Structural Aspects of Decreasing the Corrosion Resistance of Zinc Coating Obtained in Baths with Al, Ni, and Pb Additives
Abstract
1. Introduction
- reduction of the oxidation intensity of the bath surface,
- limiting the effect of Si content on steel on coating growth, and
- improving the ability of liquid zinc to flow from the surface of the product when emerging from the bath.
2. Experimental Research
2.1. Materials for Research
2.2. Research Scope and Methodology
3. Results of the Research
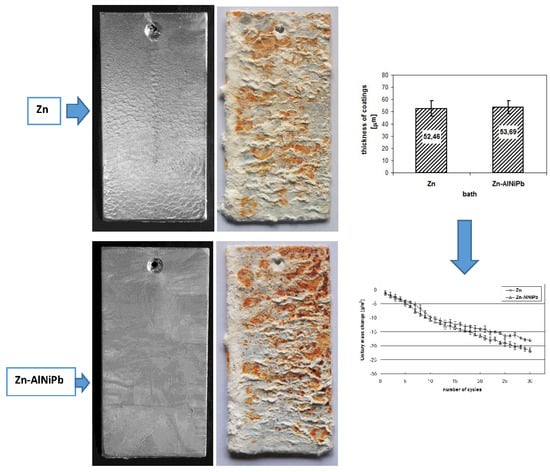
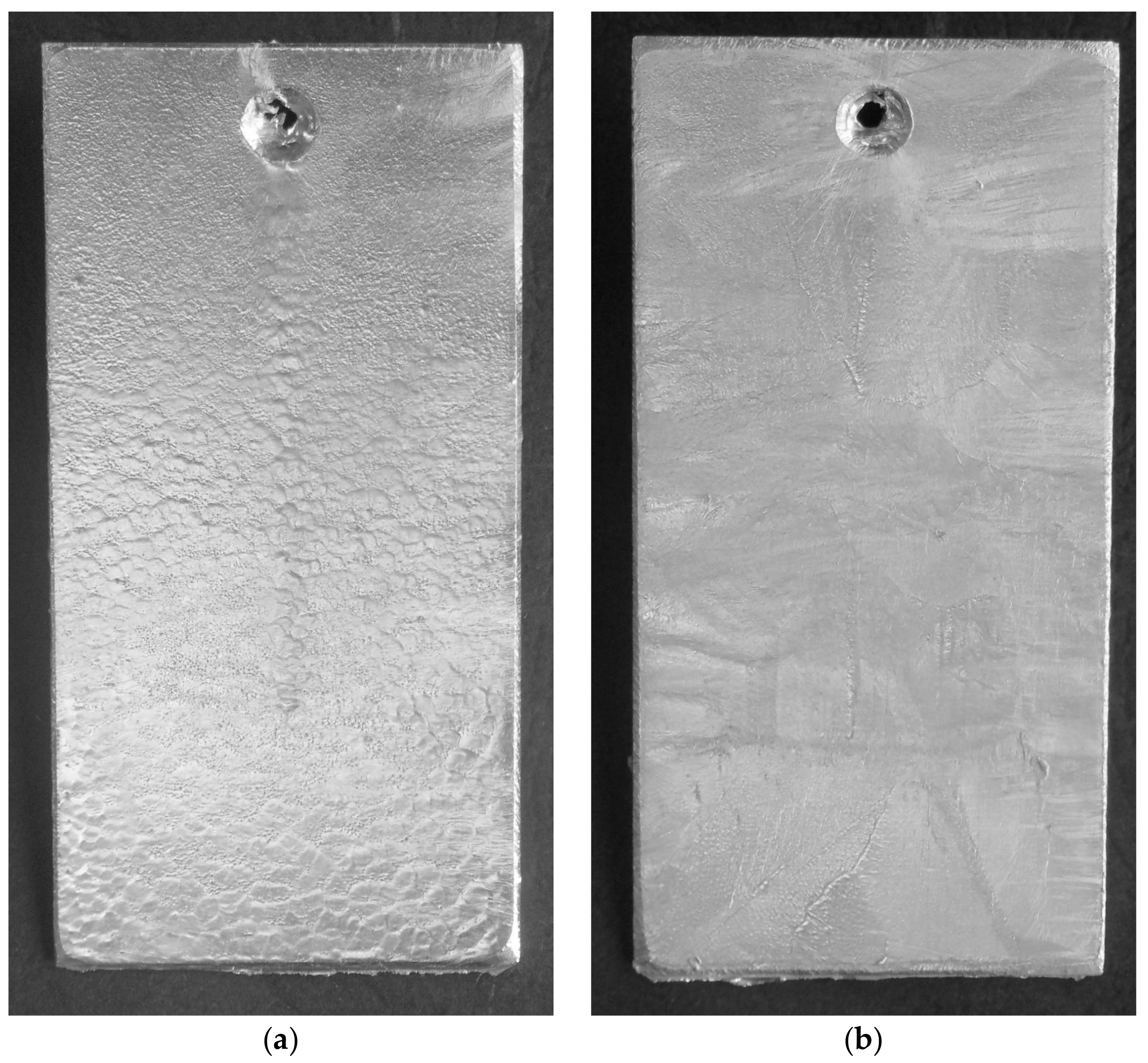
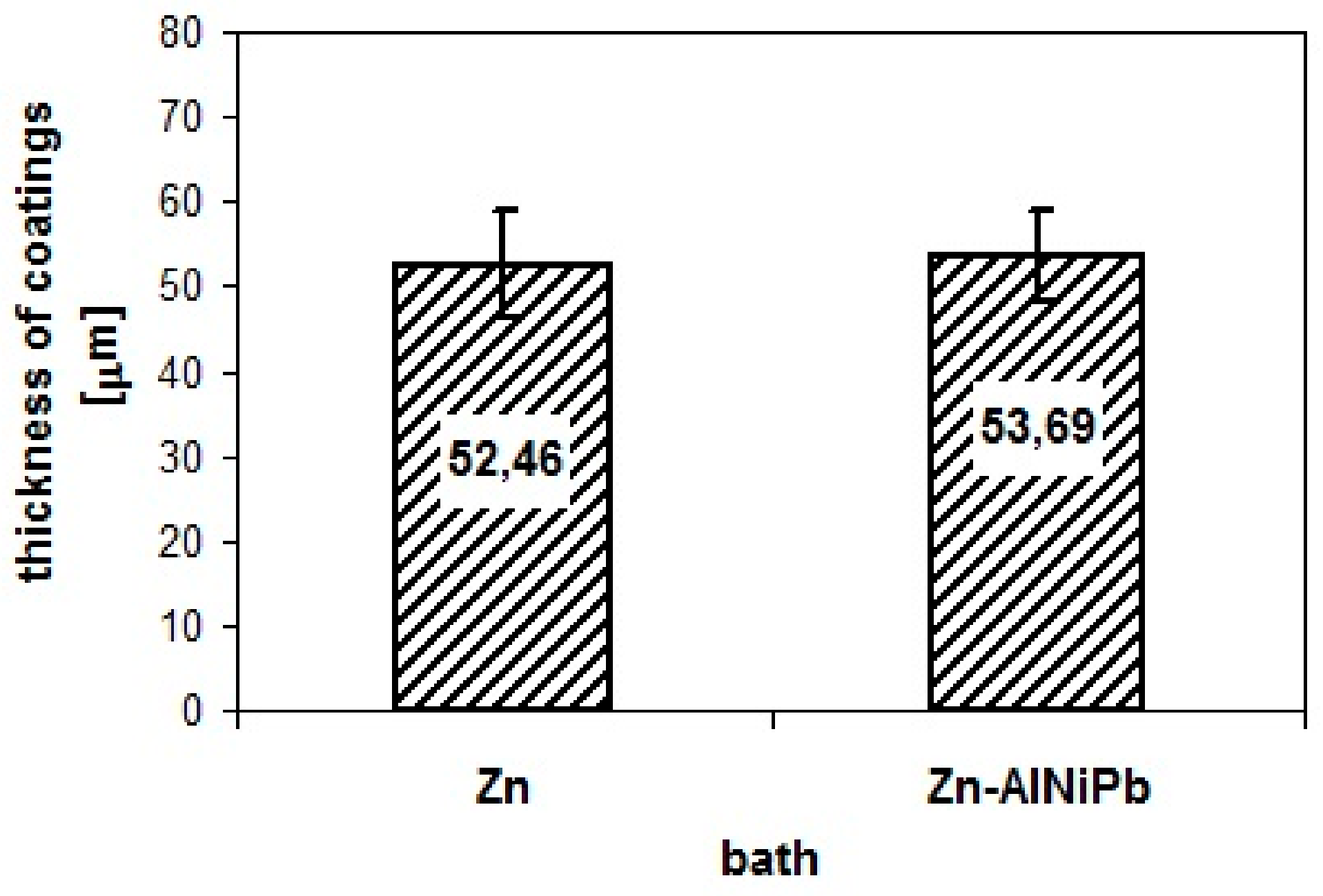
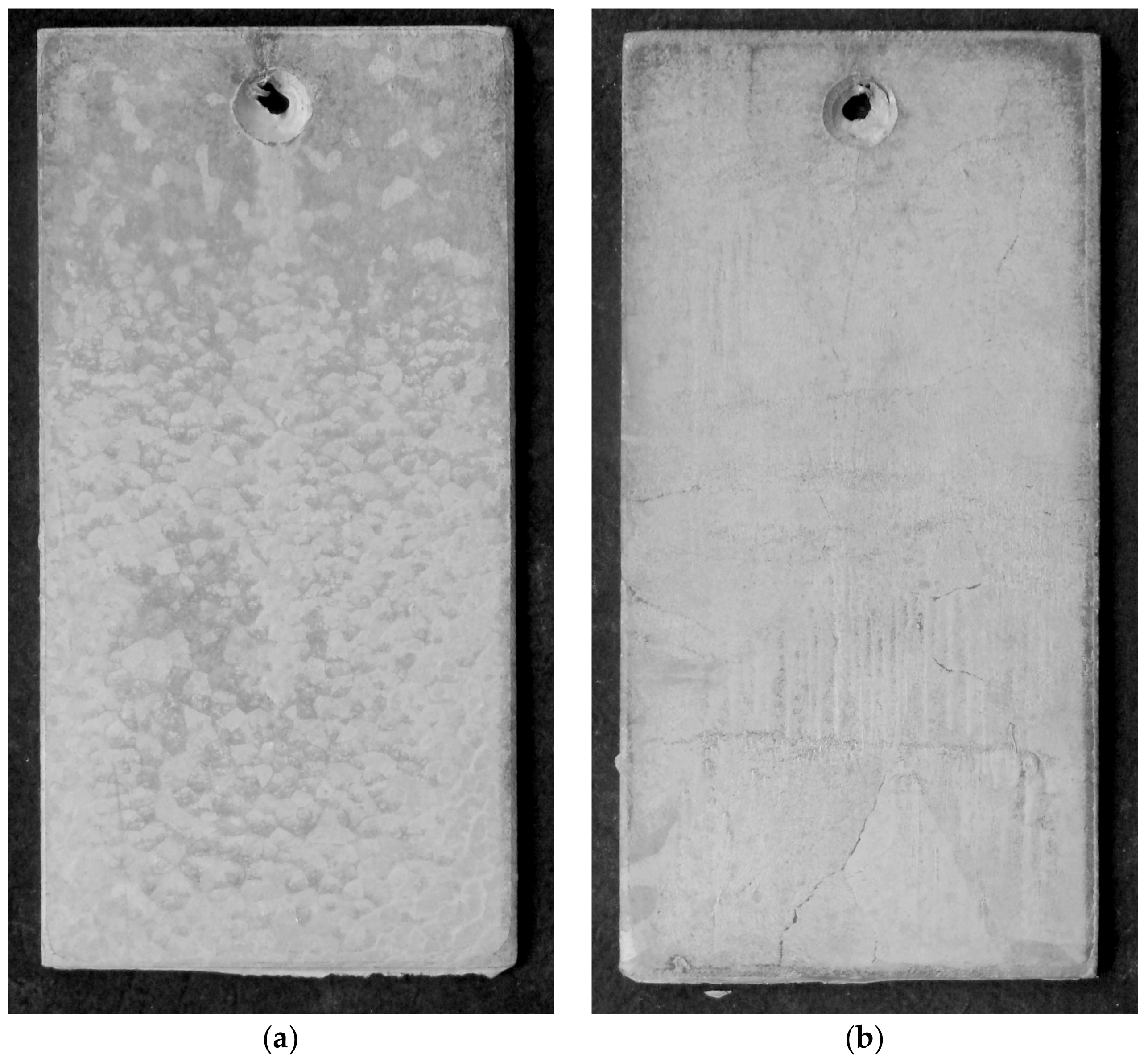
3.1. Coating Characteristics before Starting Corrosion Tests
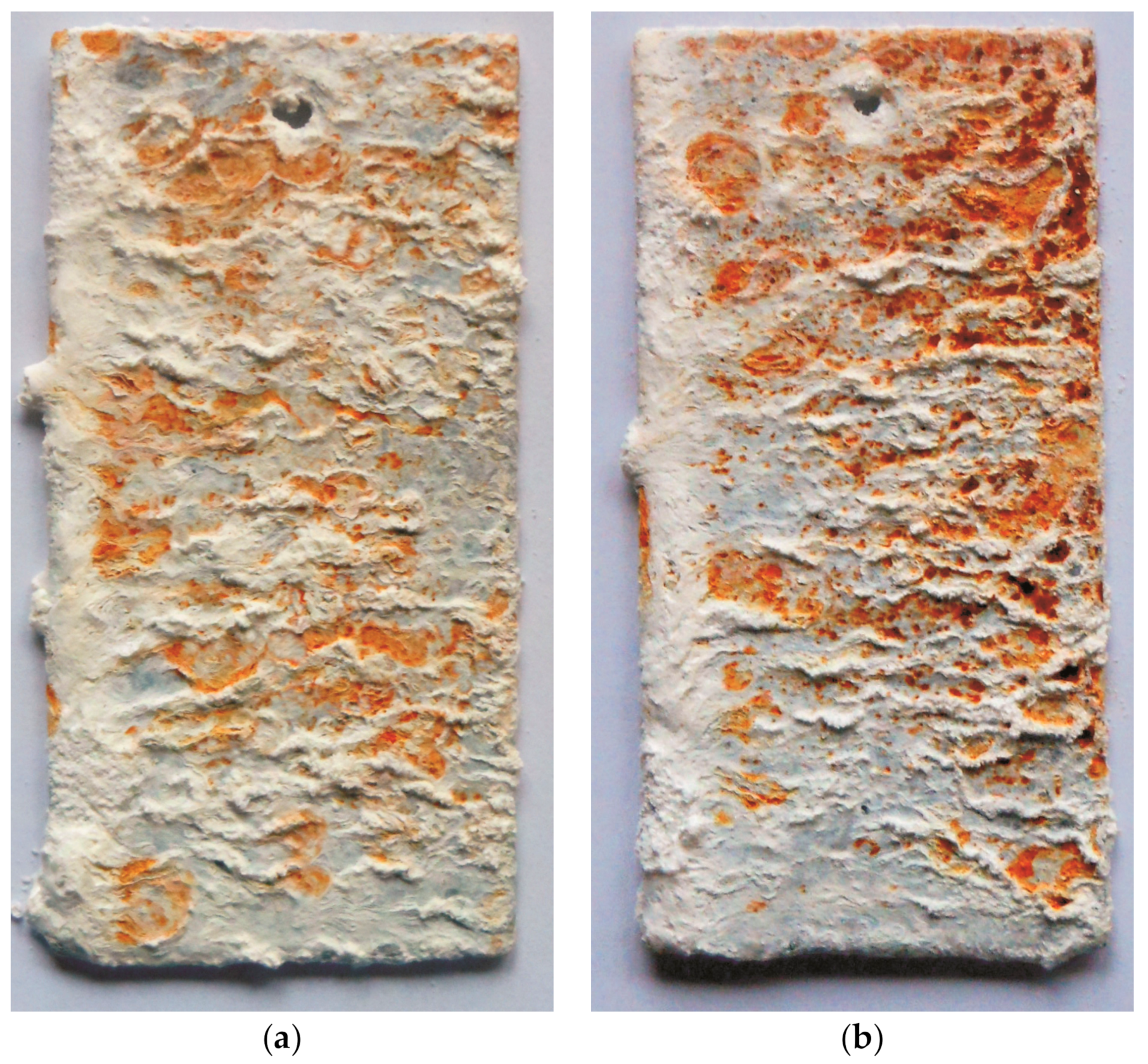
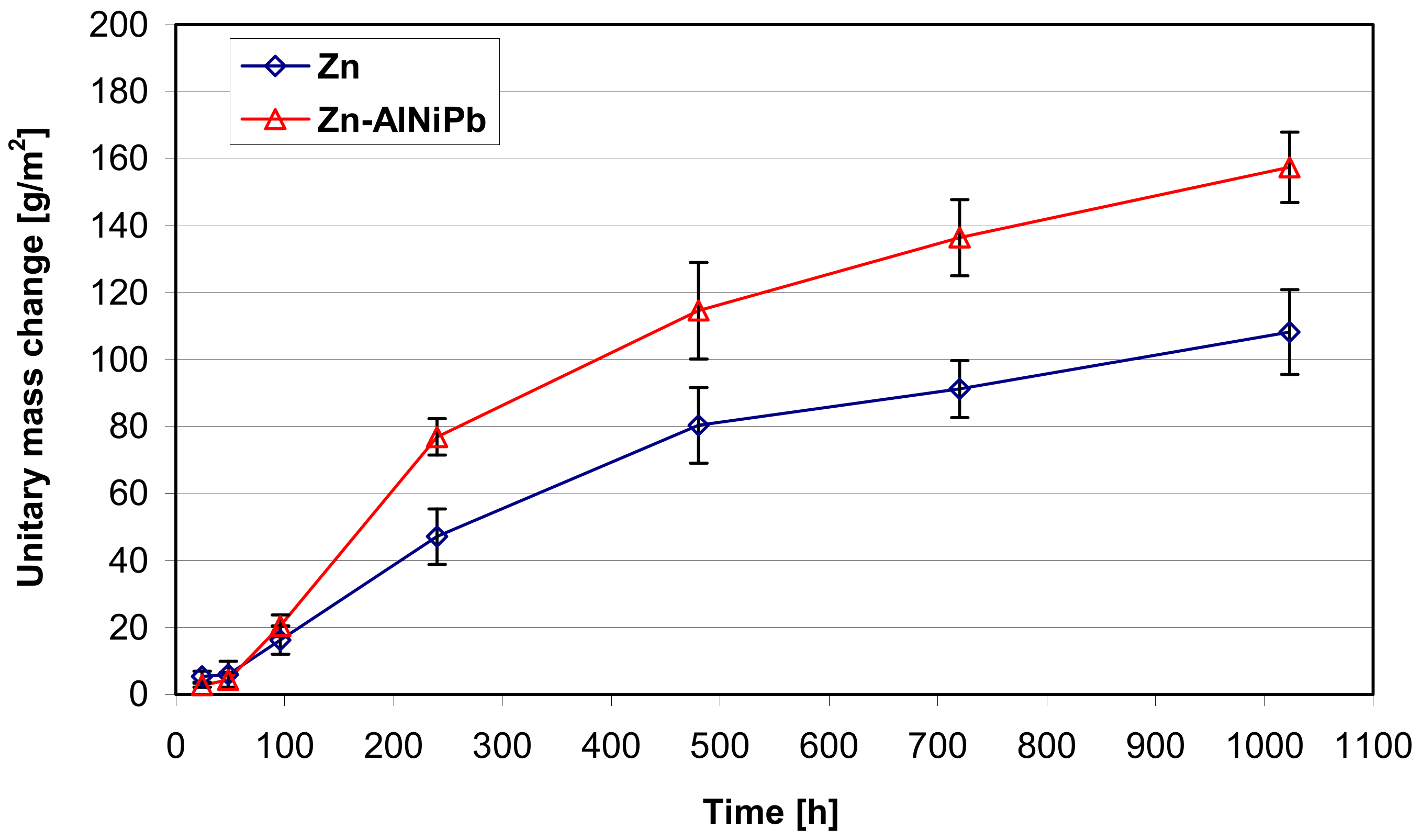
3.2. The Corrosion Resistance of Coatings in Neutral Salt Spray
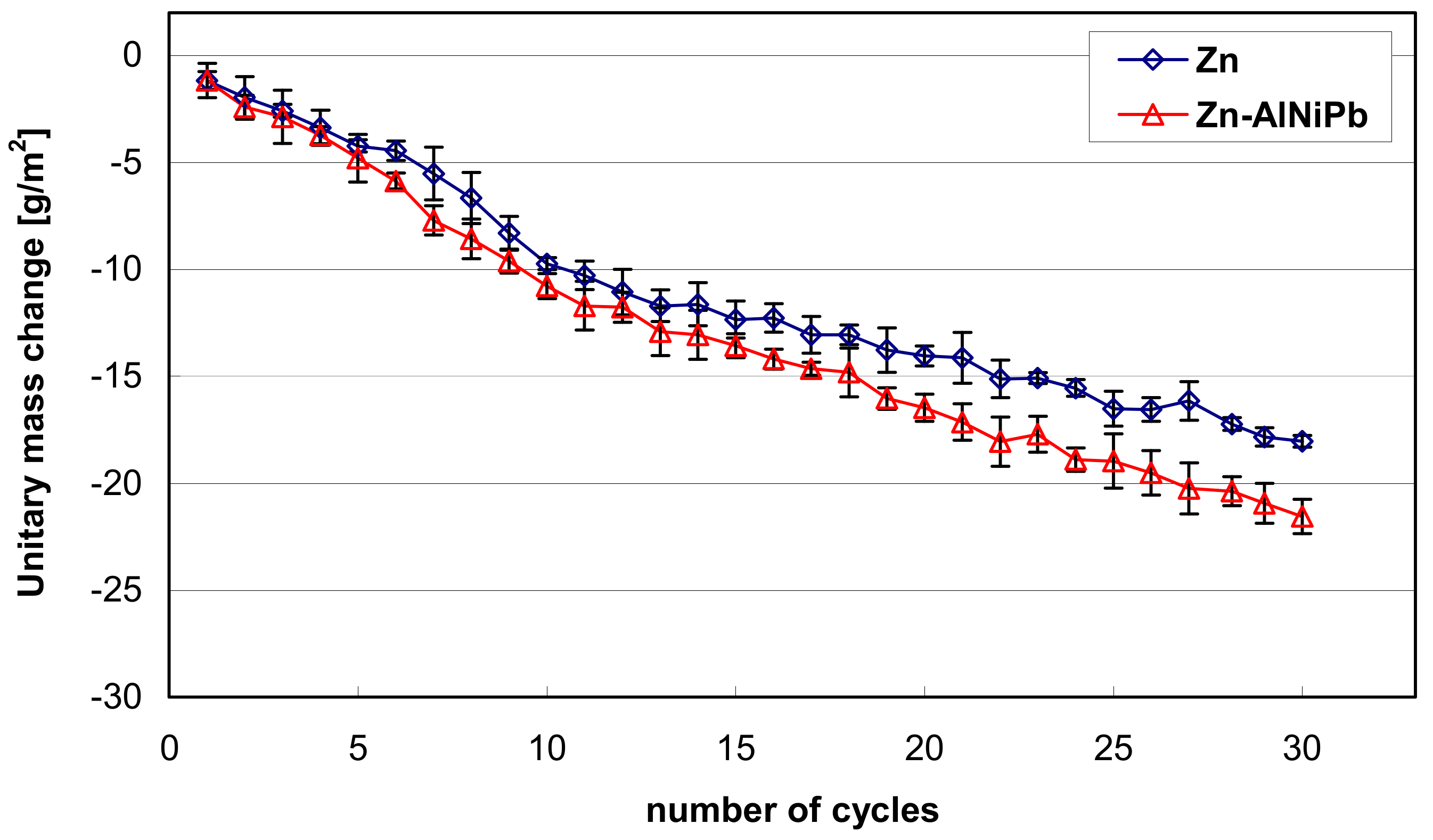
3.3. The Corrosion Resistance of Coatings in a Humid Atmosphere Containing SO2
3.4. Microstructure (SEM) and Microanalysis (EDS) of the Coating Surface
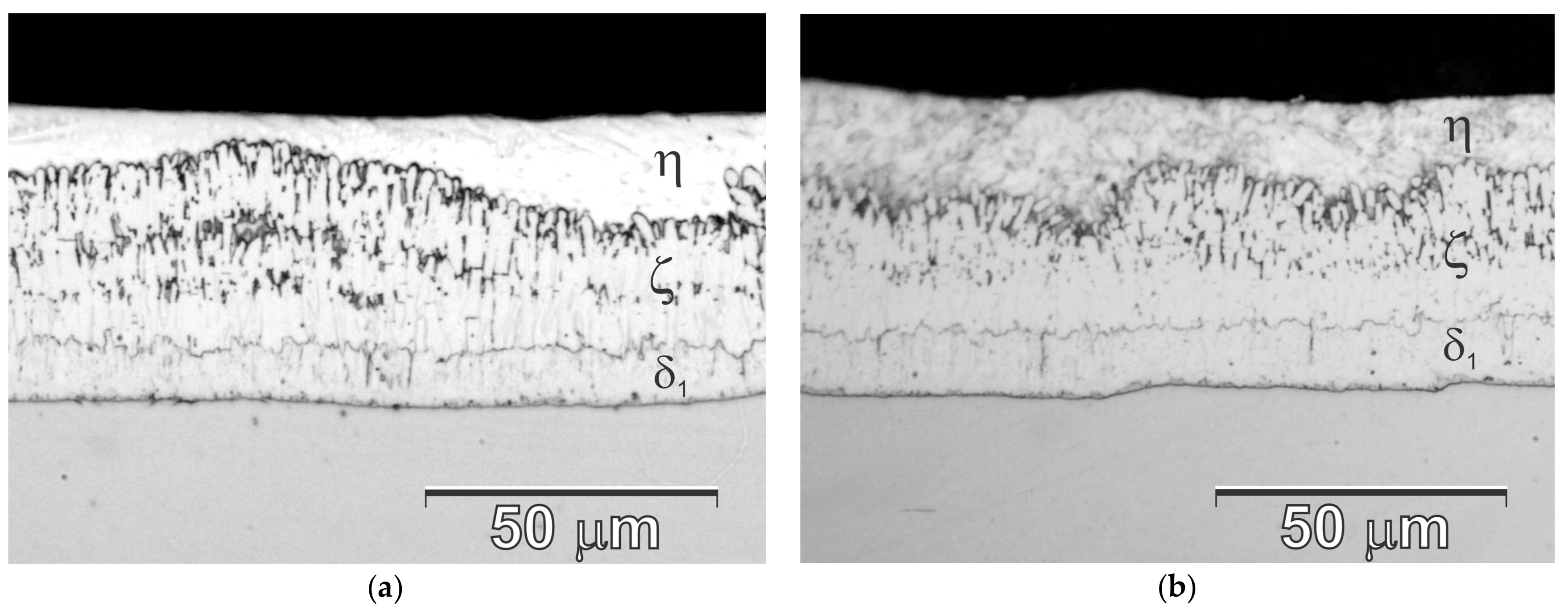
3.5. Microstructure (SEM) and Microanalysis (EDS) on the Coating Cross-Section
4. Discussion of Research Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liberski, P. Antykorozyjne Metalowe Powłoki Zanurzeniowe; Politechnika Śląska: Gliwice, Poland, 2013. [Google Scholar]
