Wood Bottom Ash and GeoSilex: A By-Product of the Acetylene Industry as Alternative Raw Materials in Calcium Silicate Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of Raw Materials
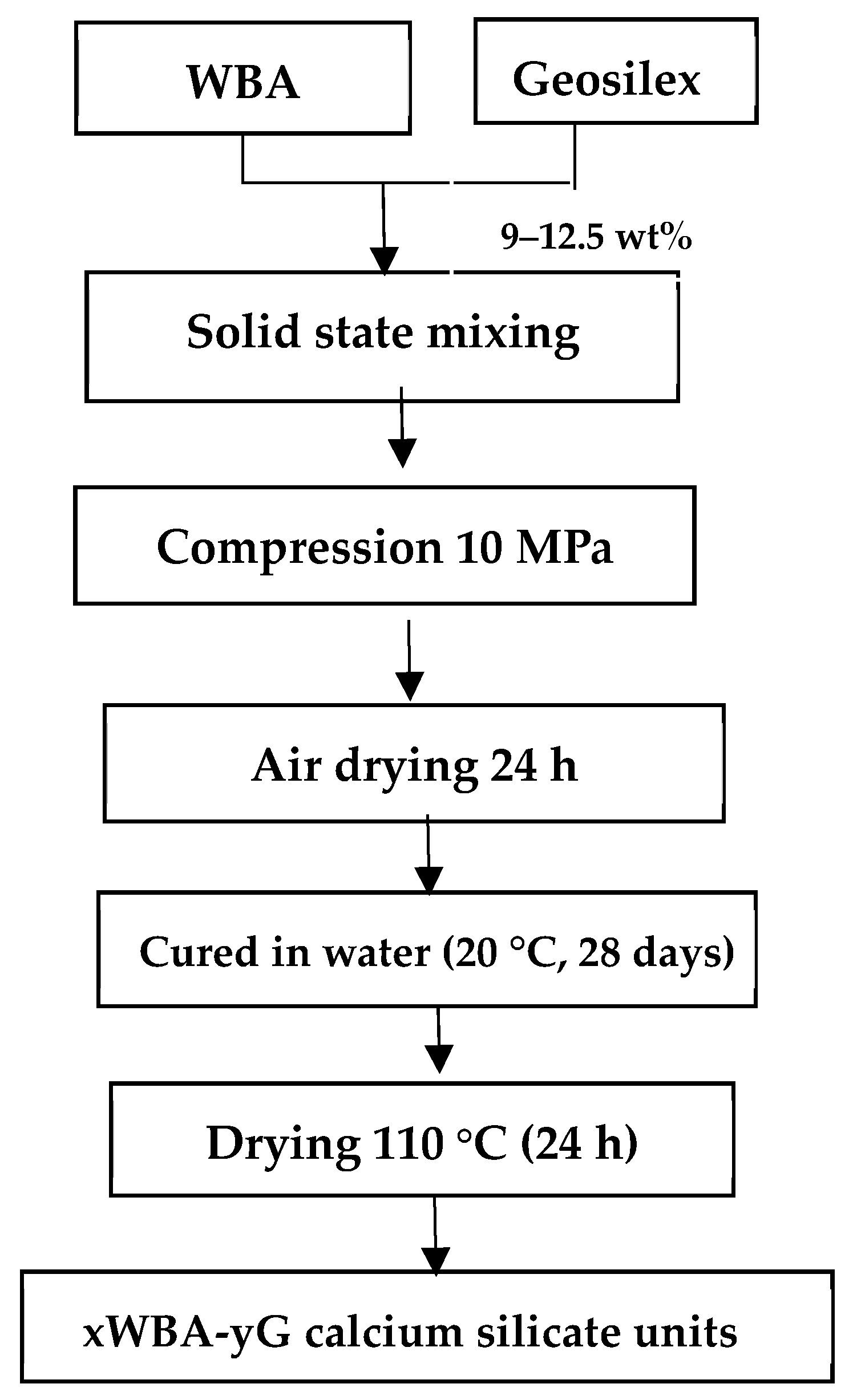
2.3. Preparation of Wood Bottom Ash-GeoSilex Calcium Silicate Units
2.4. Characterization of Calcium-Silicate Units
3. Results and Discussion
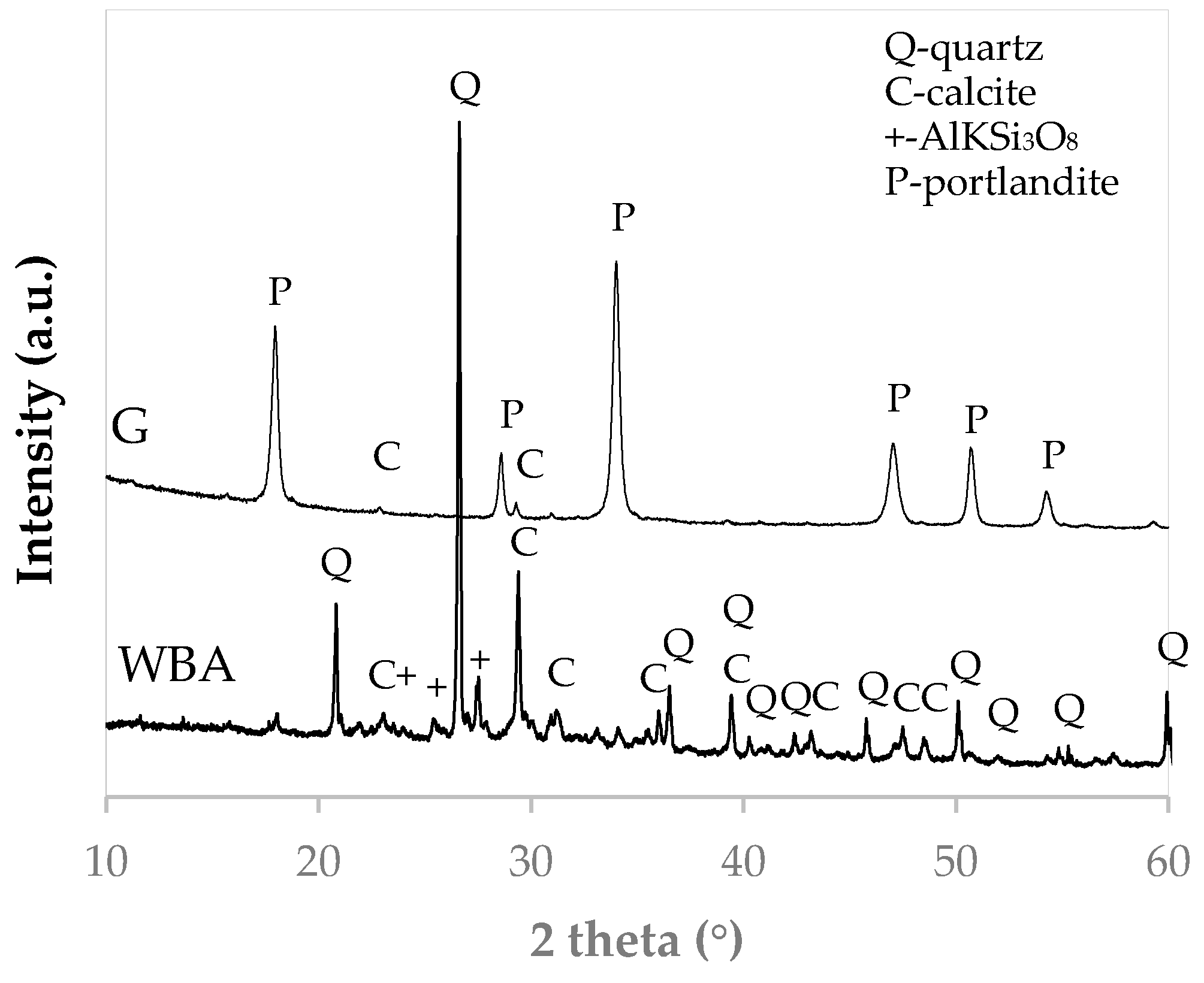
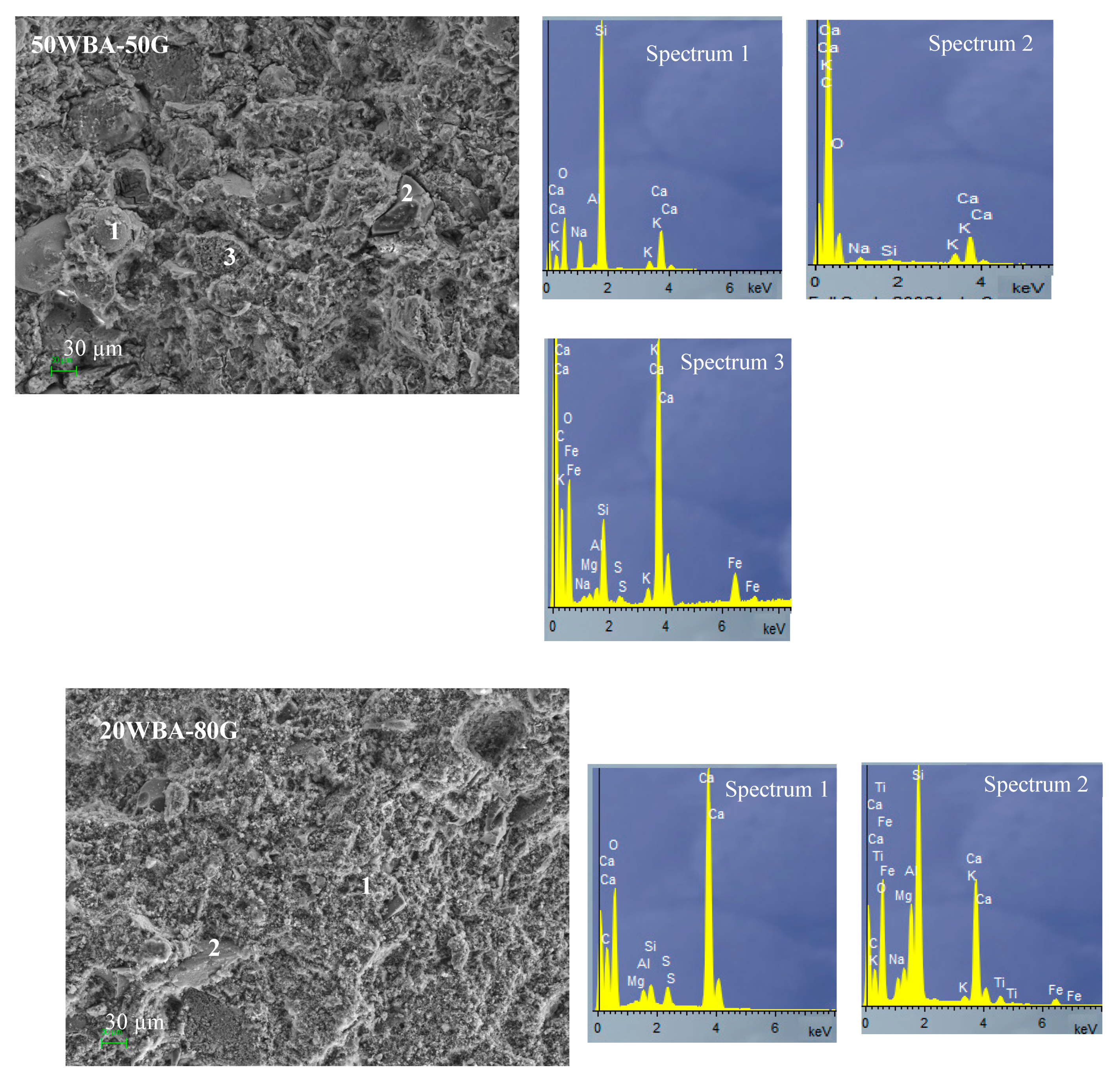
3.1. Characterization of Raw Materials
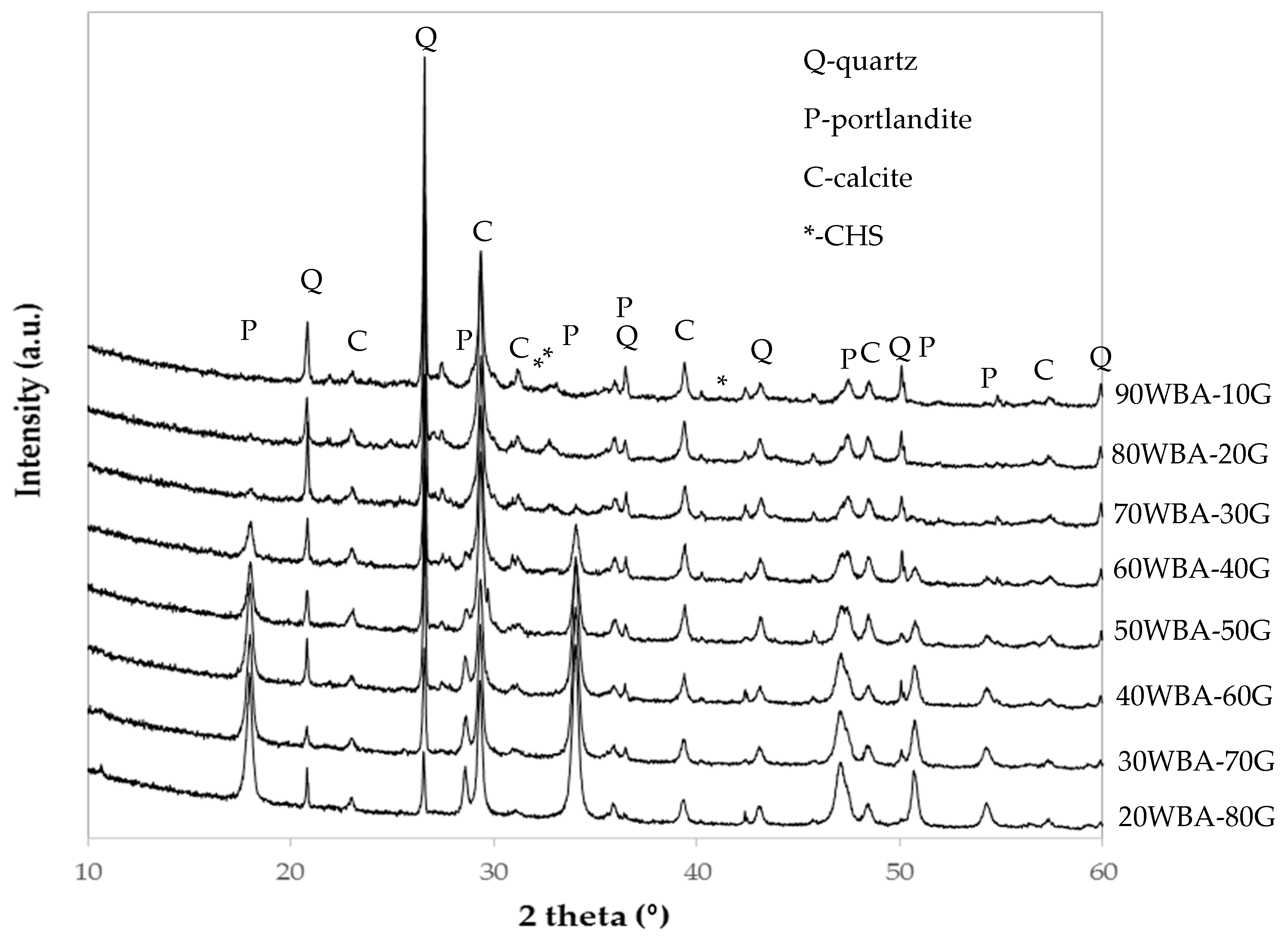
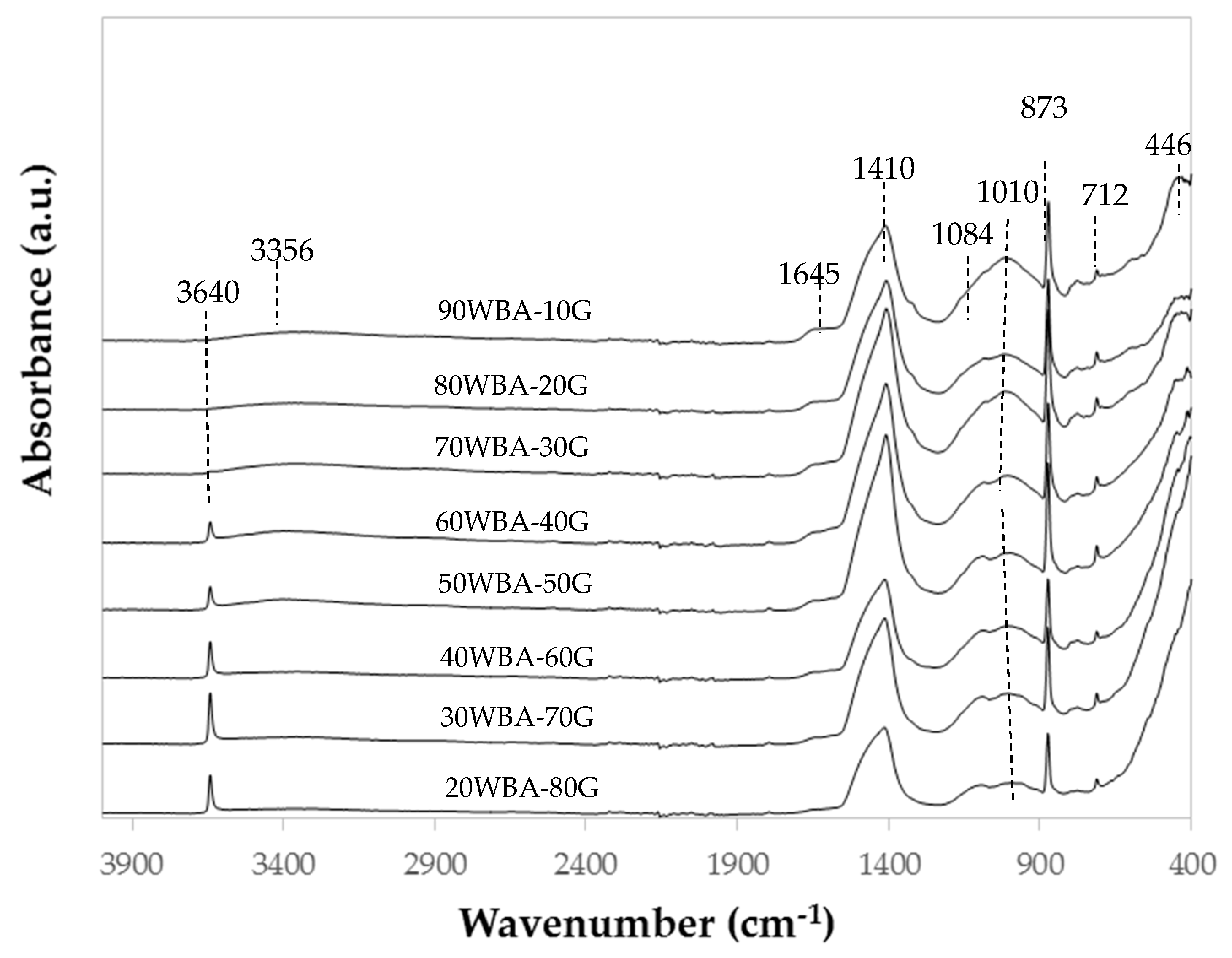
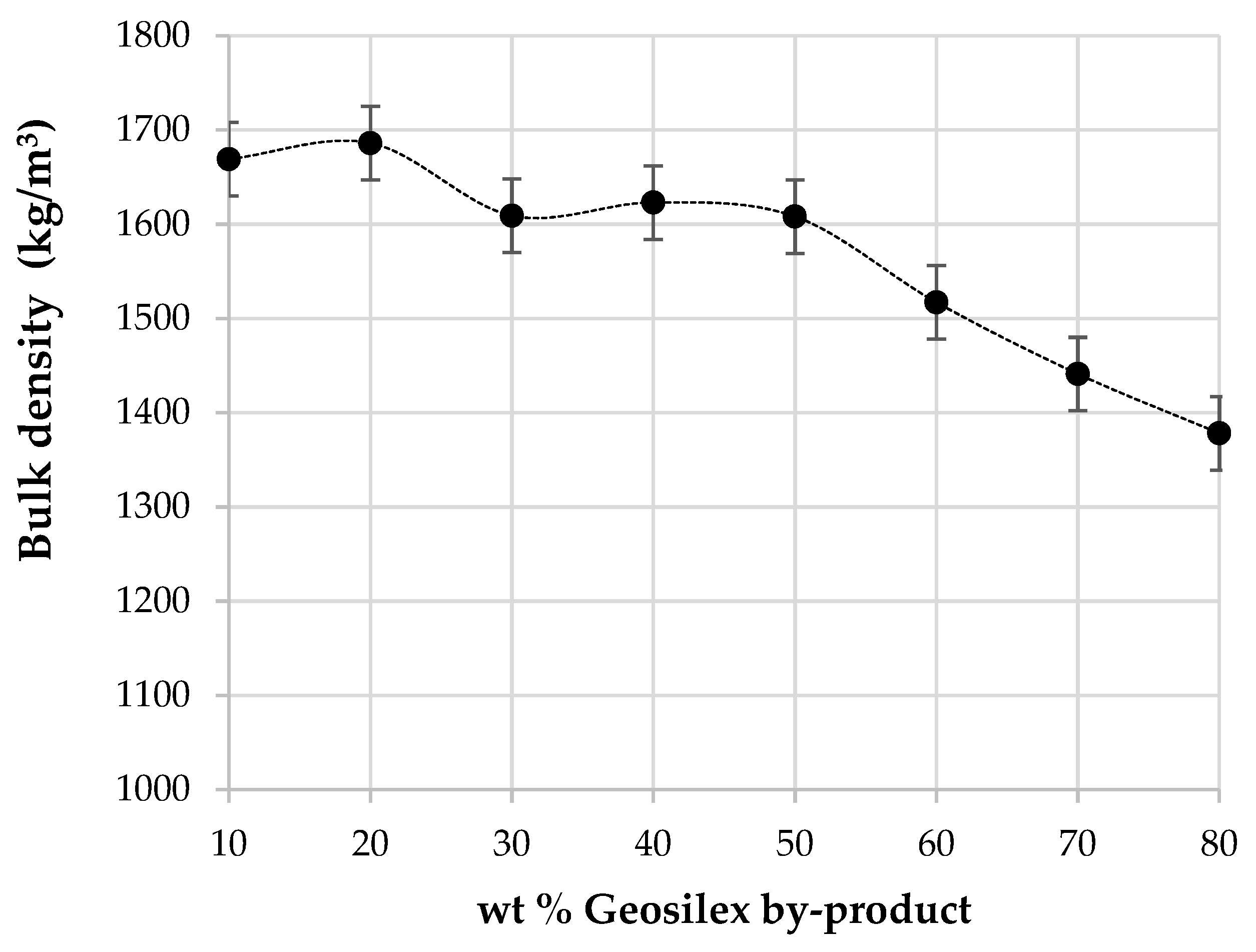
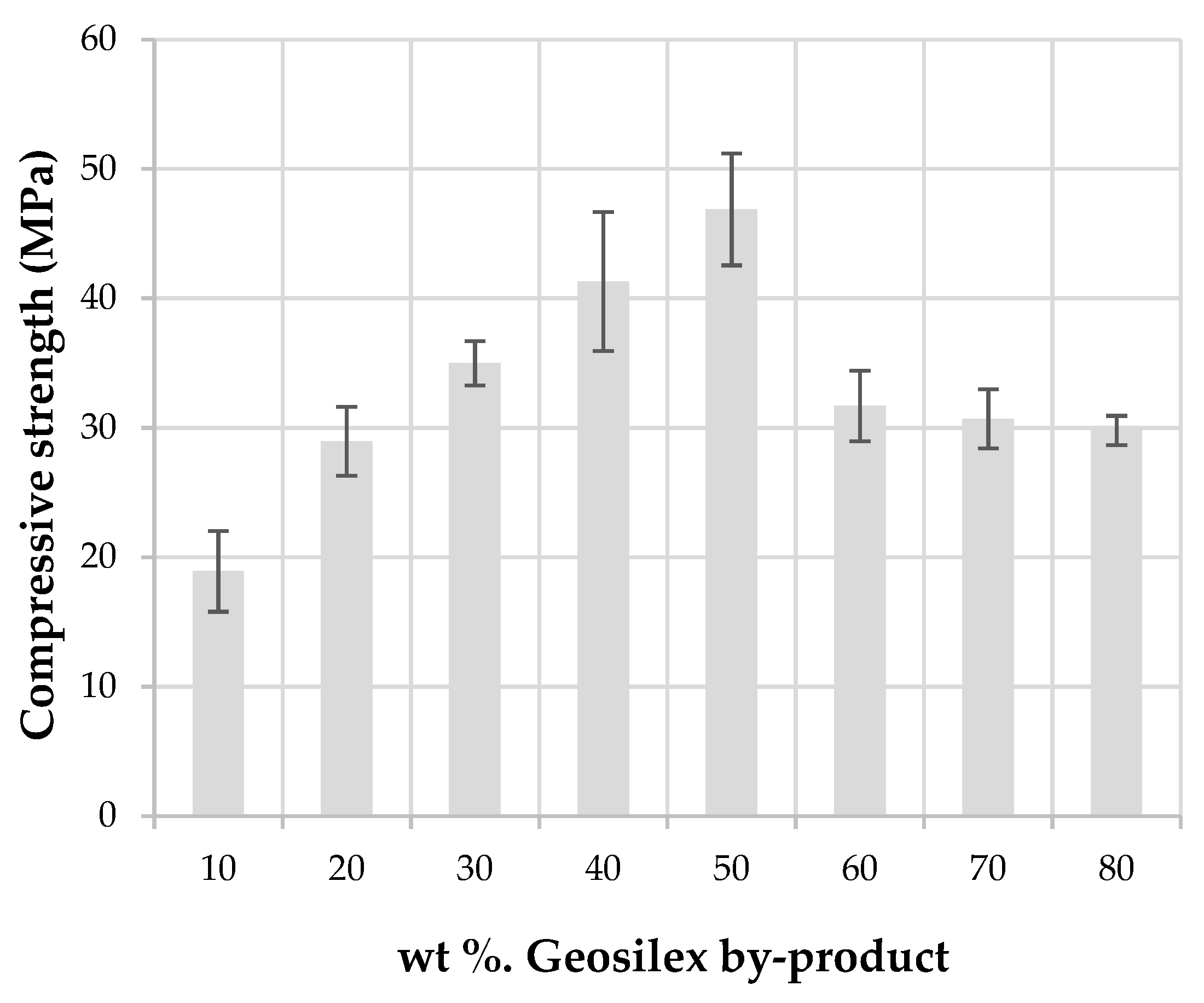
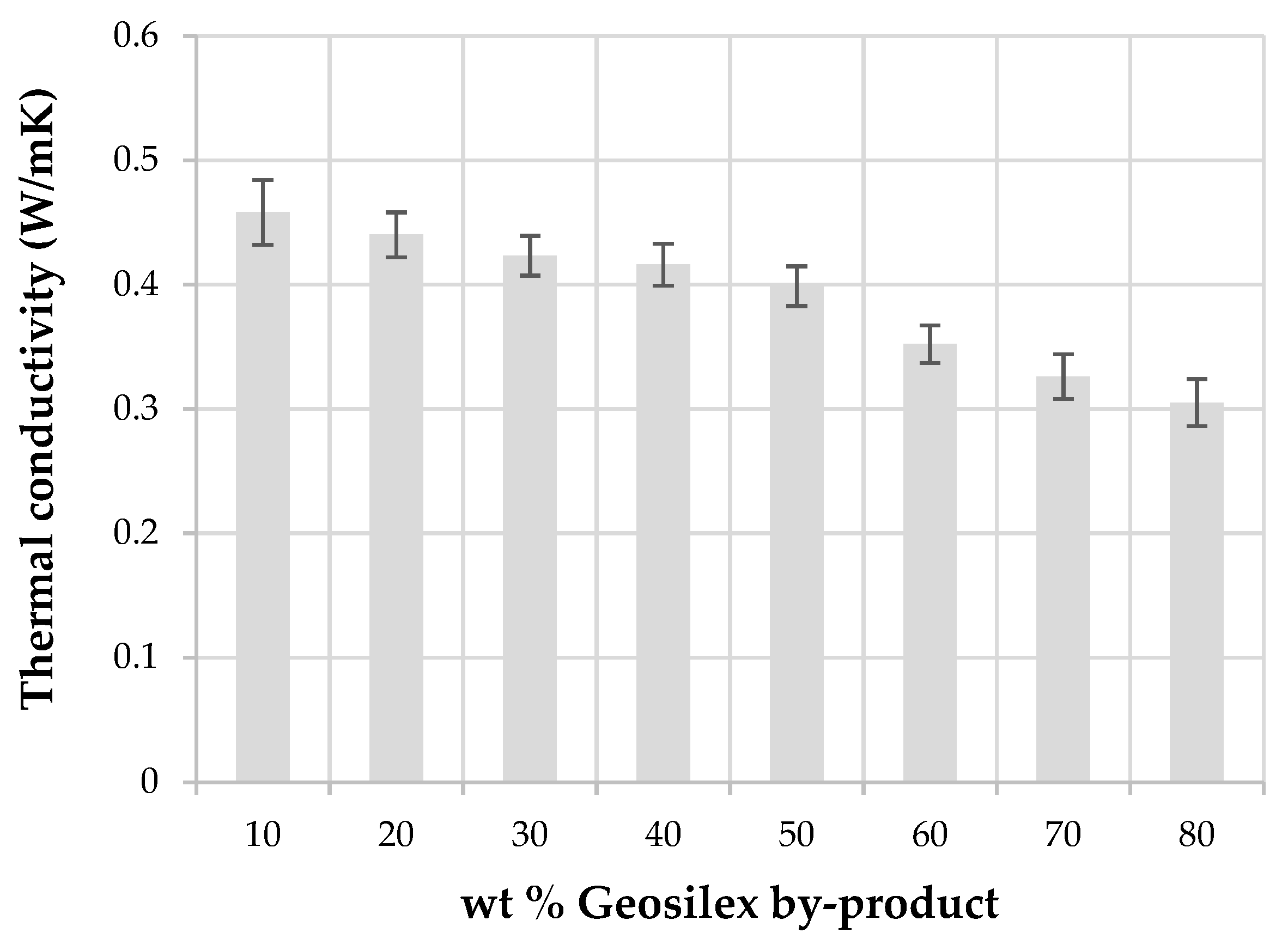
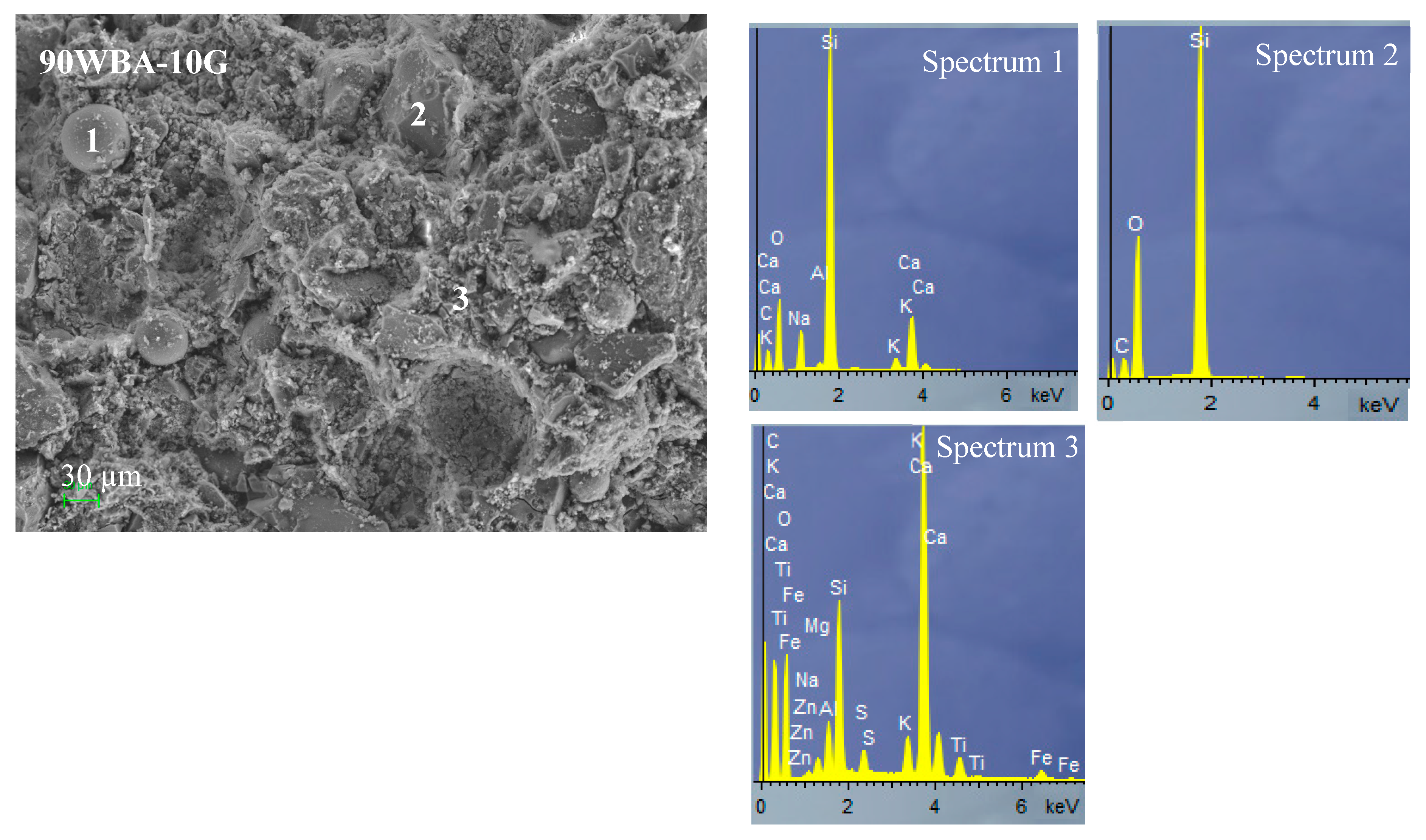
3.2. Characterization of the Calcium-Silicate Units
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Eurostat Waste Statistics, 2016. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Waste_statistics#Total_waste_generation (accessed on 10 December 2019).
- Hoornweg, D.; Bhada-Tata, P. What a waste: A global review of solid waste management. In Urban Development Series; Knowledge Papers no. 15.; World Bank: Washington, DC, USA, 2012; Available online: https://openknowledge.worldbank.org/handle/10986/17388 (accessed on 10 December 2019).
- European Commission. Environment Action Programme to 2020. Available online: http://ec.europa.eu/environment/action-programme/index.htm (accessed on 10 December 2019).
- European Commission. Eurostat. Environmental Data Centre on Natural Resources 2016. Energy from Biomass, 2018. Available online: https://ec.europa.eu/eurostat/web/environmental-data-centre-on-natural-resources/ natural resources / energy-resources/energy-from-biomass (accessed on 10 December 2019).
