Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
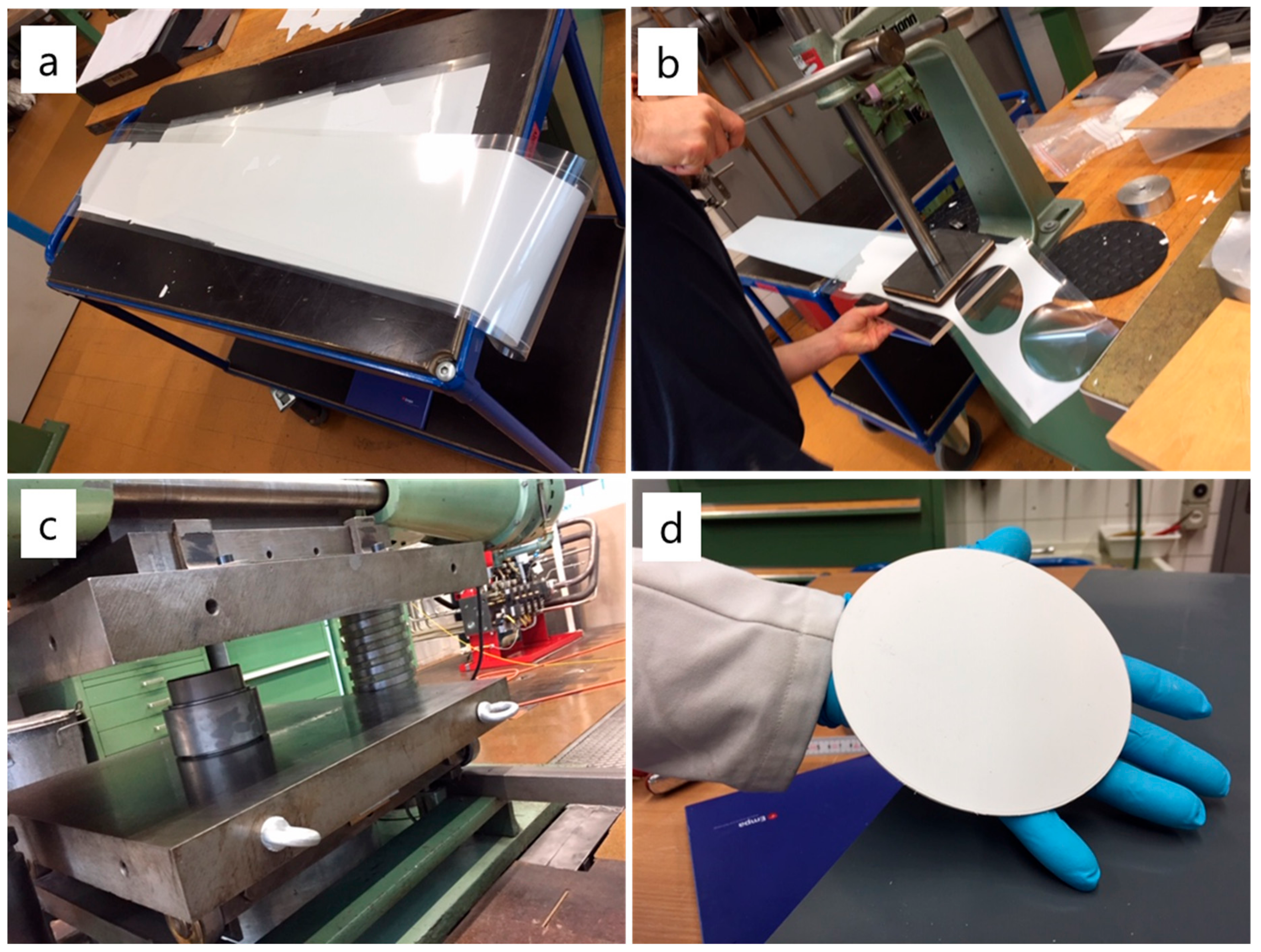
2.2. Slurry Preparation and Tape Casting
2.3. Punching and Lamination
2.4. Debinding and Sintering
2.5. Na+ Conductivity Measurement
2.6. Sample Dimensions and Tolerance Determination
3. Results and Discussion
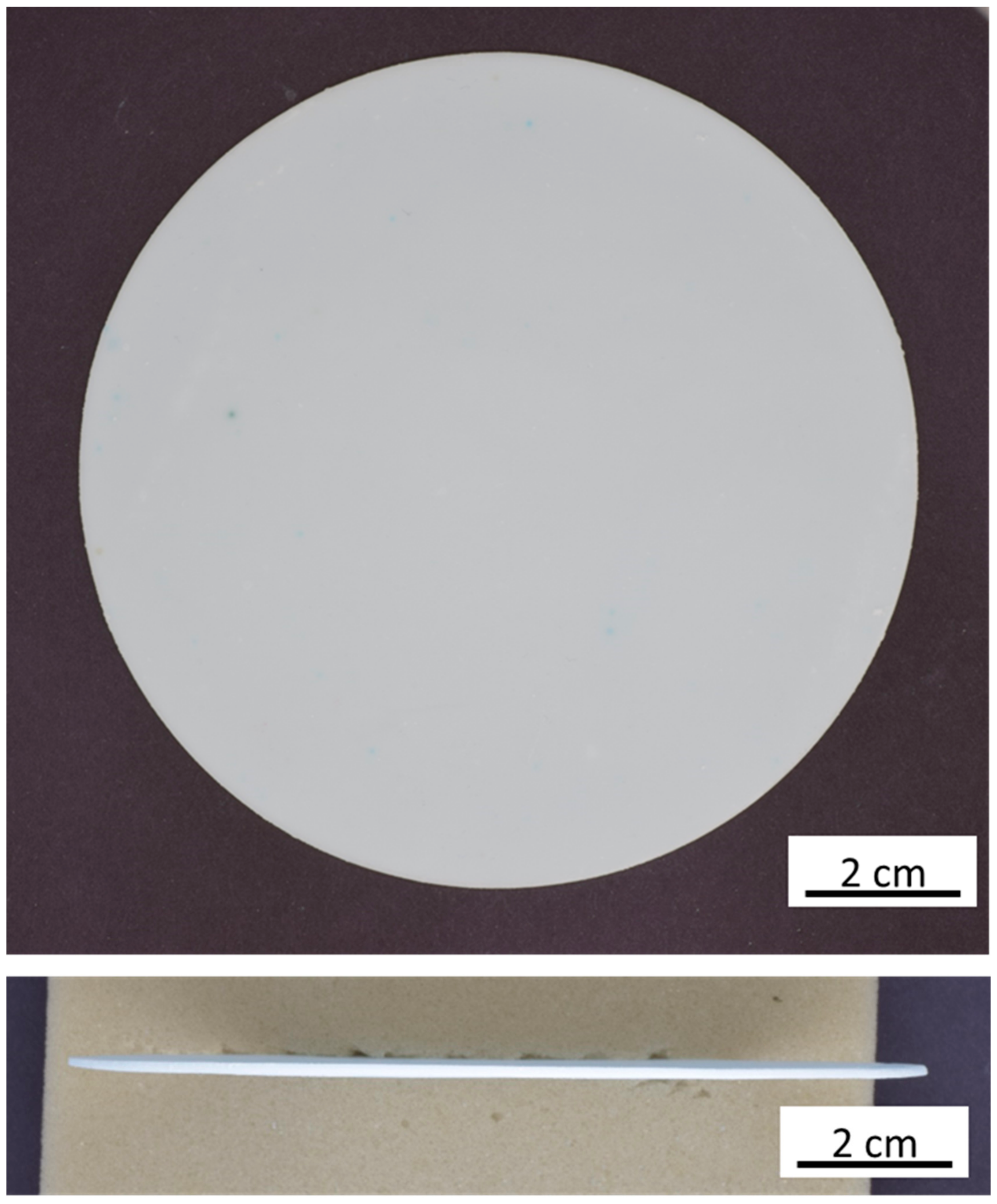
3.1. Tape Casting and Warm Lamination
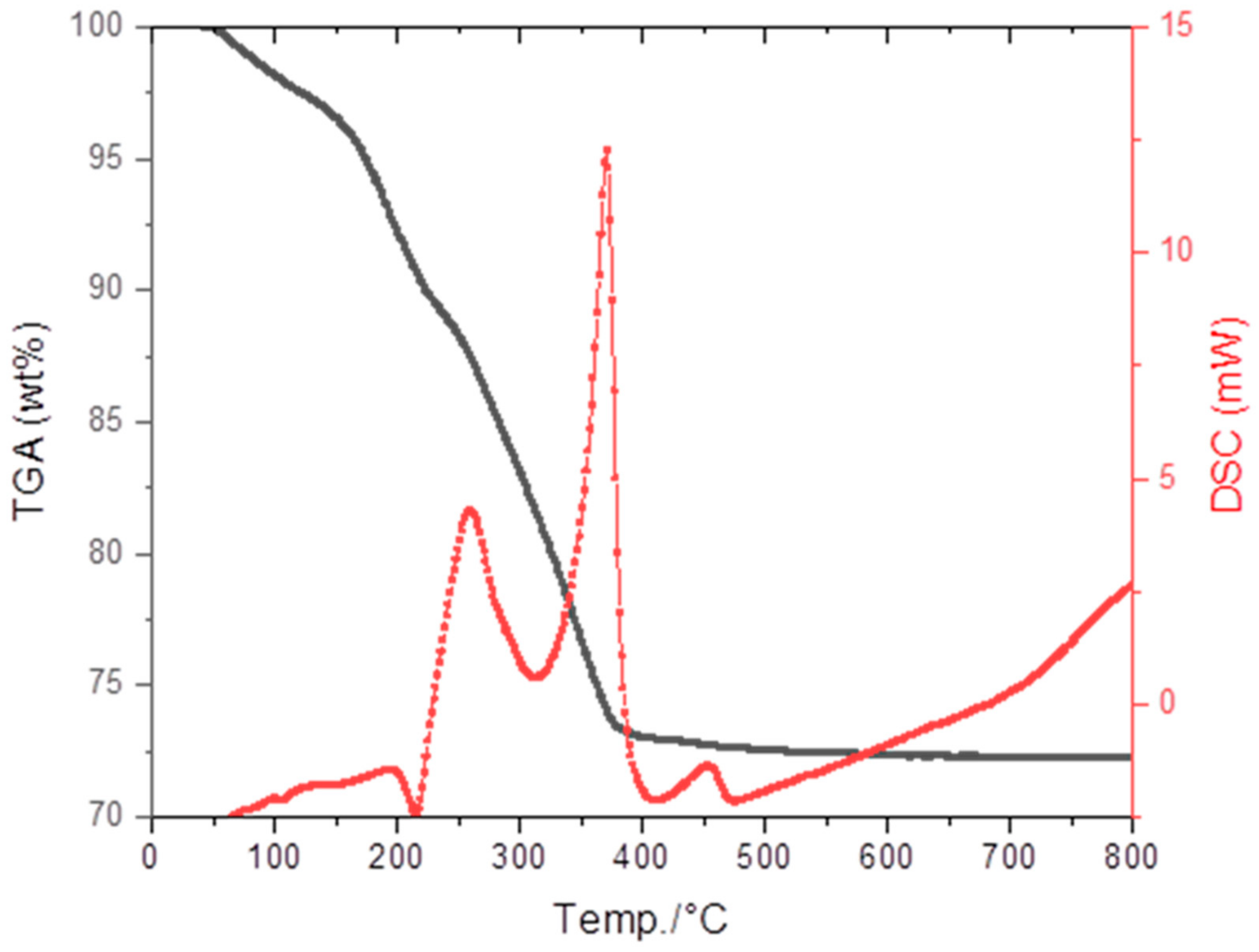
3.2. Debinding and Sintering
