Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low-Frequency Microwave Absorbing Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Plating of Nickel Cover on Carbon Fiber Surface
2.2. Preparation of Carbon Fiber–Silica Aerogel Nanocomposite
2.3. Characterization Methods
3. Results and Discussion
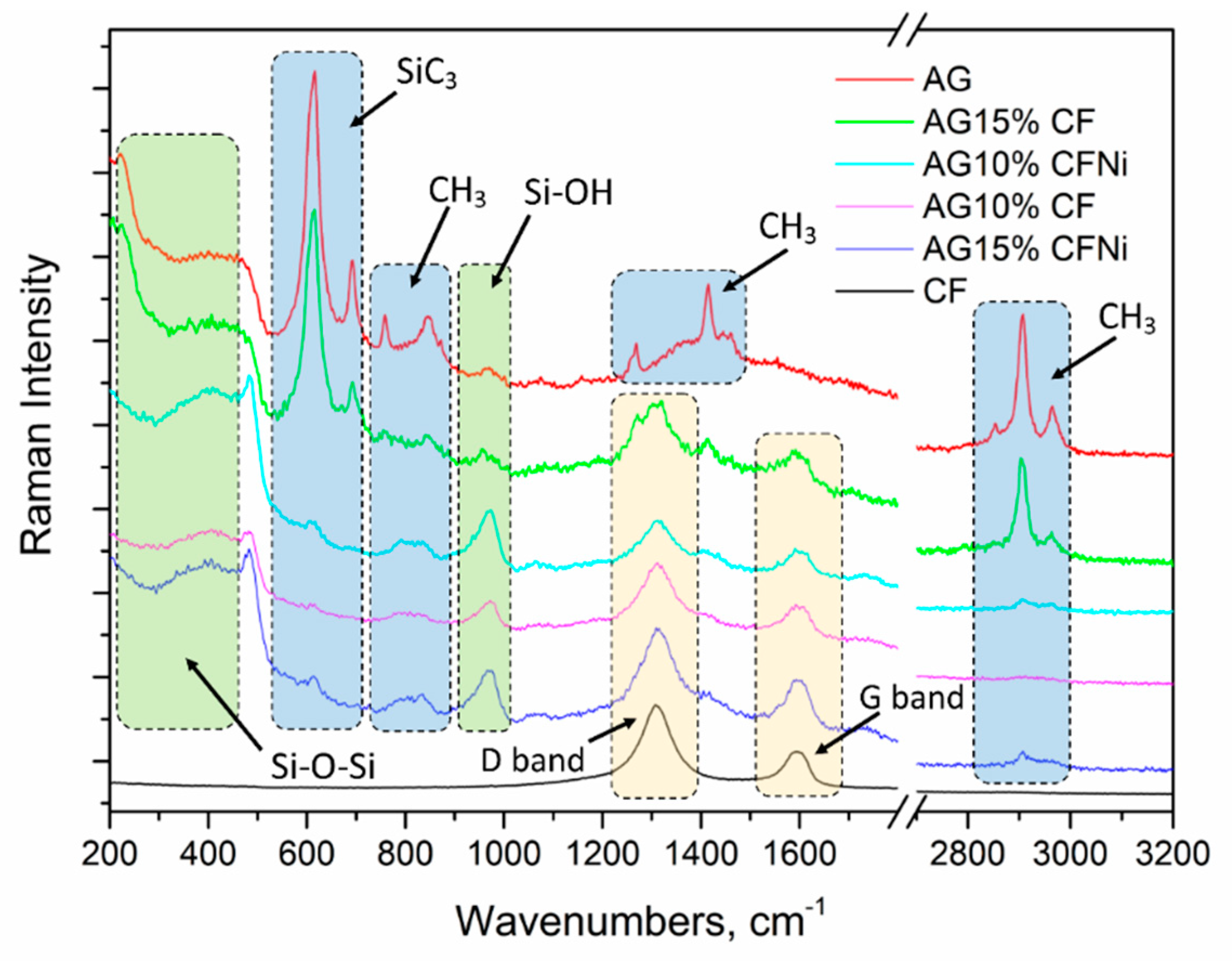
3.1. Physical and Chemical Characterization of Carbon Fibers, Nickel-Coated Carbon Fibers, and Pure Silica Aerogel
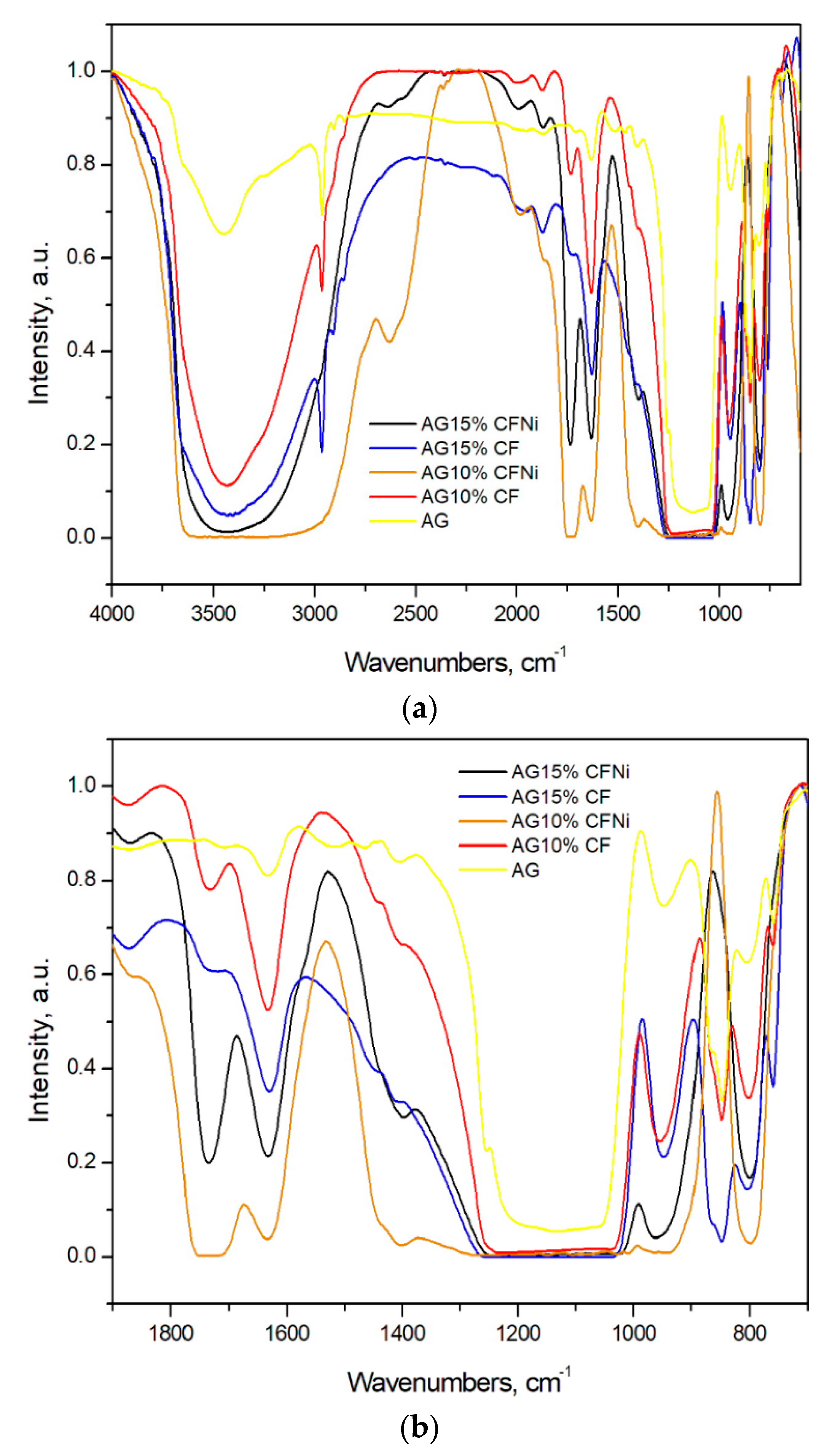
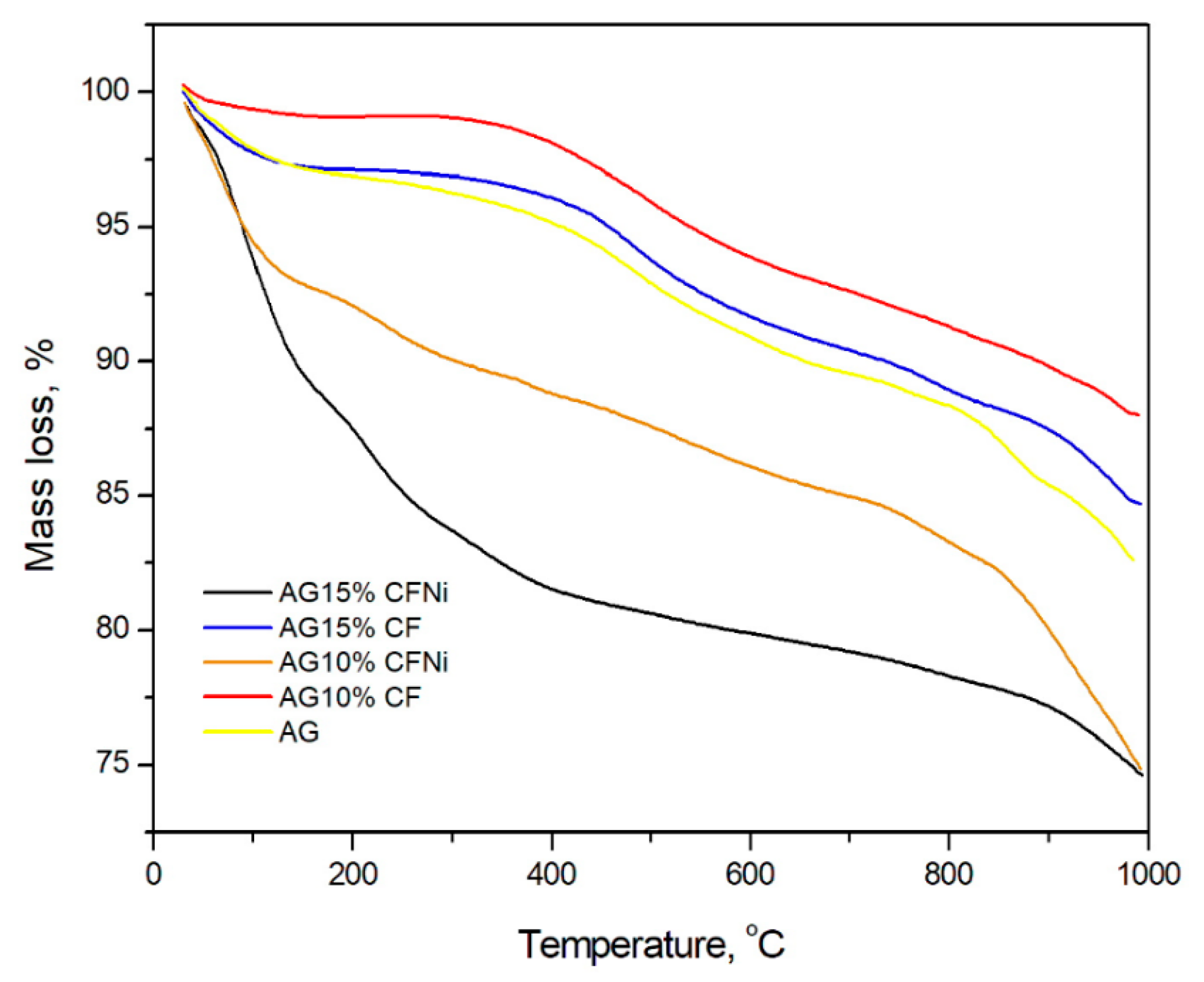