- Modolo, R.C.E.; Tarelho, L.A.C.; Teixeira, E.R.; Ferreira, V.M.; Labrincha, J.A. Treatment and use of bottom bed waste in biomass fluidized bed combustors. Fuel Process. Technol. 2014, 125, 170–181. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Picco, D. Technical Assistance for the Development and Improvement of Technologies, Methodologies and Tools for Enhanced Use of Agricultural Biomass Residues; Energy Plant Report; Central European Initiative: Trieste, Italy, 2010; p. 53. [Google Scholar]
- James, A.K.; Thring, R.W.; Rutherford, P.M.; Helle, S.S. Characterization of biomass bottom ash from an industrial scale fixed-bed boiler by fractionation. Energy Environ. Res. 2013, 3, 21–32. [Google Scholar] [CrossRef]
- Modolo, R.C.E.; Ferreira, V.M.; Tarelho, L.A.; Labrincha, J.A.; Senff, L.; Silva, L. Mortar formulations with bottom ash from biomass combustion. Constr. Build. Mater. 2013, 45, 275–281. [Google Scholar] [CrossRef]
- Maschio, S.; Tonello, G.; Piani, L.; Furlani, E. Fly and bottom ashes from biomass combustion as cement replacing components in mortars production: Rheological behaviour of the pastes and materials compression strength. Chemosphere 2011, 85, 666–671. [Google Scholar] [CrossRef]
- Agrela, F.; Cabrera, M.; Martín-Morales, M.; Zamorano, M.; Alshaaer, M. Biomass fly ash and biomass bottom ash. In New Trends in Eco-efficient and Recycled Concrete; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Cambridge, UK, 2019; pp. 23–58. [Google Scholar]
- García Calvo, J.L.; Hidalgo, A.; Alonso, M.C.; Luxán, M.P.; Fernández Luco, L. Characterization of Waste from Biomass Combustion Processes, Viability of Use as Construction Materials; XI National Congress of Materials: Zaragoza, Spain, 2010. [Google Scholar]
- Rajamma, R. Biomass Fly Ash Incorporation in Cement Based Materials. Ph.D. Thesis, Universidad de Aveiro, Aveiro, Portugal, 2011. [Google Scholar]
- Masia, A.T.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Proc. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Rajamma, R.; Ball, R.J.; Tarelho, L.A.; Allen, G.C.; Labrincha, J.A.; Ferreira, V.M. Characterisation and use of biomass fly ash in cement-based materials. J. Hazard. Mater. 2009, 172, 1049–1060. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Obernberger, I.; Biedermann, F.; Widmann, W.; Riedl, R. Concentrations of inorganic elements in biomass fuels and recovery in the different ash fractions. Biomass Bioenergy 1997, 12, 211–224. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Jensen, P.A.; Frandsen, F.; Glarborg, P. Release of Inorganic Elements during Wood-firing on a Grate. Impact of Fuel Quality on Power Production. In Proceedings: Impacts of Fuel Quality on Power Production; Electric Power Research Institute: Palo Alto, CA, USA, 2006; p. 1014551. [Google Scholar]
- Pels, J.R.; Sarabèr, A.J. Utilization of Biomass Ashes, Solid Biofuels for Energy: A Lower Greenhouse Gas Alternative. In Green Energy and Technology; Grammelis, P., Ed.; Springer: London, UK, 2011; pp. 219–235. [Google Scholar]
- Siddique, R. Utilization of wood ash in concrete manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Pavlíková, M.; Zemanová, L.; Záleská, M.; Pokorný, J.; Lojka, M.; Jankovský, O.; Pavlík, Z. Ternary Blended Binder for Production of a Novel Type of Lightweight Repair Mortar. Materials 2019, 12, 996. [Google Scholar] [CrossRef]
- Tosti, L.; Van Zomeren, A.; Pels, J.R.; Dijkstra, J.J.; Comans, R.N.J. Assessment of biomass ash applications in soil and cement mortars. Chemosphere 2019, 223, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Reuse of woody biomass fly ash in cement based materials. Constr. Build. Mater. 2015, 76, 286–296. [Google Scholar] [CrossRef]
- Cabrera, M.; Galvin, A.P.; Agrela, F.; Carvajal, M.D.; Ayuso, J. Characterisation and technical feasibility of using biomass bottom ash for civil infrastructures. Constr. Build. Mater. 2014, 58, 234–244. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash: Part 2. Potential utilisation, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Richaud, R.; Herod, A.A.; Kandiyoti, R. Comparison of trace element contents in low temperature and high-temperature ash from coals and biomass. Fuel 2004, 83, 2001–2012. [Google Scholar] [CrossRef]