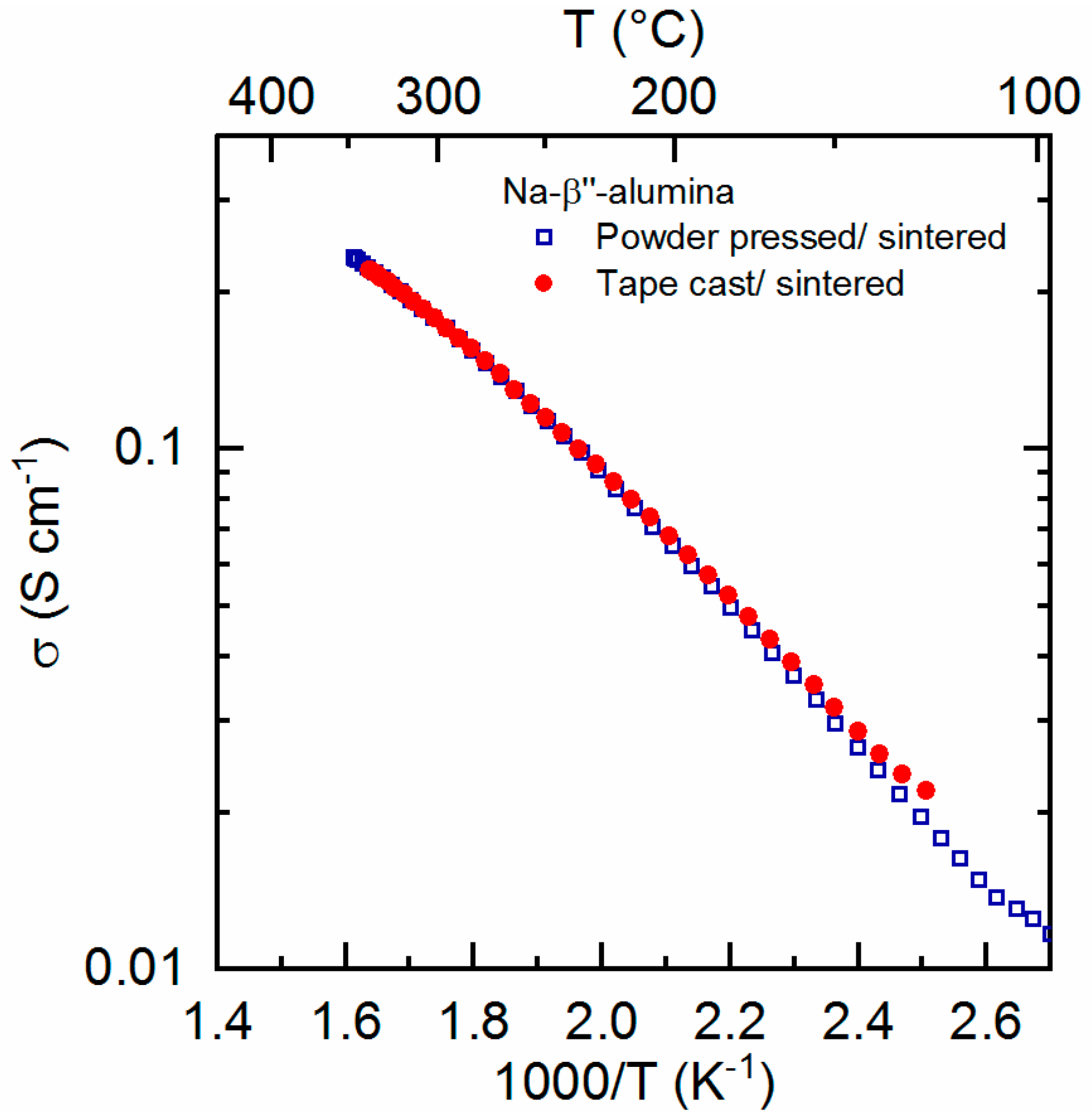
3.3. Mechanical and Electrical Properties
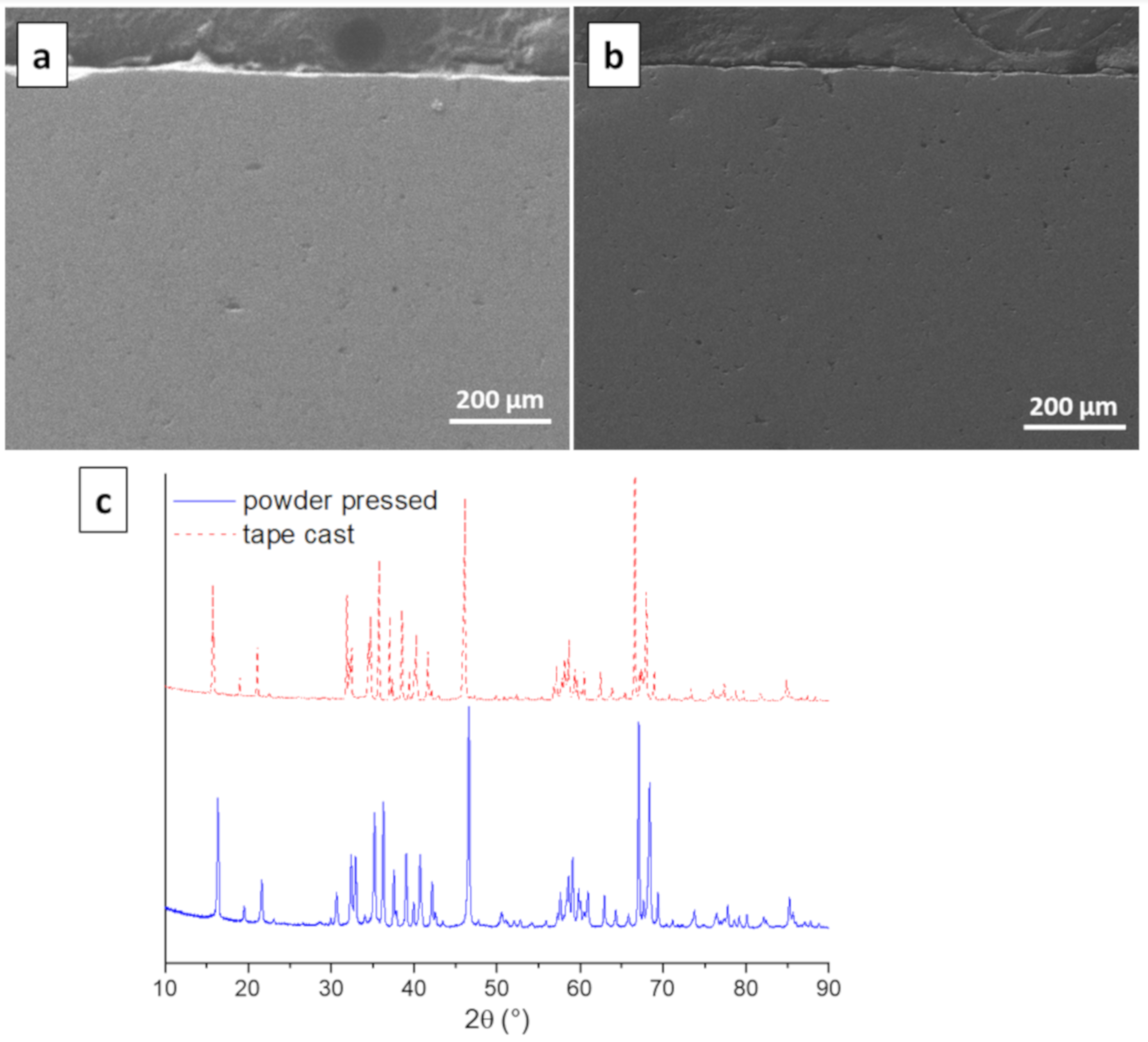
3.4. Morphology and Microstructure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dustmann, C.-H. Advances in ZEBRA batteries. J. Power Sources 2004, 127, 85–92. [Google Scholar] [CrossRef]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Nikiforidis, G.; van de Sanden, M.C.M.; Tsampas, M.N. High and intermediate