- Suliga, M.; Wartacz, R. The influence of the angle of working part of die on the zinc coating thickness and mechanical properties of medium carbon steel wires. Arch. Metall. Mater. 2019, 64, 1295–1299. [Google Scholar]
- Kania, H.; Liberski, P. Synergistic Influence of the Addition of Al, Ni and Pb to a Zinc Bath upon Growth Kinetics and Structure of Coatings. Solid State Phenom. 2014, 212, 115–120. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P. Synergistic influence of Al, Ni, Bi and Sn Addition to a Zinc Bath upon Growth Kinetics and the Structure of Coatings. In Proceedings of the IOP Conf. Series: Materials Science and Engineering, Katowice, Poland, 18 May 2012. No 012004. [Google Scholar]
- Porter, F.C. Zinc Handbook: Properties Processing and Use in Design; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Marder, A.R. The Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Reumont, G.; Perrot, P.; Foct, J. Thermodynamic Study of the Galvanizing Process in a Zn-0.1%Ni Bath. J. Mater. Sci. 1998, 33, 4759–4768. [Google Scholar] [CrossRef]
- Tang, N.Y. Alternative Description of Dross Formation When Galvanizing Steels in Zinc-Nickel Baths. J. Ph. Equilib. 1995, 16, 110–112. [Google Scholar] [CrossRef]