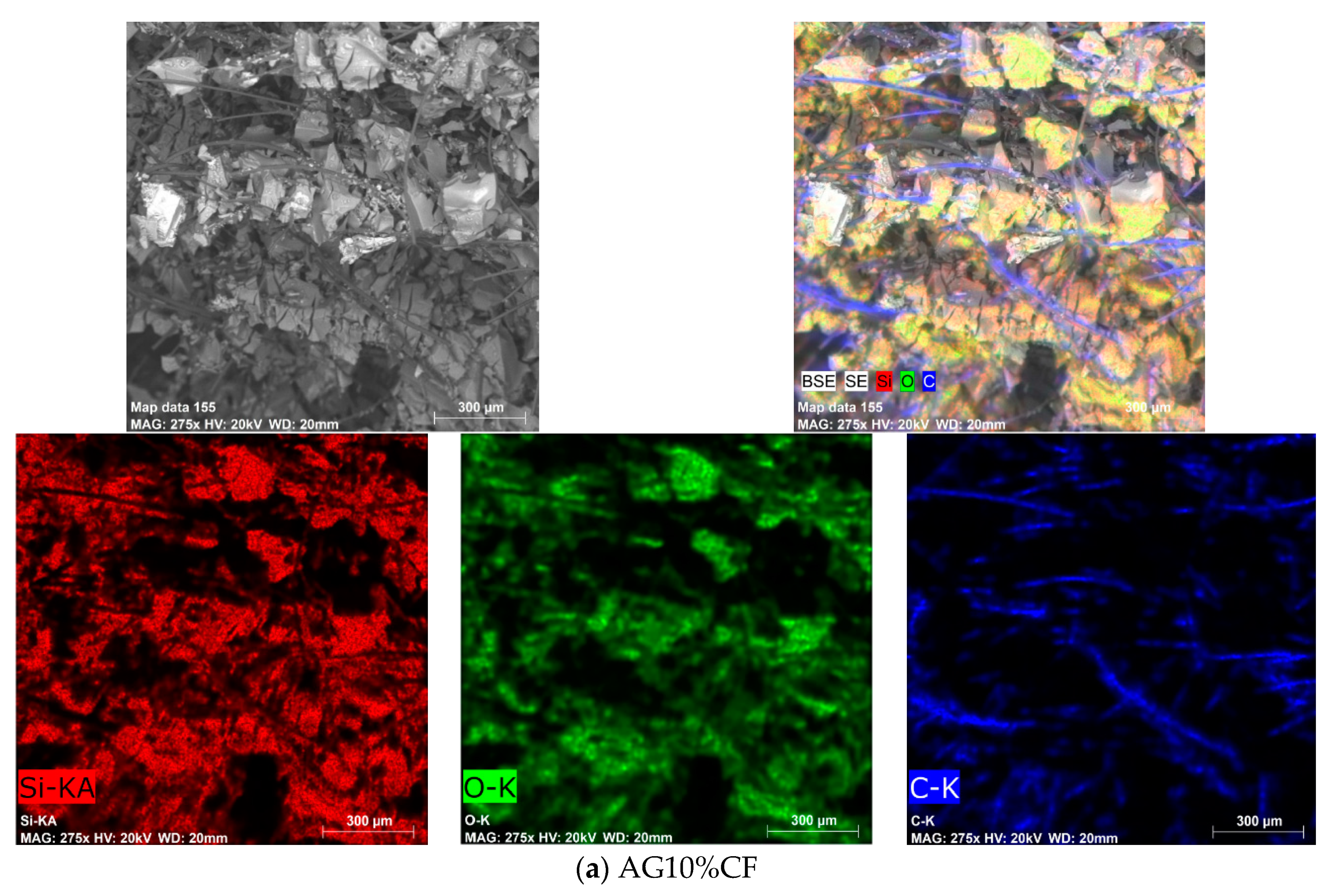
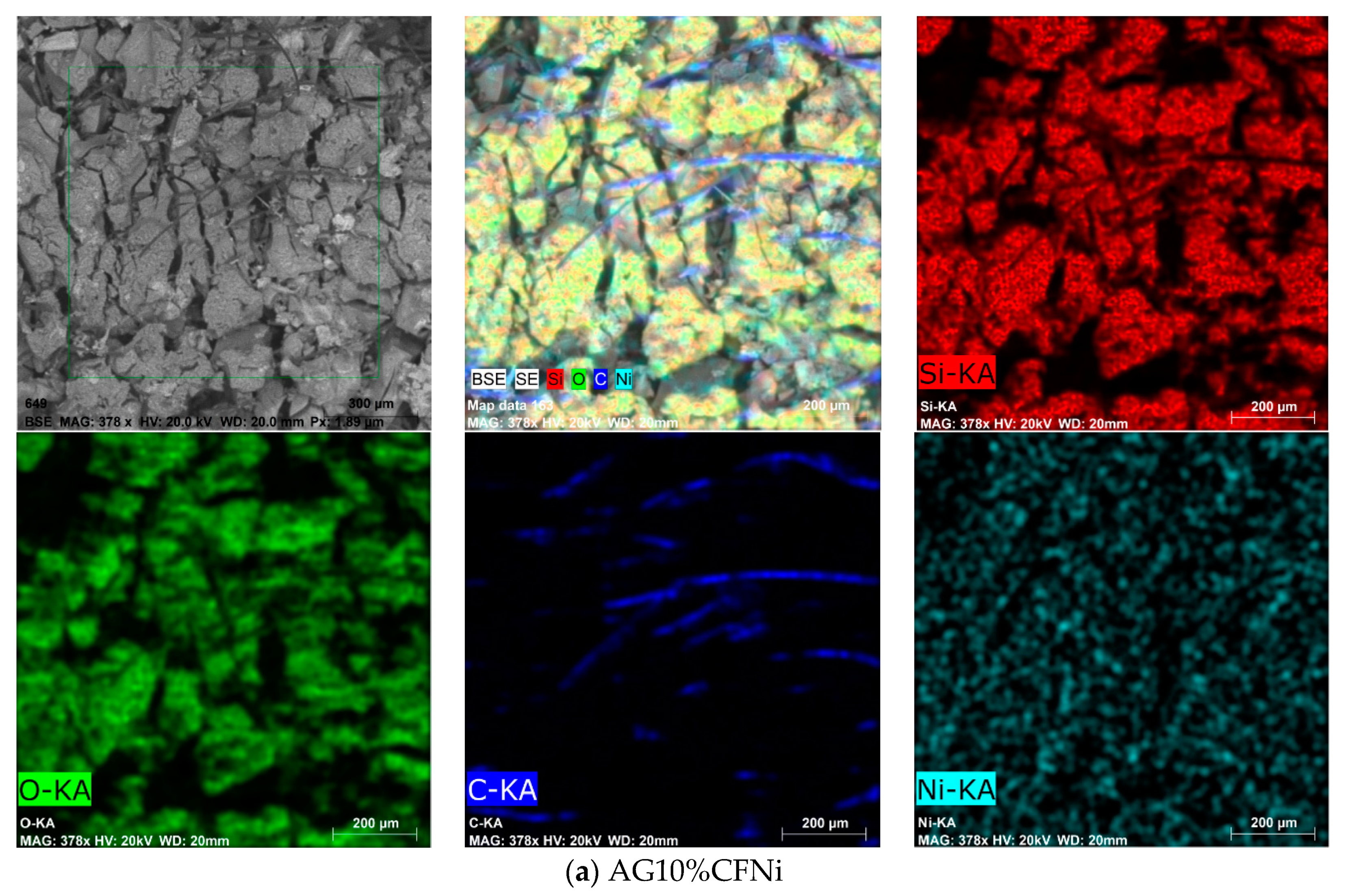
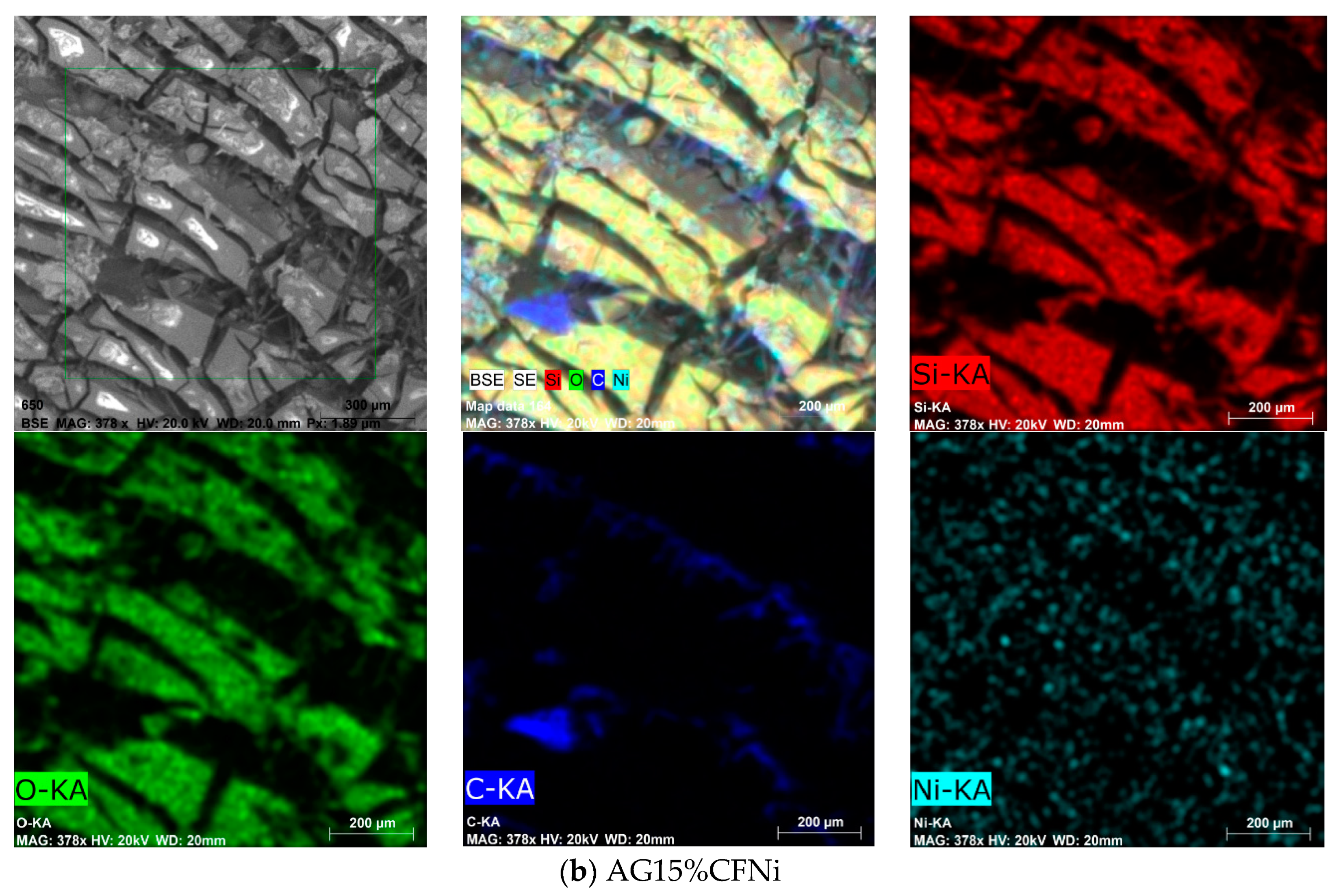
3.2. Physical and Chemical Characterization of Carbon Fiber–Silica Aerogel Nanocomposite
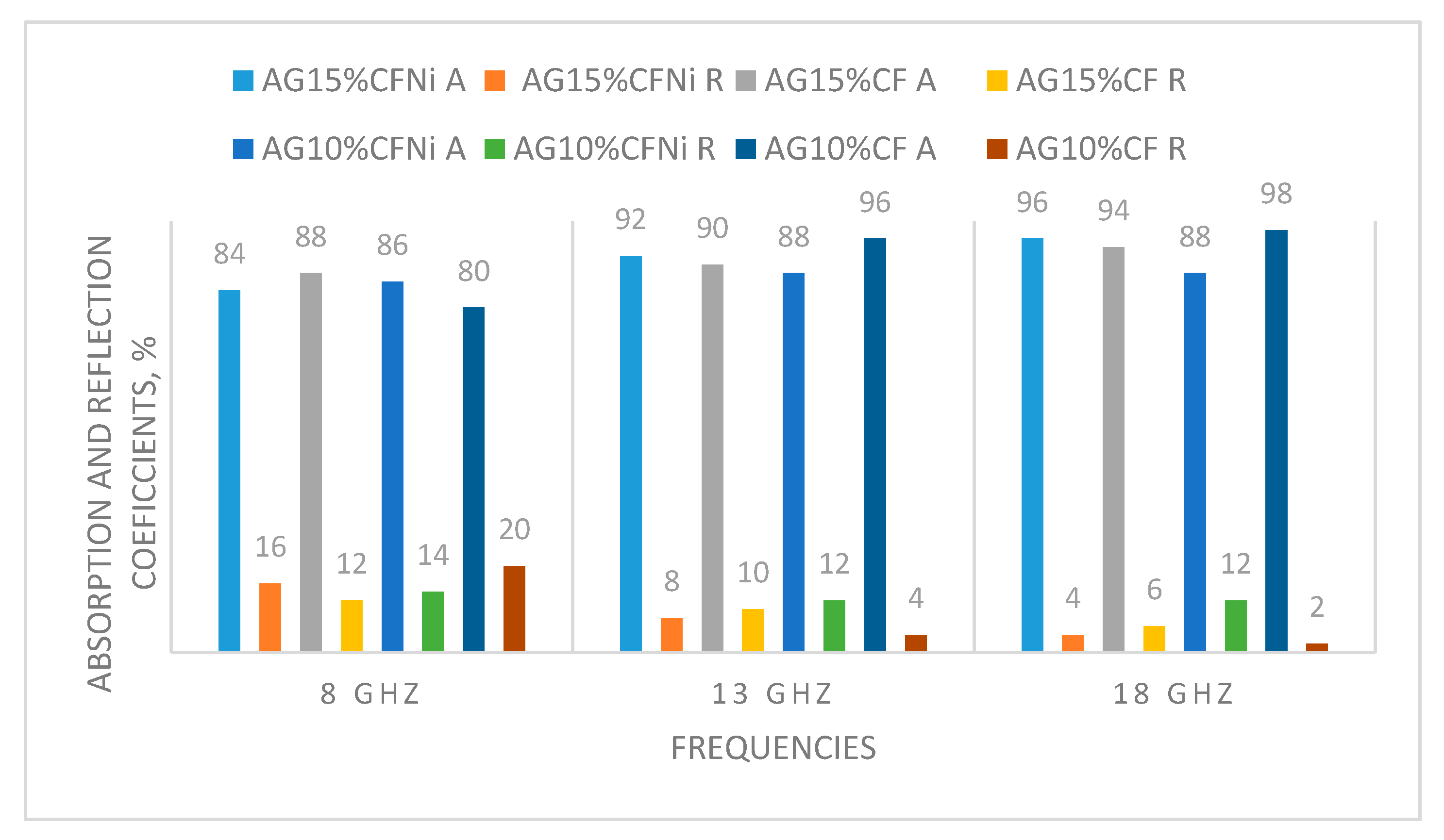
3.3. Electromagnetic Interference Shielding
4. Conclusions
- -
- Non-modified carbon fibers incorporated within the silica aerogel matrix improved the structural parameters and thermal stability of the silica aerogel composite, while, Ni-coated carbon fibers induced a deterioration of all factors.
