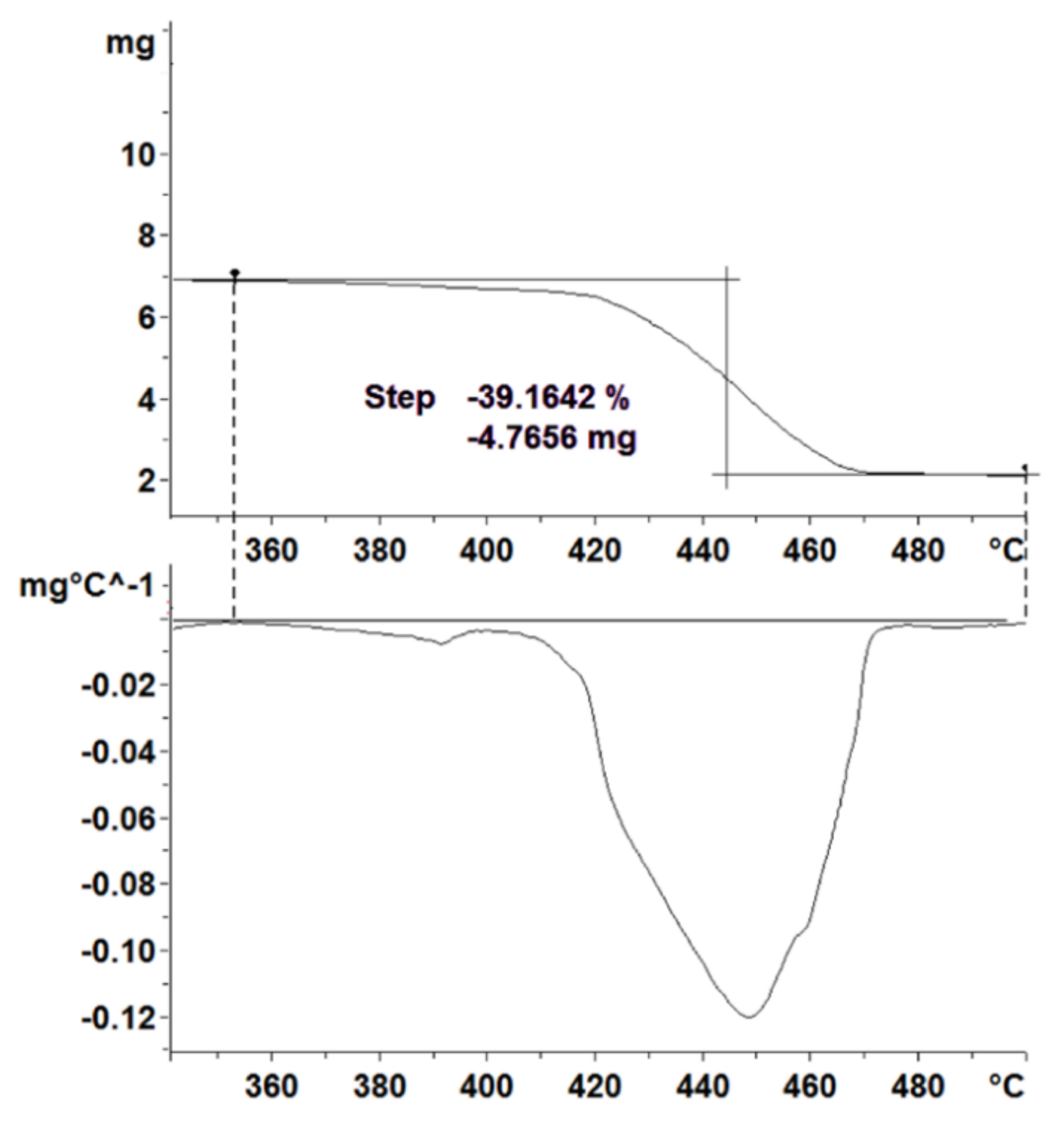
| PCM | Mass Loss (%) | Mean (%) | Standard Deviation (%) | CV (%) |
|---|---|---|---|---|
| 1-Tetradecanol | 99.95 | 99.93 | 0.02 | 0.02 |
| 99.92 | ||||
| 99.92 | ||||
| Methyl Hexadecanoate | 98.73 | 98.38 | 0.34 | 0.35 |
| 98.04 | ||||
| 98.39 | ||||
| PEG 10.000 | 97.63 | 97.66 | 0.16 | 0.16 |
| 97.84 | ||||
| 97.52 | ||||
| SAT | 32.76 | 33.09 | 0.31 | 0.93 |
| 33.36 | ||||
| 33.15 |
| Reference | PCM | Deviation Onset Temperature (%) | Deviation Temperature Maxmium Mass Loss Rate (%) |
|---|---|---|---|
| Xian Van et al. [46] | PEG 6000 | - | −3.36 |
| Zhi Chen et al. [47] | Stearic acid | 141.82 | 11.10 |
| Yaxue Lin et al. [48] | Stearic acid | 45.53 | 0.61 |
| Fang Tang et al. [25] | Octadecane | - | −15.62 |
| Hao Wang et al. [49] | Octadecane | 26.94 | - |
| Chaoen Li et al. [50] | Octadecane | 35.40 | - |
| PCM | CAS | PCM Class | Melting Enthalpy (J/g) | Melting Onset (°C) | Melting Peak (°C) | Crystallization Onset (°C) | Max. Operating Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Methanoic acid | 64-18-6 | Fatty Acid | 233.81 ± 0.95 | −50.4 ± 0.02 | −49.4 ± 0.01 | −38.74–−33.74 | n/a 1 |
| Decane | 124-18-5 | Paraffin | 207.32 ± 2.99 | −30.2 ± 0.02 | −27.8 ± 0.24 | −40.33–−36.97 | n/a 1 |
| Propionic acid | 79-09-4 | Fatty Acid | 143.7 ± 1.44 | −25.7 ± 0.15 | −22.2 ± 0.08 | −46.6–−44.49 | n/a 1 |
| Dodecane | 112-40-3 | Paraffin | 209.14 ± 1.45 | −10.2 ± 0.94 | −8 ± 0.25 | −14.69–−13.45 | n/a 1 |
| Hexanoic acid | 142-62-1 | Fatty Acid | 141.21 ± 0.58 | −4.7 ± 0.2 | −1.2 ± 0.06 | −14.59–−12.09 | 55.4 ± 1.43 |
| 1-Undecanol | 112-42-5 | Alcohol | 206.62 ± 3.94 | 14.5 ± 0.27 | 17.5 ± 0.15 | 10.35–11.54 | 82.5 ± 11.71 |
| Crodatherm 17 | n/a | Organic | 184.74 ± 5.91 | 16 ± 0.04 | 18.6 ± 0.1 | 14.48–14.51 | 143.8 ± 13.44 |
| Methyl hexadecanoate | 112-39-0 | Ester | 205.03 ± 1.12 | 28.9 ± 0.05 | 31.5 ± 0.33 | 25.32–28.31 | 111.5 ± 10.32 |
| CaCl2·6H2O | 7774-34-7 | Salt Hydrate | 197.97 ± 6.22 | 29.2 ± 0.64 | 31.7 ± 0.73 | −24.1–−16.4 | n/a 1 |
| Ethyl octadecanoate | 111-61-5 | Ester | 188.74 ± 1.49 | 32.3 ± 0.08 | 34.5 ± 0 | 30.74–30.79 | 149.4 ± 17.28 |
| CaBr2·6H2O | 71626-99-8 | Salt Hydrate | 125.1 ± 2.78 | 32.7 ± 0.08 | 35.4 ± 0.09 | 23.37–24.46 | 46.1 ± 0.26 |