- Lewis, G.P.; Pedersen, J.G. Optimizing the Nickel-Zinc Process for Hot Dip Galvanizing. In Proceedings of the 3rd Asian Pacific General Galvanizing Conference, Queensland, Australia, 1996. [Google Scholar]
- Fasoyino, F.A.; Weinberg, F. Spangle Formation in Galvanized Sheet Steel Coatings. Met. Trans. B-Process Met. 1990, 21, 549–558. [Google Scholar] [CrossRef]
- Strutzenberger, J.; Faderl, J. Solidification and Spangle Formation of Hot-Dip-Galvanized Zinc Coatings. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 1998, 29, 631–646. [Google Scholar] [CrossRef]
- Krepski, R.P. The Influence of Lead in After-Fabrication Hot Dip Galvanizing. In Proceedings of the 14th International Galvanizing Conference, Zinc Development Association, London, UK, 6–12 June 1986. [Google Scholar]
- Beguin, P.; Bosschaerts, M.; Dhaussy, D.; Pankert, R.; Gilles, M. Galveco a Solution for Galvanizing Reactive Steel. In Proceedings of the 19th International Galvanizing Conference, EGGA, Berlin, Germany, 1–8 March 2000. [Google Scholar]
- Reumont, G.; Perrot, P. Fundamental Study of Lead Additions in Industrial Zinc. In Proceedings of the 18th International Galvanizing Conference, EGGA, Birmingham, UK; 1997. [Google Scholar]
- Gagne, M. Hot-dip galvanizing with zinc-bismuth alloys. Metall 1999, 53, 269–271. [Google Scholar]
