1. Introduction
Hip arthroplasty is an innovative subject in orthopedic surgery, as it permits a significant advancement in pain and functional capabilities, in addition to a high patient justification level with the embedded prosthesis. The primary goal of this process is the long-time continuation of the implant while maintaining steady obsession of the parts with negligible wear of the contact surfaces [
1]. The introduction of reliability and failure probability concepts can play an important role in this context [
2,
3]. Cemented and cementless stem implantation results in considerable changes in femoral load transfer, in which the femoral bone responds to these changes with a process known as versatile bone remodeling that is controlled by Wolff’s law [
4].
As a matter of fact, bone remodeling relies on mechanical and biological elements [
5]. The mechanical part is associated with stress distribution enforced by the femoral stem, as well as implant features, i.e., size, shape, alloy, and also the kind of bone fixation [
2,
6,
7,
8,
9,
10,
11]. When using the deterministic optimization strategies for prosthesis design, empirical safety factors can be proposed without including the effects of uncertainties concerning materials, geometry, and loading. This way, the optimized configuration may present a lower reliability level and may lead to a higher failure rate. A reliability analysis can be carried out in order to assess the reliability level of the resulting prosthesis design [
1]. Here, the purpose of the reliability analysis is to evaluate the reliability levels in the structural design stages—taking the uncertainties of the material properties and loading into consideration.
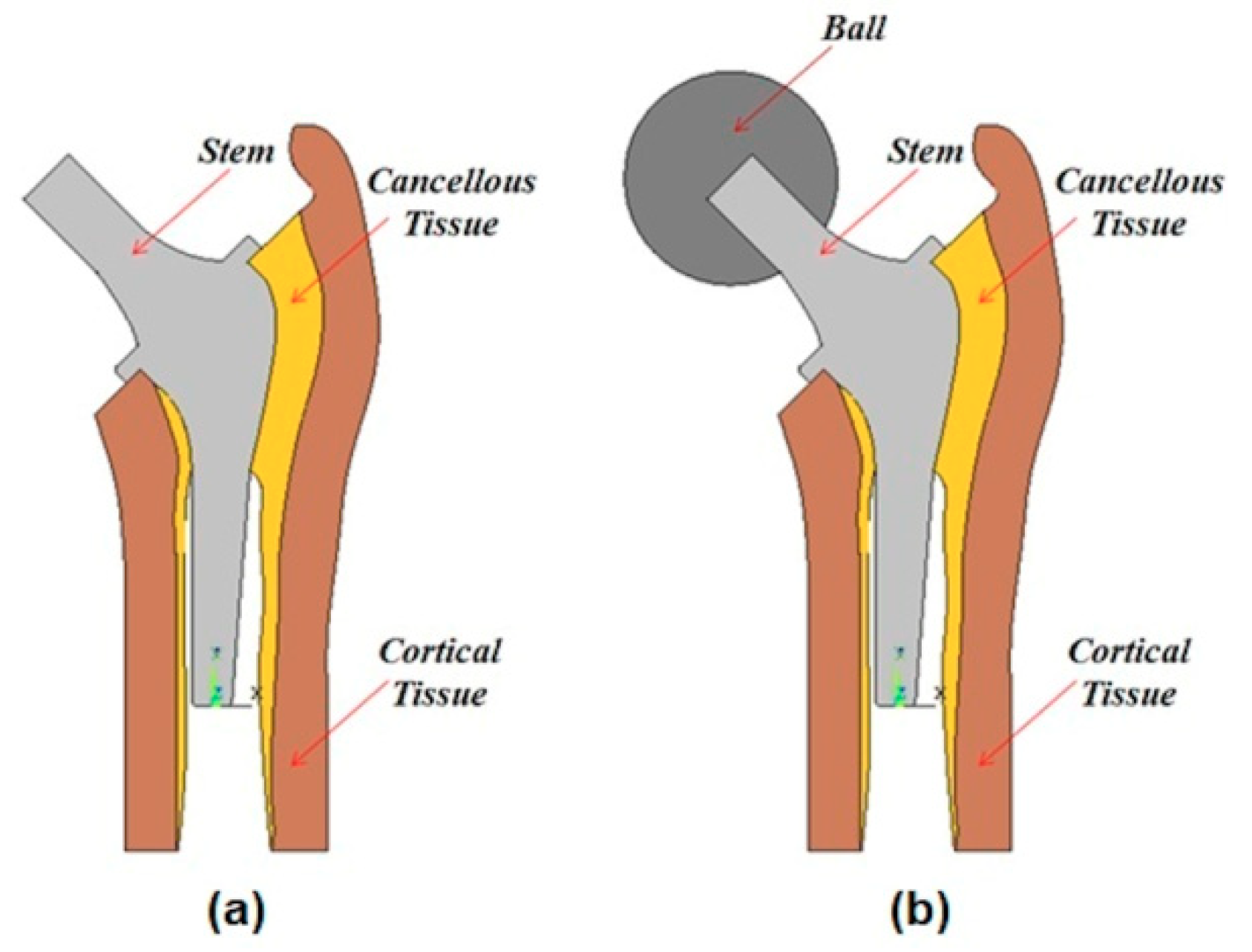
On the one hand, the living tissue modeling difficulty is related to the change in the loading and bone material properties after prosthesis arthroplasty. Considering bone as an anisotropic material, the elastic and strength properties in the axial direction are different than those in the radial direction, in which the bone is found to have higher strength values when applied in the diaphyseal axis (longitudinal direction) compared to those values obtained in the radial or circumferential “transverse” directions. Relatively, the variations in the computed modulus and strength in the radial and circumferential directions have been found to be small, so human bone can be considered and modeled as transversely isotropic. A possible reason is that the growth and adaptation of the bone is most efficiently related to the uniaxial stresses that develop along the diaphyseal axis during habitual activities, such as gait [
12]. These properties are correlated to the bone densities, which change after a prosthesis arthroplasty, and must be treated as orthotropic materials in certain cases. In brief, cortical and cancellous bone are treated as anisotropic materials, while the stem and the ball are considered isotropic materials due to the fact that their properties remain unchanged after prosthesis arthroplasty.