| Eicosane | 112-95-8 | Paraffin | 246.44 ± 5.02 | 36 ± 0.03 | 37.8 ± 0.06 | 35.94–35.96 | 154.5 ± 6.88 |
| 1-Tetradecanol | 112-72-1 | Alcohol | 226.26 ± 2.47 | 37.3 ± 0.04 | 39.7 ± 0.1 | 37.66–37.77 | 110.8 ± 5.15 |
| Methyl octadecanoate | 112-61-8 | Ester | 229.16 ± 4.26 | 37.4 ± 0.03 | 40.1 ± 0.06 | 33.76–34.76 | 141 ± 4.06 |
| Myristic acid-Stearic acid (50wt%/50wt%) | n/a | Organic Eutectic | 179.97 ± 6.1 | 45.7 ± 0.07 | 48.2 ± 0.12 | 43.58–43.96 | 147.5 ± 1.33 |
| 1-Octadecanol | 112-92-5 | Alcohol | 245.93 ± 1.09 | 56.8 ± 0.05 | 59.1 ± 0.07 | 57.19–57.26 | 147.6 ± 5.39 |
| SAT | n/a | Salt Hydrate | 228.28 ± 13.17 | 57.5 ± 0.11 | 60.4 ± 0.26 | 33.18–46.26 | 204.6 ± 1.87 |
| (MgCl2-Mg(NO3)2)·6H2O (41wt%/59wt%) | n/a | Inorganic Eutectic | 151.66 ± 0.12 | 58.1 ± 0.01 | 60.2 ± 0 | 31.85–32.89 | 60.5 ± 1.68 |
| PEG 10000 | 25322-68-3 | Organic | 179.8 ± 3.03 | 59.3 ± 0.3 | 62.1 ± 0.12 | 46.52–50.14 | 364.8 ± 1.18 |
| Hexadecanoic acid | 57-10-3 | Fatty Acid | 216.72 ± 1.39 | 62.1 ± 0.01 | 63.7 ± 0.07 | 59.55–60.65 | 171.7 ± 2.68 |
| Octadecanoic acid | 57-11-4 | Fatty Acid | 228.26 ± 1.01 | 67.8 ± 0.2 | 70 ± 0.1 | 63.66–66.31 | 195.4 ± 1.4 |
| meso-Erythritol | 149-32-6 | Sugar Alcohol | 337.76 ± 7.51 | 118.7 ± 0.21 | 120.4 ± 0.17 | 22.99–32.29 | 192.3 ± 1.84 |
| Dulcitol | 608-66-2 | Sugar Alcohol | 352.91 ± 8.18 | 186.6 ± 0.51 | 189 ± 0.31 | 105.35–119.2 | 242.4 ± 1.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, L.; Rubio-Pérez, G.; Bach, A.; Muñoz-Rujas, N.; Aguilar, F.; Worlitschek, J. Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials 2020, 13, 4486. https://doi.org/10.3390/ma13204486
Müller L, Rubio-Pérez G, Bach A, Muñoz-Rujas N, Aguilar F, Worlitschek J. Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials. 2020; 13(20):4486. https://doi.org/10.3390/ma13204486
Chicago/Turabian StyleMüller, Lukas, Gabriel Rubio-Pérez, Andreas Bach, Natalia Muñoz-Rujas, Fernando Aguilar, and Jörg Worlitschek. 2020. "Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials" Materials 13, no. 20: 4486. https://doi.org/10.3390/ma13204486
APA StyleMüller, L., Rubio-Pérez, G., Bach, A., Muñoz-Rujas, N., Aguilar, F., & Worlitschek, J. (2020). Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials, 13(20), 4486. https://doi.org/10.3390/ma13204486