- Tatarek, A.; Saternus, M. Badanie Zjawisk Rozpuszczania Dyfuzyjnego Stali Reaktywnych w Kąpieli Cynkowej z Dodatkiem Bizmutu. Ochrona przed korozją 2018, 7, 186–190. (In Polish) [Google Scholar]
- Mendala, J. Liquid Metal Embrittlement of Steel with Galvanized Coatings. In Proceedings of the IOP Conf. Ser.: Materials Science and Engineering, Katowice, Poland, 18 May 2012. No. 012002. [Google Scholar]
- Mendala, J. The Possibility of the LME Phenomenon in Elements Subjected to Metallization in Zn Bath with Bi Addition. Solid State Phenom. 2015, 226, 167–172. [Google Scholar] [CrossRef]
- Mendala, J.; Liberski, P. Liquid Metal Embrittlement of Steel with a Coating Obtained by Batch Hot Dip Method in a Zn + 2% Sn Bath. Solid State Phenom. 2014, 212, 107–110. [Google Scholar] [CrossRef]
- Huckshold, M. Improving Design Guidance to Avoid Cracking of Galvanized Structural Steelwork. In Proceedings of the 22th International Galvanizing Conference, Session: Steel and Galvanizing, EGGA, Madrid, Spain; 2009. [Google Scholar]
- Wołczyński, W.; Guzik, E.; Janczak-Rusch, J.; Kopyciński, D.; Golczewski, J.; Mo Lee, H.; Kloch, J. Morphological Characteristics of Multi-Layer/Substrate Systems. Mater. Charact. 2006, 56, 274–280. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Springer-Verlag: New York, NY, USA, 2013. [Google Scholar]