temperature sodium–sulfur batteries for energy storage: Development, challenges and perspectives. RSC Advances 2019, 9, 5649–5673. [Google Scholar] [CrossRef]
- Hueso, K.B.; Armand, M.; Rojo, T. High temperature sodium batteries: Status, challenges and future trends. Energy Environ. Sci. 2013, 6, 734. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent progress on sodium ion batteries: Potential high-performance anodes. Energy Env. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Lu, X.; Lemmon, J.P.; Sprenkle, V.; Yang, Z. Sodium-beta alumina batteries: Status and challenges. JOM 2010, 62, 31–36. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, J.; Ji, G. Effect of Na2O content on properties of beta alumina solid electrolytes. Mater. Sci. Semicond. Process. 2015, 31, 487–492. [Google Scholar] [CrossRef]
- Ghadbeigi, L.; Szendrei, A.; Moreno, P.; Sparks, T.D.; Virkar, A.V. Synthesis of iron-doped Na-β”-alumina + yttria-stabilized zirconia composite electrolytes by a vapor phase process. Solid State Ionics 2016, 290, 77–82. [Google Scholar] [CrossRef]
- Lu, X.; Coffey, G.; Meinhardt, K.; Sprenkle, V.; Yang, Z.; Lemmon, J.P. High Power Planar Sodium-Nickel Chloride Battery. ECS Trans. 2010, 28, 7–13. [Google Scholar]
- Kim, G.; Park, Y.-C.; Lee, Y.; Cho, N.; Kim, C.-S.; Jung, K. The effect of cathode felt geometries on electrochemical characteristics of sodium sulfur (NaS) cells: Planar vs. tubular. J. Power Sources 2016, 325, 238–245. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-M.; Jung, K.; Park, Y.-C.; Cho, N.-u.; Kim, H.-S. Feasibility Study of a Planar-type Sodium–Nickel Chloride Battery. Bull. Korean Chem. Soc. 2016, 37, 695–699. [Google Scholar] [CrossRef]
- Ionotec Conductive Ceramics. Available online: www.ionotec.com (accessed on 23 September 2019).
