Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Extraction of Collagen Fibrils
2.3. Amino Acid Analysis
2.4. SDS-PAGE Measurement
2.5. FTIR Spectra Measurement
2.6. CD Spectra Measurement
2.7. UV–Vis Absorption Spectra Measurement
2.8. X-Ray Diffraction Measurement
2.9. Morphology Observations
3. Results and Discussion
3.1. Amino Acid Components
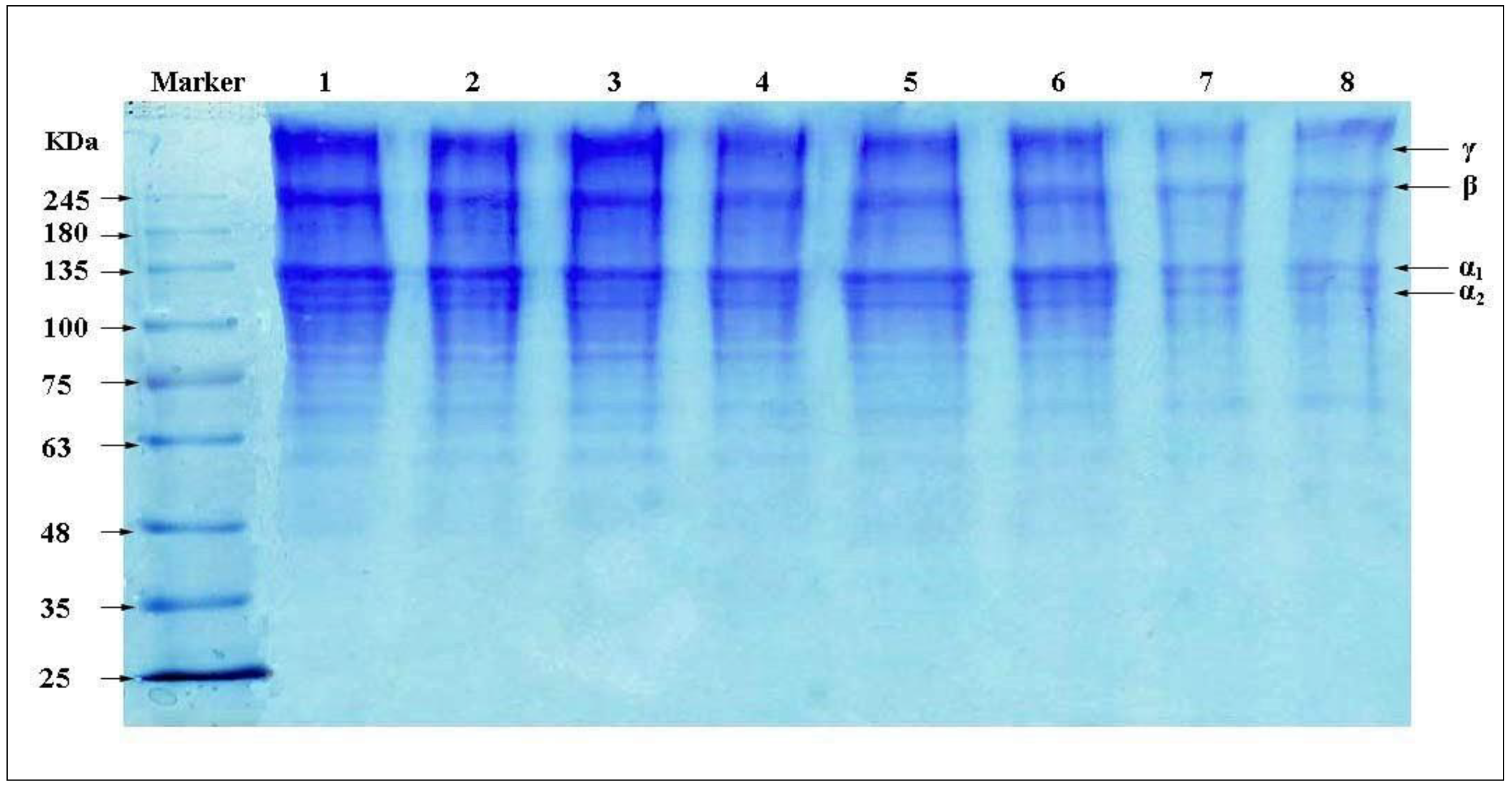
3.2. SDS-PAGE Pattern
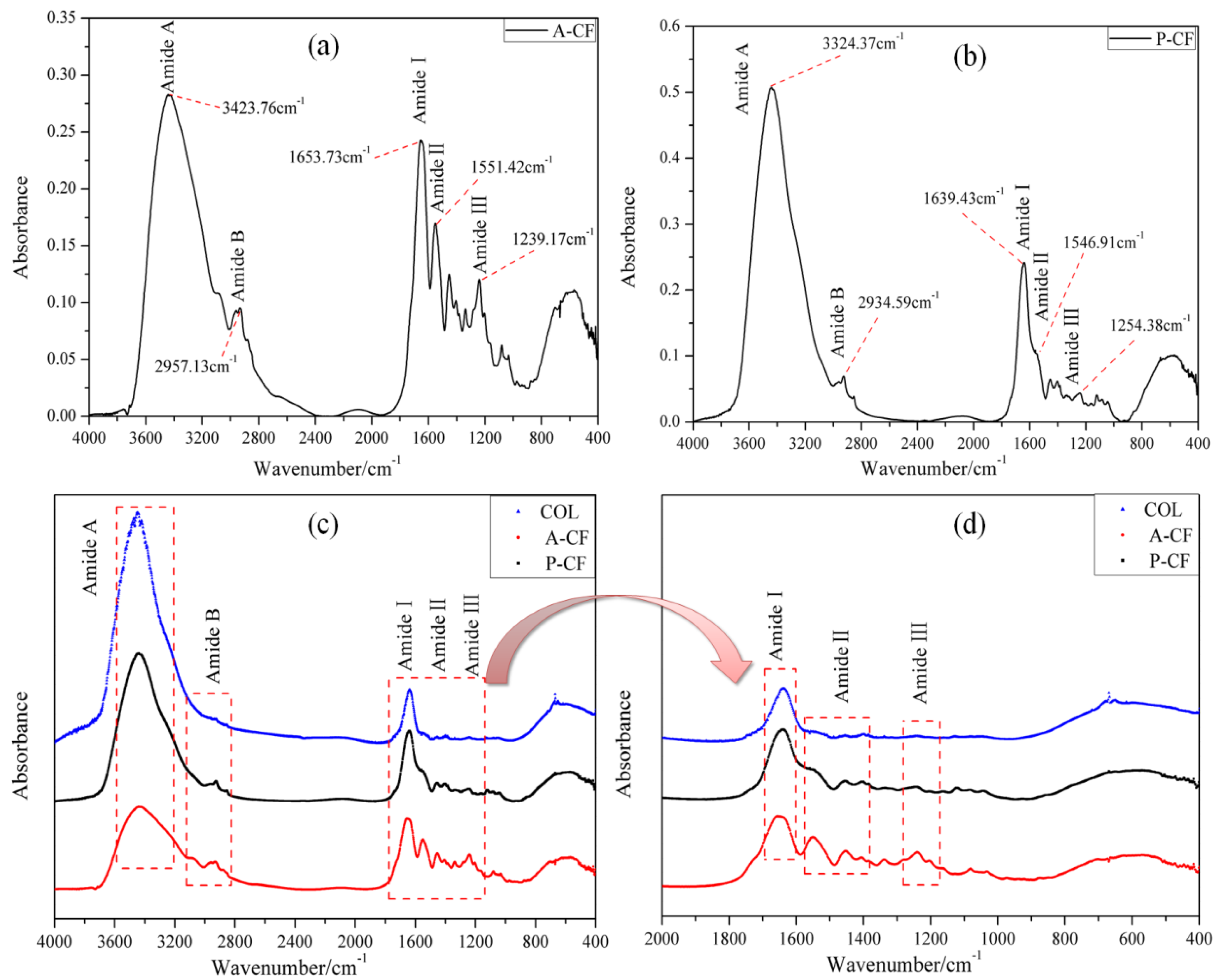
3.3. FTIR Spectra
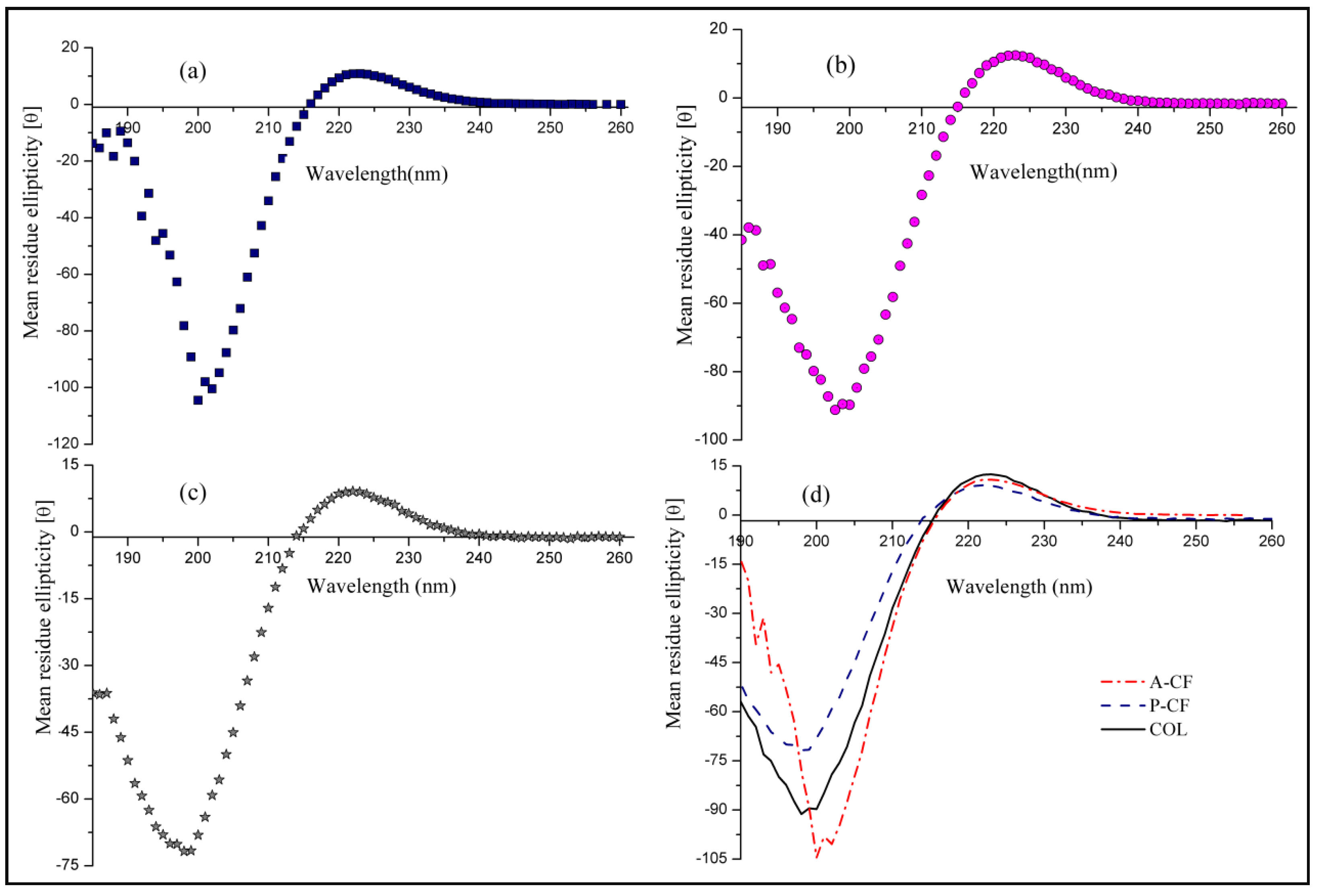
3.4. CD Spectroscopy
3.5. UV–Vis Absorption Spectra
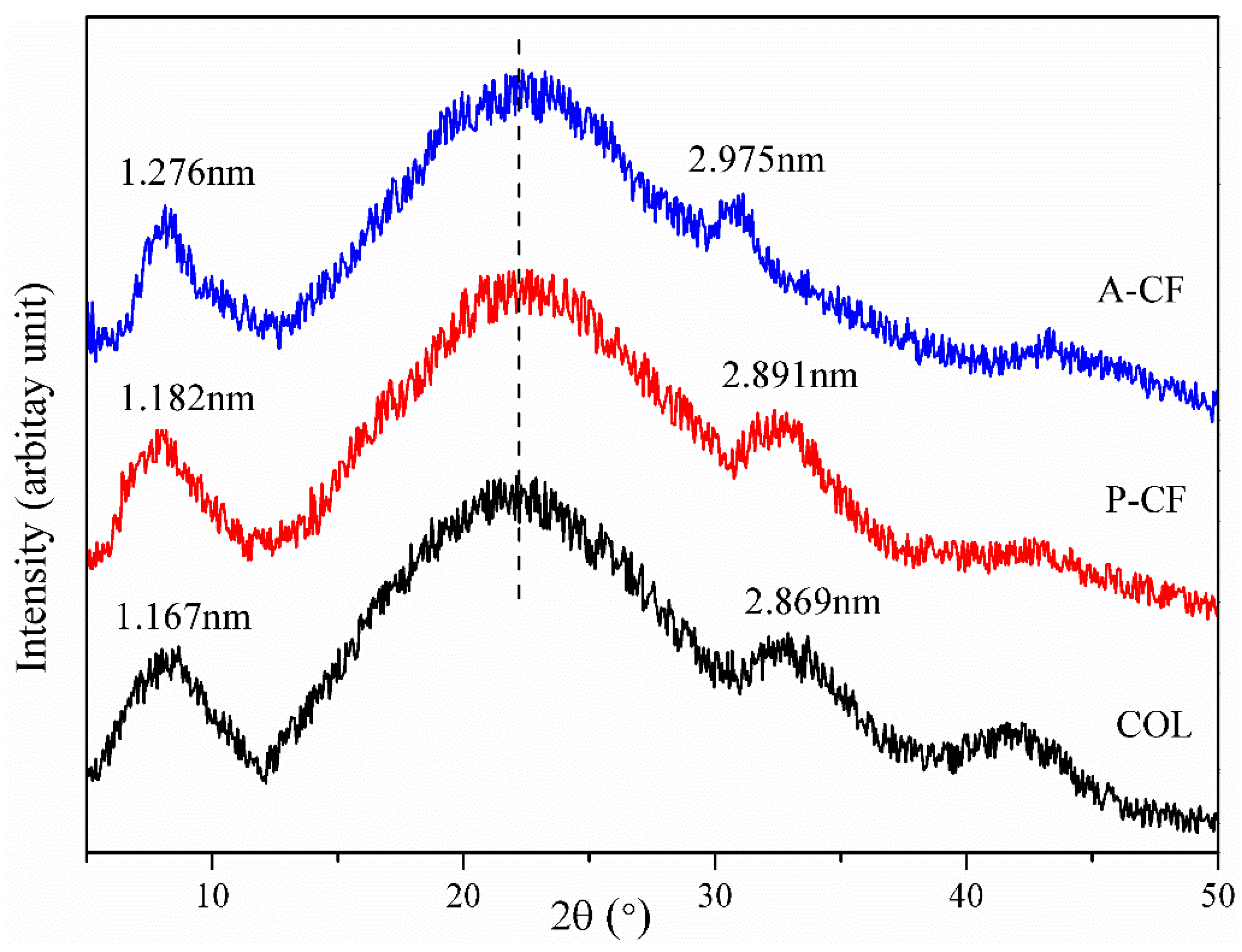
3.6. X-Ray Diffraction
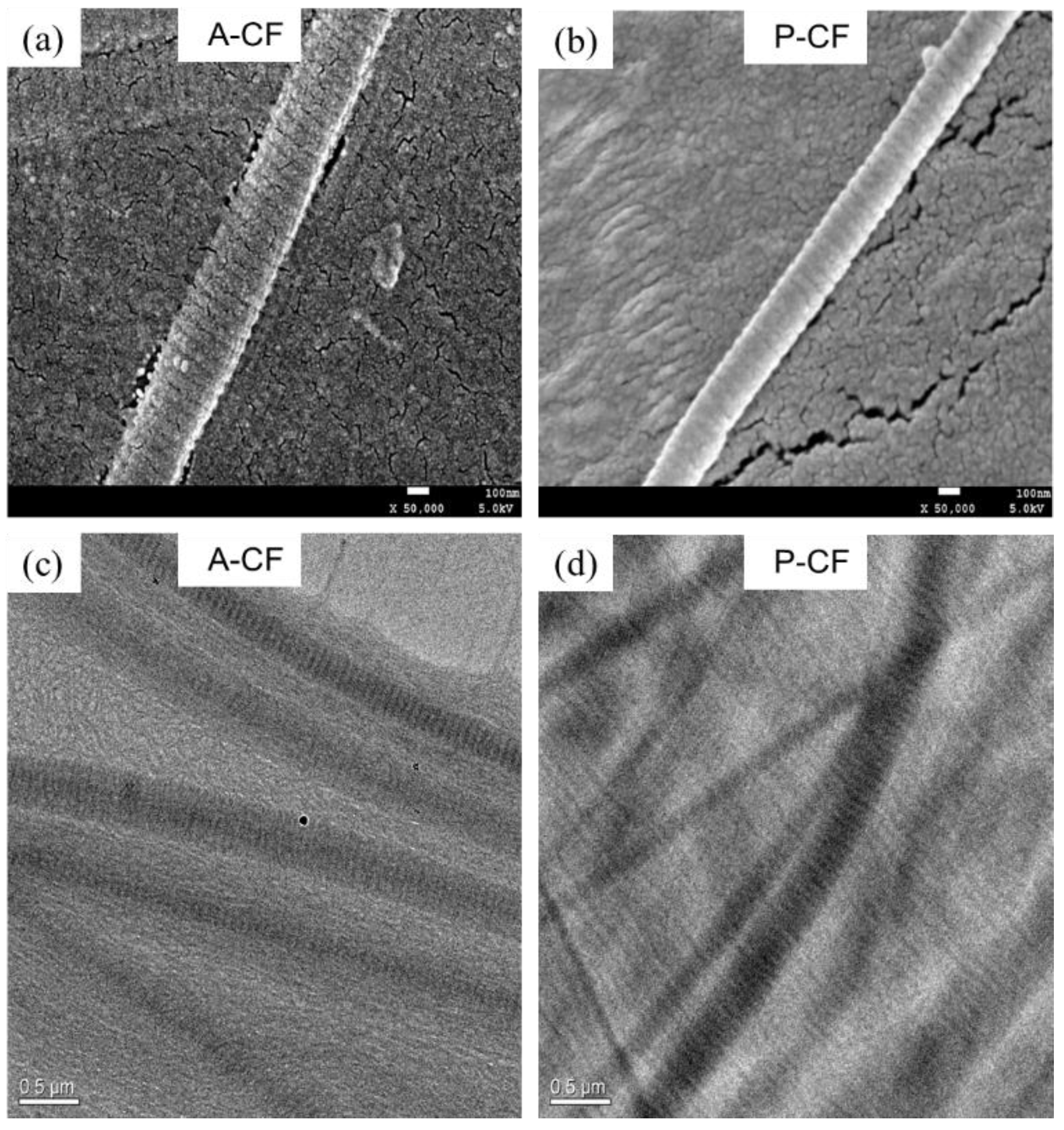
3.7. Morphology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stamov, D.R.; Nguyen, T.A.K.; Evans, H.M.; Pfohl, T.; Werner, C.; Pompe, T. The impact of heparin intercalation at specific binding sites in telopeptide-free collagen type I fibrils. Biomaterials 2011, 32, 7444–7453. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Hou, H.; Li, B.F.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia. Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Minary-Jolandan, M. Alginate-collagen fibril composite hydrogel. Materials 2015, 8, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Sridharan, I.; Ma, Y.; Zhu, B.; Chi, N.; Kobak, W.; Rotmensch, J.; Schieber, J.D.; Wang, R. Identifying distinct nanoscopic features of native collagen fibrils towards early diagnosis of pelvic organ prolapse. Nanomed. Nanotechnol. 