- -
- The percolation threshold was gained when 10 vol.% of carbon fibers was introduced to silica aerogel matrix; this amount of fiber created a conductive net in the dielectric material and electrical conductivity of nanocomposite achieved 1.213 mS/cm. Greater amount of fibers (15 vol.%) resulted in over two times higher electrical conductivity, which further was doubled by coating the carbon fiber surface with a Ni-nanolayer.
- -
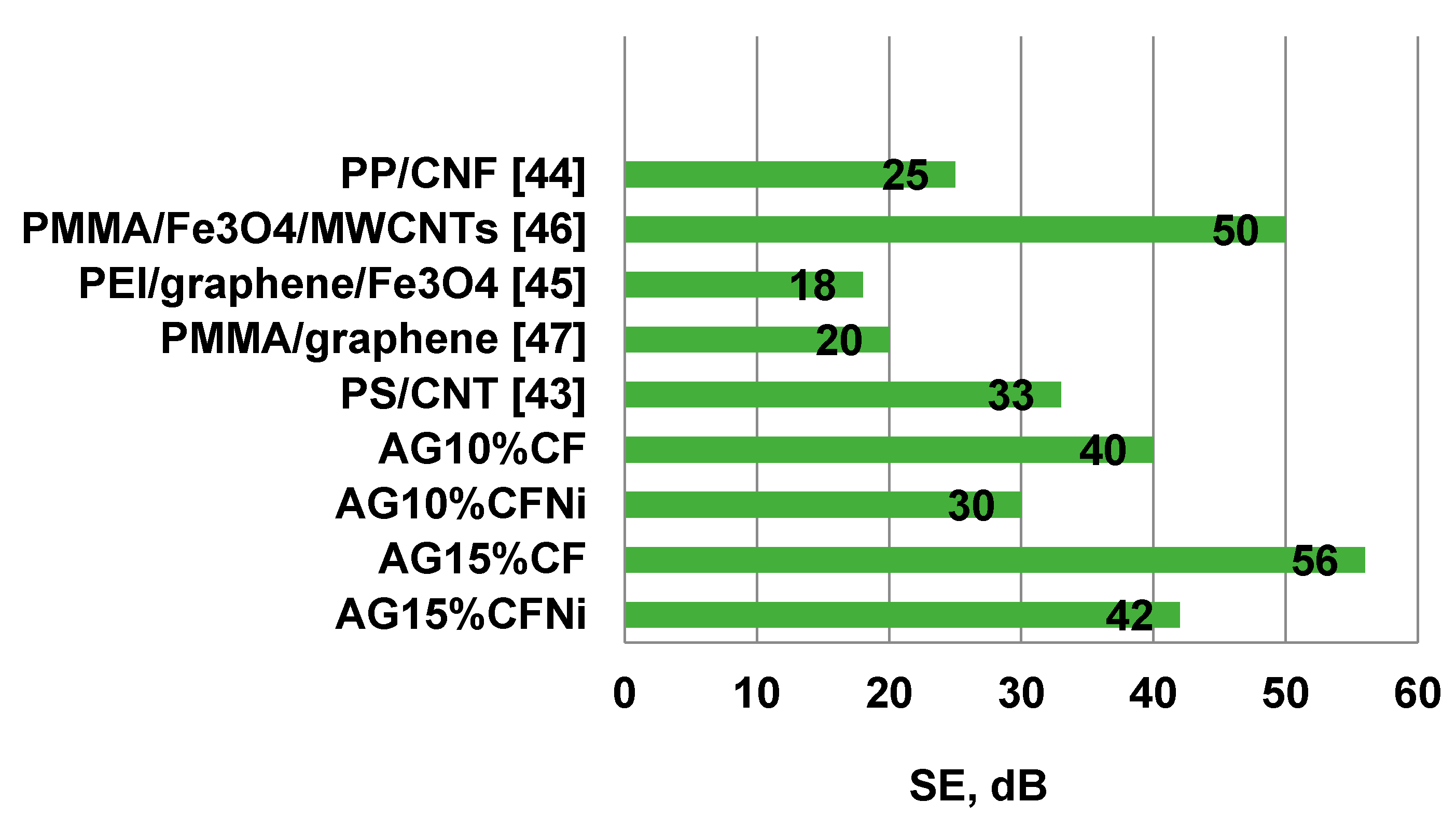
- Effectiveness of shielding properties depends strongly on the composition of the silica aerogel-carbon fiber composite. Damping was mainly due to the effect of absorbing the electromagnetic radiation that achieved values close to 90% in the tested frequency range. Composite silica aerogel–carbon fiber in the amount of 15% of volume, exhibited the best characteristics of absorption. Herein, the reflection parameters damping at 56 dB in the frequency range of 8–13 GHz, was one of the best results, in comparison to literature reports of the use of lightweight porous composites with electromagnetic shielding properties.
Author Contributions
Funding
Conflicts of Interest
References
- Calabrò, E. Introduction to the Special Issue “Electromagnetic Waves Pollution”. Sustainability 2018, 10, 3326. [Google Scholar] [CrossRef]
- Redlarski, G.; Lewczuk, B.; Żak, A.; Koncicki, A.; Krawczuk, M.; Piechocki, J.; Jakubiuk, K.; Tojza, P.; Jaworski, J.; Ambroziak, D.; et al. The influence of electromagnetic pollution on living organisms: Historical trends and forecasting changes. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Jagatheesan, K.; Ramasamy, A.; Das, A.; Basu, A. Electromagnetic shielding behavior of conductive filler composites and conductive fabrics—A review. Indian J. Fibre Text. Res. 2014, 39, 329–342. [Google Scholar]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Khadheer, B.S.K. Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Geetha, S.; Sathees Kumar, K.K.; Chepuri, R.K.R.; Vijayan, D.C.T. EMI shielding: Methods and materials—A review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- Abdalla, I.; Yu, J.; Li, Z.; Ding, B. Nanofibrous membrane constructed magnetic materials for high-efficiency electromagnetic wave absorption. Compos. Part B Eng. 2018, 155, 397–404. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, D.; Cal, J.; Yvan, L. Electromagnetic shielding effectiveness and mechanical property of polymer-matrix composites containing metallized conductive porous flake-shaped diatomite. Compos. Part B Eng. 2014, 67, 132–137. [Google Scholar] [CrossRef]
- Gorriti, A.G.; Marin, P.; Cortina, D.; Hernando, A. Microwave attenuation with composite of copper microwires. J. Magn. Magn. Mater. 2010, 322, 1505–1510. [Google Scholar] [CrossRef]
- Marin, P.; Cortina, D.; Hernando, A. Electromagnetic wave absorbing material based on magnetic microwires. IEEE Trans. Magn. 2008, 44, 3934–3937. [Google Scholar] [CrossRef]