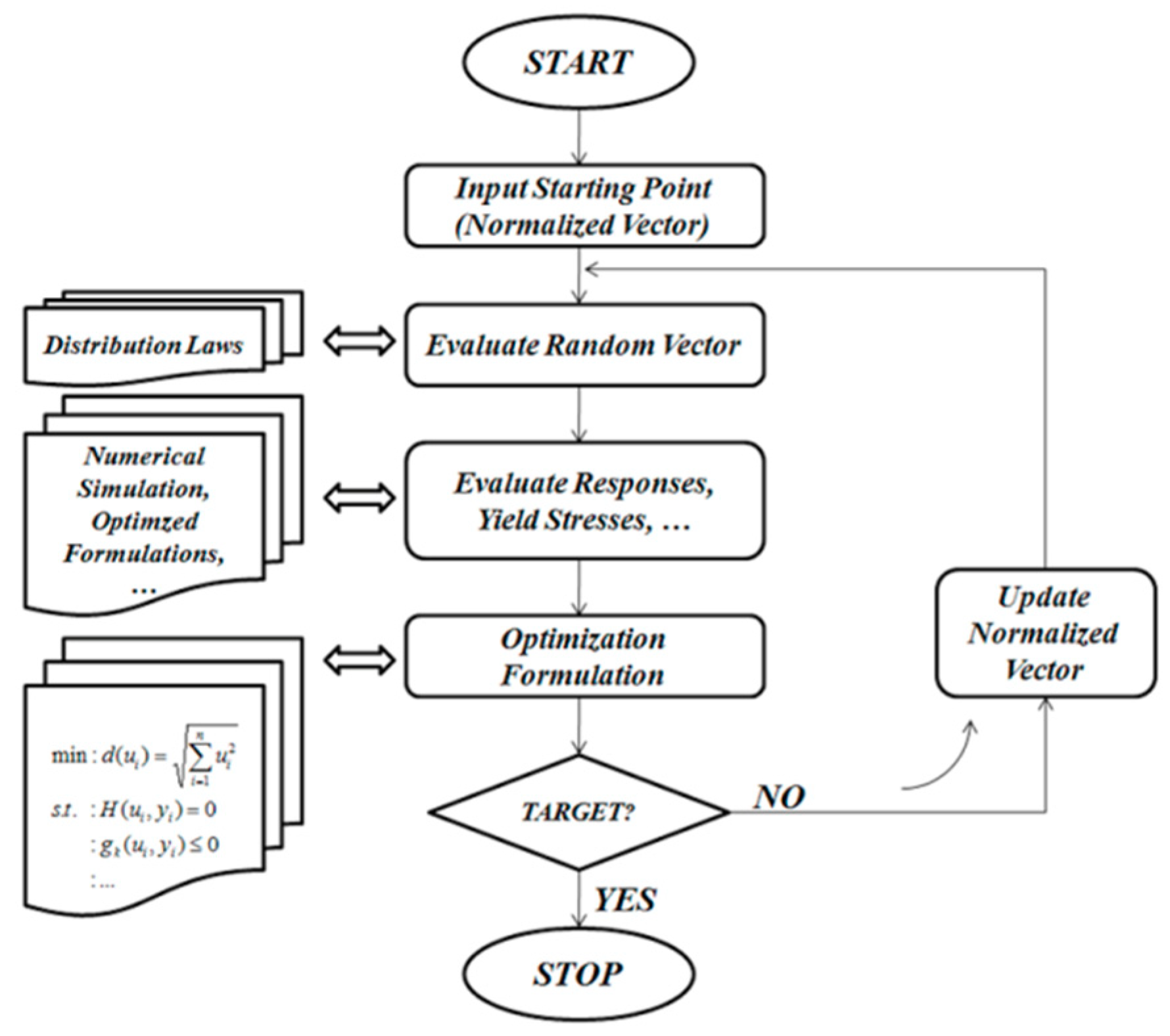
In addition to that, the prosthesis design is considered a large-scale optimization problem due to the consideration of a large number of constraints and variables. A reliability analysis of such a large problem should be performed using a particular optimization procedure [
13], and it is compulsory to develop efficient computational algorithms to solve it. The classical algorithms such as Rackwitz–Fiessler consider a single failure mode [
14]. These algorithms employ gradient optimization methods and, hence, a single search direction only is required to minimize the objective function [
13]. Therefore, the multiple failure case necessitates dealing with several optimization problems, where each of these problems has a single failure mode only. However, in this work, the non-gradient optimization method (zero-order method), so-called curve fitting method, is used to overcome this drawback. Thus, the optimization process is performed according to an approximation technique. In this study, the integration of the reliability concept [
14] is carried out considering the variation effect of the limit state functions.
This way, a direct assessment may lead to an unsuitable reliability level; therefore, it is very important to use the concept of the reliability-based design optimization (RBDO). In Kharmanda et al. [