2016, 12, 667–675. [Google Scholar] [CrossRef]
- Liu, X.H.; Dan, W.H.; Ju, H.Y.; Dan, N.H.; Gong, J.X. Preparation and evaluation of a novel pADM-derived micro- and nano-electrospun collagen membrane. RSC Adv. 2015, 5, 5279–5287. [Google Scholar] [CrossRef]
- Pezzoli, D.; di Paolo, J.P.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef]
- Böhm, S.; Strauß, C.; Stoiber, S.; Kasper, C.; Charwat, V. Impact of source and manufacturing of collagen matrices on fibroblast cell growth and platelet aggregation. Materials 2017, 10, 1086. [Google Scholar] [CrossRef]
- Shen, L.R.; Bu, H.H.; Yang, H.; Liu, W.T.; Li, G.Y. Investigation on the behavior of collagen self-assembly in vitro via adding sodium silicate. Int. J. Biol. Macromol. 2018, 115, 635–642. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.I.; Park, C.H.; Kim, C.S. Design of a modified electrospinning for the in-situ fabrication of 3D cotton-like collagen fiber bundle mimetic scaffold. Mater. Lett. 2019, 236, 521–525. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Aladin, D.M.K.; Cheung, K.M.C.; Ngan, A.H.W.; Chan, D.; Leung, V.Y.L.; Lim, C.T. Nanostructure of collagen fibrils in human nucleus pulposus and its correlation with macroscale tissue mechanics. J. Orthop. Res. 2010, 28, 497–502. [Google Scholar] [CrossRef]
