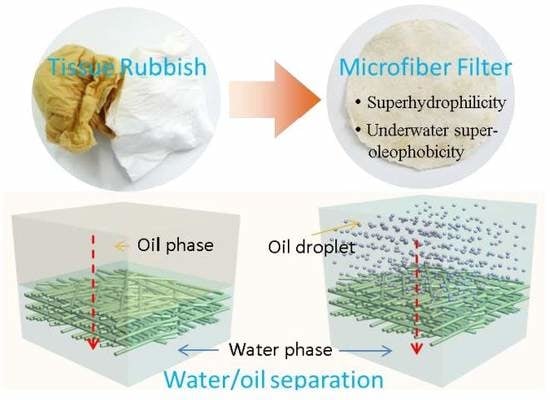
Turning Tissue Waste into High-Performance Microfiber Filters for Oily Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Microfiber Filters
2.3. Characterization of the Microfiber Filters
2.4. Performance Evaluation of the Microfiber Filters
3. Results and Discussion
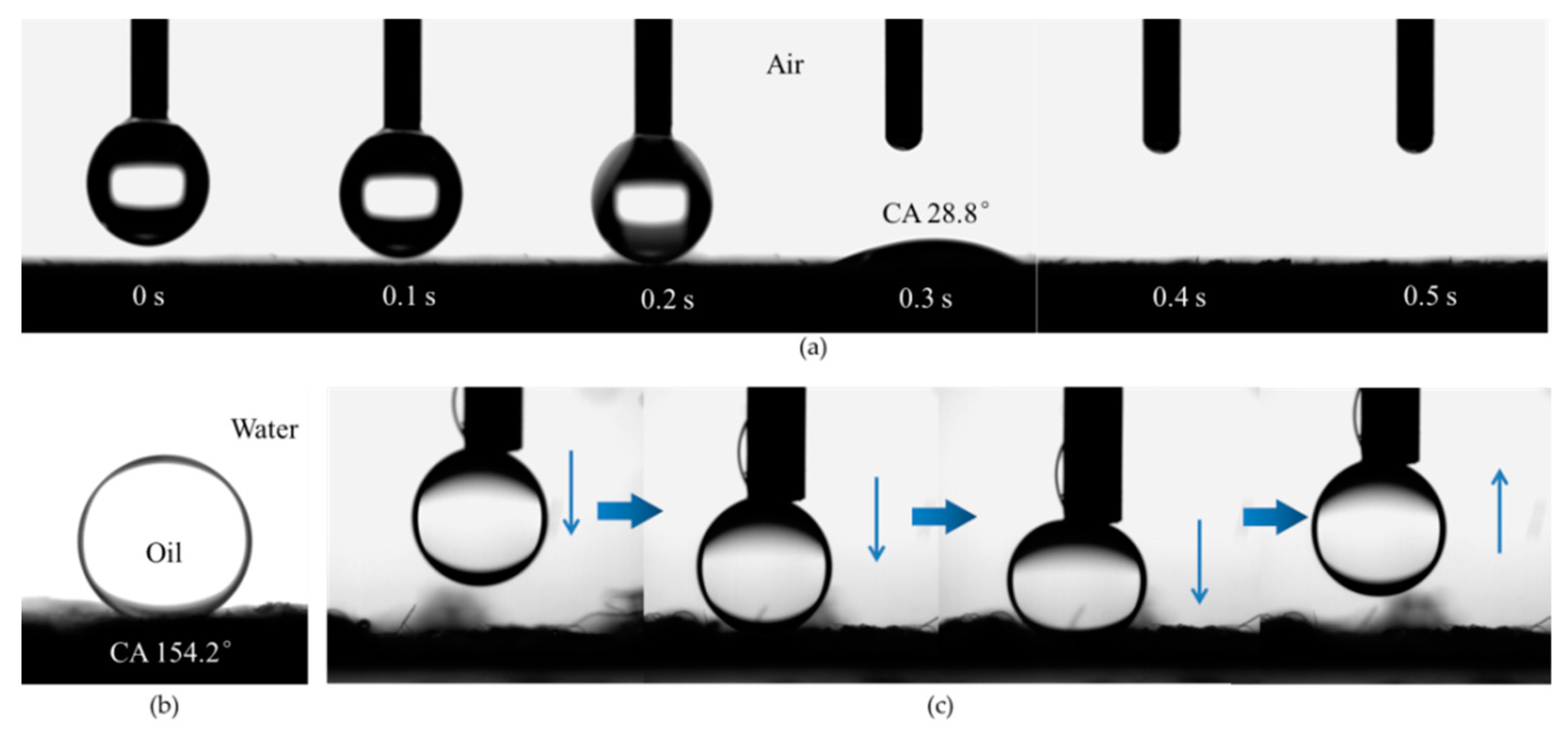
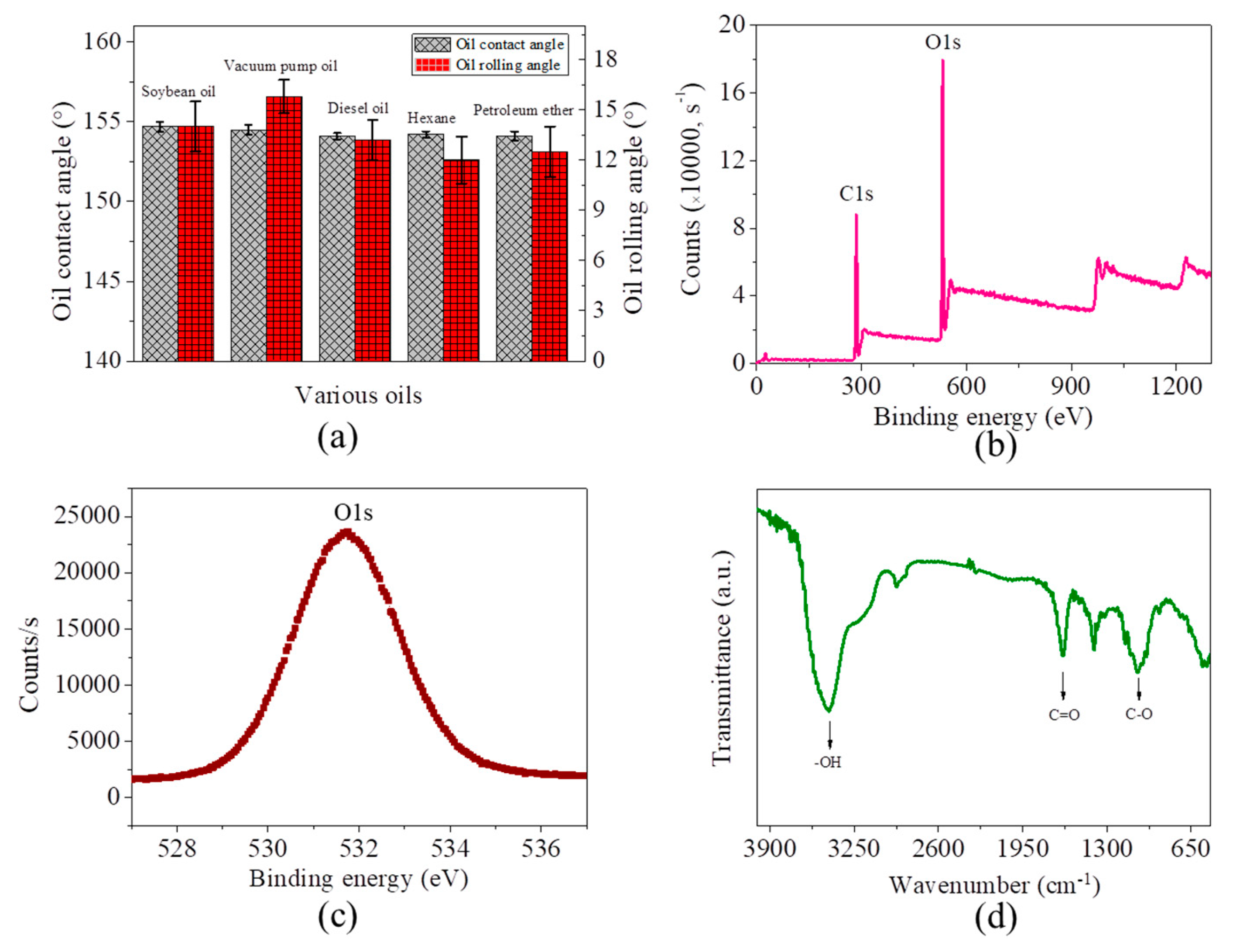
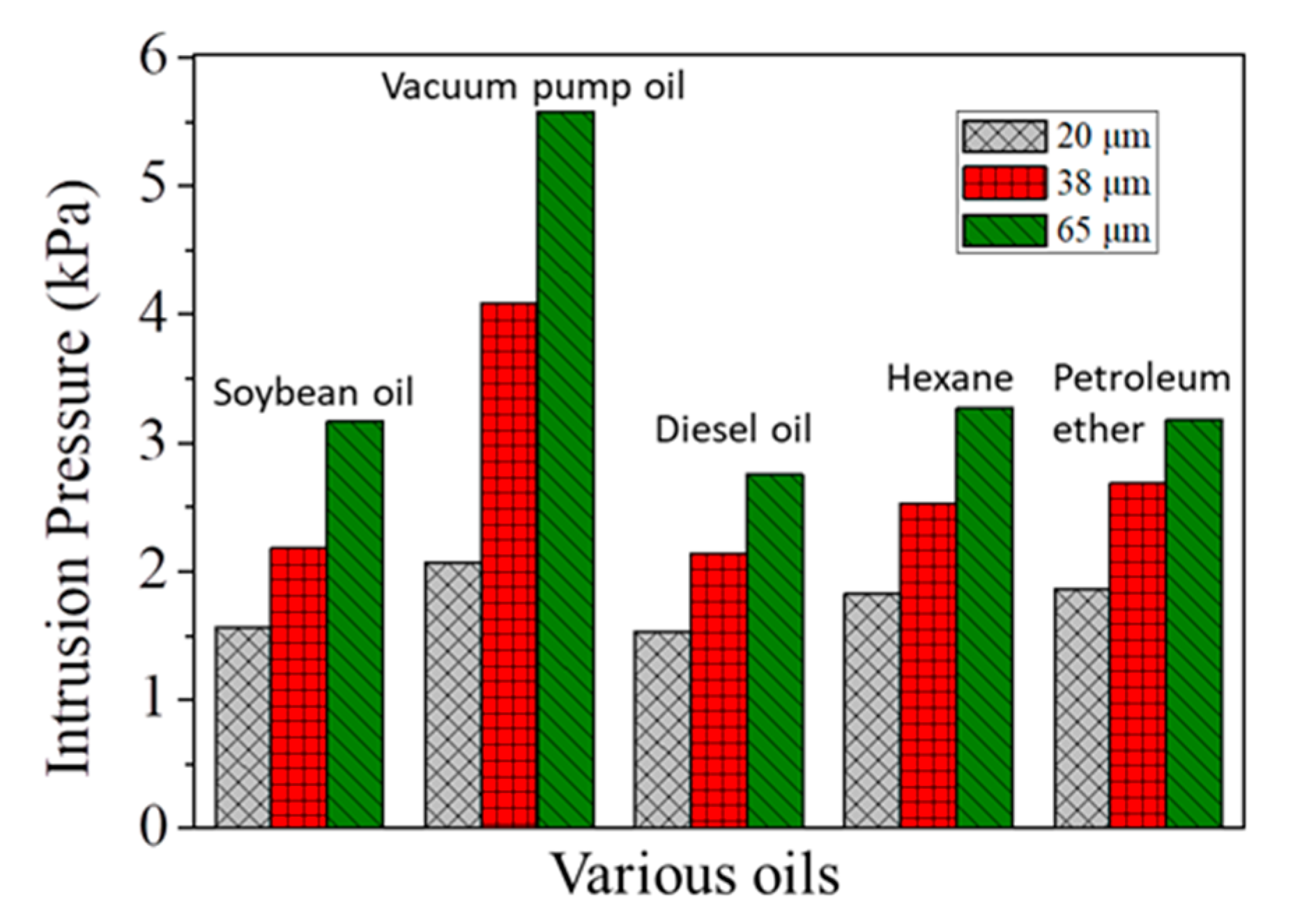
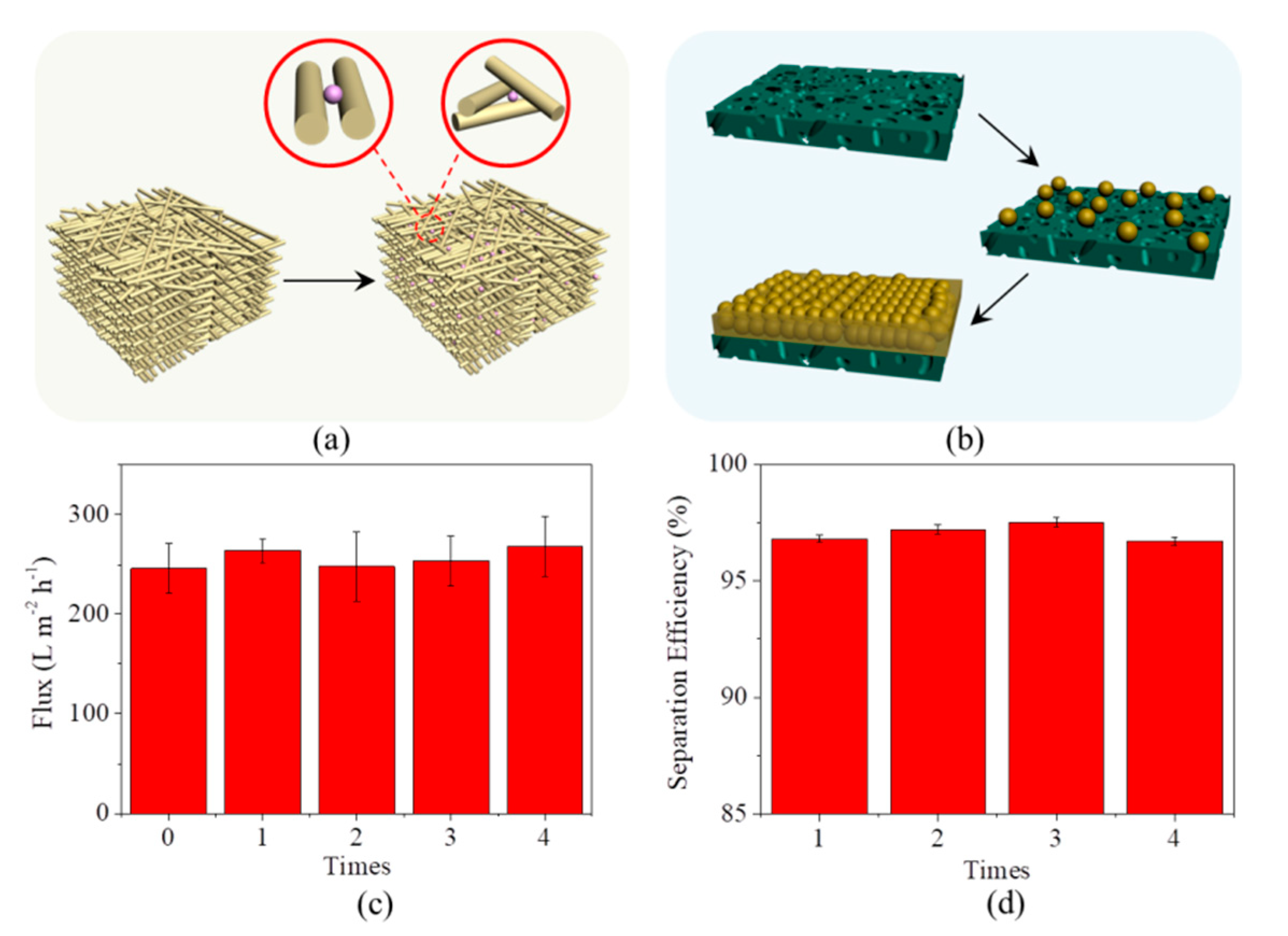
3.1. Preparation and Characterization of the Microfiber Filters
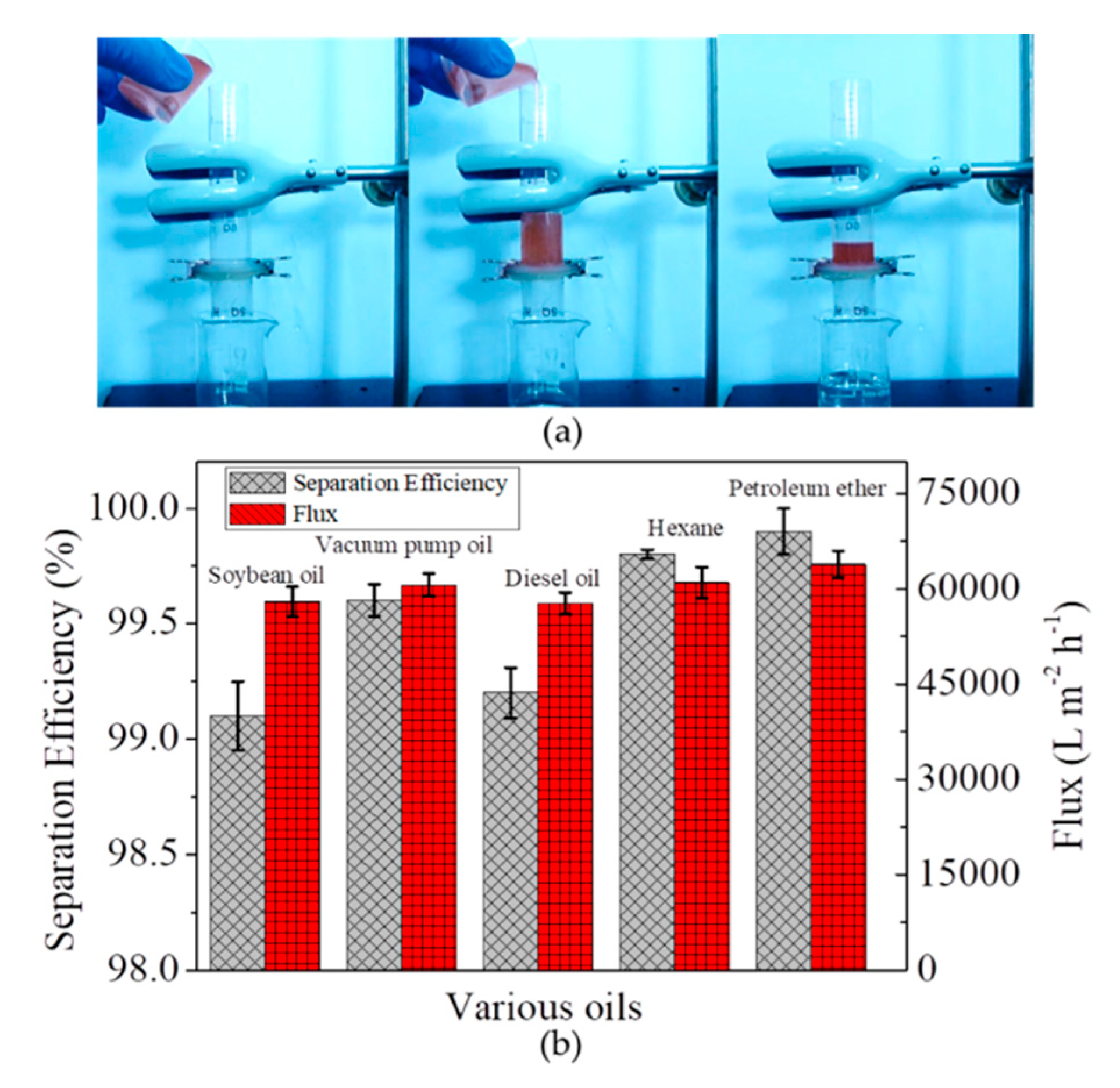
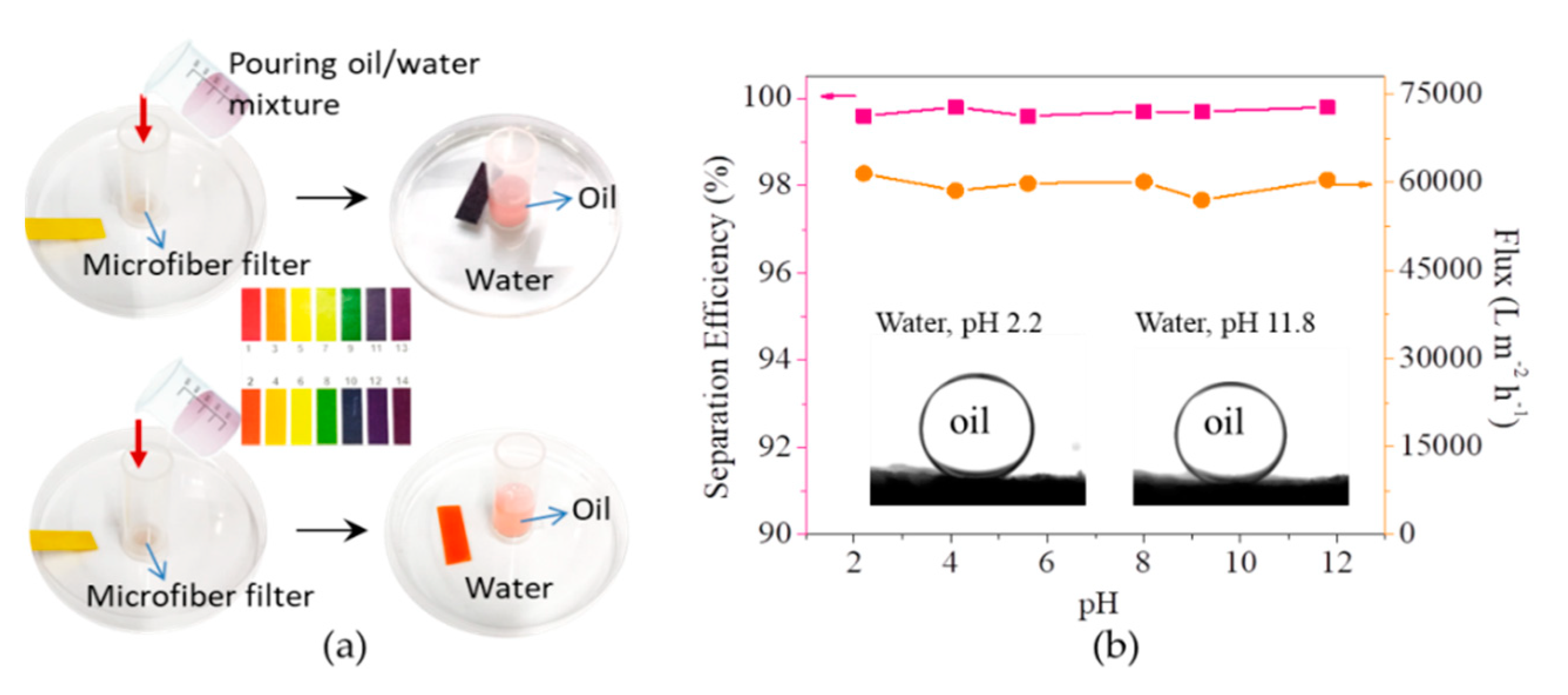
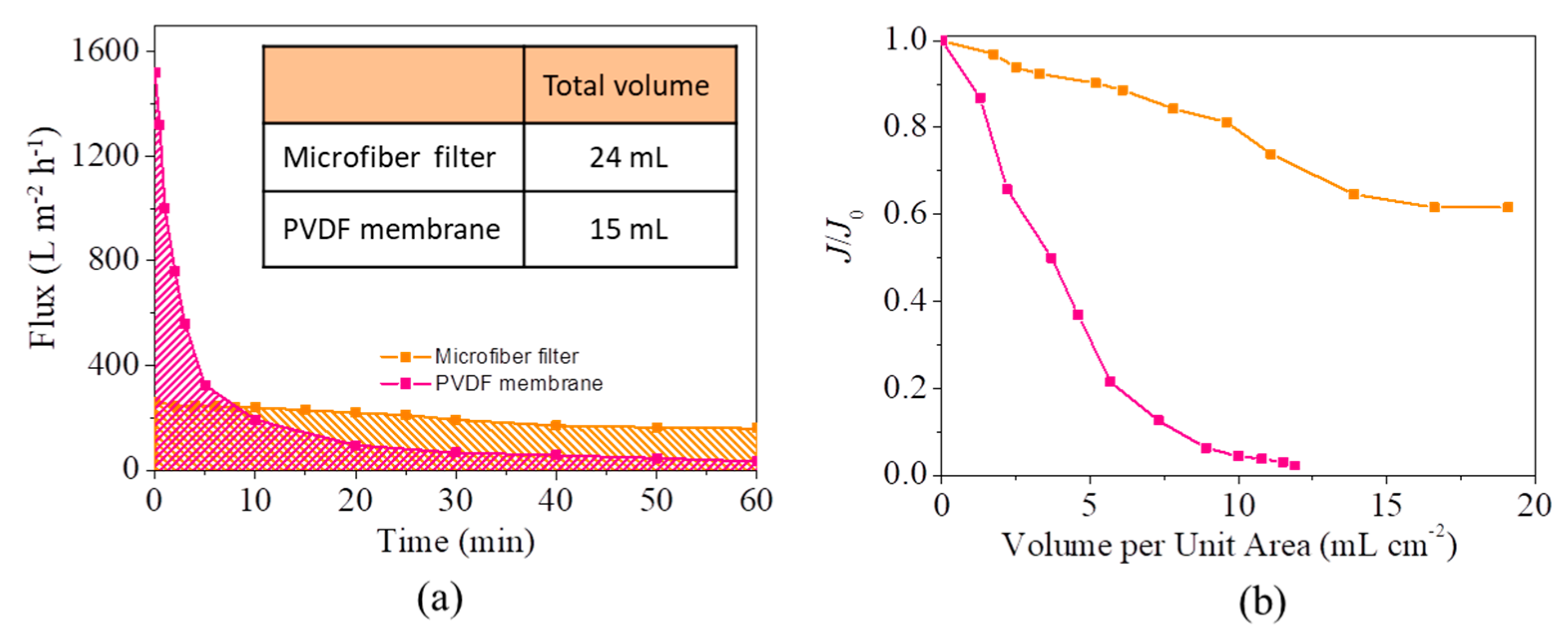
3.2. Separation of Free Oil/Water Mixtures by the Microfiber Filters
3.3. Separation of Emulsion by the Microfiber Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmadun, F.R.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar]