- Cabrera, M.; Galvin, A.P.; Agrela, F.; Beltrán, M.G.; Ayuso, J. Reduction of Leaching Impacts by Applying Biomass Bottom Ash and Recycled Mixed Aggregates in Structural Layers of Roads. Materials 2016, 9, 228. [Google Scholar] [CrossRef]
- Grau, F.; Choo, H.; Wan Hu, J.; Jung, J. Engineering Behavior and Characteristics of Wood Ash and Sugarcane Bagasse Ash. Materials 2015, 8, 6962–6977. [Google Scholar] [CrossRef]
- El Moudni El Alami, S.; Moussaoui, R.; Monkade, M.; Lahlou, K.; Hasheminejad, N.; Margaritis, A.; Van den bergh, W.; Vuye, C. Lime Treatment of Coal Bottom Ash for Use in Road Pavements: Application to El Jadida Zone in Morocco. Materials 2019, 12, 2674. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Beltrán, M.G.; López, M.; Agrela, F. Effects of treatments on biomass bottom ash applied to the manufacture of cement mortars. J. Clean. Prod. 2017, 154, 424–435. [Google Scholar] [CrossRef]
- Pavlíková, M.; Zemanová, L.; Pokorný, J.; Záleská, M.; Jankovský, O.; Lojka, M.; Pavlík, Z. Influence of Wood-Based Biomass Ash Admixing on the Structural, Mechanical, Hygric, and Thermal Properties of Air Lime Mortars. Materials 2019, 12, 2227. [Google Scholar]
- Beltrán, M.G.; Agrela, F.; Barbudo, A.; Ayuso, J.; Ramírez, A. Mechanical and durability properties of concretes manufactured with biomass bottom ash and recycled coarse aggregates. Constr. Build. Mater. 2014, 72, 231–238. [Google Scholar] [CrossRef]
- Modolo, R.C.E.; Silva, T.; Senff, L.; Tarelho, L.A.C.; Labrincha, J.A.; Ferreira, V.M.; Silva, L. Bottom ash from biomass combustion in BFB and its use in adhesive-mortars. Fuel Process. Technol. 2005, 129, 192–202. [Google Scholar] [CrossRef]
- Anh Vu, V.; Cloutier, A.; Bissonnette, B.; Blanchet, P.; Duchesne, J. The Effect of Wood Ash as a Partial Cement Replacement Material for Making Wood-Cement Panels. Materials 2019, 12, 2766. [Google Scholar]