- Ju, H.Y.; Wu, N.; Dan, W.H.; Dan, N.H. Network structure and thermotropic property of type I collagen fibrils. J. Soc. Leath. Tech. Chem. 2012, 96, 234–238. [Google Scholar]
- Pallela, R.; Bojja, S.; Janapala, V.R. Biochemical and biophysical characterization of collagens of marine sponge, Ircinia fusca (Porifera: Demospongiae: Irciniidae). Int. J. Biol. Macromol. 2011, 49, 85–92. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Meng, D.; Tanaka, H.; Kobayashi, T.; Hatayama, H.; Zhang, X.; Ura, K.; Yunoki, S.; Takagi, Y. The effect of alkaline pretreatment on the biochemical characteristics and fibril-forming abilities of types I and II collagen extracted from bester sturgeon by-products. Int. J. Biol. Macromol. 2019, 131, 572–580. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, B.; Shiau, C.; Chen, H. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish. Food Hydrocoll. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Szleifer, I.; Dhaher, Y. Mechanical properties of a collagen fibril under simulated degradation. J. Mech. Behav. Biomed. 2017, 75, 549–557. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Kishimura, H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). Food Sci. Technol. 2015, 63, 71–76. [Google Scholar] [CrossRef]
- Skierka, E.; Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chem. 2007, 105, 1302–1306. [Google Scholar] [CrossRef]
- Sinthusamrana, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef]
- Vallejos, N.; González, G.; Troncoso, E.; Zúñiga, R.N. Acid and Enzyme-Aided Collagen Extraction from the Byssus of Chilean Mussels (Mytilus Chilensis): Effect of Process Parameters on Extraction Performance. Food Biophys. 2014, 9, 322–331. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature 1970, 277, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; Mello, R.O.; Demiate, I.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manage 2020, 10, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.C.; Qiao, Y.Y.; Tian, Y.Y.; Liu, J.H.; Qin, S.; Wu, W.H. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101–115115. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohyd. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef]
- Kandamchira, A.; Selvam, S.; Marimuthu, N.; Kalarical, J.S.; Fathima, N.N. Influence of functionalized nanoparticles on conformational stability of type I collagen for possible biomedical applications. Mater. Sci. Eng. C 2013, 33, 4985–4998. [Google Scholar] [CrossRef]