- Wang, B.; Liang, W.X.; Guo, Z.G.; Liu, W.M. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Chu, Z.L.; Feng, Y.J.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.H.; Hu, R.; Zhao, J.Q.; Zhang, X.F.; Li, Q.Y.; Xia, T.; Zhang, W.; Lu, C.H. Reusable, salt-tolerant and superhydrophilic cellulose hydrogel-coated mesh for efficient gravity-driven oil/water separation. Chem. Eng. J. 2018, 338, 271–277. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Sun, T.L.; Feng, L.; Gao, X.F.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Liu, M.J.; Wang, S.T.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Sun, T.L.; Wang, G.J.; Feng, L.; Liu, B.Q.; Ma, Y.M.; Jiang, L.; Zhu, D.B. Reversible switching between superhydrophilicity and superhydrophobicity. Angew. Chem. Int. Ed. 2004, 43, 357–360. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.Q.; Lai, X.J.; Su, X.J.; Liang, T.; Zeng, X.R. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.L.; Gong, L.X.; Zhao, L.; Li, S.N.; Tang, L.C. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Cao, C.Y.; Cheng, J. A novel Cu(OH)2 coated filter paper with superhydrophobicity for the efficient separation of water-in-oil emulsions. Mater. Lett. 2018, 217, 5–8. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Lyu, S.S.; Fu, Y.X.; Heng, Y.; Mo, D.C. The Janus effect on superhydrophilic Cu mesh decorated with Ni-NiO/Ni(OH)2 core-shell nanoparticles for oil/water separation. Appl. Surf. Sci. 2017, 409, 431–437. [Google Scholar] [CrossRef]
- Gunatilake, B.U.; Bandara, J. Fabrication of highly hydrophilic filter using natural and hydrothermally treated mica nanoparticles for efficient waste oil-water separation. J. Environ. Manag. 2017, 191, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Di, J.C.; Jiang, L.; Yu, J.H.; Xu, R.R. Zeolite-coated mesh film for efficient oil–water separation. Chem. Sci. 2013, 4, 591–595. [Google Scholar] [CrossRef]