- Yim, Y.; Rhee, K.; Park, S.J. Electromagnetic interference shielding effectiveness of nickel-plated MWCNTs/high-density polyethylene composites. Compos. Part B Eng. 2016, 98, 120–125. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Al-Ghamdi, A.T.; Al-Turki, Y.; Yakuphanoglu, F.; El-Tantawy, F. Electromagnetic shielding properties of graphene/acrylonitrile butadiene rubber nanocomposites for portable and flexible electronic devices. Compos. Part B Eng. 2016, 88, 212–219. [Google Scholar] [CrossRef]
- Singh, A.; Shinkin, A.; Koppel, T.; Gupta, N. A review of porous lightweight composite materials for electromagnetic interference shielding. Compos. Part B Eng. 2018, 148, 188–197. [Google Scholar] [CrossRef]
- Fricke, J. Aerogels—Recent progress in production techniques and novel applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Carlson, G.; Lewis, D.; McKinley, K.; Richardson, J.; Tillotson, T. Aerogel commercialization: Technology, markets and costs. J. Non-Cryst. Solids 1995, 186, 372–379. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Pierre, A.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef]
- Patel, R.P. An overview of silica aerogels. Int. J. Chem. Tech. Res. 2009, 1, 1052–1057. [Google Scholar]
- Suh, D.J. Catalytic applications of composite aerogels. J. Non-Cryst. Solids 2004, 350, 314–319. [Google Scholar] [CrossRef]
- Alnaief, M.; Smirnova, I. Effect of surface functionalization of silica aerogel on their adsorptive and release properties. J. Non-Cryst. Solids 2010, 356, 1644–1649. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Pajonk, G.M. Catalytic aerogels. Catal. Today 1997, 35, 319–337. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical properties of silica aerogels. J. Non-Cryst. Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Ni, X.; Wu, G.; Zhou, B.; Yang, M.; Gu, X.; Qian, M.; Wu, Y. Hydrophobic silica aerogels strengthened with nonwoven fibers. J. Macromol. Sci. Part A 2006, 43, 1663–1670. [Google Scholar] [CrossRef]
- Bangi, U.; Hirashima, H.; Parvathy-Rao, A.; Venkatesvara Rao, A. Physicochemical properties of ambiently dried sodium silicate based aerogels catalyzed with various acids. J. Sol-Gel Sci. Technol. 2009, 50, 87–97. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Barełkowski, S.; Niemier, J.; Jakubowska, P. Synthesis and characterisation of silica aerogel/carbon microfibers nanocomposites dried in supercritical and ambient pressure conditions. J. Sol-Gel Sci. Technol. 2015, 76, 227–232. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Wojciech, S.; Piotr, Z.; Paulina, J. Synthesis and characterization of carbon fiber/silica aerogel nanocomposites. J. Non-Cryst. Solids 2015, 416, 1–3. [Google Scholar] [CrossRef]