- Eliche-Quesada, D.; Felipe-Sesé, M.A.; López-Pérez, J.A.; Infantes-Molina, A. Characterization and evaluation of rice husk ash and wood ash in sustainable clay matrix bricks. Ceram. Int. 2017, 43, 463–475. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Felipe-Sesé, M.A.; Moreno-Molina, A.J.; Franco, F.; Infantes-Molina, A. Investigation of using bottom or fly pine-olive pruning ash to produce environmental friendly ceramic materials. Appl. Clay Sci. 2017, 135, 333–346. [Google Scholar] [CrossRef]
- Demis, S.; Tapali, J.G.; Papadakis, V.G. An investigation of the effectiveness of the utilization of biomass ashes as pozzolanic materials. Constr. Build. Mater. 2014, 68, 291–300. [Google Scholar] [CrossRef]
- Carrasco-Hurtado, B.; Corpas-Iglesias, F.A.; Cruz-Pérez, N.; Terrados-Cepeda, J.; Pérez-Villarejo, L. Addition of bottom ash from biomass in calcium silicate masonry units for use as construction material with thermal insulating properties. Contr. Build. Mater. 2014, 52, 155–165. [Google Scholar] [CrossRef]
- León, N.A.; Rojas-Reyes, N.R.; Umbarilla-Suárez, B.; Bustamante, R.M.O. Experimental Evaluation of silicon-calcareous units from blast furnace slags and hydraulic lime for masonry. Dyna 2009, 76, 247–254. [Google Scholar]
- Turgut, T. Manufacturing of building bricks without Portland cement. J. Clean. Prod. 2012, 37, 361–367. [Google Scholar] [CrossRef]
- Filipponi, P.; Polettini, A.; Pomi, R.; Sirini, P. Physical and mechanical properties of cement-based products containing incineration bottom ash. Waste Manag. 2003, 23, 145–156. [Google Scholar] [CrossRef]
- Liu, D.S.; Wang, C.Q.; Mei, X.D.; Zhang, C. Environmental performance, mechanical and microstructure analysis of non-fired bricks containing water-based drilling cuttings of shale gas. Constr. Build. Mater. 2018, 183, 215–225. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Sandalio-Pérez, J.A.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Investigation of use of coal fly ash in eco-friendly construction materials: Fired clay bricks and silica-calcareous non fired bricks. Ceram. Int. 2018, 44, 4400–4412. [Google Scholar] [CrossRef]