- Virkar, A.V.; Miller, G.R.; Gordon, R.S. Resistivity-microstructure relations in lithia-stabilized polycrystalline β”-alumina. J. Am. Ceram. Soc. 1978, 61, 250. [Google Scholar] [CrossRef]
- Duncan, G.K.; West, A.R. Formation of beta aluminas in the system Li2ONa2OAl2O3. Solid State Ionics 1983, 9–10, 259–264. [Google Scholar] [CrossRef]
- Ligon, S.C.; Blugan, G.; Bay, M.-C.; Battaglia, C.; Heinz, M.V.F.; Graule, T. Performance analysis of Na-β″-Al2O3/YSZ solid electrolytes produced by conventional sintering and by vapor conversion of α-Al2O3/YSZ. Solid State Ionics 2020, 345, 115169. [Google Scholar] [CrossRef]
- Lu, X.; Evans, J.R.G.; Heavens, S.N. A comparison of the tape casting of α- and β-alumina. J. Eur. Ceram. Soc. 2012, 32, 4219–4228. [Google Scholar] [CrossRef]
- Hu, Y.; Heavens, S.N.; Blackburn, J.S.; Blackburn, S. Extrusion process for the manufacture of beta’’-alumina solid electrolyte tubes. J. Ceram. Sci. Tech. 2017, 8, 25–30. [Google Scholar]
- Mahmud, L.S.; Muchtar, A.; Somalu, M.R. Challenges in fabricating planar solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2017, 72, 105–116. [Google Scholar]
- Fleischhauer, F.; Bermejo, R.; Danzer, R.; Mai, A.; Graule, T.; Kuebler, J. High temperature mechanical properties of zirconia tapes used for electrolyte supported solid oxide fuel cells. J. Power Sources 2015, 273, 237–243. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ma, J.S. Preparation of Green Tapes for LTCC/Cu Multilayer Substrates. Mater. Sci. Forum 2007, 546–549, 2279–2282. [Google Scholar] [CrossRef]
- Bay, M.-C.; Heinz, M.V.F.; Figi, R.; Schreiner, C.; Basso, D.; Zanon, N.; Vogt, U.F.; Battaglia, C. Impact of liquid phase formation on microstructure and conductivity of Li-stabilized Na-β″-alumina ceramics. ACS Appl. Energy Mater. 2019, 2, 687–693. [Google Scholar] [CrossRef]
| Sintering Conditions | Diameter (mm) | Thickness (mm) | PP (mm) |
|---|---|---|---|
| Atop fresh β″-Al2O3 powder | 109.15 ± 0.15 | 1.61 ± 0.079 | 0.93 |
| Atop fresh β″-Al2O3 powder | 109.17 ± 0.23 | 1.41 ± 0.098 | 1.6 |
| Atop presintered β″-Al2O3 powder | 110.44 ± 0.16 | 1.63 ± 0.098 | 1.8 |
| Atop fresh/presintered β″-Al2O3 mix | 111.29 ± 0.19 | 1.38 ± 0.038 | 1.0 |
| Beneath fresh β″-Al2O3 powder | 110.04 ± 0.08 | 1.59 ± 0.055 | 1.0 |
| Beneath presintered β″-Al2O3 powder | 109.99 ± 0.15 | 1.16 ± 0.023 | 0.13 |
| Powder Pressing | Tape Casting/Lamination | |
|---|---|---|
| Sinter shrinkage (x/y) | 23.2% | 22.9% |
| Sinter shrinkage (z) | 23.1% | 27.3% |
| Relative density (%) | 96.8% | 98.1% |
| Flexural strength (MPa) | 127 ± 13 | 144 ± 14 |
| Conductivity at 300 °C (S cm−1) | 0.18 | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligon, S.C.; Bay, M.-C.; Heinz, M.V.F.; Battaglia, C.; Graule, T.; Blugan, G. Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries. Materials 2020, 13, 433. https://doi.org/10.3390/ma13020433
Ligon SC, Bay M-C, Heinz MVF, Battaglia C, Graule T, Blugan G. Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries. Materials. 2020; 13(2):433. https://doi.org/10.3390/ma13020433
Chicago/Turabian StyleLigon, Samuel Clark, Marie-Claude Bay, Meike V. F. Heinz, Corsin Battaglia, Thomas Graule, and Gurdial Blugan. 2020. "Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries" Materials 13, no. 2: 433. https://doi.org/10.3390/ma13020433
APA StyleLigon, S. C., Bay, M.-C., Heinz, M. V. F., Battaglia, C., Graule, T., & Blugan, G. (2020). Large Planar Na-β″-Al2O3 Solid Electrolytes for Next Generation Na-Batteries. Materials, 13(2), 433. https://doi.org/10.3390/ma13020433