- Ślosarczyk, A. Synthesis and characterization of silica aerogel–based nanocomposites with carbon fibers and carbon nanotubes in hybrid system. J. Sol-Gel Sci. Technol. 2017, 84, 16–22. [Google Scholar] [CrossRef]
- Ślosarczyk, A. Recent advances in research on the synthetic fibers based silica aerogel nanocomposites. Nanomaterials 2017, 7, 44. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Facile construction of MoO3@ZIF-8 core-shell nanorods for efficient photoreduction of aqueous Cr (VI). Appl. Catal. B Environ. 2019, 240, 92–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Stabilizing CuPd bimetallic alloy nanoparticles deposited on holey carbon nitride for selective hydroxylation of benzene to phenol. J. Catal. 2019, 379, 154–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Formation of hollow MoO3/SnS2 heterostructured nanotubes for efficient light-driven hydrogen peroxide production. J. Mater. Chem. A 2018, 6, 20304–20312. [Google Scholar] [CrossRef]
- Dobiasova, L.; Stary, V.; Glogar, P.; Valvoda, V. Analysis of carbon fibers and carbon composites by asymmetric X-ray diffraction technique. Carbon 1999, 37, 421–425. [Google Scholar] [CrossRef]
- Tzeng, S.; Chang, F.-Y. EMI shielding effectiveness of metal-coated carbon fiber-reinforced ABS composites. Mater. Sci. Eng. A 2001, 302, 258–267. [Google Scholar] [CrossRef]
- De Boer, H. The Structure and Properties of Porous Materials; Butterworth: London, UK, 1958. [Google Scholar]
- Rao, A.V.; Amalnerkar, D.P.; Kulkarni, M.; Seth, T. Surface chemical modification of silica aerogels using various alkyl–alkoxy/chloro silanes. Appl. Surf. Sci. 2003, 206, 262–270. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)(4) and R”Si(OR’)(3) precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Wu, G.; Yu, Y.; Cheng, X.; Zhang, Y. Preparation and surface modification mechanism of silica aerogels via ambient pressure drying. Mater. Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Zielińska, M.; Buchwald, T.; Marańda, M.; Voelkel, A. Siliceous-based monolithic materials coated with a hydroxyapatite layer: Preparation and investigation of drug affinity by Raman spectroscopy. J. Raman Spectrosc. 2019, 50, 1722–1730. [Google Scholar] [CrossRef]
- Hard, A.; Parker, S.; Jayasooriya, U. Vibrational Spectroscopic Analysis of Chlorosilanes and Siloxane Oligomers: Implications for the Spectra of Polydimethylsiloxanes. Appl. Spectrosc. 2007, 61, 314–320. [Google Scholar] [CrossRef]
- Lota, G.; Krawczyk, P.; Lota, K.; Sierczyńska, A.; Kolanowski, Ł.; Baraniak, M.; Buchwald, T. The application of activated carbon modified by ozone treatment for energy storage. J. Solid State Electrochem. 2016, 20, 2857–2864. [Google Scholar] [CrossRef]
- Chung, D.D.L. Cement reinforced with short carbon fibers: A multifunctional material. Compos. Part B Eng. 2000, 31, 511–526. [Google Scholar] [CrossRef]
