Effect of Imidazoline Inhibitor on the Rehabilitation of Reinforced Concrete with Electromigration Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
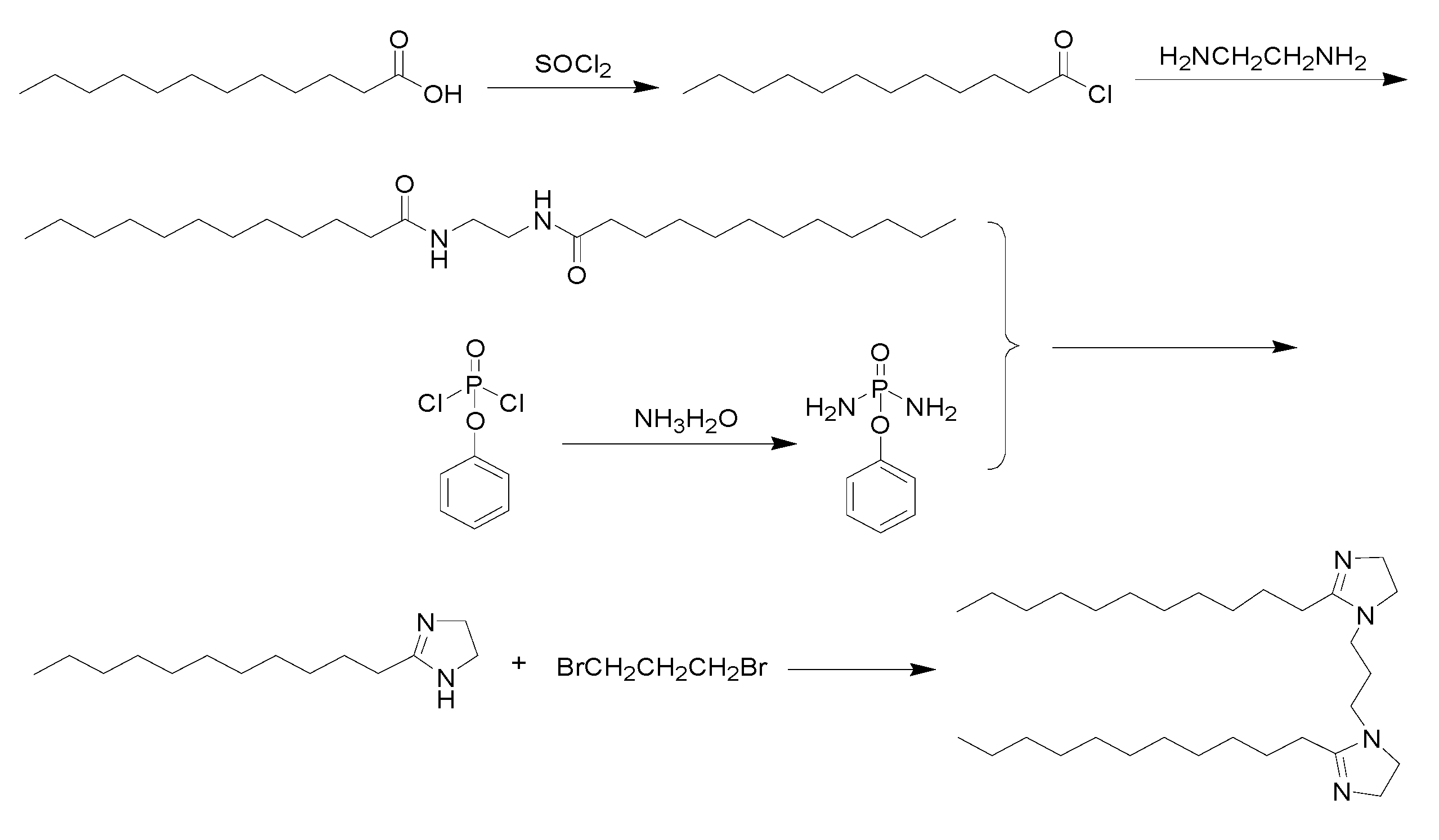
2.2. Principle and Route of Imidazoline Inhibitor Synthesis
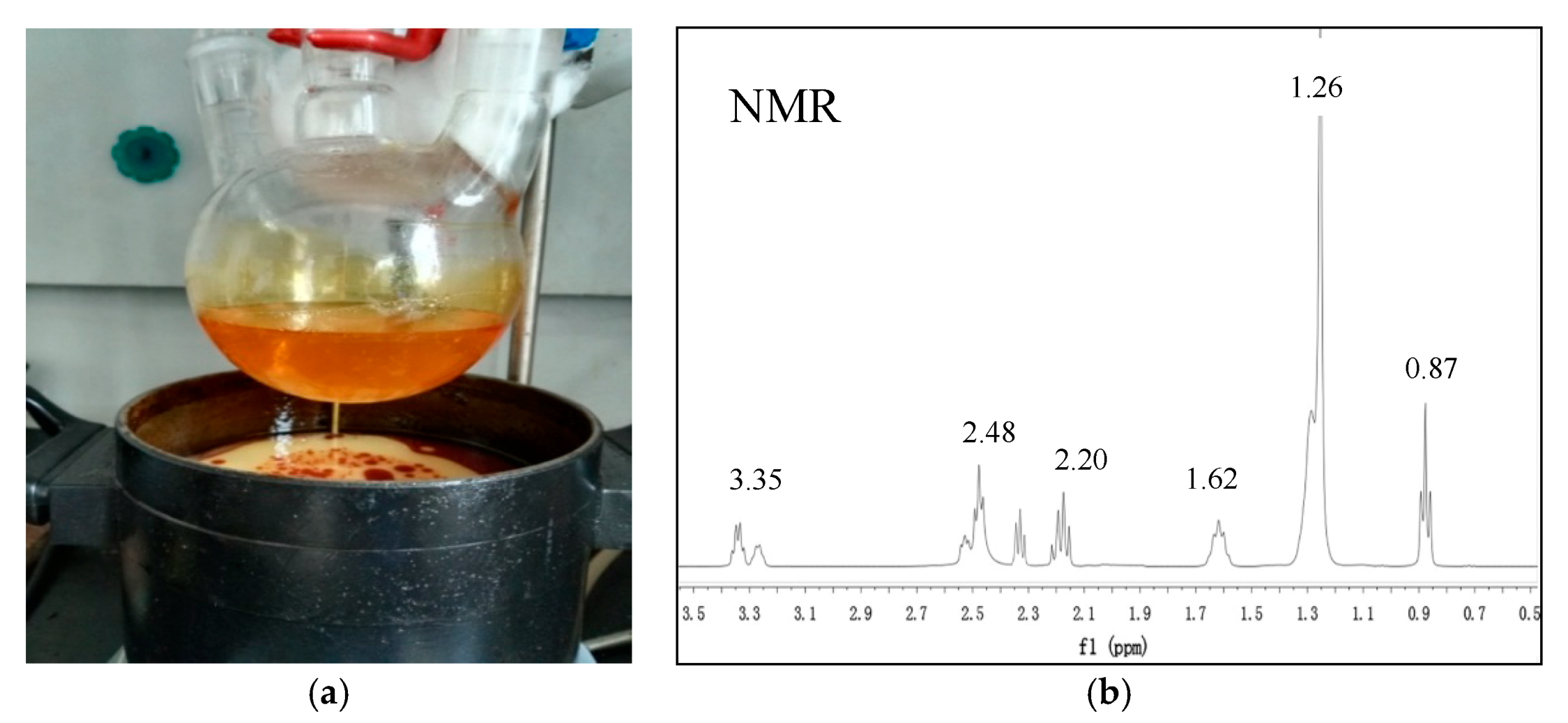
2.3. Synthesis of the Imidazoline Inhibitor
2.4. Feasibility Assessment of Imidazoline Inhibitor