- Tao, M.M.; Xue, L.X.; Liu, F.; Jiang, L. An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef]
- Yi, G.; Chen, S.; Quan, X.; Wei, G.L.; Fan, X.F.; Yu, H.T. Enhanced separation performance of carbon nanotube–polyvinyl alcohol composite membranes for emulsified oily wastewater treatment under electrical assistance. Sep. Purif. Technol. 2018, 197, 107–115. [Google Scholar] [CrossRef]
- Ge, Q.C.; Amy, L.; Chung, T.S. Forward osmosis for oily wastewater reclamation: Multi-charged oxalic acid complexes as draw solutes. Water Res. 2017, 122, 580–590. [Google Scholar] [CrossRef]
- Lu, D.W.; Zhang, T.; Gutierrez, L.; Ma, J.; Croué, J.P. Influence of surface properties of filtration-layer metal oxide on ceramic membrane fouling during ultrafiltration of oil/water emulsion. Environ. Sci. Technol. 2016, 50, 4668–4674. [Google Scholar] [CrossRef]
- Han, G.; de Wit, J.S.; Chung, T.S. Water reclamation from emulsified oily wastewater via effective forward osmosis hollow fiber membranes under the PRO mode. Water Res. 2015, 81, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tian, J.Y.; Gao, S.S.; Zhang, Z.B.; Cui, F.Y.; Tang, C.Y. In situ surface modification of thin film composite forward osmosis membranes with sulfonated poly (arylene ether sulfone) for anti-fouling in emulsified oil/water separation. J. Membr. Sci. 2017, 527, 26–34. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Chang, C.C.; Kasemset, S.; Emrick, T.; Freeman, B.D. Fouling-resistant ultrafiltration membranes prepared via co-deposition of dopamine/zwitterion composite coatings. J. Membr. Sci. 2017, 541, 300–311. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Xie, W.; Zhang, F.; Xing, T.L.; Jin, J. Superhydrophilic in-situ-cross-linked zwitterionic polyelectrolyte/PVDF-blend membrane for highly efficient oil/water emulsion separation. ACS Appl. Mater. Interfaces 