- Andrews, M.E.; Murali, J.; Muralidharan, C.; Madhulata, W.; Jayakumar, R. Interaction of collagen with corilagin. Colloid Polym. Sci. 2003, 281, 766–770. [Google Scholar] [CrossRef]
- He, L.; Mu, C.; Shi, J.; Shi, B.; Zhang, Q.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kunii, S.; Morimoto, K.; Nagai, K.; Saito, T.; Sato, K.; Tonomura, B. Actinidain-hydrolyzed type I collagen reveals a crucial amino acid sequence in fibril formation. J. Biol. Chem. 2011, 285, 17465–17470. [Google Scholar] [CrossRef] [PubMed]
- Plepis, A.M.G.; Goissis, G.; Das-Gupta, D.K. Dielectric and pyroelectric characterization of anionic and native collagen. Polym. Eng. Sci. 1996, 36, 2932–2938. [Google Scholar] [CrossRef]
- Yang, L.; Fitié, C.F.C.; Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.L. Electro-spinning of pure collagen nano-fibres-Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Bharathy, H.; Fathima, N.N. Exploiting oleuropein for inhibiting collagen fibril formation. Int. J. Biol. Macromol. 2017, 101, 179–186. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H. Graphene oxide—A tool for the preparation of chemically crosslinking free alginate-chitosan-collagen scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef]