- ASTM D-2974. Standard Test Method for Moisture, Ash, Organic Matter of Peat and Other Organic Soils; American Society for Testing and Material: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Cebrián, J.L.; Pisonero, F. Determinación de la superficie específica por el método Blaine en cenizas volantes y cementos puzolánicos. Mater. Constr. 1987, 142, 81–91. [Google Scholar]
- UNE-EN 12390-2. Testing Hardened Concrete—Part 2: Making and Curing Specimens for Strength Tests; Asociación Española de Normalización (AENOR): Madrid, Spain, 2009. [Google Scholar]
- UNE-EN 772-16. Methods of Test for Masonry Units—Part 16: Determination of Dimensions; Asociación Española de Normalización (AENOR): Madrid, Spain, 2011. [Google Scholar]
- UNE-EN 772-21. Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption; Asociación Española de Normalización (AENOR): Madrid, Spain, 2011. [Google Scholar]
- UNE-EN 772-1. Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength; Asociación Española de Normalización (AENOR): Madrid, Spain, 2011. [Google Scholar]
- ISO 8302:1991. Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties—Guarded Hot Plate Apparatus; Asociación Española de Normalización (AENOR): Madrid, Spain, 1991. [Google Scholar]
- Restrepo, J.C.; Restrepo, O.J.; Tobón, J.I. Effects of the addition of metakaolin in Portland cement. Dyna 2006, 150, 131–141. [Google Scholar]
- Qin, L.; Gao, X.; Zhang, A. Potential application of portland cement-calcium sulfoaluminate cement blends to avoid early age frost damage. Constr. Build. Mater. 2018, 190, 363–372. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C-S-H gels. FTIR analysis. Cement Concr. Res. 2009, 3, 147–153. [Google Scholar] [CrossRef]