15], a RBDO algorithm based on the hybrid method has been elaborated to integrate the RBDO model into the orthopedic prosthesis design such as hip design. In this case, the integration of the hybrid method leads to a high computing time because the total number of optimization variables is the sum of the design variables and the random variables. In this work, the optimum safety factors (OSF) are integrated to reduce the computing time and simplify the RBDO process.
In summary, for the hip prosthesis design process, there is a considerable need to take the changes of the material properties of the different bone tissues and the corresponding elasticity modulus and yield stress values into account. On the other hand, some muscles can be cut or harmed during surgery operation, and consequently, they cannot operate at their maximum capacity. Accordingly, it is important to introduce the uncertainty to the loading during the prosthesis design.
In this study, optimized formulations for yield stress against the elasticity modulus relationship are developed to dynamically consider the changes in the material properties. The uncertainty to loading cases is studied in order to take the maximum loading capacity of the joint into account. In addition, an integrated reliability model that considers the variation effect of the limit state functions is developed to provide an integrated design solution to the addressed problem.
4. Discussion
The different results can be discussed according to three aspects: Simulation, reliability, and reliability-based design optimization aspects. For the simulation aspect, when comparing the studied model to the previous works, two different changes can be noted: Geometry and material properties. In the works of Kharmanda [
2] and Kharmanda et al. [
15], the studied model was analyzed considering that the bone material properties were isotropic and the body forces were applied directly on the stem (without ball). Studying the stem without the existence of the ball would entirely change the study as the force values were extracted from Beaupré et al. [
17] whom carried out their study on the femoral part of a real bone. In addition, in the works of Kharmanda et al. [
11] and Kharmanda et al. [
27], the studied models were analyzed considering only that the bone material properties were isotropic. The consideration of isotropic bone material properties may affect the accuracy of the study as there is a significant difference between the values of Young’s modulus in the axial and the radial directions.
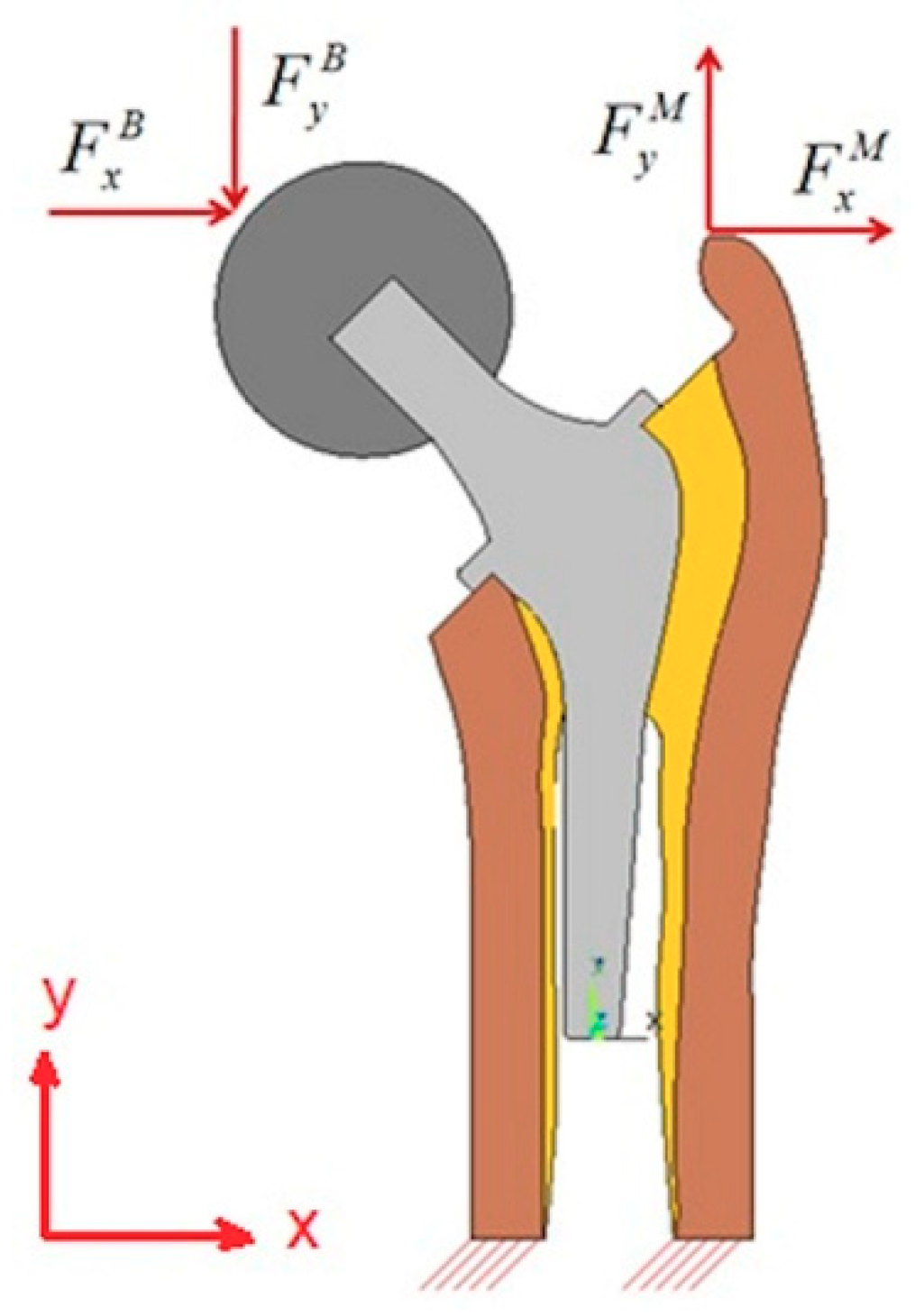
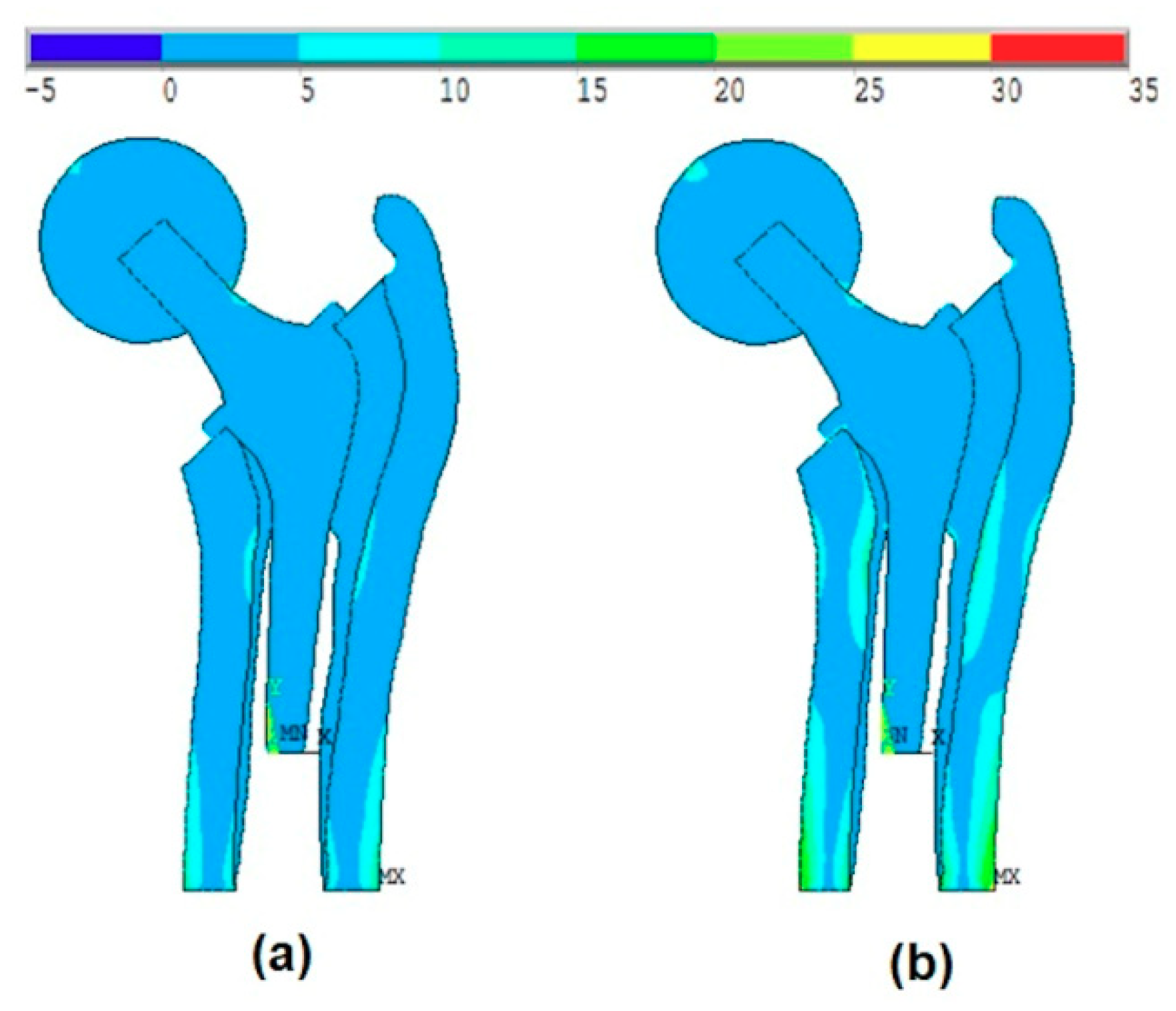
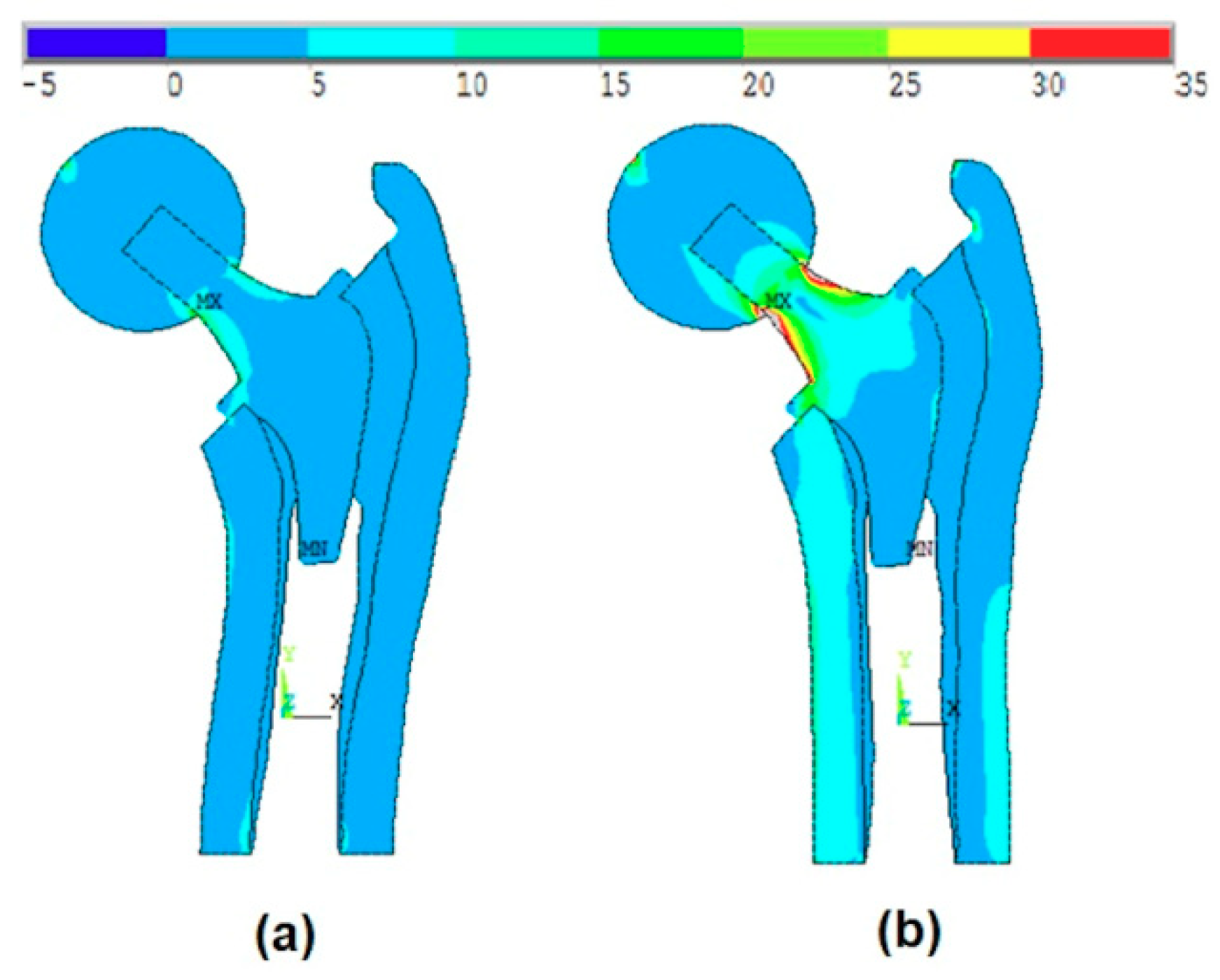
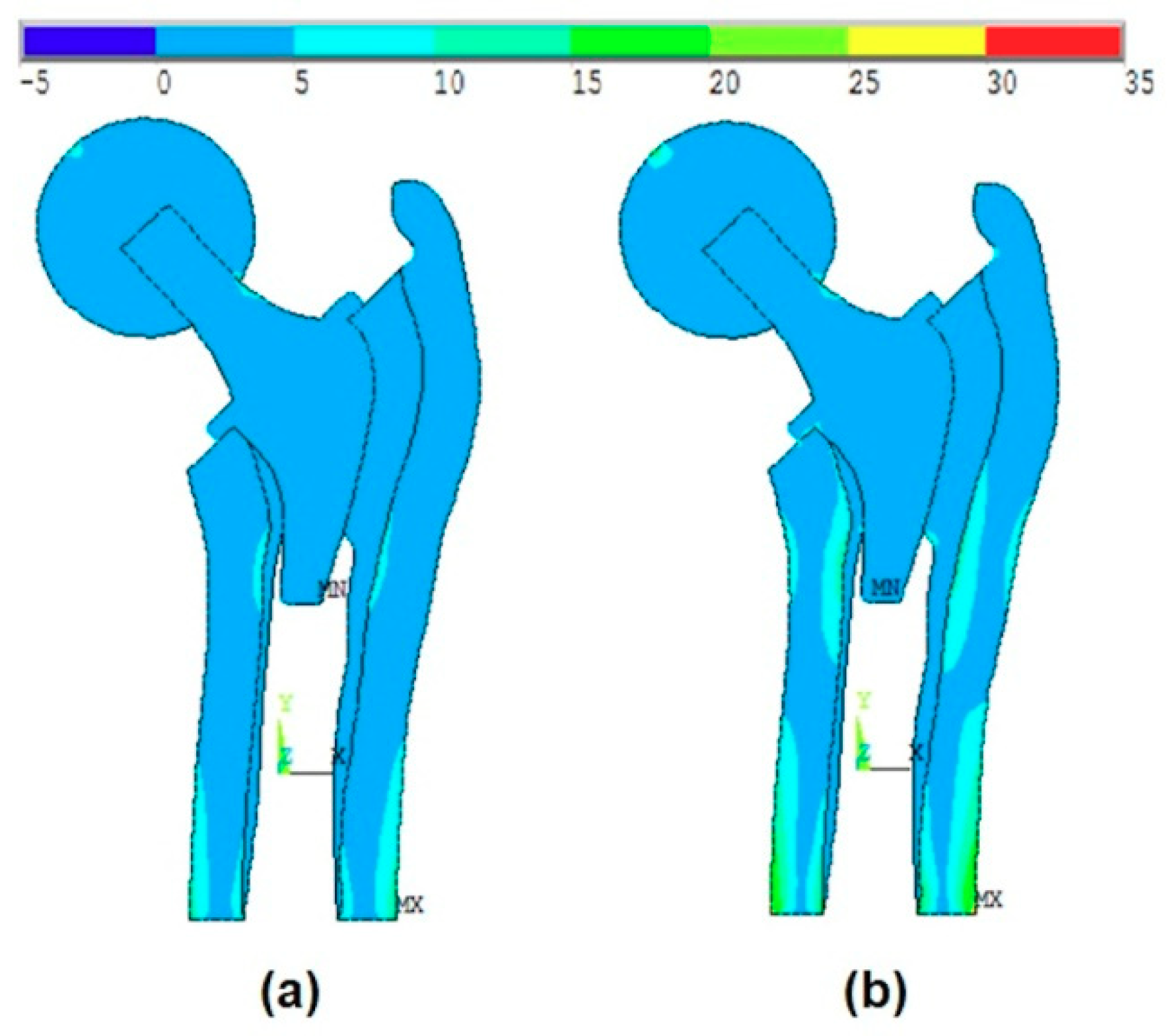
In this work, the studied model is analyzed considering the ball and the anisotropy of bone material properties. The effect of the ball was much more remarkable than the anisotropy of the bone material properties. The von-Mises stress distribution was very different especially when it concerns the maximum stress values.