- m0 and m1-quality of steel bar before and after corrosion, respectively (g)
- s-Exposure area (m2)
- t-Soaking time (h)
- V0 and V1-corrosion rate in the absence and presence of inhibitor, respectively (g/m2 h)
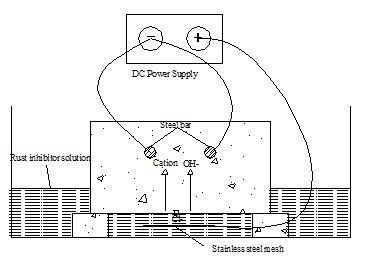
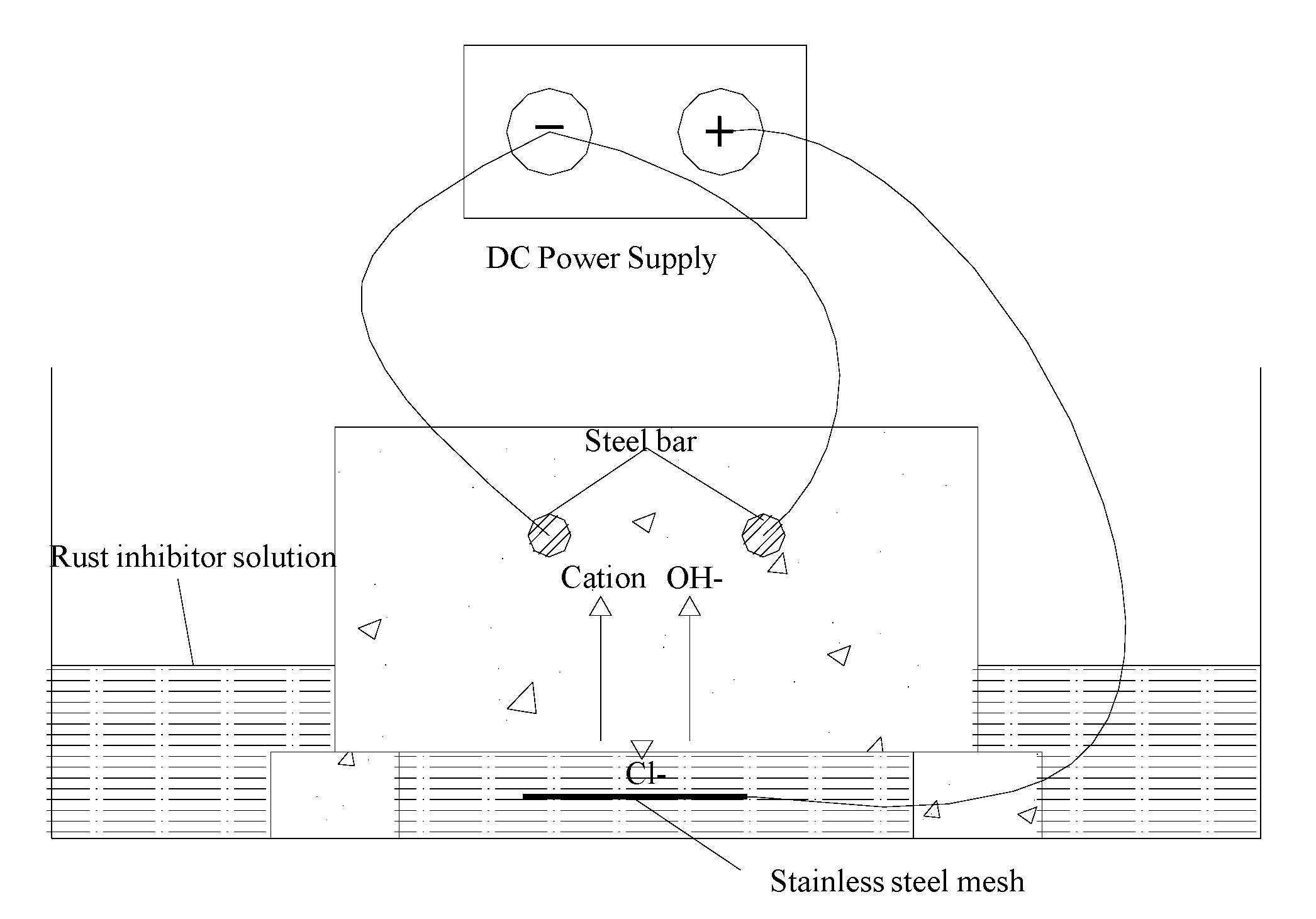
2.5. Principle Underlying Bidirectional Electromigration Rehabilitation