- Lecome, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. Microstructural comparison between gepolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 16, 3789–3797. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, Q. Upcycling carbon dioxide to improve mechanical strength of Portland cement. J. Clean. Prod. 2018, 196, 726–738. [Google Scholar] [CrossRef]
- Ylmén, R.; Wadsö, L.; Panas, I. Insights into early hydration of Portland limestone cement from infrared spectroscopy and isothermal calorimetry. Cem. Concr. Res. 2010, 40, 1541–1546. [Google Scholar] [CrossRef]
- Geng, L.Y.; Pei, W.M.; Xiaohua, Z. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar]
- Rai, U.S.; Singh, R.K. Effect of polyacrylamide on the different properties of cement and mortar. Mater. Sci. Eng. A 2005, 392, 42–50. [Google Scholar] [CrossRef]
- Russell, J.D. Infrared methods. In A Handbook of Determinative Methods in Clay Mineralogy; Wilson, M.J., Ed.; Chapman and Hall: London, UK, 1987. [Google Scholar]
- Cultrone, G.; Cazalla, O.; Rodríguez, C.; de la Torre, M.J.; Sebastián, E. Técnicas no destructivas aplicadas a la conservación del patrimonio arquitectónico. Colorimetría. PH Boletín del Instituto Andaluz del Patrimonio Histórico 2005, 53, 6–10. [Google Scholar] [CrossRef]
- ASTM C67–07. Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile; American Society for Testing and Material: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Demirboga, R. Influence of mineral admixtures on thermal conductivity and compressive strength of mortar. Energy Build. 2003, 35, 189–192. [Google Scholar] [CrossRef]
- Olmeda, J.; Sánchez de Roja, M.I.; Frías, M.; Donatello, S.; Cheeseman, C.R. Effect of petroleum (pet) coke addition on the density and thermal conductivity of cement pastes and mortars. Fuel 2003, 107, 138–146. [Google Scholar] [CrossRef]




| Sample | WBA(g) | G(g) | Water (g) | WBA wt% | G wt% | Water wt% Total |
|---|---|---|---|---|---|---|
| 90WBA-10G | 360 | 40 | 50 | 90 | 10 | 12.5 |
| 80WBA-20G | 320 | 80 | 48 | 80 | 20 | 12.0 |
| 70WBA-30G | 280 | 120 | 44 | 70 | 30 | 11.0 |