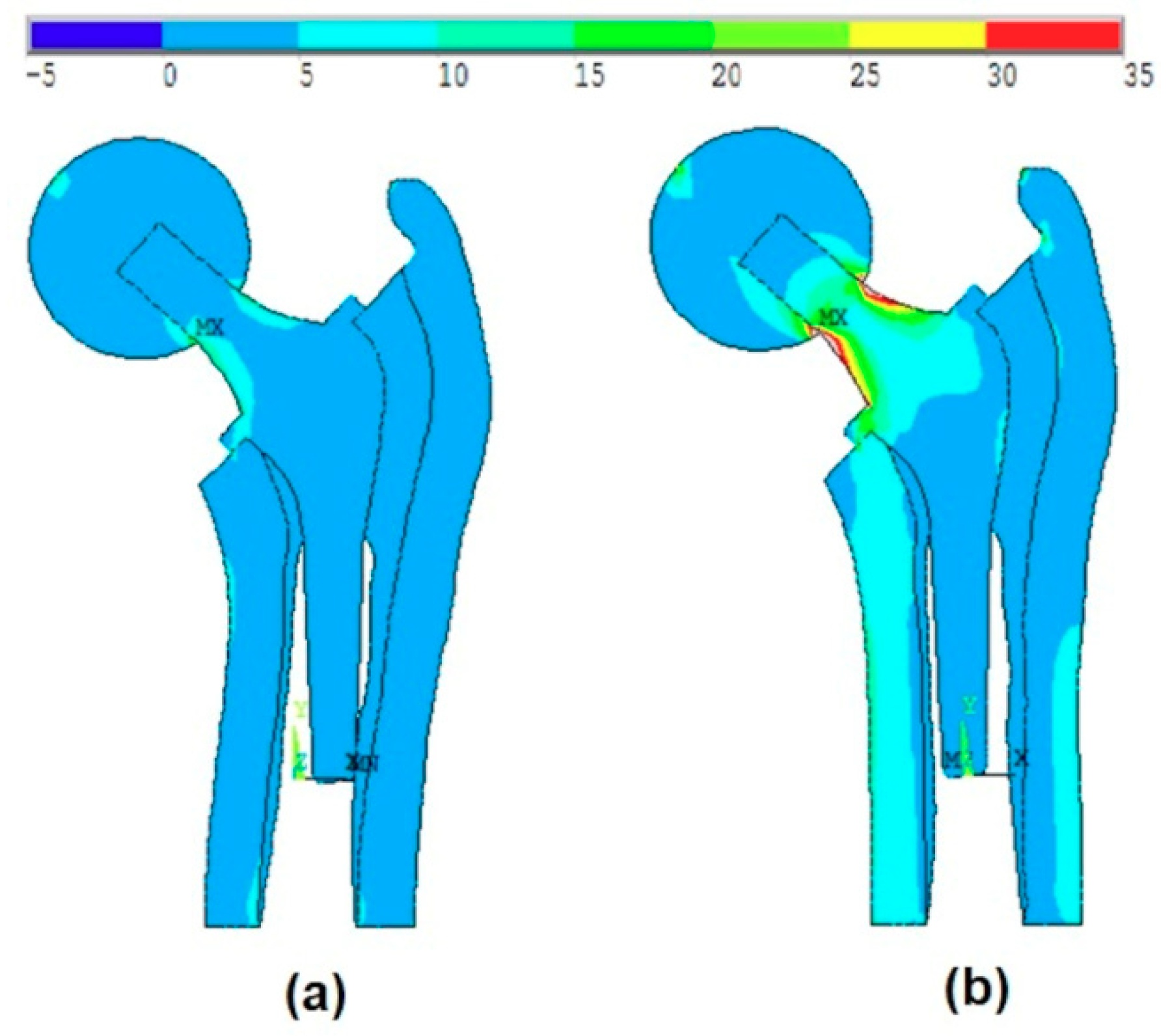
Figure 7a and
Figure 8a show that the maximum stress values were located at the intersection between the ball and the head of the stem, while when applying the body forces on the stem (without ball, as shown in Kharmanda [
2]), these values were located on the neck of the stem. On the other hand, when considering the anisotropy of the bone material properties, there were small differences in the resulting von-Mises stresses on the different layers. The resulting values in
Table 4 are compared to the studied models in Kharmanda et al. [
11] and Kharmanda et al. [
27]. Therefore, the role of the ball is much more important than the anisotropy during the simulation study.
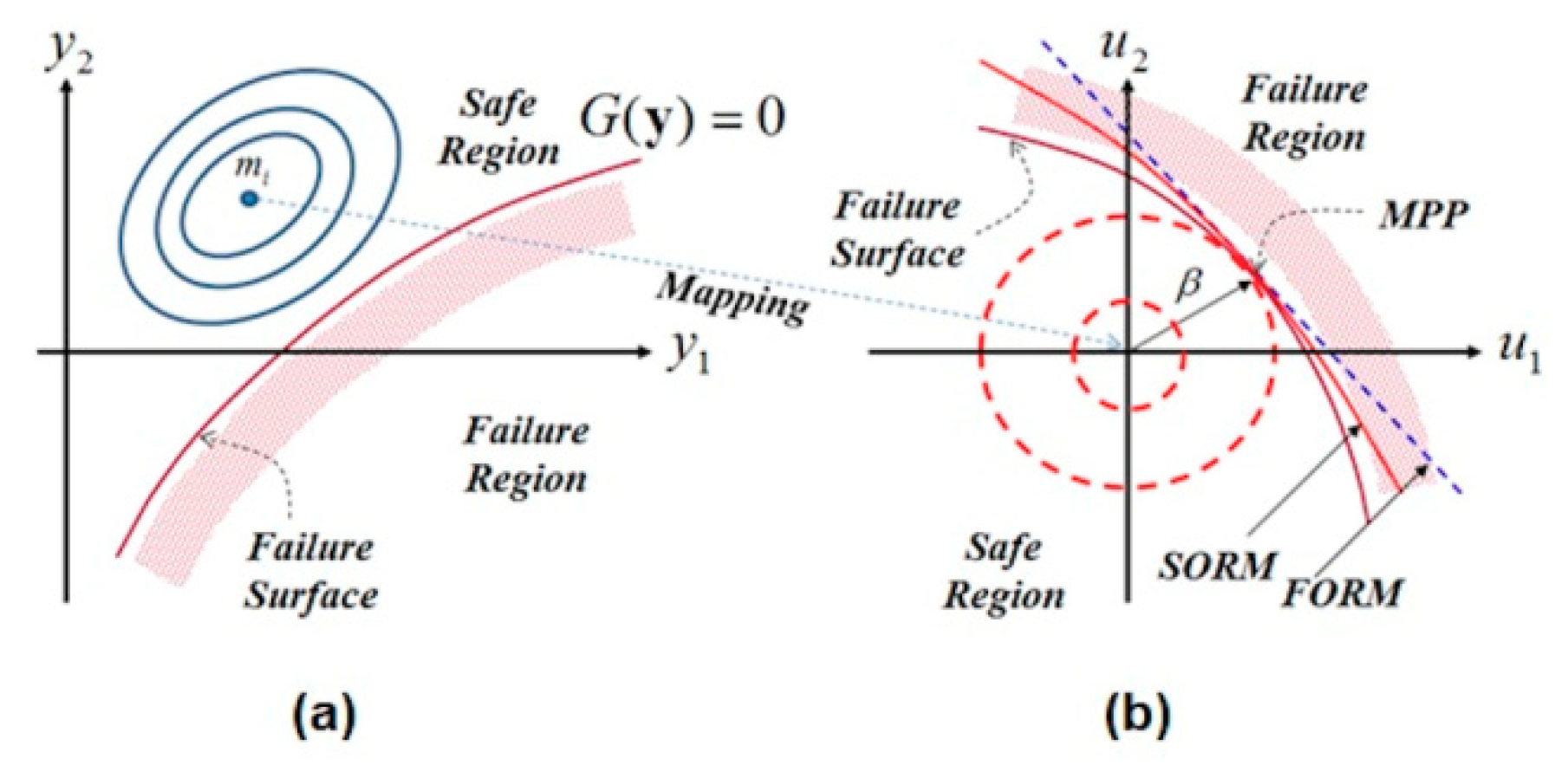
For the reliability aspect, two categories of reliability strategies can be distinguished in the literature: Dystem reliability analysis and component reliability. When considering a single failure scenario, the reliability algorithm leads to a single index, while when several failure cases are considered, the reliability algorithm leads to several indices [
31]. This way, the application of these classical system reliability algorithms leads to eight reliability indices considering six failure modes (scenarios): Two for the isotropic materials (ball and stem) and six for the anisotropic materials (cortical and cancellous tissues in transversal and axial directions). However, the developed algorithm directly provides the most critical reliability index in the system reliability problems. Equation (20) presents the different constrains
as inequality constraints while the most critical one is considered as a limit state function
. In addition, when treating the system reliability problem using the classical algorithm [
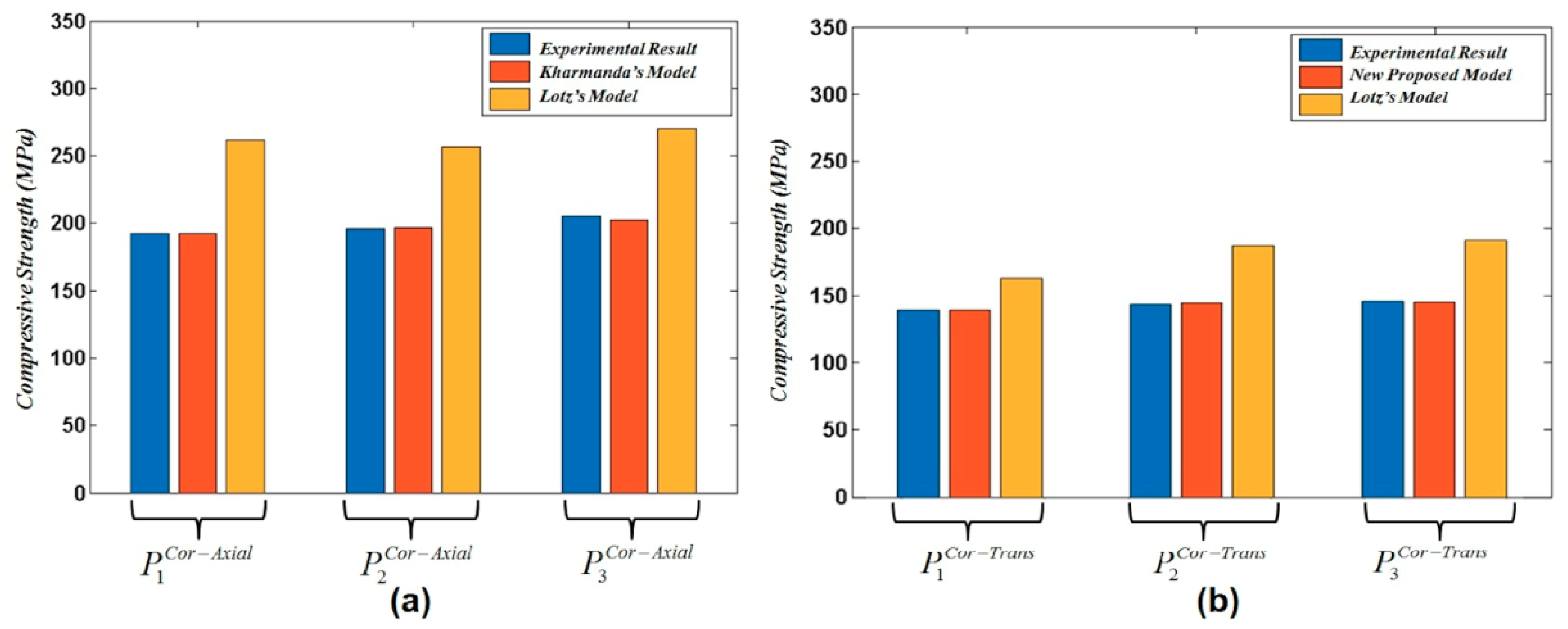
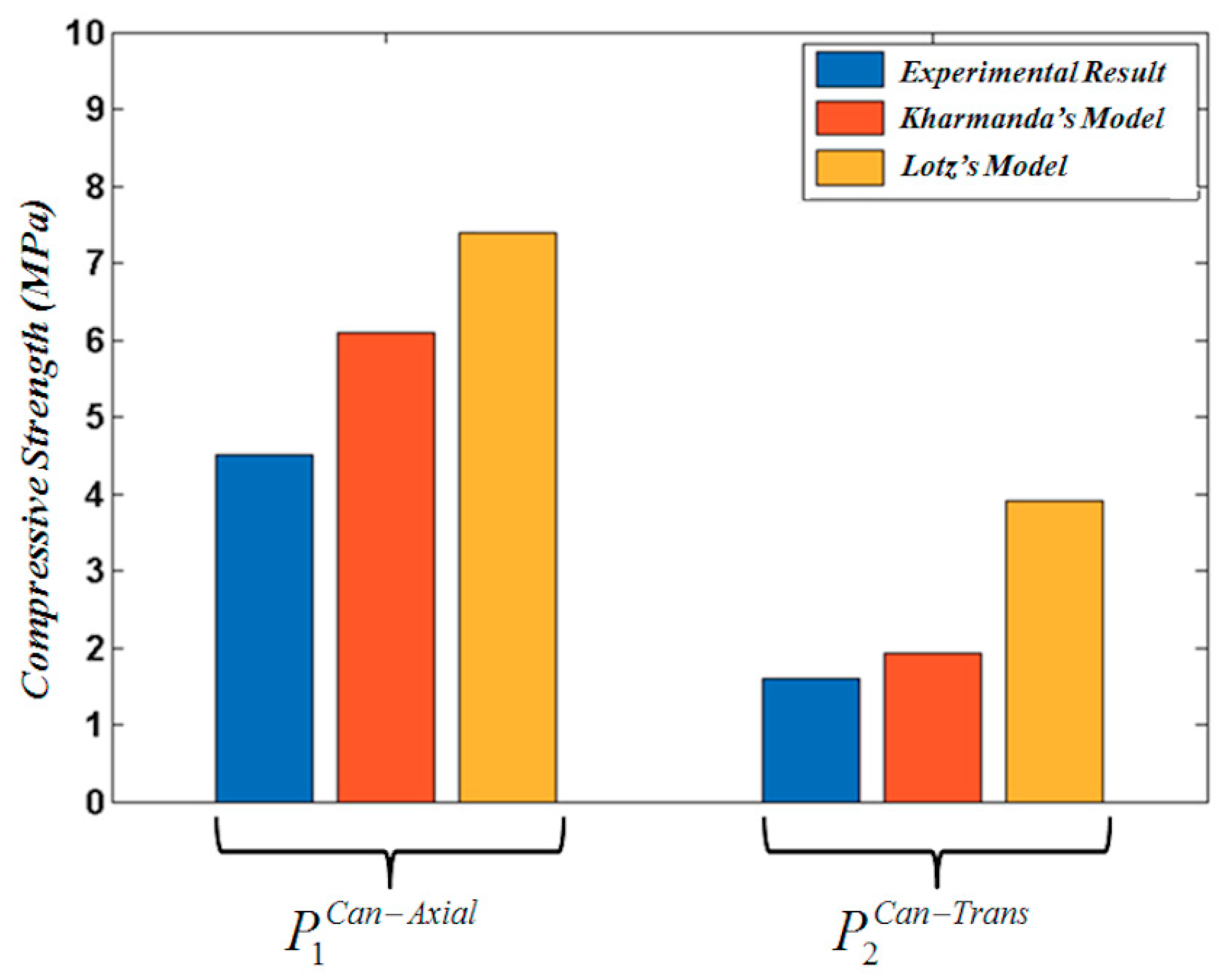