2017, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wang, L.L.; Xu, Y. ZrO2 solid superacid porous shell/void/TiO2 core particles (ZVT)/polyvinylidene fluoride (PVDF) composite membranes with anti-fouling performance for sewage treatment. Chem. Eng. J. 2015, 260, 258–268. [Google Scholar] [CrossRef]
- Li, C.; Song, C.W.; Tao, P.; Sun, M.H.; Pan, Z.L.; Wang, T.H.; Shao, M.H. Enhanced separation performance of coal-based carbon membranes coupled with an electric field for oily wastewater treatment. Sep. Purif. Technol. 2016, 168, 47–56. [Google Scholar] [CrossRef]
- Youngblood, J.P.; McCarthy, T.J. Ultrahydrophobic polymer surfaces prepared by simultaneous ablation of polypropylene and sputtering of poly(tetrafluoroethylene) using radio frequency plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Zhang, X.Y.; Tao, L.; Li, K.; Xue, Z.X.; Feng, L.; Wei, Y. Mussel-inspired chemistry and michael addition reaction for efficient oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 4438–4442. [Google Scholar] [CrossRef]
- Crick, C.R.; Gibbins, J.A.; Parkin, I.P. Superhydrophobic polymer-coated copper-mesh; membranes for highly efficient oil–water separation. J. Mater. Chem. A 2013, 1, 5943–5948. [Google Scholar] [CrossRef]
- Matin, A.; Baig, U.; Gondal, M.A.; Akhtar, S.; Zubair, S.M. Superhydrophobic and superoleophilic surfaces prepared by spray-coating of facile synthesized Cerium(IV) oxide nanoparticles for efficient oil/water separation. Appl. Surf. Sci. 2018, 462, 95–104. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kawakatsu, T.; Nakajima, M.; Kikuchi, Y. Visualization of filtration phenomena of a suspended solution including O/W emulsion or solid particle and membrane separation properties of the solution. Water Res. 1999, 33, 929–936. [Google Scholar] [CrossRef]