- Boday, D.; Muriithi, B.; Stover, R.J.; Loy, D.A. Polyaniline nanofiber-silica composite aerogels. J. Non-Cryst. Solids 2012, 358, 1575–1580. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel carbon nanotube-polystyrene foam composites for electromagnetic interference shielding. Nano Lett. 2005, 5, 2131–3134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Conducting carbon nanofiber-polymer foam structures. Adv. Mater. 2005, 17, 1999–2003. [Google Scholar] [CrossRef]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, T. Plasma activation of carbon fibres for polyarylacetylene composites. Surf. Coat. Technol. 2017, 201, 4965–4968. [Google Scholar] [CrossRef]
- Zhang, H.B.; Yan, Q.; Zheng, W.G.; He, Z.; Yu, Z.Z. Tough graphene-polymer microcellular foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2011, 3, 918–924. [Google Scholar] [CrossRef]
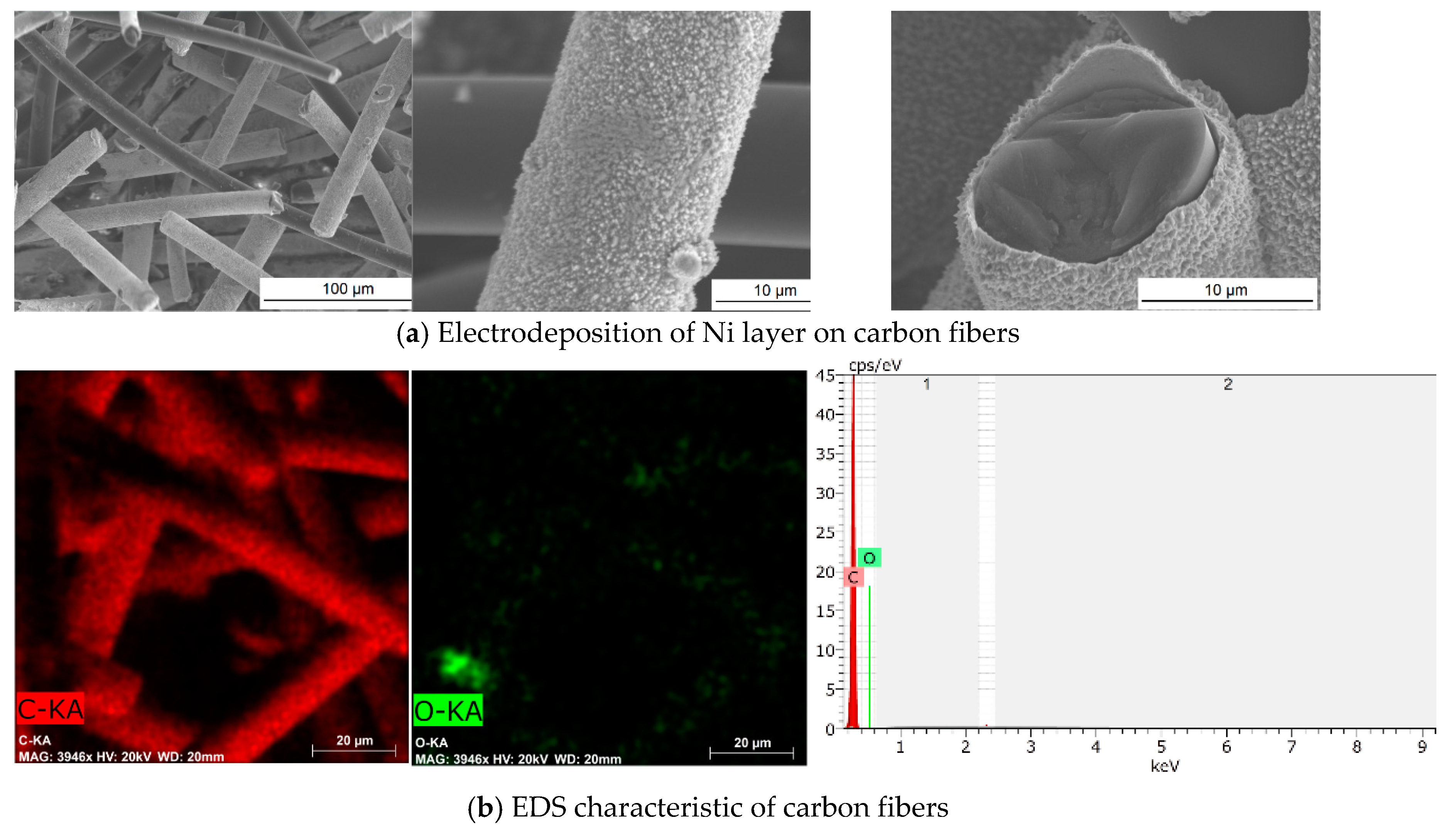
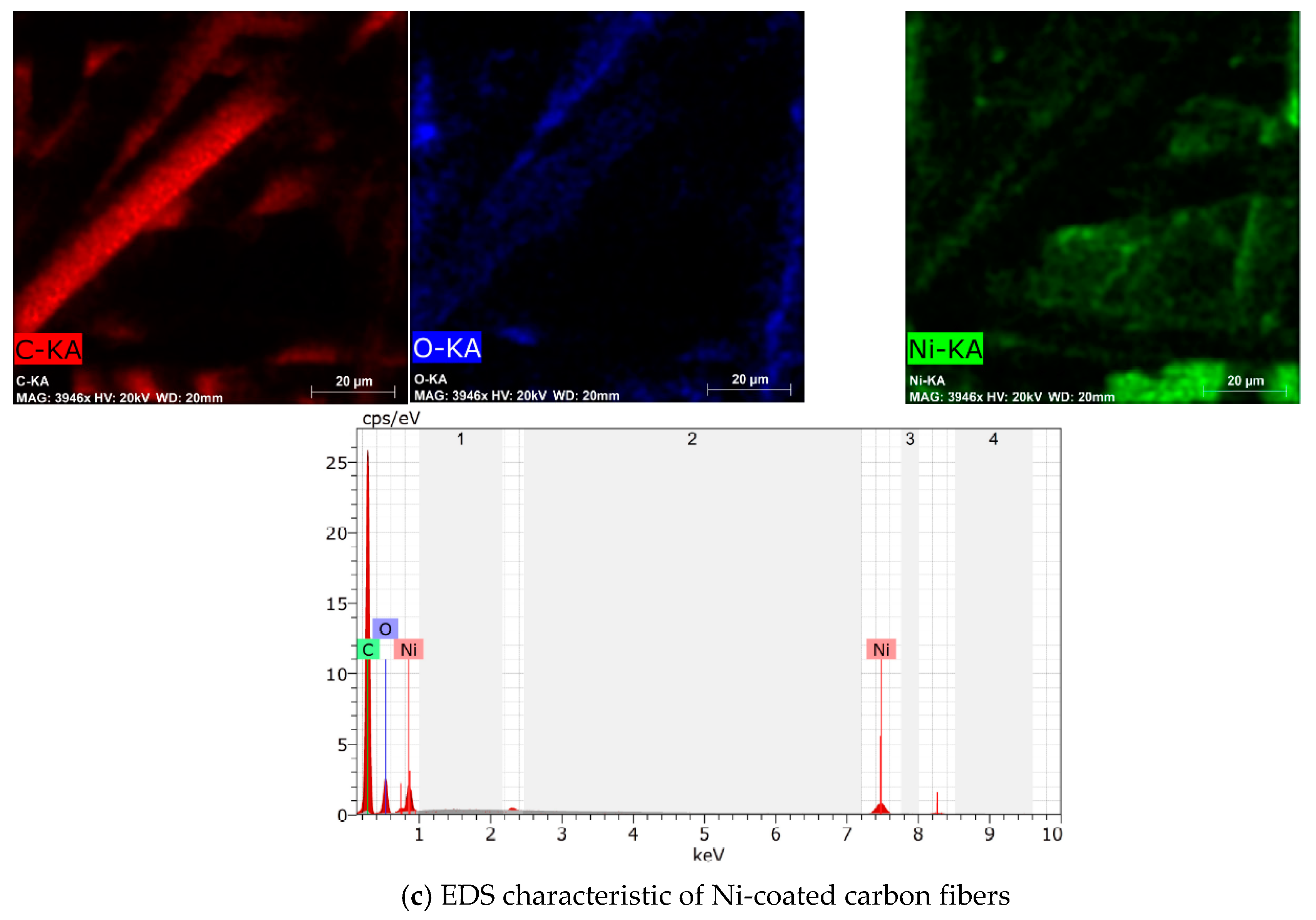

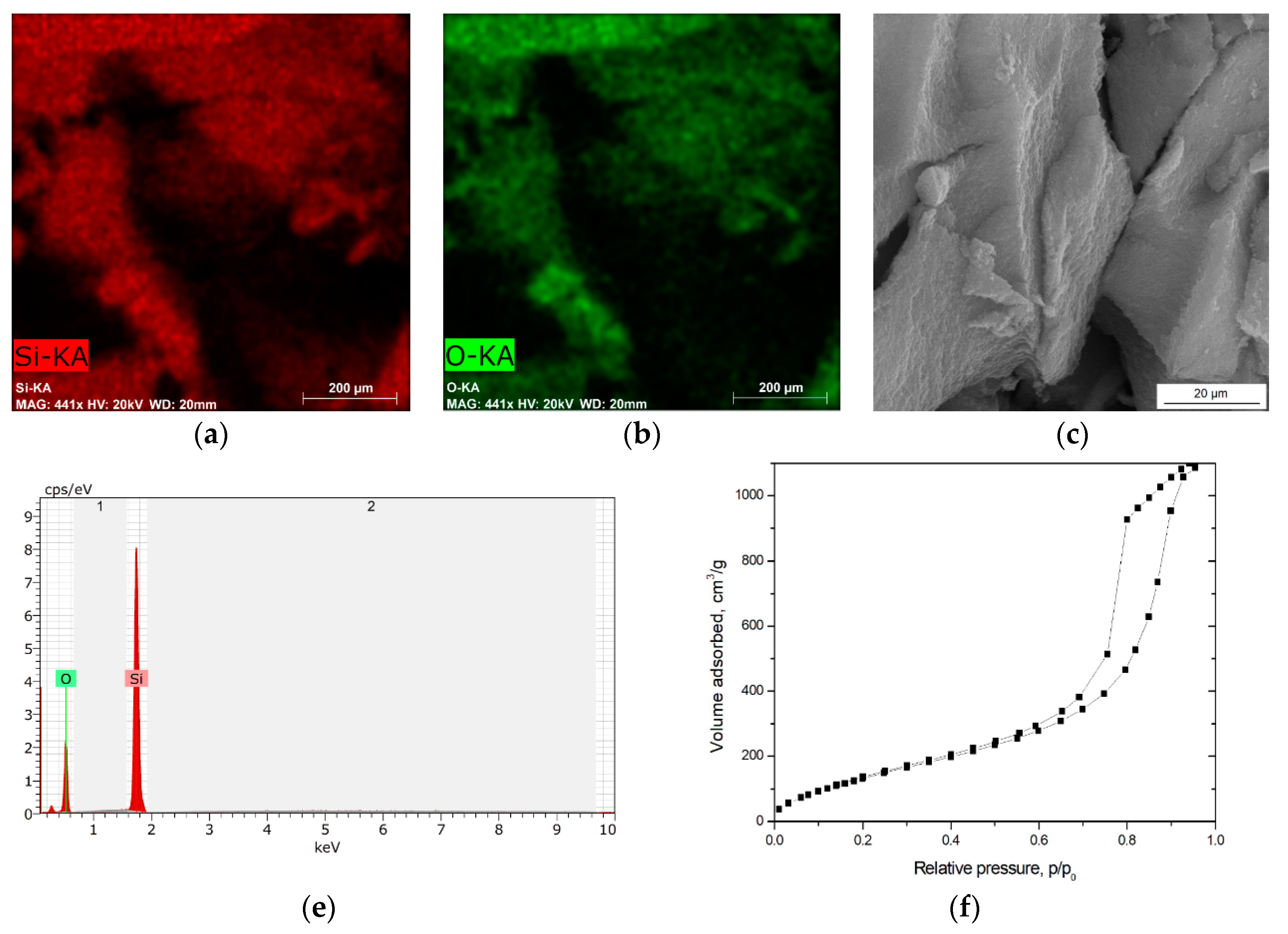


| Material | Form | Density, g/cm3 | Porosity, % | Specific Surface Area, m2/g | Average Pore Diameter, nm | Average Micropore Volume, cm3/g |
|---|---|---|---|---|---|---|
| Pure AG | granulate | 0.201 | 90.9 | 496.5 | 10.2 | 1.27 |
| Parameter | AG15%CFNi | AG15%CF | AG10%CFNi | AG10%CF |
|---|---|---|---|---|
| Form | monolith | monolith | monolith | monolith |
| Density, g/cm3 | 0.270 | 0.225 | 0.248 | 0.199 |
| Porosity, % | 88.0 | 90.0 | 89.0 | 91.0 |
| Specific surface area, m2/g | 295.7 | 467.0 | 317.4 | 474.6 |
| Average pore diameter, nm | 8.4 | 12.7 | 13.6 | 14.5 |
| Average micropore volume, cm3/g | 0.561 | 1.486 | 0.996 | 1.724 |
| Conductivity, mS/cm (LSV method) | 4.602 | 2.019 | 3.726 | 1.065 |
| Conductivity, mS/cm (EIS method) | 4.820 | 2.226 | 3.722 | 1.213 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślosarczyk, A.; Klapiszewski, Ł.; Buchwald, T.; Krawczyk, P.; Kolanowski, Ł.; Lota, G. Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low-Frequency Microwave Absorbing Materials. Materials 2020, 13, 400. https://doi.org/10.3390/ma13020400
Ślosarczyk A, Klapiszewski Ł, Buchwald T, Krawczyk P, Kolanowski Ł, Lota G. Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low-Frequency Microwave Absorbing Materials. Materials. 2020; 13(2):400. https://doi.org/10.3390/ma13020400
Chicago/Turabian StyleŚlosarczyk, Agnieszka, Łukasz Klapiszewski, Tomasz Buchwald, Piotr Krawczyk, Łukasz Kolanowski, and Grzegorz Lota. 2020. "Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low-Frequency Microwave Absorbing Materials" Materials 13, no. 2: 400. https://doi.org/10.3390/ma13020400
APA StyleŚlosarczyk, A., Klapiszewski, Ł., Buchwald, T., Krawczyk, P., Kolanowski, Ł., & Lota, G. (2020). Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low-Frequency Microwave Absorbing Materials. Materials, 13(2), 400. https://doi.org/10.3390/ma13020400