14], the resulting reliability indices may not take the correlation between the different failure scenarios into account, while the developed algorithm in this work simultaneously deals with the different constraints. An additional difference between the other structural reliability algorithms and the developed one is the integration of optimized formulations of the living tissues (cortical and cancellous), which leads to new boundaries at each iteration (new optimization variable space at each iteration). Here, accurate bone formulations for yield stress against the elasticity modulus relationship are needed to be integrated into the developed algorithm. In the literature, there were no bone formulations for the yield stress against the elasticity modulus relationship in the transversal isotropic cases. The existing formulations are Lotz’s formulations for the elasticity modulus against the density relationship and for the yield stress against the density relationship [
19]. After having developed formulations 12, 13, 14, and 15 considering Lotz’s formulations 1, 2, 3, and 4 for cortical tissue, and 5, 6, 7, and 8 for cancellous tissue, all the developed formulations 12, 13, 14, and 15 were compared to experimental results for the axial and transversal directions. The results showed a big difference between Lotz’s formulations and the experimental data for cortical and cancellous bone tissues. Thus, there is a strong need to develop the new formulations providing the optimum fitness curves of the existing experimental results. Therefore, optimized formulations 16 and 17 were developed and compared to Lotz’s formulations. These optimized formulations showed a good accuracy relative to Lotz’s formulations (see
Table 2,
Table 3 and
Table 4). In order to integrate these optimized formulations into the developed reliability algorithm, the ultimate tensile strength of bone tissue was considered as a fraction of the compression strength. Thus, formulations 18 and 19 were integrated into the reliability algorithm.