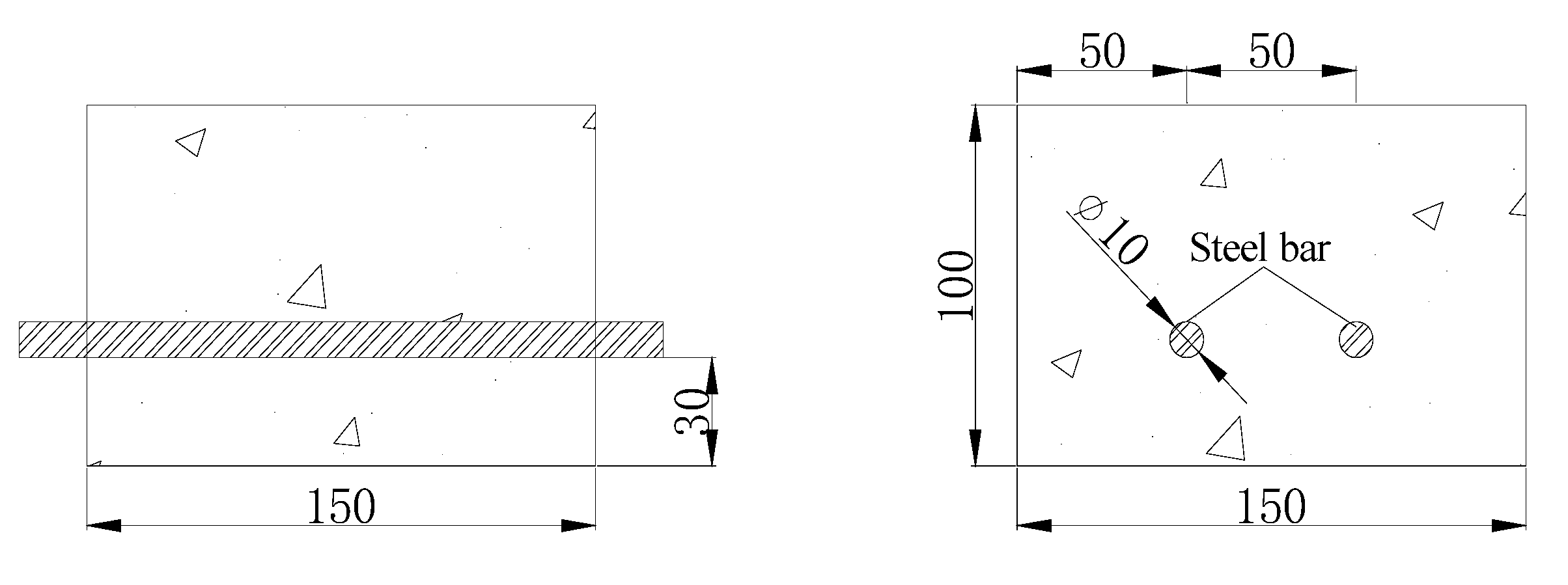
2.6. Designing and Preparation of Concreteblocks
2.7. Experimental Procedure
2.8. Dry-Wet Cycle Test of Concrete with Imidazoline Inhibitors
3. Results and Discussion
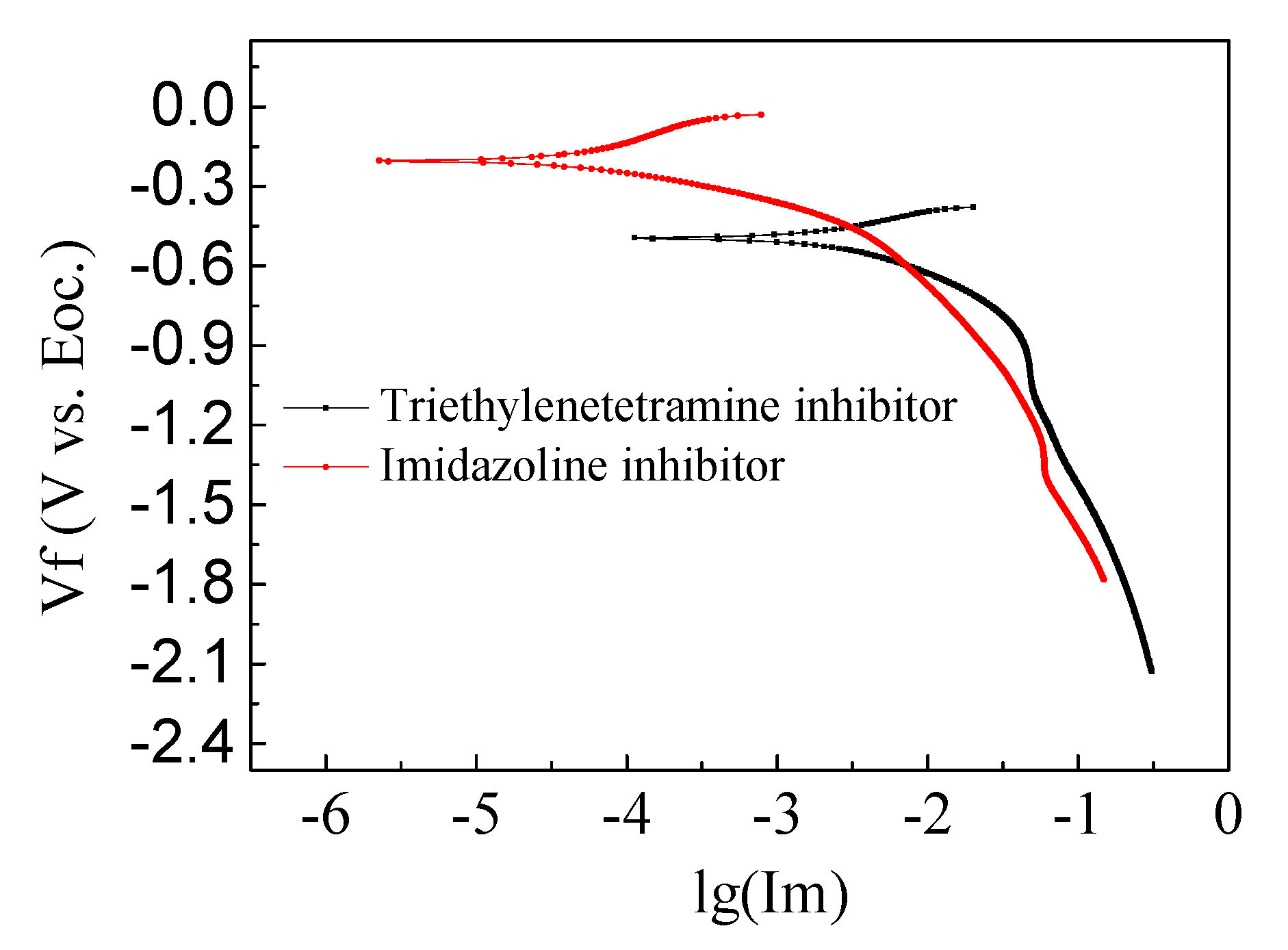
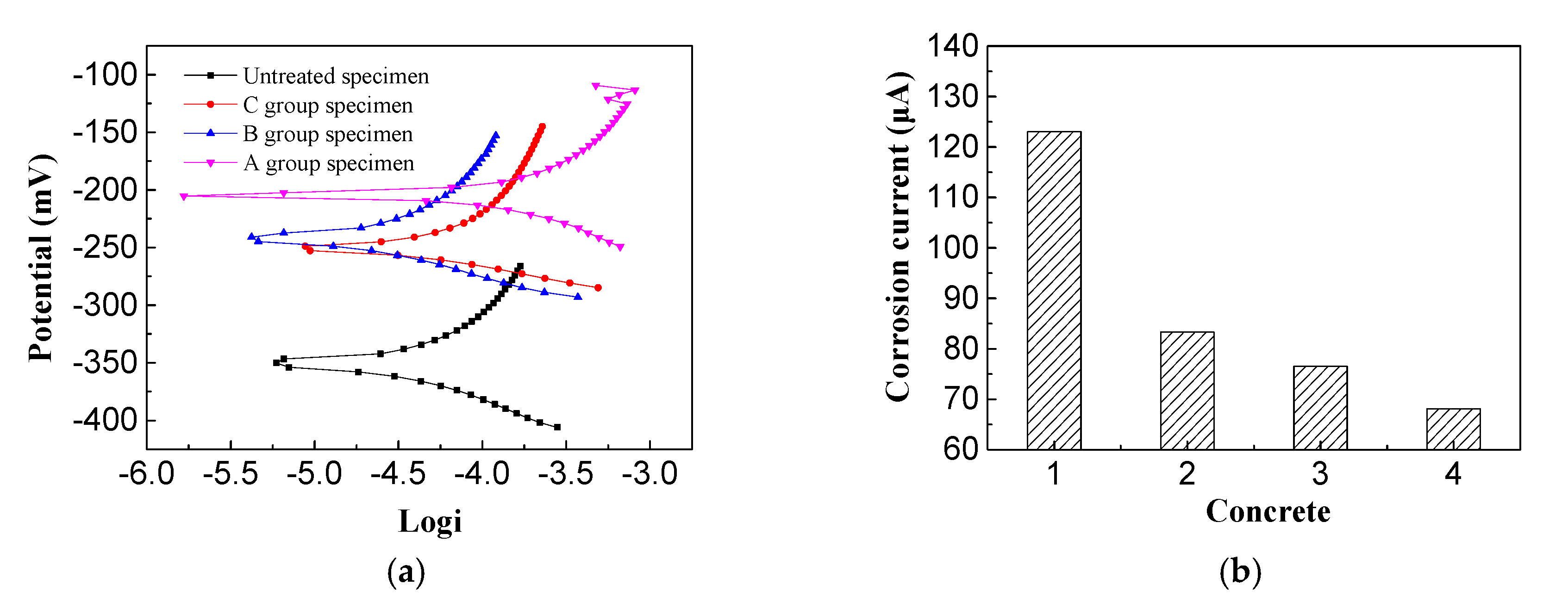
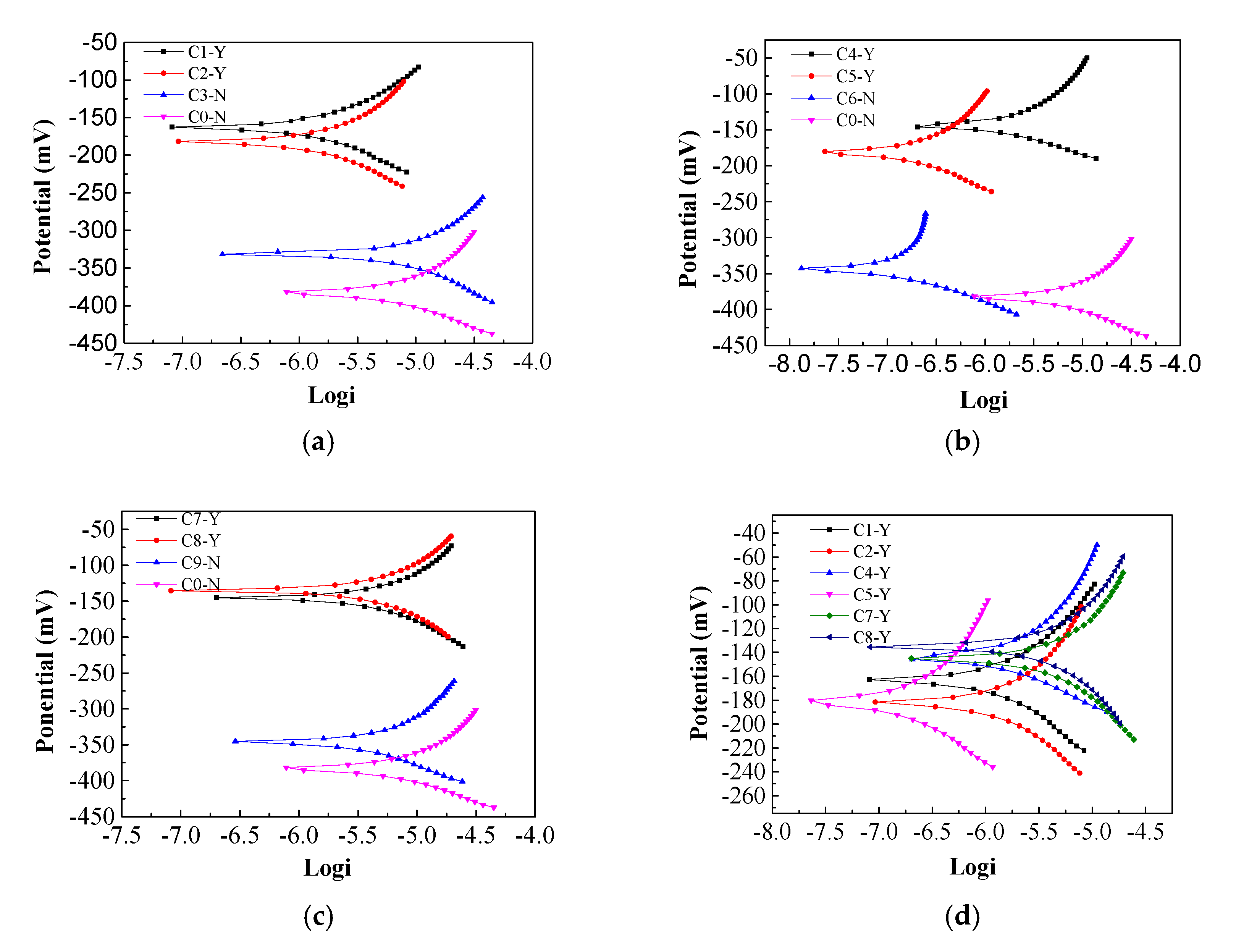