- Waseda, Y.; Suzuki, S. Characterization of Corrosion Products on Steel Surfaces; Series: Advances in Materials Research; Springer: Berlin, Germany, 2006; Volume 7. [Google Scholar]
- Kania, H.; Sipa, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained on High-Strength Grade 10.9 Bolts Using a New Thermal Diffusion Process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Vala, U. Effect of Lead on the Hot Dip Galvanized Steel as Barrier/Sacrificial Coating. In Proceedings of the Seminar on Resurgence of Metallic Materials the Current Scenario (ROMM-2002), National Metallurgical Laboratory, London, UK, 24–25 October 2002. [Google Scholar]
- Moser, Z.; Zabdyr, L.; Gasior, W.; Salawa, J.; Zakulski, W. The Pb-Zn (Lead-Zinc) System. J. Ph. Equilib. 1994, 15, 643–649. [Google Scholar] [CrossRef]
- Mendala, J. Influence of the cooling method on the structure of 55AlZn coatings. In Proceedings of the IOP Conf. Ser.: Materials Science and Engineering, Katowice, Poland, 20 May 2011. No. 012004. [Google Scholar]
- Stephen, D.C.; Bernard, S.C., Jr. ASM Handbook. Corrosion: Fundamentals, Testing, and Protection; ASM International: Cleveland, OH, USA, 1992. [Google Scholar]
- Tang, N.-Y.; Adams, G.R.; Kolisnyk, P.S. On Determining Effective Al in Continuous Galvanizing Baths. In Proceedings of the Galvatech’95 Conference: The Use and Manufacture of Zinc and Zinc Alloy Coated Sheet Steel Products into the 21st Century, Chicago, IL, USA, 17–21 September 1995. [Google Scholar]