- Yousefi, M.; Ariffin, F.; Huda, N. An alternative source of type I collagen based on by-product with higher thermal stability. Food Hydrocoll. 2017, 63, 372–382. [Google Scholar] [CrossRef]
- Schmitt, F.O.; Gross, J.; Highberger, J.H. Tropocollagen and the properties of fibrous collagen. Exp. Cell. Res. 1955, 3, 326–334. [Google Scholar]
- Stinson, R.H.; Sweeny, P.R. Skin collagen has an unusual d-spacing. Biochim. Biophys. Acta 1980, 621, 158–161. [Google Scholar] [CrossRef]

| Amino Acid | A-CF | P-CF | COL |
|---|---|---|---|
| Threonine | 16 | 17 | 16 |
| Serine | 29 | 27 | 30 |
| Glutamic acid | 69 | 69 | 65 |
| Glycine | 331 | 332 | 330 |
| Alanine | 118 | 121 | 119 |
| Valine | 18 | 20 | 21 |
| Methionine | 8 | 6 | 6 |
| Proline | 135 | 137 | 136 |
| Leucine | 24 | 22 | 25 |
| Tyrosine | 6 | 7 | 4 |
| Lysine | 23 | 24 | 28 |
| Histidine | 5 | 6 | 5 |
| Arginine | 51 | 50 | 52 |
| Hydroxyproline | 96 | 91 | 87 |
| Phenylalanine | 11 | 10 | 11 |
| Hydroxylysine | 7 | 9 | 7 |
| Isoleucine | 10 | 11 | 12 |
| Aspartic acid | 43 | 41 | 46 |
| Imino acids * | 231 | 228 | 223 |
| Region | Wavenumber (cm−1) | Characteristic | |
|---|---|---|---|
| A-CF | P-CF | ||
| Amide A | 3323.76 | 3324.57 | N–H stretch, coupled with hydrogen bonding |
| Amide B | 2957.13 | 2934.59 | C–N stretch |