3.1. Corrosion Potential and Resistance of Steel
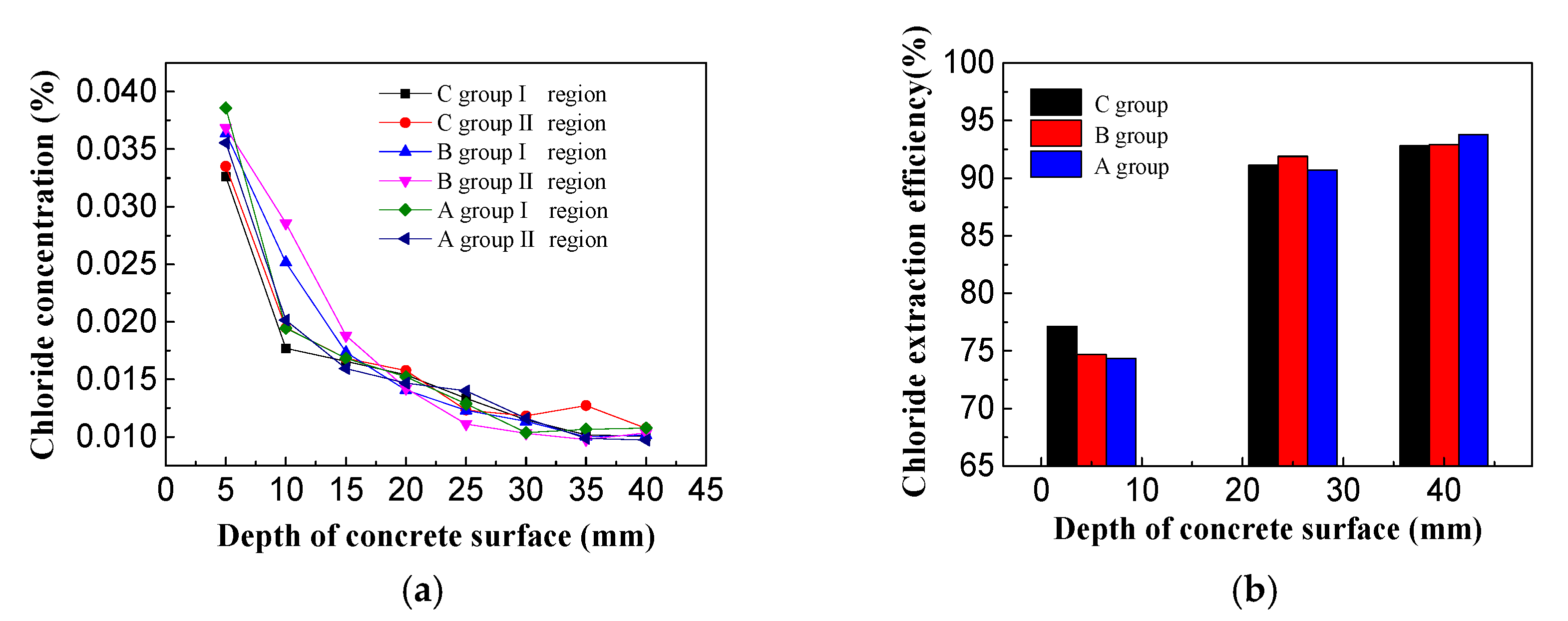
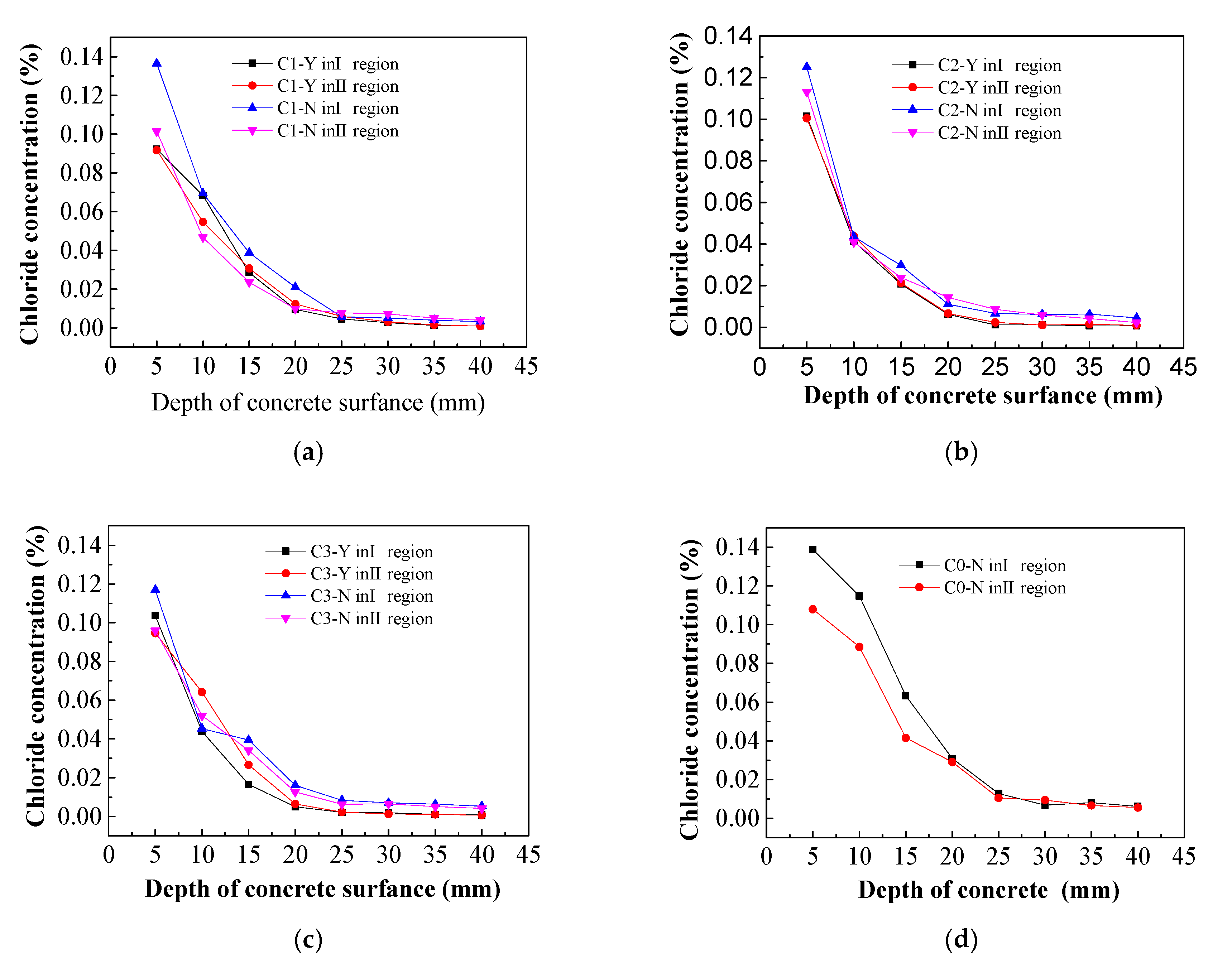
3.2. Chloride Ion Concentrations in Concrete
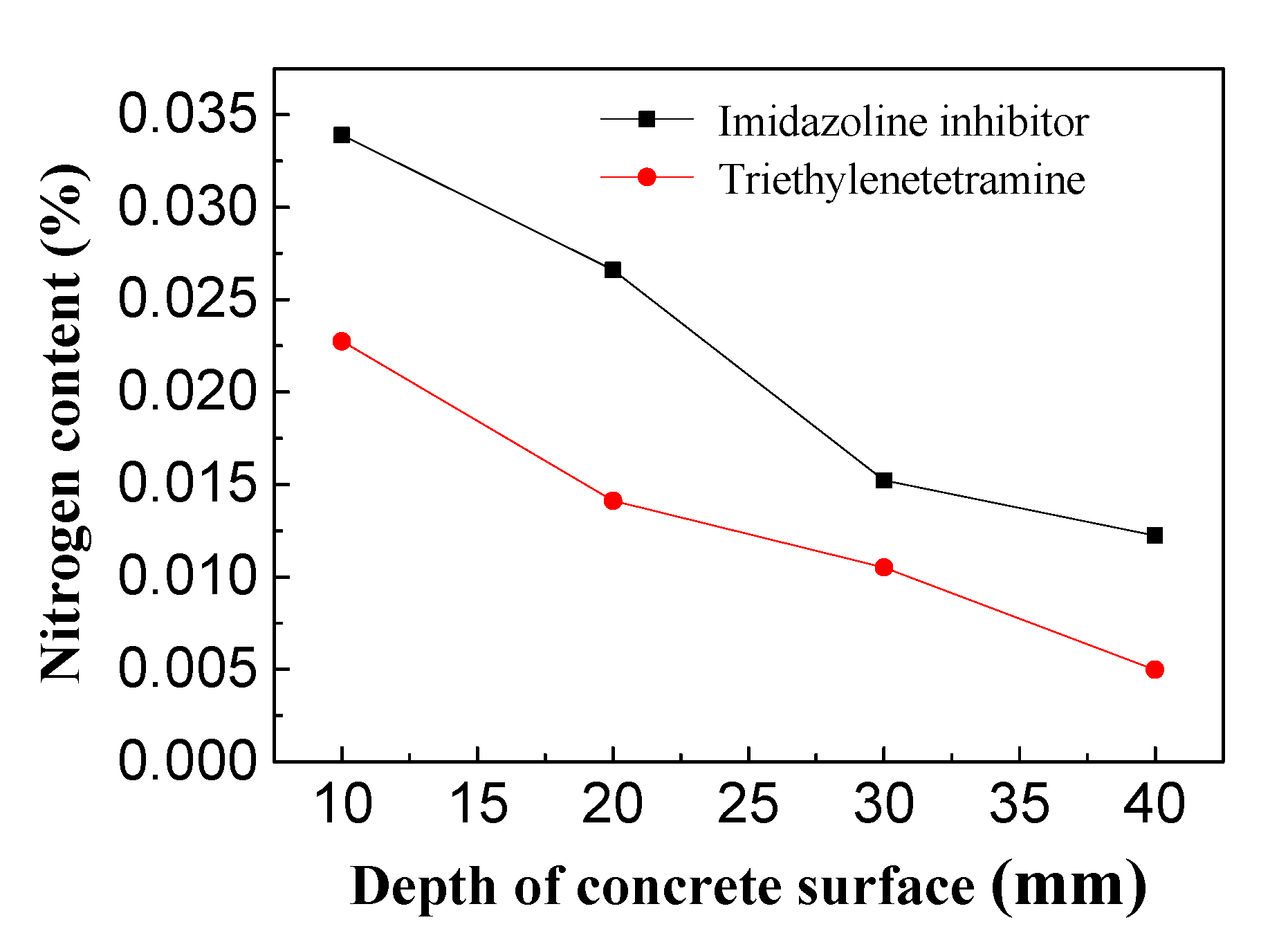
3.3. Inhibitor Concentrations in Depth of Concrete
3.4. Potential of Reinforcement Bar after Dry-Wet Cycle
3.5. Characteristics of Chloride Ion Diffusion in Concrete
