Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wood Impregnation Treatment
2.3. Scanning Electron Microscope
2.4. Cone Calorimetric Test
2.5. Thermogravimetric Analysis
3. Results and Discussion
3.1. Microstructure Analysis
3.2. Cone Calorimetric Analysis
3.2.1. Time to Ignition and Fire Performance Index
3.2.2. Heat Release
3.2.3. Smoke Production and Yield of Carbon Oxide
3.2.4. Mass Loss and Residual Char
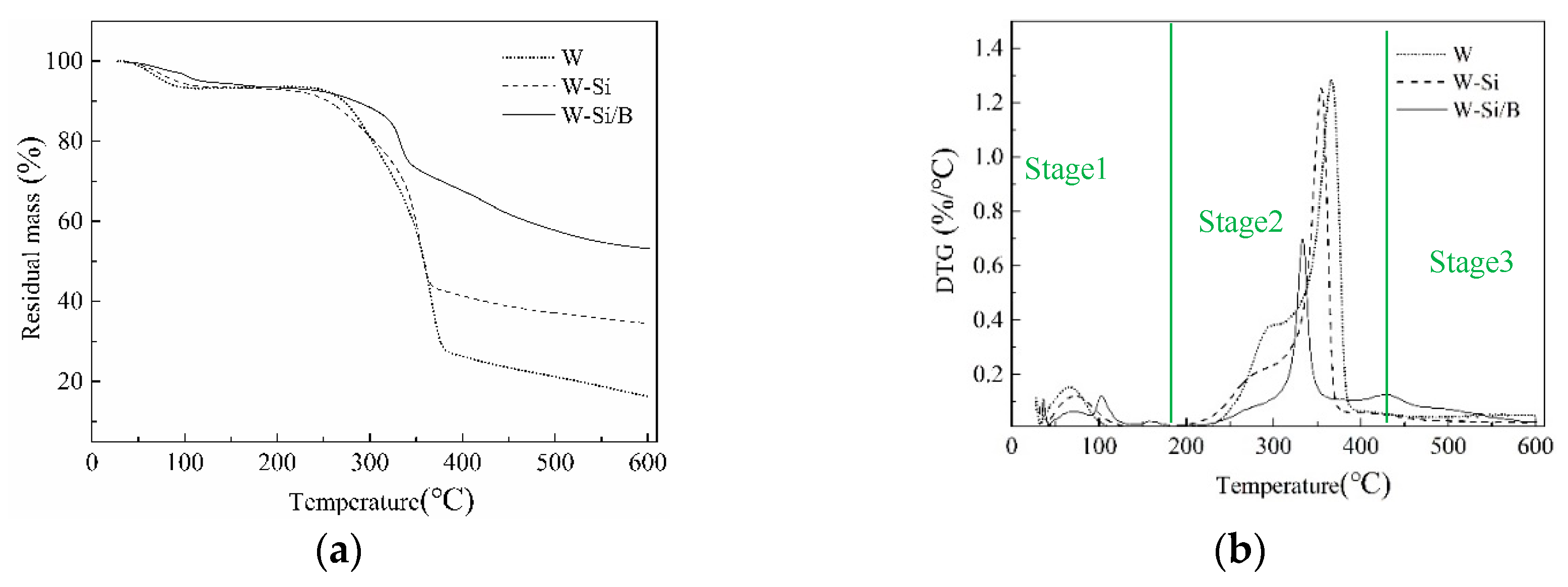
3.3. Thermogravimetric Analysis
3.4. Kinetic Analysis
4. Conclusions
- (1)
- Through the full-cell method, modifiers can fill in the vessel and fiber of poplar wood and can effectively enter and be fixed in the wood.
- (2)
- The cone calorimeter analysis shows that the ignition time, second peak of heat release rate, total heat release and mass loss of the W-Si/B are obviously delayed. Composite silicon modification has a positive effect on carbonization.
- (3)
- Thermogravimetric analysis found that the residual mass of the modified wood increased, and the thermal degradation rate of W-Si/B was significantly lower than others.
- (4)
- The Ea of modified wood has increased, and the flame-retardant effect of wood is enhanced. Compared to W-Si, the Ea (in the conversion range of 20 to 50%) of W-Si/B decreased, because boric acid catalyzed the thermal degradation and carbonization of poplar wood.