| 2878.45 | 2893.11 | CH2 symmetric and asymmetric stretch | |
| Amide I | 1653.73 | 1639.43 | C=O stretch, coupled with hydrogen bonding |
| Amide II | 1551.42 | 1546.91 | N-H bend coupled with C-N stretch |
| 1453.18 | 1447.80 | CH2 bend | |
| 1402.76 | 1398.45 | COO− symmetric stretch | |
| Amide III | 1239.17 | 1254.38 | CH2 wagging of proline |
| 1082.52 | 1036.77 | C–O stretch/C–N–C stretch | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.; Liu, X.; Zhang, G.; Liu, D.; Yang, Y. Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction. Materials 2020, 13, 358. https://doi.org/10.3390/ma13020358
Ju H, Liu X, Zhang G, Liu D, Yang Y. Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction. Materials. 2020; 13(2):358. https://doi.org/10.3390/ma13020358
Chicago/Turabian StyleJu, Haiyan, Xiuying Liu, Gang Zhang, Dezheng Liu, and Yongsheng Yang. 2020. "Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction" Materials 13, no. 2: 358. https://doi.org/10.3390/ma13020358
APA StyleJu, H., Liu, X., Zhang, G., Liu, D., & Yang, Y. (2020). Comparison of the Structural Characteristics of Native Collagen Fibrils Derived from Bovine Tendons Using Two Different Methods: Modified Acid-Solubilized and Pepsin-Aided Extraction. Materials, 13(2), 358. https://doi.org/10.3390/ma13020358