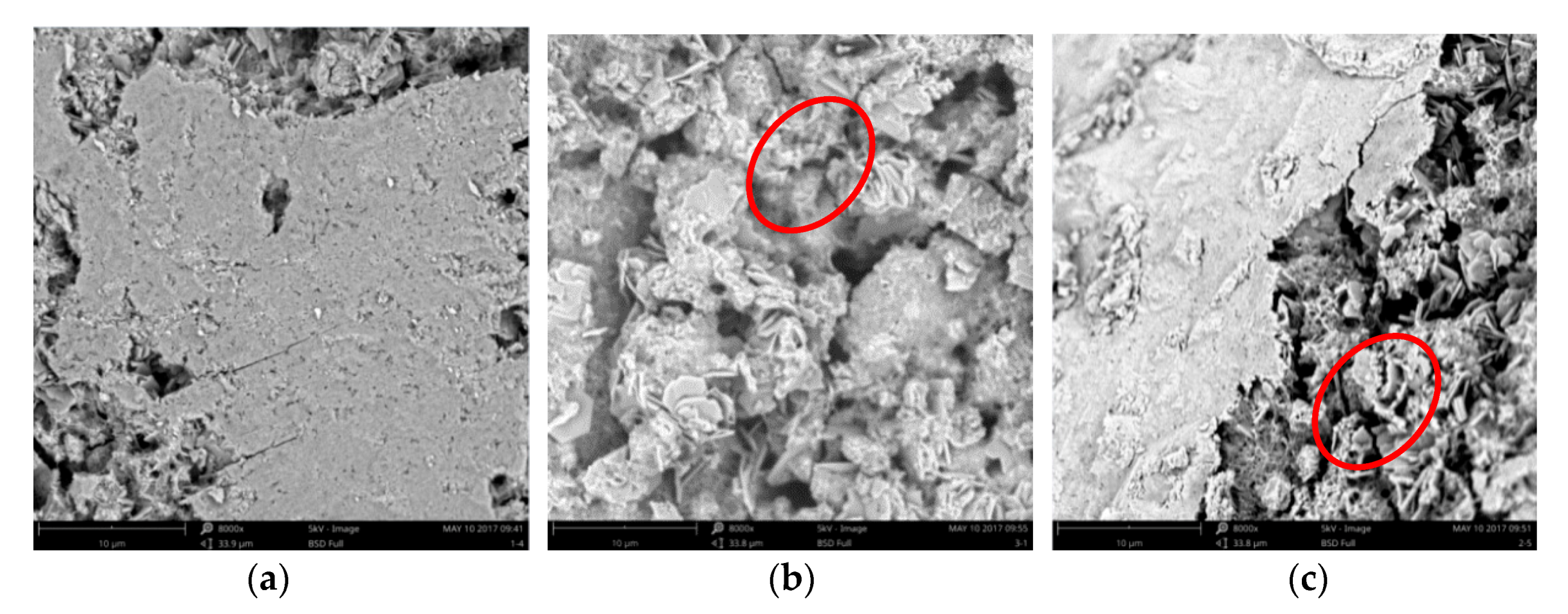
3.6. Hydration Productanalysis of Treated Samples
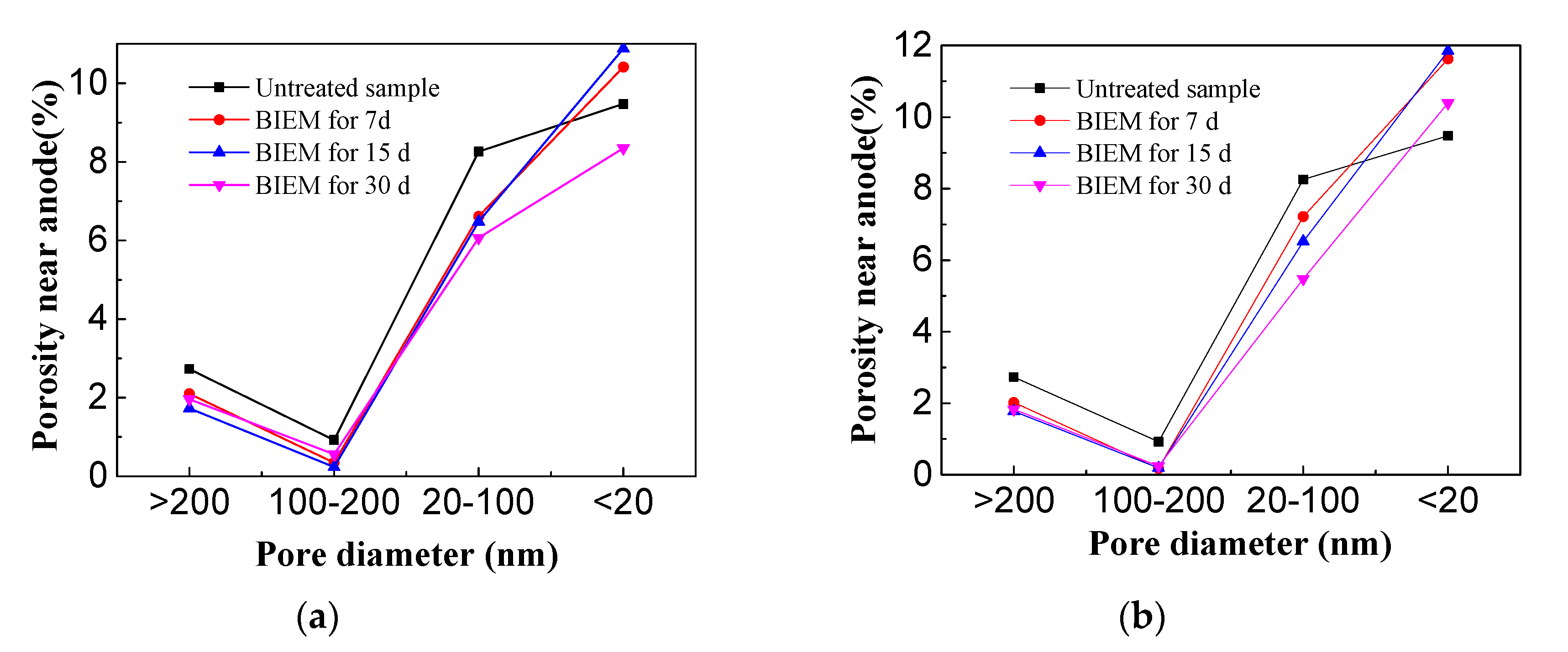
3.7. Pore Distribution of BIEM Treated Samples
4. Conclusions
- (1)
- The effect of electrochemical treatment with different inhibitors on chlorine salt erosion of reinforced concrete corrosion potential and corrosion current were studied. It was found that imidazoline and triethylenetetramine inhibitors can increase the corrosion potential to some extent. The imidazoline corrosion potential shifted positively to the amplitude ratio of triethylenetetramine. The two inhibitors reduced the corrosion current, where the imidazoline corrosion current displayed a more significant reduction than triethylenetetramine.