| 60WBA-40G | 240 | 160 | 40 | 60 | 40 | 10.0 |
| 50WBA-50G | 200 | 200 | 38 | 50 | 50 | 9.5 |
| 40WBA-60G | 160 | 240 | 36 | 40 | 60 | 9.0 |
| 30WBA-70G | 120 | 280 | 34 | 30 | 70 | 8.5 |
| 20WBA-80G | 80 | 320 | 32 | 20 | 80 | 8.0 |
| Raw Material | Organic Matter (a) (%) | Carbonate Content (%) | pH | Specific Surface Area (cm2/g) | Relative Density (kg/m3) |
|---|---|---|---|---|---|
| WBA | 10.41 ± 0.09 | 17.25 ± 0.76 | 11.1 | 3600 | 2731 |
| G | 3.12 ± 0.12 | 16.6 ±0.55 | 12.5 | 6224 | 2378 |
| Oxide Content (%) | WBA | G |
|---|---|---|
| SiO2 | 48.6 | 1.9 |
| Al2O3 | 5.9 | 1.1 |
| Fe2O3 | 3.3 | 0.12 |
| CaO | 18.1 | 67.2 |
| MgO | 3.2 | 0.09 |
| MnO | 0.05 | - |
| Na2O | 0.92 | - |
| K2O | 1.9 | - |
| TiO2 | 1.4 | 0.04 |
| P2O5 | 0.5 | 0.01 |
| SO3 | 0.14 | 1.6 |
| ZnO | 0.29 | - |
| SrO | 0.04 | - |
| Cl | 0.06 | 0.03 |
| LOI | 15.6 | 27.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felipe-Sesé, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Wood Bottom Ash and GeoSilex: A By-Product of the Acetylene Industry as Alternative Raw Materials in Calcium Silicate Units. Materials 2020, 13, 489. https://doi.org/10.3390/ma13020489
Felipe-Sesé MA, Pérez-Villarejo L, Castro E, Eliche-Quesada D. Wood Bottom Ash and GeoSilex: A By-Product of the Acetylene Industry as Alternative Raw Materials in Calcium Silicate Units. Materials. 2020; 13(2):489. https://doi.org/10.3390/ma13020489
Chicago/Turabian StyleFelipe-Sesé, Manuel Angel, Luis Pérez-Villarejo, Eulogio Castro, and Dolores Eliche-Quesada. 2020. "Wood Bottom Ash and GeoSilex: A By-Product of the Acetylene Industry as Alternative Raw Materials in Calcium Silicate Units" Materials 13, no. 2: 489. https://doi.org/10.3390/ma13020489
APA StyleFelipe-Sesé, M. A., Pérez-Villarejo, L., Castro, E., & Eliche-Quesada, D. (2020). Wood Bottom Ash and GeoSilex: A By-Product of the Acetylene Industry as Alternative Raw Materials in Calcium Silicate Units. Materials, 13(2), 489. https://doi.org/10.3390/ma13020489