- Iritani, E.; Mukai, Y.; Katagiri, N.; Yoshii, I. Formation of gel emulsions by filtration-consolidation of O/W emulsions. J. Chem. Eng. Jpn. 2003, 36, 590–596. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Dong, J.; Bai, J.; Zhao, Y.; Qin, C. Turning Tissue Waste into High-Performance Microfiber Filters for Oily Wastewater Treatment. Materials 2020, 13, 378. https://doi.org/10.3390/ma13020378
Wei G, Dong J, Bai J, Zhao Y, Qin C. Turning Tissue Waste into High-Performance Microfiber Filters for Oily Wastewater Treatment. Materials. 2020; 13(2):378. https://doi.org/10.3390/ma13020378
Chicago/Turabian StyleWei, Gaoliang, Jun Dong, Jing Bai, Yongsheng Zhao, and Chuanyu Qin. 2020. "Turning Tissue Waste into High-Performance Microfiber Filters for Oily Wastewater Treatment" Materials 13, no. 2: 378. https://doi.org/10.3390/ma13020378
APA StyleWei, G., Dong, J., Bai, J., Zhao, Y., & Qin, C. (2020). Turning Tissue Waste into High-Performance Microfiber Filters for Oily Wastewater Treatment. Materials, 13(2), 378. https://doi.org/10.3390/ma13020378