- (2)
- Inhibitor from the external solution was found to discharge the majority of chloride ions in the concrete. The chloride extraction effect and electrochemical chloride extraction made no significant difference on the surface of the concrete, where chlorine removal efficiency and the efficiency of chlorine has reached the requirement around the location of steel, which revealing that the electromigration inhibitor did not affect the migration of chloride ions in the concrete.
- (3)
- The inhibitor content in the concrete specimens was evaluated after BIEM. After a dry-wet cycle test, the potential of the concrete increased by about 200 mV by mixing the imidazoline inhibitor, and the inhibitor content in the concrete specimens decreased along the thickness of the concrete protective layer. The imidazoline inhibitor content in the concrete was higher than that of triethylenetetramine, which indicates that the electric migration ability of the imidazoline inhibitor was stronger than the other compounds under an electric field.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kang, W.N. Investigation of corrosion status of reinforced concrete reinforcement and its causes. Technol. Enter. 2013, 8, 147. [Google Scholar]
- Jiang, J.Y.; Wang, D.Q.; Chu, H.Y.; Ma, H.; Liu, Y. The passive film growth mechanism of new Corrosion resistant steel rebar in simulated concrete pore solution:nanometer structure and electrochemical study. Materials 2017, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.Y. The design and synthesis of a new type of electro-migrating inhibitor and its influence on the composition, structure and performances of reinforced concrete. South. Chin. Univ. Technol. 2013, 30–36. [Google Scholar]
- Zhang, M. Study on the Durability of High-Pile Whart-Test Study about the Effect of Concrete Carbonizaion Degree on Chloride Diffusion Coefficient; Tongji University: Shanghai, China, 2007. [Google Scholar]
- Ze, L.; Pan, C.G. Research on properties and preparation of resist seawater and corrosion inhibitior. New Build. Mater. 2014, 26, 1787–1790. [Google Scholar]
- Fajardo, G.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction (ECE) from steel-reinforced concrete specimens contaminated by ‘‘artificial” seawater. Corros. Sci. 2006, 48, 110–125. [Google Scholar] [CrossRef]
- Ghahari, S.A.; Ramezanianpour, A.M.; Ramezanianpour, A.A.; Esmaeili, M. An accelerated test method of simultaneous carbonation and chloride ion ingress: Durability of silica fume concrete in severe environments. Adv. Mater. Sci. Eng. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Yei, W.; Chang, J.J.; Hung, C.C. Selecting an adequate procedure for the electrochemical chloride removal. Cem. Concr. Res. 2006, 36, 562–570. [Google Scholar] [CrossRef]
- Jin, W.L.; Huang, N.; Xu, C. Experimental research on effect of bidirectional eletromigration rehabilitation on reinforced concrete-Concentration changes of inhibitor, chloride ions and total alkalinity. J. Zhejiang Univ. 2014, 48, 1586–1594. [Google Scholar]
- Xu, C.; Jin, W.L.; Huang, N. Experimental on repair effect of bidirectional eletromigration rehabilitation on reinforced concrete: Changing rule of protective layer strength. J. Zhejiang Univ. 2015, 6, 1128–1138. [Google Scholar]
- Liu, Z.Y.; Liao, C.W.; Zhou, W.L. Maintenance and improvement of durability of reinforced concrete using migrating inhibitors. J. Chin. Ceram. Soc. 2008, 36, 1494–1500. [Google Scholar]
- Pan, C.G.; Jin, W.L.; Mao, J.H.; Zhang, H.; Sun, L.H.; Wei, D. Influence of Reinforcement Mesh Configuration for Improvement of Concrete Durability. Chin. Ocean. Eng. 2017, 31, 1–8. [Google Scholar] [CrossRef]
- Chen, C.C.; Cai, J.S.; Liu, J.Z. Use of Aminoalcohol as inhibitor in Chloride-contaminated Steel Reinforced Concrete. J. Chin. Ceram. Soc. 2015, 4, 393–399. [Google Scholar]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Fei, F.L.; Hu, J.; Yu, Q.J.; Wei, J.X.; Nong, Y.B. The effect of a tailored electro-migrating corrosion inhibitor on the corrosion performance of chloride-contaminated reinforced concrete. Mater. Corros. 2015, 66, 1039–1050. [Google Scholar] [CrossRef]
- Okafor, P.C.; Zheng, Y.G. Synergistic inhibition behavior of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros. Sci. 2009, 51, 850–859. [Google Scholar] [CrossRef]
- Gu, T.; Su, P.C.; Liu, X.Y.; Zou, J.C.; Zhang, X.Y.; Hu, Y. A composite inhibitor used in oilfield: MA-AMPS and imidazoline. J. Petrol. Sci. Eng. 2013, 102, 41–46. [Google Scholar] [CrossRef]
- Wang, J.H.; Shu, F.C. Preparation and Evaluation of Imidazoline Derivative as Inhibitor. Adv. In. Fine. Petro. 2004, 5, 22–24. [Google Scholar]
- Kang, H.Y.; Li, S.J. Prelinminary investigations synthesis and anticorrosive per for mances of imidazoline drivatives form zanthoxylum bungeanoleum and dietyleneiriamine. Spec. Petro. 2004, 5, 33–35. [Google Scholar]
- Fei, F.L. The investigation on the preparation of a tailored electro-migrating inhibitor and its corrosion inhibition performance and mechanism. South. Chin. Univ. Technol. 2015, 50–57. [Google Scholar]
- Mu, G.N. Corrosion of pure aluminium in hydrochloric acid solution. Chemistry 1986, 2, 38–39. [Google Scholar]
- Pan, C.G.; Geng, J.; Ding, Q.J. Stray Current Affects the Release of Bound Chloride Ions in Hydrated Cement Paste. Int. J. Electrochem. Sci. 2018, 13, 6098–6111. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Ren, B.; Xu, C.X. Applications and Prospectus of Electrochemical in_situ Raman Spectroscopy. Electrochemistry 1995, 5, 1. [Google Scholar]
- Liu, J.P.; Li, Z.F. Electrochemical behavior of an imidazoline film formed on carbon steel surface. Corros. Sci. Prot. Technol. 2003, 15, 263–265. [Google Scholar]