The developed reliability algorithm in this work deals with multiple failure modes, while the previous reliability algorithm in Kharmanda [
2] treated a single failure mode. In addition, the anisotropy and geometry affect the probabilistic model such as the mean point, which is considered a starting point for the current developed reliability algorithm. The reliability results are much more realistic than the previous results that were presented in Kharmanda [
2] because of the consideration of the anisotropy on the bone material properties, as well as the application of body forces on the ball instead of directly applying them to the stem, and the consideration of multiple failure modes. According to the obtained reliability results, the failure scenario for all cases was related to the cancellous tissue in the transversal and/or axial directions. In the first and second cases, L1 and L2, the failure was found in the transversal direction of the cancellous tissue, while in the third case L3, it was found in the axial direction of the cancellous tissue. The yield stress in the cortical and cancellous tissues is related to Young’s modulus values (Equations (18) and (19)). The maximum stress value should not exceed these yield stresses values. In
Table 5, the maximum values of von-Mises stress were presented for the different parts. In addition, the maximum stress values in both transversal and axial directions were presented for the cortical and cancellous tissues. The minimum value of the resulting reliability index was
. As it is less than the lowest value in the reliability index interval for structural engineering [
29], there is a strong need to improve this value to meet the standard interval. Accordingly, a RBDO procedure was then implemented to provide the suitable design with a controlled reliability index. In addition to considering bone isotropic material and the non-existence of the ball, the used RBDO method in our previous work [