Author Contributions
Funding
Conflicts of Interest
References
- Yue, K.; Wu, J.; Xu, L.; Tang, Z.; Chen, Z.; Liu, W.; Wang, L. Use impregnation and densification to improve mechanical properties and combustion performance of Chinese fir. Constr. Build. Mater. 2020, 24, 1118101. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.L.; LV, W.H. Thermal degradation and fire performance of wood treated with PMUF resin and boron compounds. Fire Mater. 2017, 41, 1051–1057. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Walinder, M.E.P.; Claesson, P.M. Wettability and swelling of acetylated and furfurylated wood analyzed by multicycle Wilhelmy plate method. Holzforschung 2016, 70, 69–77. [Google Scholar] [CrossRef]
- Gao, J.; Kim, J.S.; Terziev, N. Decay resistance of softwoods and hardwoods thermally modified by the Thermovouto type thermo-vacuum process to brown rot and white rot fungi. Holzforschung 2016, 70, 877–884. [Google Scholar] [CrossRef]
- Merk, V.; Chanana, M.; Gaan, S. Mineralization of wood by calcium carbonate insertion for improved flame retardancy. Holzforschung 2016, 70, 867–876. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, J.; Chu, D. Synthesis of fire retardants based on N and P and poly (sodium silicate-aluminum dihydrogen phosphate) (PSADP) and testing the flame-retardant properties of PSADP impregnated poplar wood. Holzforschung 2016, 70, 341–350. [Google Scholar] [CrossRef]
- Yu, X.; Sun, D.; Li, X. Preparation and characterization of urea-formaldehyde resin-sodium montmorillonite intercalation-modified poplar. J. Wood Sci. 2011, 57, 501–506. [Google Scholar] [CrossRef]
- Klueppel, A.; Mai, C. The influence of curing conditions on the chemical distribution in wood modified with thermosetting resins. Wood Sci. Technol. 2013, 47, 643–658. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Khalil, H.P.S.A.; Hale, M.D. A study of the potential of acetylation to improve the properties of plant fibres. Ind. Crop. Prod. 1998, 8, 53–63. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, J.; Wang, W. Characteristics of wood-silica composites influenced by the pH value of silica sols. Holzforschung 2018, 72, 311–319. [Google Scholar] [CrossRef]
- Unger, B.; Bücker, M.; Reinsch, S.; Hübert, T. Chemical aspects of wood modification by sol-gel-derived silica. Wood Sci. Technol. 2012, 47, 83–104. [Google Scholar] [CrossRef]
- Kirilovs, E.; Kukle, S.; Gravitis, J.; Gusovius, H.J. Moisture absorption properties of hardwood veneers modified by a sol-gel process. Holzforschung 2017, 71, 645–648. [Google Scholar] [CrossRef]
- Schorr, D.; Blanchet, P. Improvement of White Spruce Wood Dimensional Stability by Organosilanes Sol-Gel Impregnation and Heat Treatment. Materials 2020, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Wu, T.L.; Wu, J.H. Long-Term Creep Behavior Prediction of Sol-Gel Derived SiO2- and TiO2-Wood Composites Using the Stepped Isostress Method. Polymers 2019, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hochmanska, P.; Bartłomiej, M.; Krystofiak, T. Hydrophobicity and weathering resistance of wood treated with silane-modified protective systems. Drewno 2014, 57, 99–111. [Google Scholar]
- Xu, E.; Zhang, Y.; Lin, L. Improvement of Mechanical, Hydrophobicity and Thermal Properties of Chinese Fir Wood by Impregnation of Nano Silica Sol. Polymers 2020, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Palanti, S.; Feci, E.; Predieri, G.; Vignali, F. A wood treatment based on siloxanes and boric acid against fungal decay and coleopter Hylotrupes bajulus. Int. Biodeterior. Biodegrad. 2012, 75, 49–54. [Google Scholar] [CrossRef]
- Ozawa, T.A. New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef]
- Li, Y.; Du, L.; Kai, C. Bamboo and high-density polyethylene composite with heat-treated bamboo fiber: Thermal decomposition properties. Bioresources 2013, 8, 900–912. [Google Scholar] [CrossRef]
- Miyafuji, H.; Saka, S. Fire-resistant properties in several TiO2 wood-inorganic composites and their topochemistry. Wood Sci. Technol. 1997, 31, 449–455. [Google Scholar]