- Zhang, S.C.; Bai, Z.M. Synthesis and performance of inhibitors with multi adsorption centers. Corros. Sci. Prot. Technol. 2003, 15, 79–81. [Google Scholar]
- Lei, L.C.; Xiao, K. Preparation and characteristics of a novel imidazoline inhibitor made from naphthenic acid and diethyllenetriamine. Corros. Prot. 2001, 22, 420–423. [Google Scholar]
- Zhu, X.; Zhou, X.Q. study on corrosion inhibition of naphthenic imidazoline derivative in H2SO4 solution. Appl. Chem. Ind. 2006, 35, 51–53. [Google Scholar]
- Zhang, S.Y.; Jin, W.L.; Xu, C. Effectiveness of an amine-based inhibitor-guanidine for steel in chloride-contaminated simulated concrete pore solutions. J. Zhejiang Univ. 2013, 47, 445–449. [Google Scholar]
- Xu, C.; Jin, W.L.; Wang, H.L.; Wu, H.T.; Huang, N.; Li, Z.Y.; Mao, J.H. Organic inhibitor of triethylenetetramine into chloride contamination concrete by eletro-injection method. Constr. Build. Mater. 2016, 115, 602–617. [Google Scholar] [CrossRef]
- Mao, J.H.; Yu, K.Q.; Xu, Y.D.; Wu, X.X.; Jin, W.L.; Xu, C.; Pan, C.G. Experimental Research on the Distribution of Chloride Ion Migration in Concrete Cover during Electrochemical Chloride Extraction Treatment. Int. J. Electrochem. Sci. 2016, 11, 4076–4083. [Google Scholar] [CrossRef]
- Da, B.; Yu, H.; Ma, H.; Tan, Y.; Mi, R.; Dou, X. Chloride diffusion study of coral concrete in a marine environment. Constr. Build. Mater. 2016, 123, 47–58. [Google Scholar] [CrossRef]
- Djerbi, A.; Bonnet, S.; Khelidj, A. Influence of traversing crack on chloride diffusion into concrete. Cem. Concr. Res. 2008, 38, 877–883. [Google Scholar] [CrossRef]
- Elsener, B.; Buchler, M.; Stalder, F. Migrating corrosion inhibitor blend for reinforced concrete: Part 1-prevention of corrosion. Corrosion 1999, 55, 1155–1163. [Google Scholar] [CrossRef]
- Pan, C.G.; Li, Y.Y.; Shu, D.D.; Wang, Z.; Zhu, S.J.; Mao, J.H.; Jin, W.L. Development of migrating corrosion inhibitor in reinforced concrete. Mater. Rep. 2016, 30, 145–149. [Google Scholar]
- Miranda, J.M.; Gonzalez, J.A.; Cobo, A. Several questions about electrochemical rehabilitation methods for reinforced concrete structures. Corros. Sci. 2006, 48, 2172–2188. [Google Scholar] [CrossRef]
- Montes-García, P.; Castellanos, F.; Vásquez-Feijoo, J.A. Assessing corrosion risk in reinforced concrete using wavelets. Corros. Sci. 2010, 52, 555–561. [Google Scholar] [CrossRef]
- Leelalerkiet, V.; Kyung, J.W.; Ohtsu, M.; Yokota, M. Analysis of half-cell potential measurement for corrosion of reinforced concrete. Constr. Build. Mater. 2004, 18, 155–162. [Google Scholar] [CrossRef]
- Şahmaran, M. Effect of flexure induced transverse crack and self-healing on chloride diffusivity of reinforced mortar. J. Mater. Sci. 2007, 42, 9131. [Google Scholar] [CrossRef]
- Hara, H.; Wakizaka, Y.; Okazaki, S. Silver chloride pre-treatment for the direct potentiometric determination of chloride in stream waters using a solid-state chloride ion-selective electrode. Analyst 1985, 110, 1087–1090. [Google Scholar] [CrossRef]
- Saremi, M.; Mahallati, E. A study on chloride-induced depassivation of mild steel in simulated concrete pore solution. Cem. Concr. Res. 2002, 32, 1915–1921. [Google Scholar] [CrossRef]
- Wu, Z.W. The recent development of concrete science and technology. J. Chin. Ceram. Soc. 1979, 7, 3–7. [Google Scholar]
- Ihekwaba, N.M.; Hope, B.B.; Hansson, C.M. Pull-out and bond degradation of steel rebars in ECE concrete. Cem. Concr. Res. 1996, 26, 267–282. [Google Scholar] [CrossRef]
- Siegwart, M.; Lyness, J.F.; Mcfarland, B.J. Change of pore size in concrete due to electrochemical chloride extraction and possible implications for the migration of ions. Cem. Concr. Res. 2003, 33, 1211–1221. [Google Scholar] [CrossRef]





| Test Conditions | Inhibitor | Time (d) | Mass Loss (g) 9 | Rate |
|---|---|---|---|---|
| Pore solution | - | 7 | 0.0710 | - |
| 14 | 0.1499 | - | ||
| Pore solution | Triethylenetetramine | 7 | 0.0585 | 17.6% |
| 14 | 0.1101 | 26.5% | ||
| Pore solution | Imidazoline | 7 | 0.0424 | 40.3% |
| 14 | 0.0916 | 38.8% |
| Strength | Water (kg/m3) | Cement (kg/m3) | Sand (kg/m3) | Gravel (kg/m3) |
|---|---|---|---|---|
| C30 | 177 | 393 | 534 | 1297 |
| Samples | Strength | Electrolyte | |
|---|---|---|---|
| New Imidazoline | TETA | ||
| A | C30 | 0.3 mol/L | - |
| B | - | 0.3 mol/L | |
| C | - | - | |
| Samples | Inhibitor | Amount of Inhibitor | Power (Y/N) |
|---|---|---|---|
| C0-N | Imidazoline | 0 | N |
| C1-Y | 0.25% | Y | |
| C2-Y | 0.25% | Y | |
| C3-N | 0.25% | N | |
| C4-Y | 0.5% | Y | |
| C5-Y | 0.5% | Y | |
| C6-N | 0.5% | N | |
| C7-Y | 0.75% | Y | |
| C8-Y | 0.75% | Y | |
| C9-N | 0.75% | N |
| Time | 0 | 7 Days | 15 Days | 30 Days | |
|---|---|---|---|---|---|
| Position | |||||
| Porosity near anode | 21.39% | 19.47% | 19.32% | 16.93% | |
| Porosity near cathode | 21.04% | 20.33% | 17.93% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Mao, J.; Jin, W. Effect of Imidazoline Inhibitor on the Rehabilitation of Reinforced Concrete with Electromigration Method. Materials 2020, 13, 398. https://doi.org/10.3390/ma13020398
Pan C, Mao J, Jin W. Effect of Imidazoline Inhibitor on the Rehabilitation of Reinforced Concrete with Electromigration Method. Materials. 2020; 13(2):398. https://doi.org/10.3390/ma13020398
Chicago/Turabian StylePan, Chonggen, Jianghong Mao, and Weiliang Jin. 2020. "Effect of Imidazoline Inhibitor on the Rehabilitation of Reinforced Concrete with Electromigration Method" Materials 13, no. 2: 398. https://doi.org/10.3390/ma13020398
APA StylePan, C., Mao, J., & Jin, W. (2020). Effect of Imidazoline Inhibitor on the Rehabilitation of Reinforced Concrete with Electromigration Method. Materials, 13(2), 398. https://doi.org/10.3390/ma13020398