15] was carried out using the hybrid method, which has several drawbacks concerning computing time, especially when dealing with multiple failure modes.
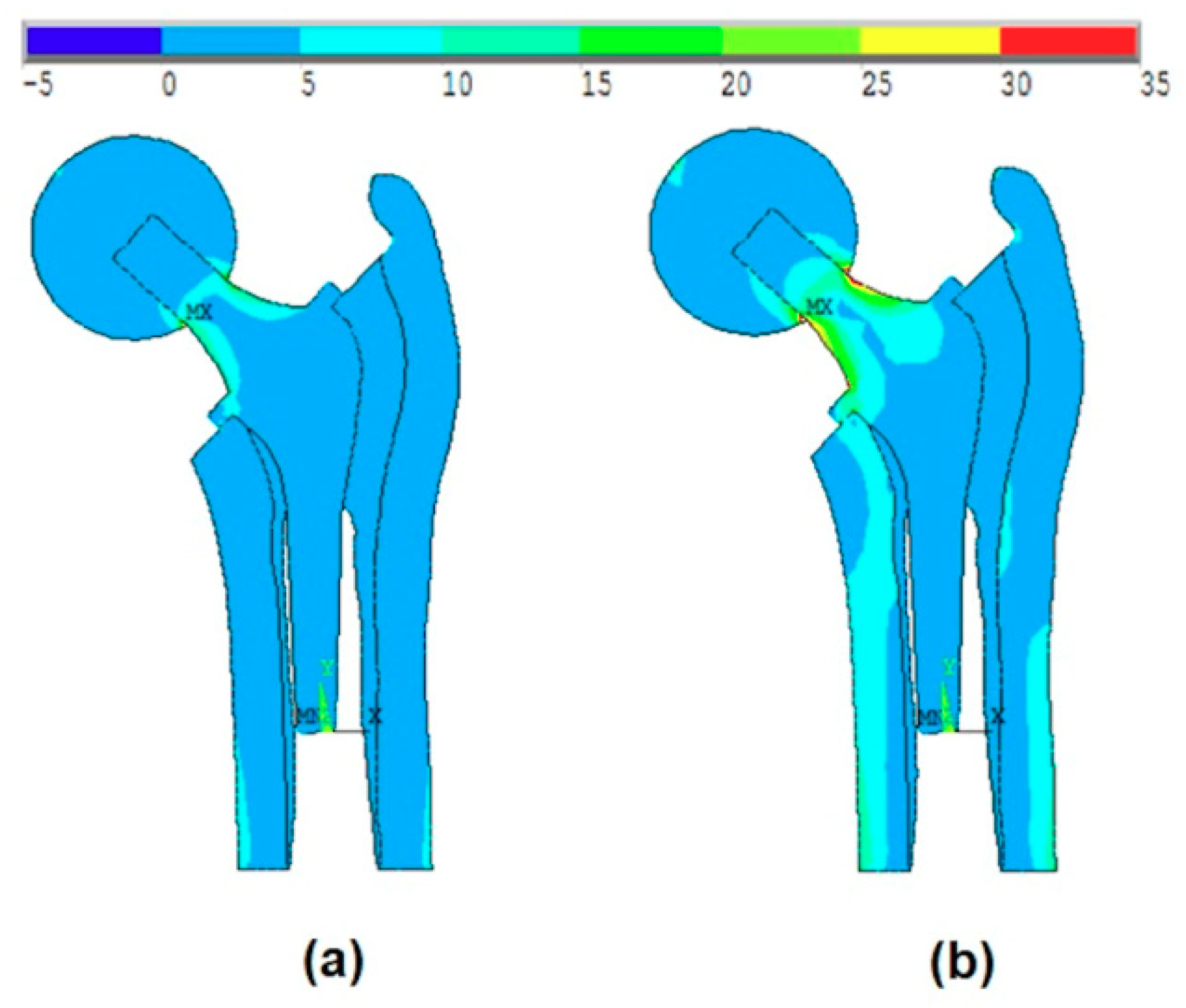
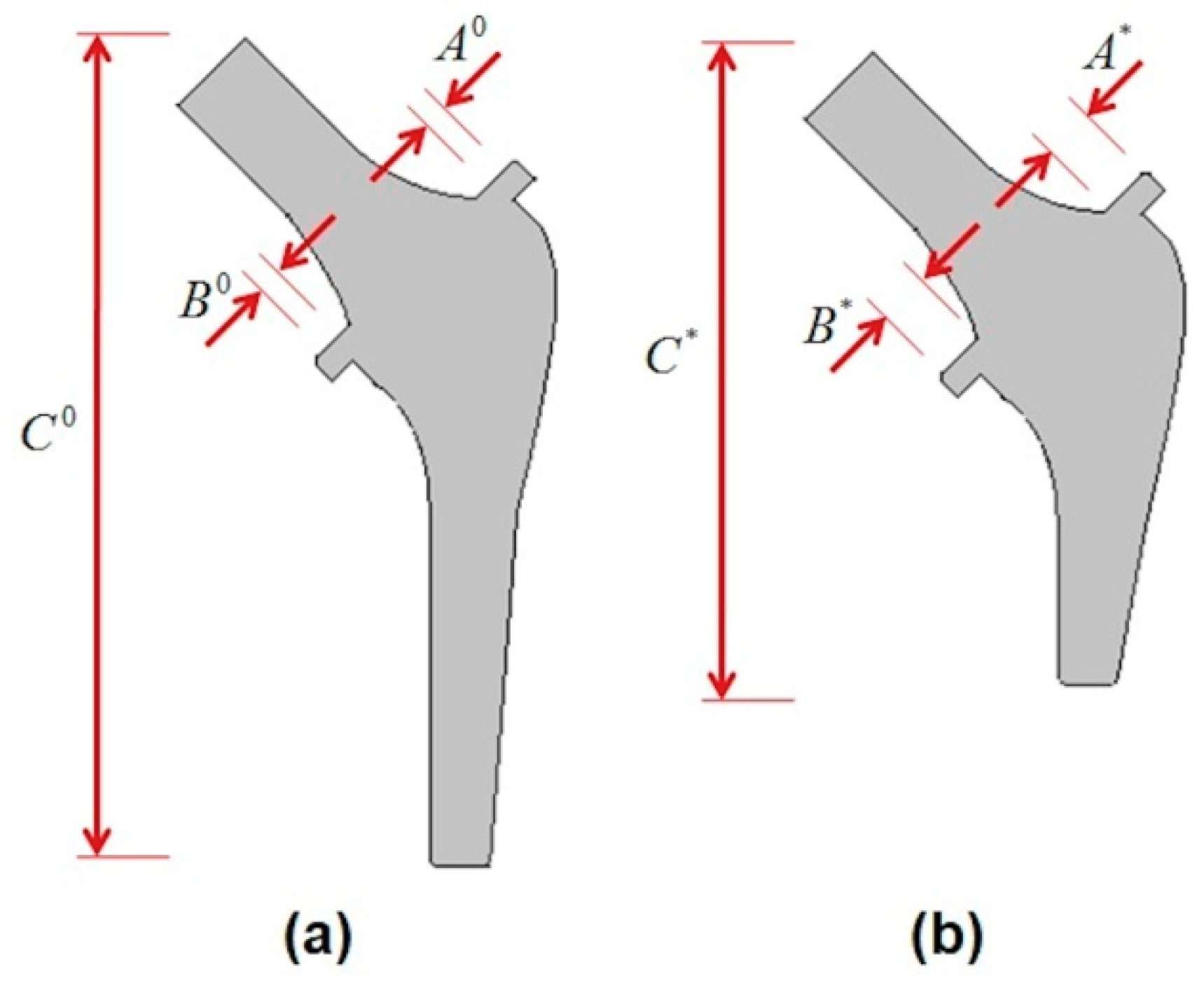
In order to overcome these drawbacks, the OSF method was efficiently used for several failure modes in this work. Here, three geometrical parameters concerning the stem were considered to be optimized in order to control the reliability level (
). The optimized stem is shown in
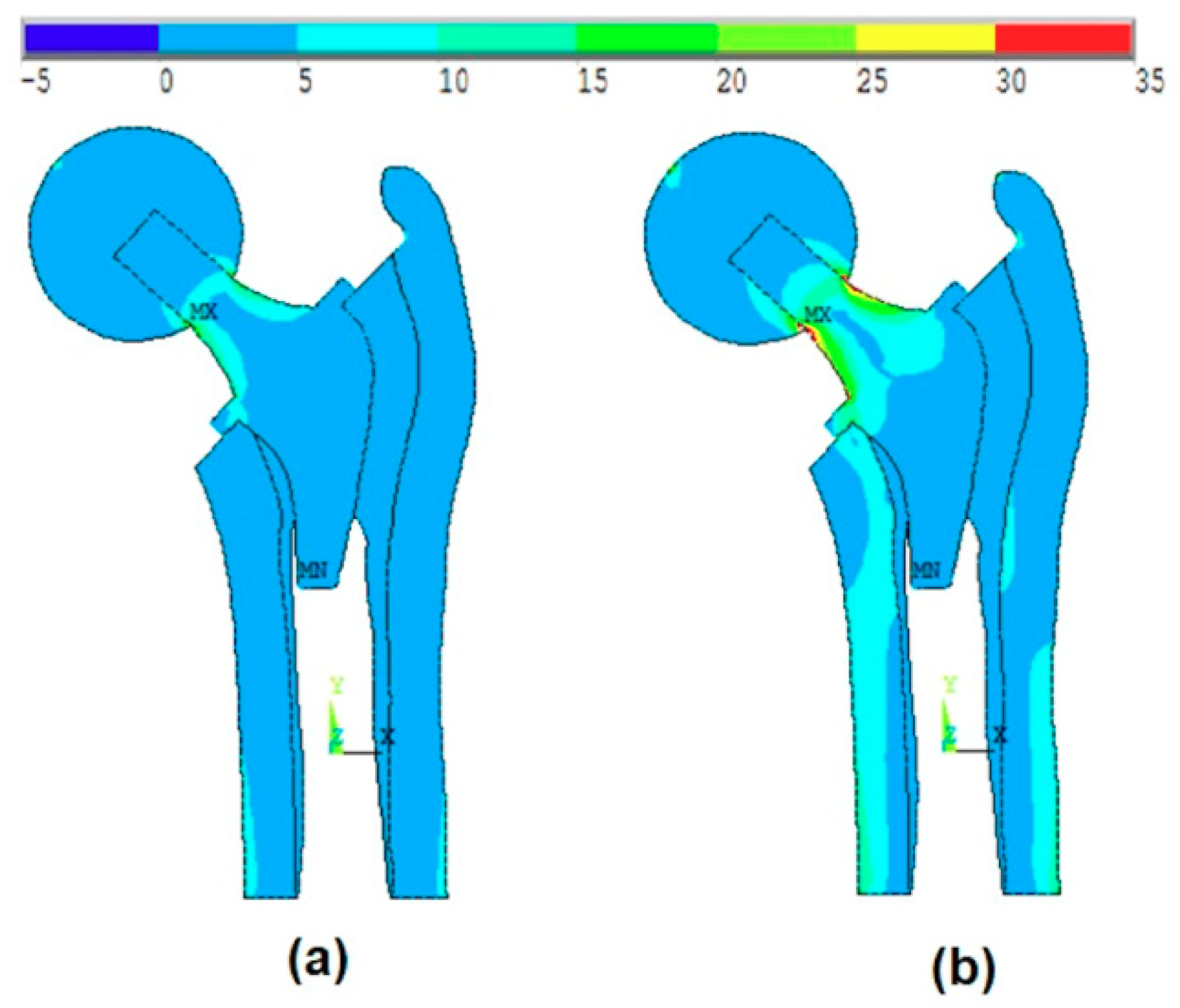
Figure 7b and its corresponding dimensions are presented in
Table 7. When performing a direct simulation of the optimal configuration and the failure point, it is exactly found that the same scenarios of failure of the MPP that are presented in
Table 5 have been obtained. The resulting values are almost close to each other (see
Table 5 and
Table 6). For both points, the failure mode for the first and second loading cases occurs in the cancellous tissue on the transversal directions and for the third loading case in the cancellous tissue in the axial direction. This result confirms the robustness of the RBDO using the OSF for multiple failure scenarios. This study is limited to the developed 2D model, which was employed in order to reduce the computing time, as well as to guarantee convergence stability.
In general, the replaced hip prosthesis may have different outcomes according to several factors. These factors are, but not limited to, hip design, surgeon experience, and implantation technique. In addition to this, patient characteristics such as age, sex, weight, activity level, and overall health affect the choice of the replaced hip prosthesis type. One of the most clinical implications of this research is to reduce the risk of bone fracture as all failure modes introduced in this research focus on the maximum von-Mises stress values, which are considered fracture indicators [
32]. Furthermore, the resulting RBDO solution can be classified as a short stem. This kind of stem could be utilized to ensure primary stability of hip prostheses [
33].