- Taylor, M.S.; Murphy, S. A decade of Technigalva. In Proceedings of the 18th International Galvanizing Conference Intergalva’97, General Galvanizing Association, Birmingham, UK, 8–11 June 1997. [Google Scholar]
- Anderson, E.A. The atmospheric corrosion of rolled zinc. In Proceedings of the ASTM 58th Annual Meeting, Symposium on Atmospheric Corrosion of Non-Ferrous Metals; ASTM International: West Conshohocken, PA, USA, 1953; Volume 175, pp. 126–134. [Google Scholar]
- Pryor, M.J. Bimetallic Corrosion. In Corrosion; George Newnes Ltd.: London, UK, 1963. [Google Scholar]
- Seré, P.R.; Culcasi, J.D.; Elsner, C.I.; Sarli, A.R. Relationship between Texture and Corrosion Resistance in Hot-Dip Galvanized Steel Sheets. Surf. Coat. Technol. 1999, 122, 143–149. [Google Scholar]
- Pyun, S.; Bae, J.; Park, S.; Kim, J.; Lee, Z. The Anodic Behavior of Hot-Galavnized Zinc Layer in Alkaline Solution. Corros. Sci. 1994, 36, 827–835. [Google Scholar] [CrossRef]
- Arenas, M.A.; Bethencourt, M.; Botana, F.J.; de Damborenea, J.; Marcos, M. Inhibition of 5083 Aluminium Alloy and Galvanised Steel by Lanthanide Salts. Corros. Sci. 2001, 143, 157–170. [Google Scholar] [CrossRef]