- Wang, X.; Liu, J.; Chai, Y. Thermal, mechanical, and moisture absorption properties of wood-TiO2 composites prepared by a sol-gel process. Bioresources 2012, 7, 893–901. [Google Scholar]
- Savas, L.A.; Dogan, M. Flame retardant effect of zinc borate in polyamide 6 containing aluminum hypophosphite. Polym. Degrad. Stab. 2019, 165, 101–109. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2010, 31, 327–354. [Google Scholar] [CrossRef]
- Wen, L.; Han, L.; Zhou, H. Factors Influencing the Charring Rate of Chinese Wood by using the Cone Calorimeter. Bioresources 2015, 10, 7263–7272. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, M.; Cai, L. Improvement of mechanical, humidity resistance and thermal properties of heat-treated rubber wood by impregnation of SiO2 precursor. Sci. Rep. 2019, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xu, J.; Mai, C. Combustion behavior of Scots pine (Pinus sylvestris L.) sapwood treated with a dispersion of aluminum oxychloride-modified silica. Holzforschung 2016, 70, 1165–1173. [Google Scholar] [CrossRef]
- Blasi, C.D.; Branca, C.; Galgano, A. Flame retarding of wood by impregnation with boric acid-Pyrolysis products and char oxidation rates. Polym. Degrad. Stab. 2007, 92, 752–764. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Winandy, J.E. Chemical mechanism of fire retardance of boric acid on wood. Wood Sci. Technol. 2004, 38, 375–389. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Huang, J. Flame retardance and thermal stability of wool fabric treated by boron containing silica sols. Mater. Des. 2015, 85, 796–799. [Google Scholar] [CrossRef]
- Qu, H.; Wu, W.; Wu, H.; Xie, J.; Xu, J. Study on the effects of flame retardants on the thermal decomposition of wood by TG-MS. J. Anal. Calorim 2010, 103, 935–942. [Google Scholar] [CrossRef]
- Gasˇparovic, L.; Labovsky´, J.; Markosˇ, J.; Jelemensky´, L. Calculation of kinetic parameters of the thermal decomposition of wood by distributed activation energy model (DAEM). Chem. Biochem. Eng. 2012, 26, 45–53. [Google Scholar]
- Gao, M.; Li, S.; Sun, C. Thermal degradation of wood in air and nitrogen treated with basic nitrogen compounds and phosphoric acid. Combust. Sci. Technol. 2004, 176, 2057–2070. [Google Scholar] [CrossRef]
- Hirata, T.; Kawamoto, S.; Nishimoto, T. Thermogravimetry of wood treated with water-insoluble retardants and a proposal for development of fire-retardant wood materials. Fire Mater. 1991, 15, 27–36. [Google Scholar] [CrossRef]






| Sample | TTI (s) | FPI (m2 s/kW) | p-HRR1 (kW/m2) | p-HRR2 (kW/m2) | THR (MJ/m2) | TSP (m²) |
|---|---|---|---|---|---|---|
| W | 9 | 0.043 | 179.4 | 207.5 | 107.4 | 2.35 |
| W-Si | 11 | 0.064 | 171.4 | 164.2 | 92.2 | 1.35 |
| W-Si/B | 15 | 0.103 | 145.4 | 109.4 | 72.9 | 0.7 |
| Degree of Conversion α | W | W-Si | W-Si/B | |||
|---|---|---|---|---|---|---|
| Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea(kJ/mol) | R2 | |
| 0.2 | 144.0 | 0.9989 | 257.27 | 0.9928 | 258.3 | 0.9757 |
| 0.3 | 170.8 | 0.9994 | 292.16 | 0.9913 | 278.3 | 0.9993 |
| 0.4 | 182.2 | 0.9786 | 308.83 | 0.9986 | 287.2 | 0.9992 |
| 0.5 | 186.6 | 0.9904 | 319.50 | 0.9966 | 286.5 | 0.9997 |
| 0.6 | 186.4 | 0.9960 | 311.13 | 0.9970 | 342.9 | 0.9994 |
| 0.7 | 184.0 | 0.9981 | 301.91 | 0.9987 | 385.1 | 0.9865 |
| 0.8 | 181.8 | 0.9998 | 282.82 | 0.9989 | 314.2 | 0.9991 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Chai, Y.; Ni, L.; Lyu, W. Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol. Materials 2020, 13, 4478. https://doi.org/10.3390/ma13204478
Liu Q, Chai Y, Ni L, Lyu W. Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol. Materials. 2020; 13(20):4478. https://doi.org/10.3390/ma13204478
Chicago/Turabian StyleLiu, Qiangqiang, Yubo Chai, Lin Ni, and Wenhua Lyu. 2020. "Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol" Materials 13, no. 20: 4478. https://doi.org/10.3390/ma13204478
APA StyleLiu, Q., Chai, Y., Ni, L., & Lyu, W. (2020). Flame Retardant Properties and Thermal Decomposition Kinetics of Wood Treated with Boric Acid Modified Silica Sol. Materials, 13(20), 4478. https://doi.org/10.3390/ma13204478