- Komorowski, L.; Królikowska, A. Effects of Bismuth and Lead Alloy Addition on the Corrosion Behavior of Hot-Dip Galvanized Coatings: Coating Morphology. Ochrona przed korozją 2015, 10, 350–357. [Google Scholar]
| Bath | Content [wt.%] | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Ni | Pb | Bi | Sn | Zn and Others | |
| Zn | 0.0002 | 0.031 | 0.0001 | 0.001 | 0.0003 | 0.0007 | residue |
| Zn-AlNiPb | 0.0048 | 0.029 | 0.049 | 0.48 | 0.0004 | 0.0006 | residue |
| Grade | Content [wt.%] | |||||
|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Fe and Others | |
| G235JRG2 | 0.138 | 0.021 | 0.743 | 0.0086 | 0.0088 | residue |
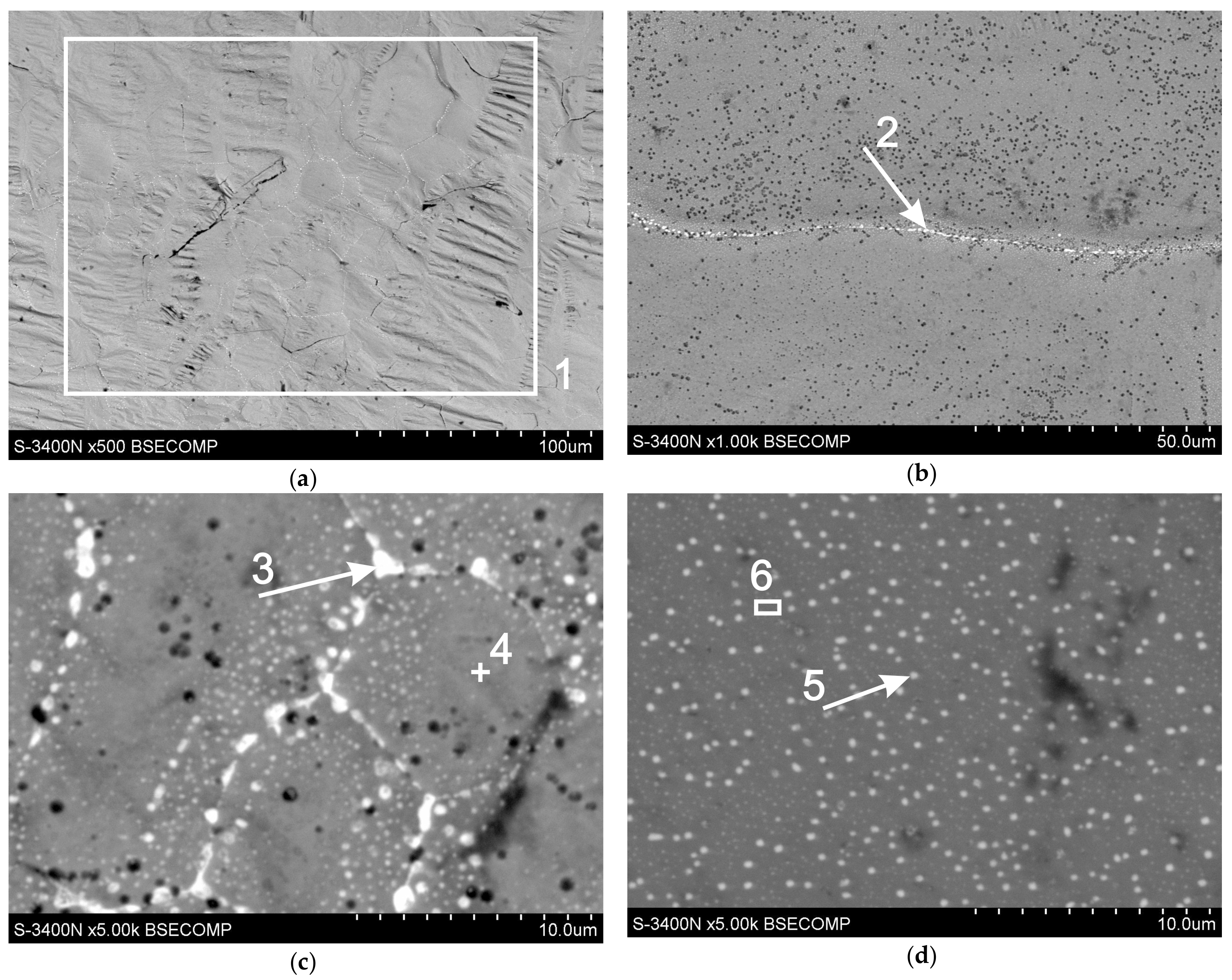
| Analyzing points | Element Contents | |||
|---|---|---|---|---|
| Zn-K | Pb-M | |||
| wt% | at% | wt% | at% | |
| point 1 | 96.5 | 98.9 | 3.5 | 1.1 |
| point 2 | 60.1 | 82.7 | 39.9 | 17.3 |
| point 3 | 34.5 | 62.5 | 65.5 | 37.5 |
| point 4 | 100 | 100 | - | - |
| point 5 | 79.3 | 92.4 | 20.7 | 7.6 |
| point 6 | 99.4 | 99.8 | 0.6 | 0.2 |
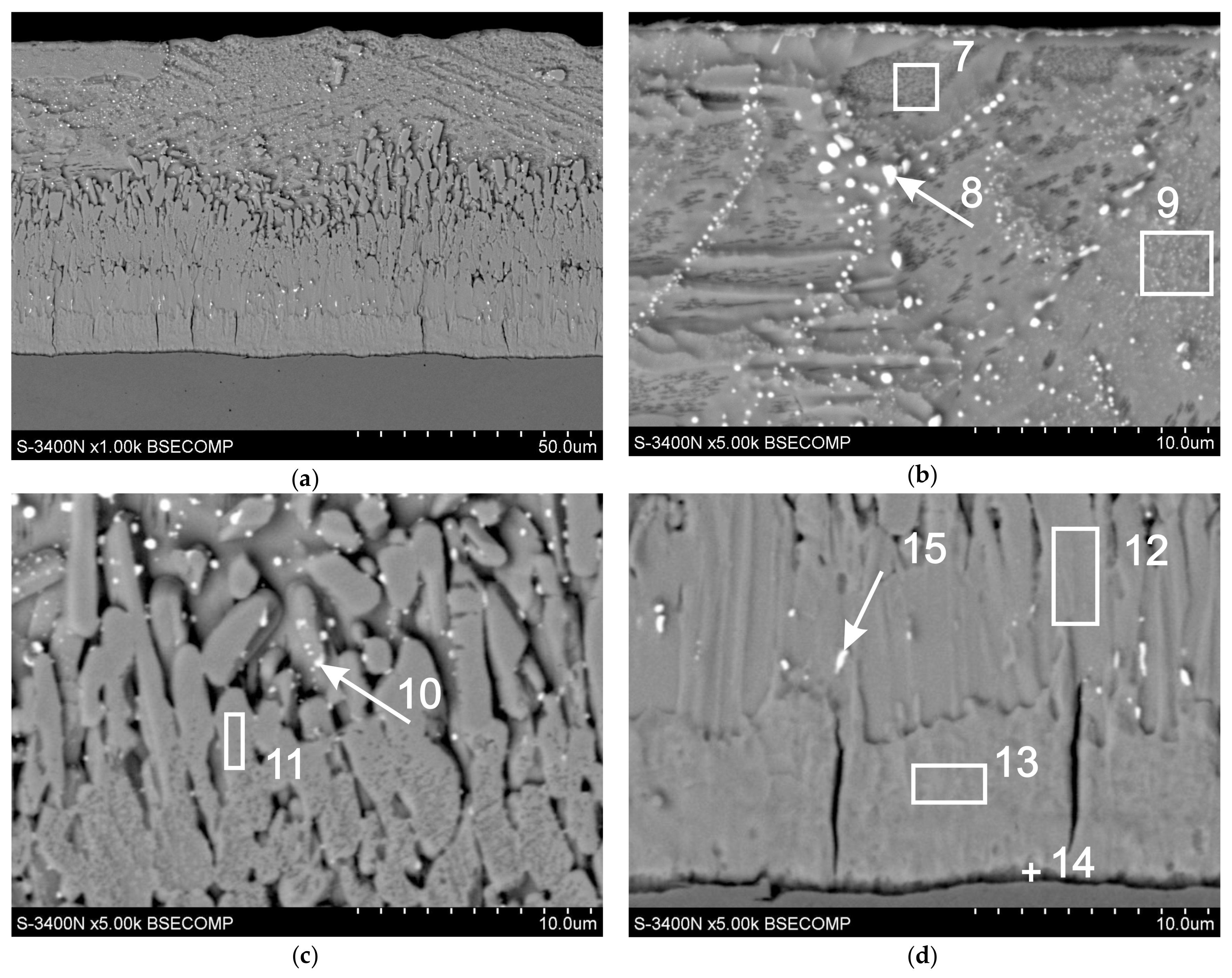
| Analysis Points | Content of Elements | |||||
|---|---|---|---|---|---|---|
| Fe-K | Zn-K | Pb-M | ||||
| wt. % | at. % | wt. % | at. % | wt. % | at. % | |
| point 7 | - | - | 100 | 100 | - | - |
| point 8 | - | - | 39.7 | 67.6 | 60.3 | 32.4 |
| point 9 | - | - | 99.6 | 99.9 | 0.4 | 0.1 |
| point 10 | 4.2 | 5.3 | 83.5 | 90.5 | 12.3 | 4.2 |
| point 11 | 5.9 | 6.8 | - | - | 94.1 | 93.2 |
| point 12 | 6.3 | 7.3 | 93.7 | 92.7 | - | - |
| point 13 | 12.5 | 14.4 | 87.5 | 85.6 | - | - |
| point 14 | 22.8 | 25.7 | 77.2 | 74.3 | - | - |
| point 15 | 5.5 | 7.6 | 70.2 | 83.3 | 23.3 | 9.1 |
| Electrode | Eo (V) |
|---|---|
| Zn/Zn2+ | −0.76 |
| Fe/Fe2+ | −0.45 |
| Pb/Pb2+ | −0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, H.; Saternus, M.; Kudláček, J. Structural Aspects of Decreasing the Corrosion Resistance of Zinc Coating Obtained in Baths with Al, Ni, and Pb Additives. Materials 2020, 13, 385. https://doi.org/10.3390/ma13020385
Kania H, Saternus M, Kudláček J. Structural Aspects of Decreasing the Corrosion Resistance of Zinc Coating Obtained in Baths with Al, Ni, and Pb Additives. Materials. 2020; 13(2):385. https://doi.org/10.3390/ma13020385
Chicago/Turabian StyleKania, Henryk, Mariola Saternus, and Jan Kudláček. 2020. "Structural Aspects of Decreasing the Corrosion Resistance of Zinc Coating Obtained in Baths with Al, Ni, and Pb Additives" Materials 13, no. 2: 385. https://doi.org/10.3390/ma13020385
APA StyleKania, H., Saternus, M., & Kudláček, J. (2020). Structural Aspects of Decreasing the Corrosion Resistance of Zinc Coating Obtained in Baths with Al, Ni, and Pb Additives. Materials, 13(2), 385. https://doi.org/10.3390/ma13020385